Abstract
Marsupial frogs have a unique reproductive mode in which females carry eggs enclosed in a sealed dorsal brood pouch. While most anurans are considered to be oviparous with lecithotrophic eggs, the extensively vascularized membrane of the brood pouch in marsupial frogs suggests potential opportunities for nutrient transfer. We tested for matrotrophy in the live-bearing Gastrotheca excubitor (Hemiphractidae), through feeding insects labelled with a 13C-fatty acid and a 15N-amino acid to brooding marsupial frogs. We observed significant increases of δ13C and δ15N in both maternal pouch tissues and embryos, suggesting nutrient transfer. Embryo dry mass also increased with developmental stage, providing further direct evidence for matrotrophy. These results suggest that in addition to gas exchange, the vascularized brood pouch membrane of G. excubitor also enables maternal nutrient transfer. This finding revealed a suspected but untested trait in the evolution of parental care in marsupial frogs, in contrast to previous work on Gastrotheca species that release tadpoles, and suggests greater complexity in reproductive and provisioning modes than previously thought.
Keywords: matrotrophy, viviparity, amphibians, reproductive mode, tetrapod evolution, stable isotopes
1. Introduction
Anurans exhibit a great diversity of reproductive modes [1], ranging from typical aquatic eggs with free-feeding tadpoles, to terrestrial eggs with direct-developing larvae, as well as live-bearing where embryos or larvae are carried internally within pouches or oviducts of parents [2,3]. A consequence of brooding embryos enclosed within the body is that parents must provide respiratory gas exchange, often via a close connection between highly vascularized parental surfaces and egg membranes or gill structures of larvae [2,4,5]. While this close connection can provide opportunities for maternal nutrient transfer (i.e. matrotrophy), the vast majority of frogs, including live-bearing species, are thought to be lecithotrophic, by which mothers provide all nutrients to larvae via yolk [2].
Across vertebrates, embryos or larvae can obtain nutrients by absorption through skin or gills, by ingesting maternal tissues from oviductal epithelia or mucosa, by cannibalizing eggs or siblings, or via placental structures [6,7]. Despite the strong potential for maternal nutrient transfer in egg brooding frogs, there have been few direct tests of matrotrophy beyond two early studies of an oviduct and a pouch brooding frog [2,4,8]. Direct evidence of matrotrophy includes shifts among eggs, embryos and hatchlings in chemical composition, increasing embryonic dry mass, or transfer of isotopically labelled molecules or nutrients [7,9–11].
In this study, we tested the hypothesis that matrotrophy occurs in live-bearing Gastrotheca excubitor (Hemiphractidae; figure 1), a marsupial frog species that gives birth to froglets from fully enclosed pouches. We directly tested for maternal nutrient transfer in G. excubitor through feeding insects enriched in carbon (13C) and nitrogen (15N) isotopes to brooding mothers, which is an effective method for tracking nutrient allocation [10,12]. We also tested for matrotrophy by assessing if dry mass increased from eggs through developing embryos. With these data coupled with a comparative exploration of Hemiphractidae, we tested the hypothesis that pouch structure and embryonic retention influence the evolution of matrotrophy among marsupial frogs.
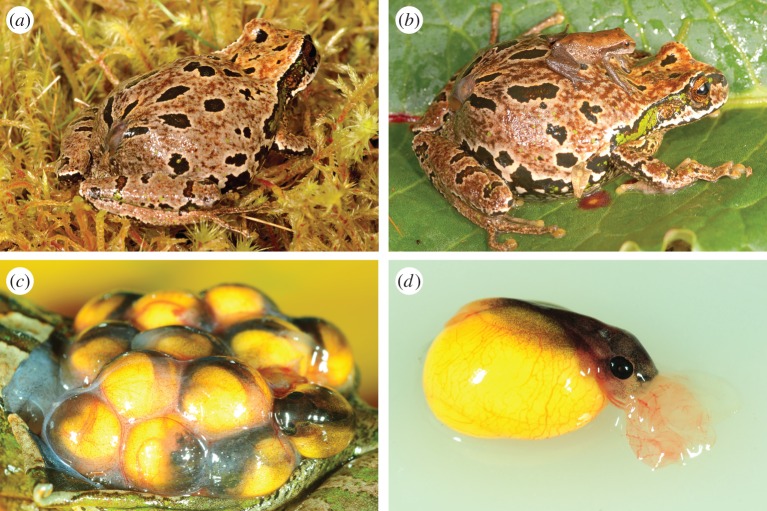
Figure 1.
(a) Marsupial frogs (Gastrotheca excubitor) brood their direct-developing larvae within a fully enclosed pouch, from which (b) froglets emerge. (c) Embryos are surrounded by a highly vascularized membrane within the brood pouch, which along with (d) external gills enables gas exchange and nutrient transfer throughout development. Photographs by A. Catenazzi.
2. Material and methods
(a). Study species and experimental design
We captured brooding G. excubitor frogs near Manu National Park, Peru [13] from 1 to 23 June 2014. We transported frogs to Wayqecha Biological Station within 4 h of capture, weighed them and transferred them to individual 1.2 l plastic containers with ventilated lids, wet paper towels and fresh leaves. We monitored the frogs daily, noting general condition and feeding. The containers were cleaned weekly and the frogs weighed. The frogs were maintained for 26–48 days until the end of the experiment on 19 July 2014.
We performed a feeding experiment among frogs randomly assigned to two groups. Frogs in the treatment (n = 5) group were fed one isotopically enriched prey per day, whereas frogs in the control group (n = 3) were fed the same amount of non-enriched prey (electronic supplementary material, table S1). We regularly sampled the feeder insects for isotope analysis. At the end of the feeding experiment, the frogs were euthanized (20% bencozaine gel applied to the pelvic patch) and dissected for embryos and maternal tissues. During dissections, all scissors and forceps were rinsed with ethanol and flame sterilized between removal of each tissue to prevent cross-contamination. After counting, measuring and staging the embryos [14], all tissues were rinsed with ethanol and then sealed in individual Whirl-Pak bags with 95% ethanol until preparation for isotope analysis.
(b). Stable isotope treatment and analysis
Treated frogs were fed insects coated with δ13C-palmitic acid (1-13C, 99%; Cambridge Isotope Labs, Inc.) dissolved in ethanol, and δ15N-leucine (15N, 98%) dissolved in water. Treated insects were isotopically enriched with δ15N (33.2 ± 7‰ Air (see below)) and δ13C (−6.8 ± 5.1‰ VPDB (see below)) compared with non-treated prey (δ15N: 4 ± 0.9‰; δ13C: −25.3 ± 0.8‰) (log δ15N: F1,23 = 22.2, p < 0.001; δ13C: F1,25 = 11.8, p = 0.002; electronic supplementary material, table S1). Dissected tissues, embryos and whole insects were freeze dried and homogenized, and 0.25 mg powdered samples were placed into tin capsules for stable isotope analysis [12]. We report all isotope values in delta notation (δX = [Rsample/Rstandard − 1] × 1000) in parts per thousand relative to the international standards (Vienna Pee Dee Belemnite (VPDB) for δ13C and Air for δ15N). Measurements were conducted on an elemental analyser and continuous flow isotope ratio mass spectrometer at Southern Illinois University Carbondale. The analytical precision of these analyses was±0.08‰ for δ13C and ±0.08‰ δ15N.
(c). Statistical analysis
To test for the effect of isotope labelling on δ13C and δ15N, we used linear models for insects and frogs, and mixed models for embryos. Both models included treatment as a fixed effect, and mixed models included embryo developmental stage as a covariate (frog identity was used as a random factor to account for non-independence of unfertilized eggs and embryos from the same female). Similarly, a mixed model was used to test for an association between embryo dry mass and developmental stage with frog identity as a random factor. To test whether developmental stage was associated with δ13C or δ15N in the embryos, we excluded control animals, and fitted linear and quadratic functions to data from treated animals. An F-test was then used to compare the fit of these linear and quadratic functions. δ13C data were squared and δ15N values were log-transformed to meet assumptions of normality. Data for this study are available via Dryad (doi:10.5061/dryad.g89s5) [15].
3. Results and discussion
We found that live-bearing females of G. excubitor provide maternally derived nutrients to their embryos, as demonstrated by two lines of evidence. First, embryos exhibited higher δ15N and δ13C in parallel with isotope enrichment of maternal pouch membranes and tissues in mothers fed isotopically labelled prey, when compared with control frogs and embryos. Maternal pouch membranes surrounding developing embryos were significantly enriched in the treated frogs (figure 2a) compared with control frogs for both δ15N (F1,5 = 9.1, p = 0.04) and δ13C (F1,5 = 61.1, p = 0.001). Embryonic δ15N values were associated with maternal isotope labelling (F1,7 = 7.0, p = 0.02), along with developmental stage (F1,5.1 = 7.7, p = 0.04). Embryo δ13C patterns only exhibited a trend with isotope treatment (F1,5.1 = 5.5, p = 0.07). The second line of evidence supporting matrotrophy in G. excubitor was increased embryo dry mass with developmental stage (figure 2b; slope = 0.76 ± 0.05), ranging from eggs to near-metamorphic larvae (F1,1.2 = 203.9, p = 0.03).
Figure 2.
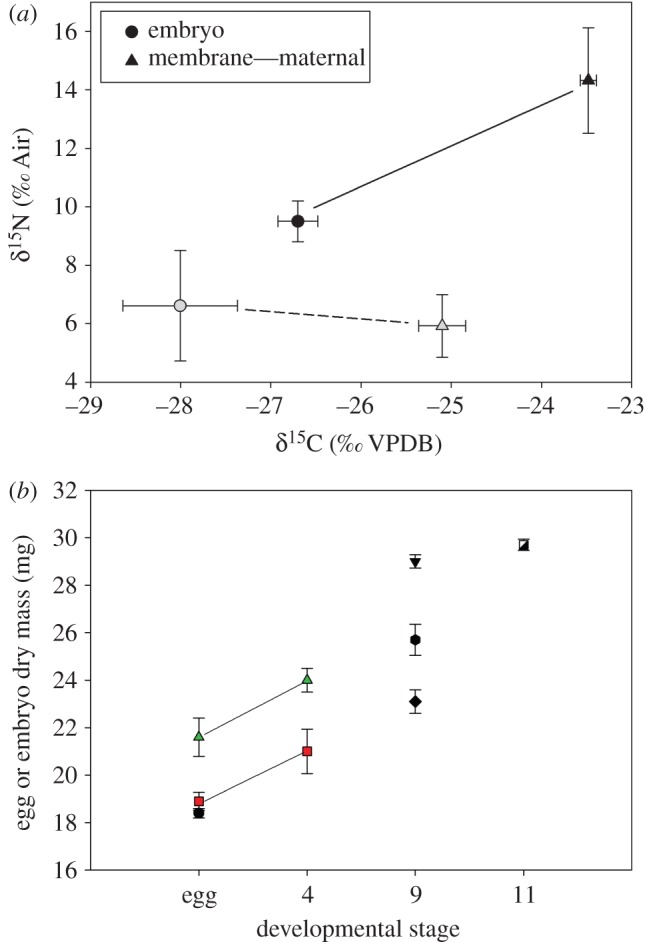
(a) Mean (±s.e.) δ15N and δ13C stable isotope values for the maternal membrane (triangles) and embryos (circles) of control (grey symbols) and labelled (black symbols) brooding marsupial frogs fed insects for six weeks; lines connect treatments. (b) Mean dry mass of embryos (±s.e.) increased with developmental stage. Each type of symbol represents embryos from an individual female (n = 7); each line connects unfertilized eggs and embryos from the same female. (Online version in colour.)
The differences in embryonic δ15N and δ13C enrichment combined with mass increases across ontogeny suggest that G. excubitor embryos absorb specific macronutrients to build additional body mass. Specifically, embryonic δ15N increased with developmental stage in a nonlinear fashion among embryos of treated frogs (electronic supplementary material, figure S1a; r2 = 0.78, d.f. = 5, p = 0.02), best described by a quadratic function (p = 0.04). By contrast, δ13C was not associated with developmental stage (electronic supplementary material, figure S1b; p > 0.56). Greater absorption of nitrogen relative to carbon nutrients parallels higher transfer of paternally derived amino acids in pouch brooding pipefish [10] and vocal sac brooding Rhinoderma darwinii [11]. In addition, the nonlinear δ15N transfer to embryonic G. excubitor suggests amino acid demand increases with development in rapidly growing embryos and then declines near metamorphosis [16–18].
Among amphibians, known forms of parental nutrient transfer beyond egg yolk include uterine epithelial nutrient secretions and larval consumption of trophic eggs or siblings [2,19,20]. In our study, G. excubitor embryos apparently used their large, fused external gills to absorb dissolved nutrients that crossed egg membranes from the pouch lumen. Matrotrophy has been suspected in marsupial frogs owing to the highly vascularized external gills and pouch membrane [2,21], but previous studies found no evidence of embryonic mass change during development in Gastrotheca riobambae [4]. Thus, our study is the first, we believe, to directly demonstrate maternal nutrient transfer in a marsupial frog.
Brood pouch structure and the degree of embryonic retention probably determine matrotrophy. Pouch structure in hemiphractid frogs ranges from simple dorsal skin patches to partially and fully enclosed pouches [5]. Frogs with fully enclosed pouches could be predisposed to transfer nutrients, because they must provide gas exchange for embryos. However, the degree of embryo retention may be a secondary determinant for matrotrophy because, while G. riobambae has a fully enclosed pouch, their embryos are released as free-feeding tadpoles [5]. By contrast, live-bearing frogs such as G. excubitor that give birth to froglets may be predisposed to matrotrophy, because of maternal and embryonic modifications necessary for sustained brooding. This hypothesis that pouch structure and embryonic retention influence the occurrence of matrotrophy in marsupial frogs is supported by comparison with other vertebrates [8,10].
Marsupial frogs are of great interest to developmental and evolutionary biologists because of the potential reversal in reproductive modes from live-bearing to tadpole development [22]. Our findings show that ancestral features for gas exchange for developing embryos may be co-opted via convergent evolution for nutrient transfer [7], and suggest that greater exploration of the diverse reproductive modes among marsupial frogs could provide insight into the evolution of matrotrophy in vertebrates.
Supplementary Material
Acknowledgements
We thank the Peruvian Ministry of Agriculture for granting permits, D. Burkart for field assistance, S. McQueen for laboratory assistance and D. Wake for comments on a draft of the manuscript.
Ethics
All institutional and national guidelines for the care and humane use of animals were followed and approved by the SIU IACUC (no. 14-009).
Data accessibility
Data for this study are available via Dryad (http://dx.doi.org/10.5061/dryad.g89s5) [15].
Authors' contributions
R.W.W. and A.C. conceived and co-designed the study, carried out the laboratory work, carried out the statistical analyses and drafted the manuscript. Both authors gave final approval for publication, and ensure the accuracy and integrity of the work.
Competing interests
The authors have no competing interests.
Funding
This work was funded by SIUC new faculty startup grants to R.W.W. and A.C. and Disney Worldwide Conservation Fund to A.C. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Gomez-Mestre I, Pyron RA, Wiens JJ. 2012. Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 66, 3687–3700. ( 10.1111/j.1558-5646.2012.01715.x) [DOI] [PubMed] [Google Scholar]
- 2.Wake MH. 2015. Fetal adaptations for viviparity in amphibians. J. Morphol. 276, 941–960. ( 10.1002/jmor.20271) [DOI] [PubMed] [Google Scholar]
- 3.Wiens JJ, Kuczynski CA, Duellman WE, Reeder TW. 2007. Loss and re-evolution of complex life cycles in marsupial frogs: does ancestral trait reconstruction mislead? Evolution 61, 1886–1899. ( 10.1111/j.1558-5646.2007.00159.x) [DOI] [PubMed] [Google Scholar]
- 4.del Pino E, Galarza ML, de Albuja CM, Humphries A. 1975. The maternal pouch and development in the marsupial frog Gastrotheca riobambae (Fowler). Biol. Bull. Mar. Biol. Lab. Woods Hole 149, 480–491. ( 10.2307/1540381) [DOI] [PubMed] [Google Scholar]
- 5.Duellman WE. 2015. Marsupial frogs: gastrotheca and allied genera. Baltimore, MD: JHU Press. [Google Scholar]
- 6.Crump ML. 2015. Anuran reproductive modes: evolving perspectives. J. Herpetol. 49, 1–16. ( 10.1670/14-097) [DOI] [Google Scholar]
- 7.Blackburn DG. 2015. Evolution of vertebrate viviparity and specializations for fetal nutrition: a quantitative and qualitative analysis. J. Morphol. 276, 961–990. ( 10.1002/jmor.20272) [DOI] [PubMed] [Google Scholar]
- 8.Xavier F. 1977. An exceptional reproductive strategy in Anura: Nectophrynoides occidentalis, an example of adaptation to terrestrial life by viviparity. In Major patterns in vertebrate evolution (ed. M Hecht), pp. 545–552. Berlin, Germany: Springer. [Google Scholar]
- 9.Marsh-Matthews E, Skierkowski P, DeMarais A, Gatten J, RE. 2001. Direct evidence for mother-to-embryo transfer of nutrients in the livebearing fish Gambusia geiseri. Copeia 2001, 1–6. ( 10.1643/0045-8511(2001)001[0001:DEFMTE]2.0.CO;2) [DOI] [Google Scholar]
- 10.Ripley JL, Foran CM. 2009. Direct evidence for embryonic uptake of paternally-derived nutrients in two pipefishes. J. Comp. Physiol. B 179, 325–333. ( 10.1007/s00360-008-0316-2) [DOI] [PubMed] [Google Scholar]
- 11.Goicoechea O, Garrido O, Jorquera B. 1986. Evidence for a trophic paternal-larval relationship in the frog Rhinoderma darwinii. J. Herpetol. 20, 168–178. ( 10.2307/1563941) [DOI] [Google Scholar]
- 12.Warne RW, Gilman CA, Garcia DA, Wolf BO. 2012. Capital breeding and allocation to life-history demands are highly plastic in lizards. Am. Nat. 180, 130–141. ( 10.1086/665995) [DOI] [PubMed] [Google Scholar]
- 13.Catenazzi A, Lehr E, von May R. 2013. The amphibians and reptiles of Manu National Park and its buffer zone, Amazon basin and eastern slopes of the Andes, Peru. Biota Neotropica 13, 269–283. ( 10.1590/S1676-06032013000400024) [DOI] [Google Scholar]
- 14.Townsend DS, Stewart MM. 1985. Direct development in Eleutherodactylus coqui: a staging table. Copeia 1985, 423–436. ( 10.2307/1444854) [DOI] [Google Scholar]
- 15.Warne RW, Catenazzi A. 2016. Data from: Pouch brooding marsupial frogs transfer nutrients to developing embryos. Dryad Digital Repository. ( 10.5061/dryad.g89s5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Files T, Lombardi J. 1993. Free amino acids in the uterine fluids of four species of viviparous sharks. Comp. Biochem. Physiol. B Comp. Biochem. 104, 583–588. ( 10.1016/0305-0491(93)90285-D) [DOI] [Google Scholar]
- 17.Korsgaard B. 1994. Proteins and amino acids in maternal–embryonic trophic relationships in viviparous teleost fishes. Isr. J. Zool. 40, 417–429. [Google Scholar]
- 18.Warne RW, Crespi EJ. 2015. Larval growth rate and sex determine resource allocation and stress responsiveness across life stages in juvenile frogs. J. Exp. Zool. A 323, 191–201. ( 10.1002/jez.1911) [DOI] [PubMed] [Google Scholar]
- 19.Gibson RC, Buley KR, Douglas M. 2004. Maternal care and obligatory oophagy in Leptodactylus fallax: a new reproductive mode in frogs. Copeia 2004, 128–135. ( 10.1643/CE-02-091R2) [DOI] [Google Scholar]
- 20.Kupfer A, Muller H, Antoniazzi MM, Jared C, Greven H, Nussbaum RA, Wilkinson M. 2006. Parental investment by skin feeding in a caecilian amphibian. Nature 440, 926–929. ( 10.1038/nature04403) [DOI] [PubMed] [Google Scholar]
- 21.Jones RE, Gerrard AM, Roth JJ. 1973. Estrogen and brood pouch formation in the marsupial frog, Gastrotheca riobambae. J. Exp. Zool. 184, 177–183. ( 10.1002/jez.1401840205) [DOI] [PubMed] [Google Scholar]
- 22.Castroviejo-Fisher S, et al. 2015. Phylogenetic systematics of egg-brooding frogs (Anura: Hemiphractidae) and the evolution of direct development. Zootaxa 4004, 1–75. ( 10.11646/zootaxa.4004.1.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are available via Dryad (http://dx.doi.org/10.5061/dryad.g89s5) [15].