Abstract
Photosynthesis is a mechanism developed by terrestrial life to utilize the energy from photons of solar origin for biological use. Subsurface regions are isolated from the photosphere, and consequently are incapable of utilizing this energy. This opens up the opportunity for life to evolve alternative mechanisms for harvesting available energy. Bacterium Candidatus Desulforudis audaxviator, found 2.8 km deep in a South African mine, harvests energy from radiolysis, induced by particles emitted from radioactive U, Th and K present in surrounding rock. Another radiation source in the subsurface environments is secondary particles generated by galactic cosmic rays (GCRs). Using Monte Carlo simulations, it is shown that it is a steady source of energy comparable to that produced by radioactive substances, and the possibility of a slow metabolizing life flourishing on it cannot be ruled out. Two mechanisms are proposed through which GCR-induced secondary particles can be utilized for biological use in subsurface environments: (i) GCRs injecting energy in the environment through particle-induced radiolysis and (ii) organic synthesis from GCR secondaries interacting with the medium. Laboratory experiments to test these hypotheses are also proposed. Implications of these mechanisms on finding life in the Solar System and elsewhere in the Universe are discussed.
Keywords: radiolysis, astrobiology, subsurface life
1. Introduction
Radiation in the form of photons is the primary way by which solar energy is transferred to living systems. The energy of a typical photon is between 2 and 3 eV (visible red, 1.8 eV and visible blue, 3.1 eV), and an abundant supply of such photons makes it convenient for life to utilize it for metabolic purposes. Even though most of the energy is lost in heat, the overall efficiency of 0.1–8% is sufficient to power metabolism [1]. Studies of ionizing radiation, on the other hand, have mostly been associated with health-effects on humans [2,3] and studies of radiation resistance on microbes [4,5]. By definition, ionizing radiation has the energy to ionize, or to eject at least one electron from a neutral atom or a molecule, which is 13.6 eV for a neutral hydrogen atom, for example. Ionizing radiation can interact with DNA and cause reparable or irreparable damage, depending on the type and energy of the radiation [6]. Such damages have the potential to modify the genetic code through mutations, alter the way DNA functions, and transfer mutations to the next generation(s) [7,8]. Radiation damage can also cause cancer as seen in a number of studies with ultraviolet (UV) and particle interactions with humans, primarily in context of oncological studies, nuclear accidents and astronaut health in outer space [9–12]. Nevertheless, a high dosage of ionizing radiation can force organisms to develop mechanisms that enable them to survive in extreme conditions [13].
Geochemical processes are well known to support subsurface life [14,15]. An important shift in our understanding came about when studies revealed that subsurface life can be independently supported by radiolysis, where the source of radiation is particles emitted from the decay of radioactive substances [16,17]. Radioactive materials present deep underground produce secondary particles such as alpha, beta and gamma radiation. Secondary particles interact with the environment and provide energy for chemical change. Organisms can use these particles indirectly for metabolic purposes. Candidatus Desulforudis audaxviator is such an example, which thrives in a radiolysis-powered ecosystem (figure 1a). Radiation from radioactive rock dissociates H2O into a number of radicals, useful for biological reactions. It is able to extract carbon from dissolved CO2 and nitrogen in the form of ammonia from the rock, and utilize them to synthesize amino acids. Such an organism can potentially thrive in subsurface environments on Mars, Moon, Europa or other planetary systems in the presence of radioactive substances.
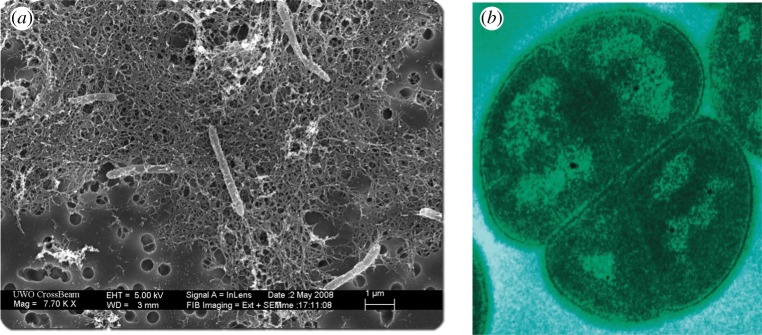
Figure 1.
(a) A colony of Ca. D. audaxviator, discovered in a 2.8 km deep gold mine near Johannesburg, South Africa. https://upload.wikimedia.org/wikipedia/commons/1/1e/Desulforudis_audaxviator.jpg (public domain), via Wikimedia Commons. (b) Transmission electron micrograph of D. radiodurans acquired in the laboratory of Michael Daly, Uniformed Services University, Bethesda, MD, USA. http://www.usuhs.mil/pat/deinococcus/index_20.htm (public domain), via Wikimedia Commons. (Online version in colour.)
Candidatus D. audaxviator thrives in a 2.8 km deep South African gold mine [18,19]. A comprehensive analysis on the availability of nuclear power for deep surface microbes has also been done [16]. Radiolytic dissociation of water due to radiogenic decay of U, Th and K in rock produces a number of radicals, and generates molecular hydrogen (H2), along with other biologically useful products. Radiolytically generated chemicals provide the necessary energy and nutrients to the system, sulfate 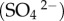 reduction is the dominant electron-accepting process and H2 and formate are primary electron donors. These provide the energy to sustain a minimal metabolism. A part of the energy is also utilized to repair damage caused by ionizing radiation. Studies have shown that H2 produced through geochemical processes can also be utilized for metabolism, independent of photosynthesis, using similar mechanisms [20].
reduction is the dominant electron-accepting process and H2 and formate are primary electron donors. These provide the energy to sustain a minimal metabolism. A part of the energy is also utilized to repair damage caused by ionizing radiation. Studies have shown that H2 produced through geochemical processes can also be utilized for metabolism, independent of photosynthesis, using similar mechanisms [20].
In the light of these findings, it is reasonable to propose that instead of or in addition to in situ energy sources, the radiation (potentially supporting radiolysis-based life) can be of cosmic origin. Galactic cosmic rays (GCRs) are charged particles, mostly protons, originating beyond the Solar System [21–23]. They have relatively lower flux but possess much higher energy than other radiation sources on Earth, and have noteworthy biological effects on terrestrial life and possibly on extrasolar planets [24–26]. Upon interaction with a planetary atmosphere or surface, GCRs produce a cascade of secondary particles that include electrons, positrons, gamma rays, neutrons and muons [21]. Muons can travel several kilometres deep below the surface depending on their energy [22,23]. These secondary particles can induce radiolysis and as described later, possibly power a subsurface ecosystem.
2. Proposed mechanisms
2.1. Galactic cosmic ray-induced radiolysis
As discussed in the previous section, radioactive rocks provide an in situ production source of energy for subsurface life. A similar mechanism is proposed in this section. However, the energy source considered here is extraterrestrial. For planets with substantial atmospheres, the primary GCR particles strike the atmosphere and produce a cascade of secondary particles, also referred to as an air shower [21]. If a planet lacks an atmosphere, particles are able to directly strike the surface and the cascade of secondary particles is able to propagate underground [27]. The secondary particles produced in the cascade, such as pions and kaons, are highly unstable, and quickly decay to other particles including beta particles (electrons and positrons) and gamma rays. It must be noted that beta particles and gamma rays are also produced during radioactive decay in underground rock. When charged pions decay muons are produced, and these can travel several kilometres deep depending on their energy. It should be emphasized that all types of charged particles such as electrons and positrons can contribute to radiolysis in regions near the surface, but muons become important at greater depths where the flux of other particles becomes nearly zero. They only undergo electromagnetic interactions and lose 2–8 MeV energy per gram per square centimetre in the material they traverse [28]. The energy loss is a combination of ionization, bremsstrahlung, pair production and inelastic nuclear scattering [29].
Energetic particles are capable of destroying organic materials present in the surface or subsurface environment of planets with thin atmospheres. A number of studies have been devoted to studying the impact of GCRs on the possible destruction of organic matter on Mars [30–32]. Mars' surface radiation dose is several orders of magnitude higher than that of the Earth due to its thin atmosphere. Exposure to high environmental radiation dose also leads to substantial energy expenditure in repairing radiation-induced damage. Bacterium Deinococcus radiodurans is the focus of numerous studies and is the most radioresistant microbe with a lethal dose (LD10) at 15 kGy (figure 1b). Deinococcus radiodurans has a specialized recombination system for DNA repair from ionizing radiation. Interestingly, many fungal species also have very high radiation resistance. Many fungi have 10% survival chance or LD10 values exceeding 5 kGy. Other species, such as Ustilago maydis are also known to have extreme radiation resistance but have a different mechanism to cope with compared with radiation D. radiodurans. For comparison, mammals have very low radiation resistance and a dose between 5 and 10 Gy is lethal. It has been found that the action of proteins is primarily responsible for the repair mechanism [33]. An abundance of highly melanized fungal spores in the early Cretaceous period deposits has been uncovered, where other plant and animal species have died out [34]. These types of fungi can be found in high-altitude terrains, Arctic and Antarctic regions, and in the Evolution Canyon in Israel.
Radiotropism is a term used to describe the growth of fungi from exposure to radionuclides [35]. Most radionuclides emit beta (electrons and positrons) and gamma (photons) radiation and studies have shown that exposure to both sources promoted directional growth of fungi [35]. Exposure to 121Sn and 137Cs sources showed that the spore germination in species increased considerably, a phenomenon also known as ‘radiostimulation’ [35]. The presence of several microorganisms has been studied in the Russian Mir Space Station as well as the International Space Station [36]. The organisms consist of both bacteria and fungi, and are exposed to about 4 cGy of ionizing radiation per year. Many bacteria and fungi in these environments have been found to be pigmented or melanized [37]. Dadachova et al. concluded that ionizing radiation changed the electron spin resonance signal of melanin, making it more efficient in acting as an ionizing radiation transducer [38]. Experiments have shown increased metabolic activity in melanized Cryptococcus neoformans relative to non-melanized ones, indicating the enhancement of the electron transfer properties of melanin. The authors concluded that melanin played a major role in protecting the organism against ionizing radiation and these radioprotective properties arise due to its chemical composition, free radical quenching and spherical spatial arrangement [39].
An abundance of highly melanized fungal spores in early Cretaceous period deposits has been uncovered, where other plant and animal species have died out [34]. These types of fungi can be found in high-altitude terrains, Arctic and Antarctic regions, and in the Evolution Canyon in Israel. The south-facing slope of the canyon receives 2–8 times higher solar radiation than those on the north, and Aspergillus niger found there contains 300% higher levels of melanin than that found in the north facing slope [40]. Several groups have studied these effects on melanized fungal species at the Chernobyl accident site [41] and in nuclear reactor pool water [42].
The discussion above shows that organisms can develop mechanisms to thrive and to repair damage under the exposure of high radiation dose and, in one case, harvest it for metabolic purposes, but it remains to be shown that the ionizing energy produced by secondary particles is comparable to energies observed in radioactive decay processes known to support radiolysis-based life.
The GEANT4 package was used to model the energy deposition rate in subsurface environments. It is a widely used package and is considered a gold standard in modelling particle interactions. The code models all particle interactions as particles traverse through a medium and has been well tested in the planetary science community. As the particles traverse in the subsurface medium, they lose energy by ionizing it. At greater depths, muons become important because of their long range, as discussed earlier. Muons transfer their large kinetic energy to the medium, and also form muonium (μ+e−), which has been found useful for various chemical and biological reactions due to its similarities with the hydrogen atom [43]. It should be noted that below 15 km, the energy deposition rate is constant due to contribution from neutrinos.
Three cases were considered here: a planetary object with no atmosphere so the entire GCR flux deposits energy in the ground; the Earth where most of the radiation is blocked by the atmosphere, and Mars. For each case, the energy deposition in pure ice with 1 g cm−3 density and in standard rock with the density of 2.65 g cm−3 was calculated. The flux of GCRs was taken from Dorman [22] and was incident directly on the planet's surface in the first case and on the planet's atmosphere in subsequent cases. The surface in case 1 is pure ice with 1 g cm−3 density and in case 2 is standard rock with density of 2.65 g cm−3. It must be noted that both pure ice and water give the same results in these simulations. In total, 109 particles were used for simulations and the energy deposition profile was obtained in each case. It must be emphasized that energy deposition calculations depend primarily on the column density of the material through which particles traverse and other factors such as temperature, pressure and atmospheric composition are not important. Figures 2 and 3 show the subsurface energy deposition rates for different cases. In figure 2, the energy deposition rate is approximately 107 eV g−1 s−1 close to the surface and drops below 105 eV g−1 s−1 in the 10 m depth range. The deposition rate falls sharply in the case of rock due to higher density compared to ice. Because the energy deposition rate is about three orders of magnitude lower in the case of the Earth, it has been displayed separately in figure 3. The atmosphere absorbs most of the radiation; however, because of the long range of muons, a small amount of radiation reaches a depth of a few kilometres.
Figure 2.
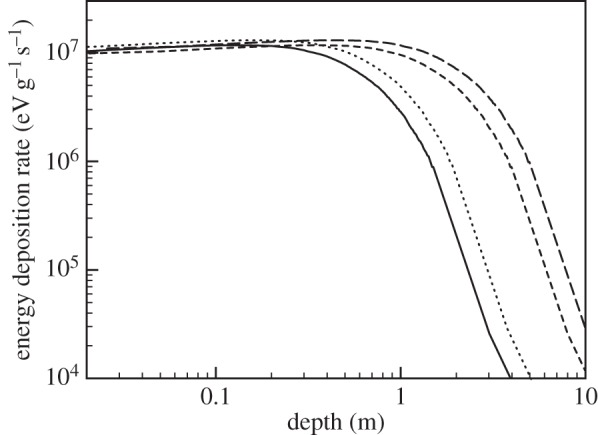
Simulation results of the subsurface energy deposition rate in different cases. Vertical axis is the energy deposition rate in units of eV g−1 s−1 and the horizontal axis is the depth in metres. Long dashes represent energy deposition in ice of a planet with no atmosphere (Europa/Enceladus), short dashes represent deposition for a planet with no atmosphere in rock with density 2.65 g cm−3 (Pluto), dots represent deposition in ice for Mars and solid line is for deposition in rock on Mars.
Figure 3.
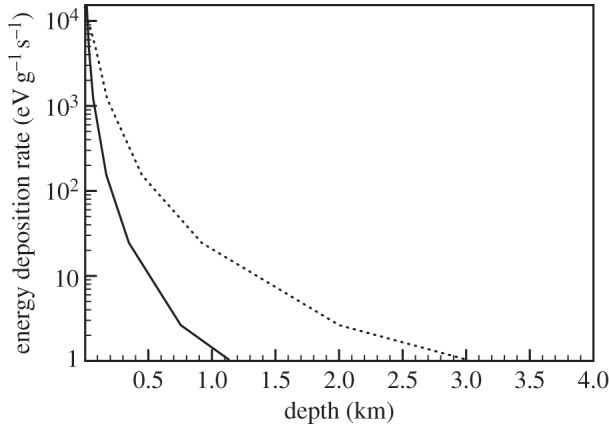
Subsurface energy deposition rate as a function of depth in rock of density 2.65 g cm−3 (solid) and water/ice (dots). Vertical axis has energy deposition rate in eV g−1 s−1 and horizontal axis has depth in kilometres.
Let us now compare these results with the energy environment of Ca. D. audaxviator. The radiolytic model calculations by Lin et al. [16] yielded the net dosage range of alpha particles between 4.25 × 105 and 8.52 × 105 eV g−1 s−1, beta particles between 6.58 × 104 and 4.27 × 105 eV g−1 s−1, and for gamma rays between 4.00 × 104 and 2.25 × 105 eV g−1 s−1 [16]. This energy is used to split water molecules into H+ and OH− forming H2O2. H2O2 in turn reacts with the surrounding medium to form sulfate, 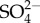 . It uses sulfate instead of O2 and obtains nitrogen from surrounding ammonia [18,19]. As one can see, the energy deposition rate is about an order of magnitude higher close to the surface in case 1 than that available to Ca. D. audaxviator.
. It uses sulfate instead of O2 and obtains nitrogen from surrounding ammonia [18,19]. As one can see, the energy deposition rate is about an order of magnitude higher close to the surface in case 1 than that available to Ca. D. audaxviator.
Life could evolve a variety of mechanisms to utilize such a large range of energy injected underground. It could produce molecular hydrogen and oxidants useful for life. Muons can both directly react with molecules present in the medium, and also indirectly through radiolysis products [44]. A detailed description of all the chemical reactions including the intermediate steps can be found elsewhere [45]. Let us now use these radiation doses to calculate familiar biologically useful processes such as the production of adenosine triphosphate (ATP). The terminal phosphate bond in ATP requires 0.304 eV per molecule [46]. As in the case of Ca. D. audaxviator, the total energy produced by radioactive rocks would be potentially divided between radiolysis-powered metabolism, radiation damage and damage repair, only a fraction of the total energy will be utilized for radiolysis. Based on our simulation results the energy availability shown in figure 2 approximately ranges between 107 and 104 eV g−1 s−1, which could potentially produce ATP molecules, and the upper limit of production is approximately 3 × 107 ATP molecules g−1 s−1 based on the 0.304 eV per molecule conversion factor and assuming 100% efficiency.
It must be mentioned that life, as we know it, requires water, which is a neutral fluid and fits perfectly with temperature variations on Earth. However, other fluids might offer similar functionality in terms of being stable; they may provide transportation of essential nutrients and remain liquid for temperature ranges for that particular planet. Underground water or other fluid sources, in combination with flux of secondary particles can provide a stable self-sustained environment for life to exist. Low energy availability can produce organisms with a very slow metabolism. There is a possibility of an ecosystem thriving on this energy source based on other biochemical bases, and might necessitate alternative approaches to detect life ‘as we don't know it’ [46,47] in subsurface environments on Earth and elsewhere.
A laboratory experiment to test this hypothesis could also be performed. It would involve gradually changing the radiation environment of Ca. D. audaxviator, by using different particle radiation beams, keeping the same chemical environment and observing its growth over a period of time. If the organism is able to adapt to gradually changing radiation environment, it can be eventually exposed to GCR secondaries proving an ultimate test for the hypothesis.
2.2. Galactic cosmic ray-induced synthesis of organics and other biologically useful products
Even though Ca. D. audaxviator is able to maintain an ecosystem independent of photosynthesis, it uses photosynthetic products. Based on experimental results and theoretical models, some authors have proposed that high-energy particles could produce the amino acid glycine on extraterrestrial ices [48]. In this section, it is proposed that biologically useful products, using a similar mechanism, can be produced in subsurface environments by GCR-induced secondaries. Charged particles directly interact with ice and produce a number of biologically useful secondary products [49–51]. Hudson & Moore irradiated different mixtures of water and CO (carbon monoxide) with 0.8 MeV protons at temperatures near 16 K in order to simulate interstellar conditions. The results of isotopic substitution and IR spectroscopy showed the formation of several hydrocarbons such as HCOOH, HCO−, H2CO and CH3OH [51]. Earlier experiments of Bernstein et al. were conducted with a larger temperature range (12–300 K), and in addition to the above-mentioned products, they discovered hexamethylenetetramine (C6H12N4), ethers, alcohols, compounds related to polyoxymethylene, ketones and amides in their samples [49]. Their subsequent experiments showed the formation of aroma-bearing ketones and carbolylic acid functional groups [52]. Other groups have also reported experimental evidence of the formation of amino acid precursors on exposure to high-energy particles [50,53]. Kobayashi et al. [50,53] irradiated several ice mixtures composed of methane, CO and ammonia with high-energy protons. The results of quadrupole mass spectrometry and ion exchange chromatography showed the formation of amino acids, such as glycine and alanine, and some hydrocarbons. Garrod & Herbst [54] conducted charged particle-induced photodissociation calculations to model chemical changes from interstellar radiation field and GCRs and reported the production of complex chemicals such as formic acid, methyl formate and dimethyl ether.
One common feature of these studies is that they all consider organic synthesis on the surface, which is true for high-energy photons such as UV, X-rays and low energy protons (approx. keV to MeV). However, for higher energy particles such as GCRs whose energies are approximately 10 GeV and beyond, the secondary particles penetrate below the surface. Particles such as electrons, positrons, neutrons and photons produced in interactions have very short ranges and are confined in a relatively small volume. Particles with the highest range are muons as shown in figure 2 and are the primary source of GCR-induced radiation in such environments. In order to validate this hypothesis, one can irradiate samples with muons produced in accelerator experiments. Muons can also be approximated with electrons in laboratory experiments to a certain extent because electrons also undergo electromagnetic interactions like muons; however, they lose energy very quickly, which makes this test possible only at low energies. Based on the studies cited above, an additional mechanism supporting subsurface life could be direct organic synthesis induced by GCR-induced secondary particles, especially muons at greater depths. There is experimental evidence of the formation of amino acid precursors on exposure to high-energy particles [50,53]. This mechanism could be especially important in the case of comets, as cosmic ray-induced ionization is believed to be the main driver of cometary organic chemistry [55]. Organic synthesis occurring at the polar regions of the Moon, Mercury and other silicate bodies has also been proposed [56]. There are studies of GCR-induced synthesis of organic molecules in Titan's atmosphere [57,58], production of oxidants on Europa [59] and the possibility of an aerial biosphere on Venus [60].
The production of O2, H2O2 and other oxidants on Europa's surface by charged particles accelerated in the Jovian magnetic field has been estimated in an earlier study [59]. They proposed that through impact gardening, these biologically useful chemicals could be transported to Europa's oceans. Ionization through 40K decay was also considered. Because GCRs are much more energetic compared with particles considered in the above study, in addition to producing these chemicals on Europa's surface, GCR secondaries can directly produce them in ice below the surface. Let us now calculate the energy deposited in the ice shell of Europa from GCRs. The average energy deposited from 0 to 1 m depth is approximately 1015 eV g−1 yr−1. For this 1 m shell for entire Europa, the total energy is approximately 1.6 × 1032 eV yr−1, which would produce 2.4 × 107 mol yr−1 of H2 and O2. This scenario is valid in other cases too where non-photosynthetic chemicals can be produced in subsurface environments using this mechanism.
3. Implications on the origin of life and possibility of finding life beyond Earth
It is believed that approximately 3.5–4 Gyr ago, when life originated on the Earth, the Sun was in a highly active phase. In this scenario, the Earth's surface was likely to be bombarded by a high flux of energetic solar particles and super coronal mass ejections. Enhanced flux of solar particles and variability in the GCR flux can also enhance the rate of lightning [61], and provide energy to the prebiotic soup to synthesize amino acids and other organic compounds forming the building blocks of life [62]. If the solar particles are sufficiently energetic (more than 10 GeV), they can produce secondary particles capable of penetrating underground and in water [63,64], providing a small source of energy away from direct exposure to high flux of harmful UV radiation on the surface. Solar particles typically reach energies of hundreds of MeV during violent eruptions and in some cases greater than 10 GeV [65]. Typical solar proton events (SPEs) produce a fluence of about 109 protons cm−2 on Earth. Because the Sun was considerably more active in the past, it is highly likely that such eruptions might have occurred on the Sun more frequently. The higher energy component of these SPEs [65], just like GCRs, is capable of penetrating underground [64], and would have increased the flux of secondary particles in subsurface environments. A combination of particle flux along with water and nutrients might provide ideal conditions for life to originate and evolve until conditions on the surface become optimal.
As independent/freely floating or ‘rogue’ planets are not tied to any stellar system, they do not receive a steady stream of photons from a parent star. A mechanism has been proposed which could support life on such planets with a combination of sufficient pressure and radioactive heat [66]. Alternatively, GCRs and radioactive materials can be a steady source of energy on such planets. The mechanisms proposed in this paper can be used to synthesize biologically useful chemicals and to power such ecosystems. Europa is believed to have an abundance of liquid water below its thick ice shell [67]. GCR-induced particles, although cannot provide energy in the ocean, as discussed earlier, they can provide ingredients and fuel to a potential ecosystem in its ice shell. Table 1 shows the energy availability from a number of sources on different planetary objects. Objects with no or negligible atmospheres have higher energy availability, about one order of magnitude higher than that available to Ca. D. audaxviator from radioactive rocks. There are no other such organisms found on Earth so far. One reason could be that due to a substantial atmosphere, most of the radiation is blocked and only a small amount is available in subsurface environments. As seen in table 1, the energy availability on Earth is three orders of magnitude smaller than that found on Mars and about two orders of magnitude smaller than used by Ca. D. audaxviator. As in the case of Ca. D. audaxviator, where the total energy produced by radioactive rocks is divided between radiolysis-powered metabolism, radiation damage and damage repair, it is possible that a fraction of this energy deposited by GCRs can be utilized for metabolic purposes.
Table 1.
Energy availability from GCR simulations and other sources. GCR results show the maximum energy deposited, which is near the surface and drops with depth. For radioactive rocks, the value is the average obtained from Lin et al. [16]. For hydrothermal vents, the energy availability is obtained from Schulze-Makuch & Irwin [46] and depends on temperature gradient. The Gibbs free energy of 145 reactions was calculated by Rogers & Amend [68] and ranged from 0 to 125 kJ mol−1 (1.3 eV) per electron transfer. The value in the table shows the maximum energy from a reaction.
| source | energy availability |
|---|---|
| GCR Mars subsurface | 1.2 × 107 eV g−1 s−1 |
| GCR Europa, Enceladus, Pluto, Moon subsurface | 1.3 × 107 eV g−1 s−1 |
| GCR Earth subsurface | 1.5 × 104 eV g−1 s−1 |
| radioactive rocks | 1.0 × 106 eV g−1 s−1 |
| hydrothermal vents | 2.5 × 106 eV/°C |
| geochemical | 1.3 eV/e− transfer |
There is growing evidence of pockets of near-surface water on Mars [69]. The presence of indigenous nitrogen in sedimentary and aeolian deposits using the SAM instrument on-board the Curiosity rover was reported recently [70]. Observations of hydrated salts, magnesium perchlorate, magnesium chlorate and sodium perchlorate on the Martian surface, were also recently announced [71]. Radiolysis-powered ecosystems can use these chemicals for metabolic processes. The possibility of methane, N2 and traces of O2 being by-products of such an ecosystem cannot be ruled out and could possibly explain the presence of methane [72,73] that cannot yet be explained by standard physics and chemistry models [74,75].
4. Conclusion
Studying the biological effects of ionizing radiation is a growing area of research. Much of the effort has been focused on examining its damaging effects on human health in context of radiation oncology, nuclear accidents and astronaut health in outer space. Several experiments have shown ionizing radiation to synthesize organic compounds on interaction with ice mixtures. The discovery of Ca. D. audaxviator thriving 2.8 km below the Earth's surface powered by radiolysis opens up new possibilities of biological interaction with (ionizing) particle radiation. GCRs produce secondary particles that deposit energy in the subsurface environment. Conceivable mechanisms have been proposed through which the energy of GCR-induced particles can be used to produce biologically useful products such as organics and utilize energy for radiolysis to power a potential subsurface ecosystem. Ionizing radiation causes damage, and just as in the case of Ca. D. audaxviator, a part of the energy deposited in subsurface environments can be used for repairing damage and the rest for chemical reactions and potential biological use.
GCRs are a steady source of ionizing radiation throughout the Galaxy and beyond. Their secondary component can deposit energy underground; muons especially can penetrate several kilometres underground [21,22,26–28,63,64]. It has been shown that GCR-induced radiolysis is a steady source of energy for subsurface environments and could potentially be a viable source of energy supporting such an ecosystem. GCR-induced particles can directly interact with the medium with essential nutrients and synthesize basic chemicals vital for life to develop, analogous to the experiments with high-energy protons and ice mixtures [48,50,51,53,54,76].
The GCR-induced radiolysis mechanisms proposed in the paper open up new possibilities of life in subsurface environments on a number of planetary bodies such as Mars, Moon, Europa, Enceladus, Pluto and especially ones with negligible atmospheres. Radiolysis-powered life can either thrive independently, or can consume a combination of sources such as heat from chemical and geological processes. GCR-induced radiolysis can produce a number of ion species leading to the production of biologically useful products such as molecular hydrogen. There is a possibility of life on icy objects in the interplanetary medium such as comets, and other bodies in the interstellar environment. This energy source could support life locked inside icy objects and facilitate efficient transportation conforming to the panspermia hypothesis. Because rogue or independent planets also receive a steady flux of this radiation, there is a possibility of a thriving subsurface ecosystem on such planets. Ground-based laboratory tests are suggested that can be conducted to validate the hypotheses presented here.
Acknowledgements
The author acknowledges Arnold Wolfendale, Andrew Karam, Adrian Melott, Jacob Haqq-Misra, Steven Hsu and the anonymous reviewers for their helpful comments, and Dipanwita Shome for her help with editing the manuscript.
Competing interests
I declare I have no competing interests.
Funding
This work made use of the Extreme Science and Engineering Discovery Environment, supported by National Science Foundation grant no. ACI-1053575.
References
- 1.Zhu X-G, Long SP, Ort DR. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin Biotechnol. 19, 153–159. ( 10.1016/j.copbio.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 2.United Nations. 2013. Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation: sources, vol. 1. United Nations Publications.
- 3.Brenner DJ, et al. 2003. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA 100, 13 761–13 766. ( 10.1073/pnas.2235592100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattimore V, Battista JR. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178, 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battista JR. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51, 203–224. ( 10.1146/annurev.micro.51.1.203) [DOI] [PubMed] [Google Scholar]
- 6.Ward JF. 1988. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 35, 95–125. ( 10.1016/S0079-6603(08)60611-X) [DOI] [PubMed] [Google Scholar]
- 7.Dubrova YE, Plumb M, Gutierrez B, Boulton E, Jeffreys AJ. 2000. Genome stability: transgenerational mutation by radiation. Nature 405, 37–37 ( 10.1038/35011135) [DOI] [PubMed] [Google Scholar]
- 8.Dubrova YE, Plumb MA. 2002. Ionising radiation and mutation induction at mouse minisatellite loci: the story of the two generations. Mutat. Res./Fundam. Mol. Mech. Mutagen. 499, 143–150. ( 10.1016/S0027-5107(01)00284-6) [DOI] [PubMed] [Google Scholar]
- 9.Cucinotta FA, Durante M. 2006. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 7, 431–435. ( 10.1016/S1470-2045(06)70695-7) [DOI] [PubMed] [Google Scholar]
- 10.Durante M, Cucinotta FA. 2008. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 8, 465–472. ( 10.1038/nrc2391) [DOI] [PubMed] [Google Scholar]
- 11.National Council on Radiation Protection and Measurements. 2002. Report No. 142, Operational Radiation Safety Program for Astronauts in Low-Earth Orbit: A Basic Framework.
- 12.National Council on Radiation Protection and Measurements. 2010. Report No. 167, Potential Impact of Individual Genetic Susceptibility and Previous Radiation Exposure on Radiation Risk for Astronauts.
- 13.Thornley MJ. 1963. Radiation resistance among bacteria. J. Appl. Bacteriol. 26, 334–345. ( 10.1111/j.1365-2672.1963.tb04784.x) [DOI] [Google Scholar]
- 14.Ghiorse WC. 1997. Subterranean life. Science 275, 789–790. ( 10.1126/science.275.5301.789) [DOI] [Google Scholar]
- 15.Fernández-Remolar DC, Prieto-Ballesteros O, Rodríguez N, Gómez F, Amils R, Gómez-Elvira J, Stoker CR. 2008. Underground habitats in the Río Tinto basin: a model for subsurface life habitats on Mars. Astrobiology 8, 1023–1047. ( 10.1089/ast.2006.0104) [DOI] [PubMed] [Google Scholar]
- 16.Lin L-H, et al. 2005. Radiolytic H2 in continental crust: nuclear power for deep subsurface microbial communities. Geochem. Geophys. Geosyst. 6, Q07003 ( 10.1029/2004GC000907) [DOI] [Google Scholar]
- 17.Onstott, et al. 2006. The origin and age of biogeochemical trends in deep fracture water of the Witwatersrand Basin, South Africa. Geomicrobiol. J. 23, 369–414. ( 10.1080/01490450600875688) [DOI] [Google Scholar]
- 18.Lin L-H, et al. 2006. Long-term sustainability of a high-energy, low-diversity crustal biome. Science 314, 479–482. ( 10.1126/science.1127376) [DOI] [PubMed] [Google Scholar]
- 19.Chivian D, et al. 2008. Environmental genomics reveals a single-species ecosystem deep within Earth. Science 322, 275–278. ( 10.1126/science.1155495) [DOI] [PubMed] [Google Scholar]
- 20.Stevens TO, McKinley JP. 1995. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270, 450–455. ( 10.1126/science.270.5235.450) [DOI] [Google Scholar]
- 21.Gaisser TK. 1990. Cosmic rays and particle physics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Dorman L. 2004. Cosmic rays in the earth's atmosphere and underground, vol. 303 Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 23.Stanev T. 2010. Cosmic rays underground. In High energy cosmic rays, pp. 137–171. Berlin, Germany: Springer; ( 10.1007/978-3-540-85148-6_7) [DOI] [Google Scholar]
- 24.Dartnell LR. 2011. Ionizing radiation and life. Astrobiology 11, 551–582. ( 10.1089/ast.2010.0528) [DOI] [PubMed] [Google Scholar]
- 25.Atri D, Hariharan B, Grießmeier J-M. 2013. Galactic cosmic ray-induced radiation dose on terrestrial exoplanets. Astrobiology 13, 910–919. ( 10.1089/ast.2013.1052) [DOI] [PubMed] [Google Scholar]
- 26.Atri D, Melott AL. 2014. Cosmic rays and terrestrial life: a brief review. Astroparticle Phys. 53, 186–190. ( 10.1016/j.astropartphys.2013.03.001) [DOI] [Google Scholar]
- 27.Mei D-M, Hime A. 2006. Muon-induced background study for underground laboratories. Phys. Rev. D 73, 053004 ( 10.1103/PhysRevD.73.053004) [DOI] [Google Scholar]
- 28.Groom DE, Mokhov NV, Striganov SI. 2001. Muon stopping power and range tables 10 MeV–100 TeV. Atomic Data and Nuclear Data Tables 78, 183–356. ( 10.1006/adnd.2001.0861) [DOI] [Google Scholar]
- 29.Heisinger B, Lal D, Jull AJT, Kubik P, Ivy-Ochs S, Neumaier S, Knie K, Lazarev V, Nolte E. 2002. Production of selected cosmogenic radionuclides by muons: 1. Fast muons. Earth Planet. Sci. Lett. 200, 345–355. ( 10.1016/S0012-821X(02)00640-4) [DOI] [Google Scholar]
- 30.Pavlov AA, Vasilyev G, Ostryakov VM, Pavlov AK, Mahaffy P. 2012. Degradation of the organic molecules in the shallow subsurface of Mars due to irradiation by cosmic rays. Geophys. Res. Lett. 39, L13202 ( 10.1029/2012GL052166) [DOI] [Google Scholar]
- 31.Pavlov AK, Blinov AV, Konstantinov AN. 2002. Sterilization of Martian surface by cosmic radiation. Planet. Space Sci. 50, 669–673. ( 10.1016/S0032-0633(01)00113-1) [DOI] [Google Scholar]
- 32.Dartnell LR, Desorgher L, Ward JM, Coates AJ. 2007. Modelling the surface and subsurface Martian radiation environment: implications for astrobiology. Geophys. Res. Lett. 34, L02207 ( 10.1029/2006GL027494) [DOI] [Google Scholar]
- 33.Holloman WK, Schirawski J, Holliday R. 2007. Towards understanding the extreme radiation resistance of Ustilago maydis. Trends Microbiol. 15, 525–529. ( 10.1016/j.tim.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 34.Hulot G, Gallet Y. 2003. Do superchrons occur without any palaeomagnetic warning? Earth Planet. Sci. Lett. 210, 191–201. ( 10.1016/S0012-821X(03)00130-4) [DOI] [Google Scholar]
- 35.Zhdanova NN, Tugay T, Dighton J, Zheltonozhsky V, Mcdermott P. 2004. Ionizing radiation attracts soil fungi. Mycol. Res. 108, 1089–1096. ( 10.1017/S0953756204000966) [DOI] [PubMed] [Google Scholar]
- 36.Alekhova TA, Aleksandrova AA, Novozhilova TYu, Lysak LV, Zagustina NA, Bezborodov AM. 2005. Monitoring of microbial degraders in manned space stations. Appl. Biochem. Microbiol. 41, 382–389. ( 10.1007/s10438-005-0065-x) [DOI] [PubMed] [Google Scholar]
- 37.Baranov VM, Polikarpov NA, Novikova ND, Deshevaia EA, Poddubko SV, Svistunova IuV, Tsetlin VV. 2006. Main results of the Biorisk experiment on the International Space Station. Aviakosm. Ekolog. Med. 40, 3–9. [PubMed] [Google Scholar]
- 38.Dadachova E, Bryan RA, Huang X, Moadel T, Schweitzer AD, Aisen P, Nosanchuk JD, Casadevall A. 2007. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2, e457 ( 10.1371/journal.pone.0000457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dadachova E, Casadevall A. 2008. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr. Opin Microbiol. 11, 525–531. ( 10.1016/j.mib.2008.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singaravelan N, Grishkan I, Beharav A, Wakamatsu K, Ito S, Nevo E. 2008. Adaptive melanin response of the soil fungus Aspergillus niger to UV radiation stress at ‘Evolution Canyon’, Mount Carmel, Israel. PLoS ONE 3, e2993 ( 10.1371/journal.pone.0002993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mironenko NV, Alekhina IA, Zhdanova NN, Bulat SA. 2000. Intraspecific variation in gamma radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: a case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol Environ. Saf 45, 177–187. ( 10.1006/eesa.1999.1848) [DOI] [PubMed] [Google Scholar]
- 42.Mal'tsev VN, Saadavi A, Aı̌iad A, El'gaui O, Shlip M. 1995. Microecology of nuclear reactor pool water. Radiatsionnaiabiologiia, Radioecologiia/Rossiiskaiaakademiianauk 36, 52–57. [PubMed] [Google Scholar]
- 43.Percival PW, Roduner E, Fischer H. 1978. Radiolysis effects in muonium chemistry. Chem. Phys. 32, 353–367. ( 10.1016/0301-0104(78)85018-6) [DOI] [Google Scholar]
- 44.Smilga VP, Belousov YuM. 1994. The muon method in science, vol. 219 New York, NY: Nova Publishers. [Google Scholar]
- 45.Hatano Y, Katsumura Y, Mozumder A (eds). 2010. Charged particle and photon interactions with matter: recent advances, applications, and interfaces. Boca Raton, FL: CRC Press. [Google Scholar]
- 46.Schulze-Makuch D, Irwin LN. 2008. Life in the universe: expectations and constraints, vol. 3 Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 47.Azua-Bustos A, Vega-Martínez C. 2013. The potential for detecting ‘life as we don't know it’ by fractal complexity analysis. Int. J. Astrobiol. 12, 314–320. ( 10.1017/S1473550413000177) [DOI] [Google Scholar]
- 48.Holtom PD, Bennett CJ, Osamura Y, Mason NJ, Kaiser RI. 2005. A combined experimental and theoretical study on the formation of the amino acid glycine (NH2CH2COOH) and its isomer (CH3NHCOOH) in extraterrestrial ices. Astrophys. J. 626, 940 ( 10.1086/430106) [DOI] [Google Scholar]
- 49.Bernstein MP, Sandford SA, Allamandola LJ, Chang S, Scharberg MA. 1995. Organic compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys. J. 454, 327 ( 10.1086/176485) [DOI] [Google Scholar]
- 50.Kobayashi K, Kasamatsu T, Kaneko T, Koike J, Oshima T, Saito T, Yamamoto T, Yanagawa H. 1995. Formation of amino acid precursors in cometary ice environments by cosmic radiation. Adv. Space Res. 16, 21–26. ( 10.1016/0273-1177(95)00188-K) [DOI] [PubMed] [Google Scholar]
- 51.Hudson RL, Moore MH. 1999. Laboratory studies of the formation of methanol and other organic molecules by water + carbon monoxide radiolysis: relevance to comets, icy satellites, and interstellar ices. Icarus 140, 451–461. ( 10.1006/icar.1999.6144) [DOI] [Google Scholar]
- 52.Bernstein MP, Moore MH, Elsila JE, Sandford SA, Allamandola LJ, Zare RN. 2003. Side group addition to the polycyclic aromatic hydrocarbon coronene by proton irradiation in cosmic ice analogs. Astrophys. J. Lett. 582, L25 ( 10.1086/345941) [DOI] [Google Scholar]
- 53.Kobayashi K, et al. 2001. Formation of bioorganic compounds in simulated planetary atmospheres by high energy particles or photons. Adv. Space Res. 27, 207–215. ( 10.1016/S0273-1177(01)00049-7) [DOI] [PubMed] [Google Scholar]
- 54.Garrod RT, Herbst E. 2006. Formation of methyl formate and other organic species in the warm-up phase of hot molecular cores. Astron. Astrophys. 457, 927–936. ( 10.1051/0004-6361:20065560) [DOI] [Google Scholar]
- 55.Cottin H, Gazeau M-C, Doussin J-F, Raulin F. 2000. An experimental study of the photodegradation of polyoxymethylene at 122, 147 and 193 nm. J. Photochem. Photobiol. A 135, 53–64. ( 10.1016/S1010-6030(00)00274-4) [DOI] [Google Scholar]
- 56.Crites ST, Lucey PG, Lawrence DJ. 2013. Proton flux and radiation dose from galactic cosmic rays in the lunar regolith and implications for organic synthesis at the poles of the Moon and Mercury. Icarus 226, 1192–1200. ( 10.1016/j.icarus.2013.08.003) [DOI] [Google Scholar]
- 57.Capone LA, Dubach J, Whitten RC, Prasad SS, Santhanam K. 1980. Cosmic ray synthesis of organic molecules in Titan's atmosphere. Icarus 44, 72–84. ( 10.1016/0019-1035(80)90056-1) [DOI] [Google Scholar]
- 58.Capone LA, Prasad SS, Huntress WT, Whitten RC, Dubach J, Santhanam K. 1981. Formation of organic molecules on Titan. Nature 293, 45–46. ( 10.1038/293045a0) [DOI] [Google Scholar]
- 59.Chyba CF, Hand KP. 2001. Life without photosynthesis. Science 292, 2026–2027. ( 10.1126/science.1060081) [DOI] [PubMed] [Google Scholar]
- 60.Dartnell LR, Nordheimb TA, Pateld MR, Masond JP, Coatesb AJ, Jonesb GH. 2015. Constraints on a potential aerial biosphere on Venus: I. Cosmic rays. Icarus 257, 396–405. ( 10.1016/j.icarus.2015.05.006) [DOI] [Google Scholar]
- 61.Erlykin AD, Wolfendale AW. 2010. Long term time variability of cosmic rays and possible relevance to the development of life on Earth. Surveys Geophys. 31, 383–398. ( 10.1007/s10712-010-9097-8) [DOI] [Google Scholar]
- 62.Miller SL, Urey HC, Oro J. 1976. Origin of organic compounds on the primitive earth and in meteorites. J. Mol. Evol. 9, 59–72. ( 10.1007/BF01796123) [DOI] [PubMed] [Google Scholar]
- 63.Khalchukov FF, Korolkova EV, Kudryavtsev VA, Malguin AS, Ryasny VG, Ryazhskaya OG, Zatsepin GT, Saavedra O. 1995. Hadrons and other secondaries generated by cosmic-ray muons underground. Il NuovoCimento C 18, 517–529. ( 10.1007/BF02506782) [DOI] [Google Scholar]
- 64.Atri D, Melott AL. 2011. Modeling high-energy cosmic ray induced terrestrial muon flux: a lookup table. Radiat. Phys. Chem. 80, 701–703. ( 10.1016/j.radphyschem.2011.02.020) [DOI] [Google Scholar]
- 65.Tylka AJ, Dietrich WF. 2009. A new and comprehensive analysis of proton spectra in ground-level enhanced (GLE) solar particle events. In Proc. 31st Int. Cosmic Ray Conf., Lódź, Poland, 7–15 July 2009.
- 66.Stevenson DJ. 1999. Life-sustaining planets in interstellar space? Nature 400, 32–32 ( 10.1038/21811) [DOI] [PubMed] [Google Scholar]
- 67.Chyba CF, Phillips CB. 2001. Possible ecosystems and the search for life on Europa. Proc. Natl Acad. Sci. USA 98, 801–804. ( 10.1073/pnas.98.3.801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers KL, Amend JP. 2005. Archaeal diversity and geochemical energy yields in a geothermal well on Vulcano Island, Italy. Geobiology 3, 319–332. ( 10.1111/j.1472-4669.2006.00064.x) [DOI] [Google Scholar]
- 69.Chojnacki M, McEwen A, Dundas C, Ojha L, Urso A, Sutton S. 2016. Geologic context of recurring slope lineae in Melas and Coprates Chasmata, Mars. J. Geophys. Res. Planets 121, 1204–1231. ( 10.1002/2015JE004991) [DOI] [Google Scholar]
- 70.Stern JC, et al. 2015. Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale crater, Mars. Proc. Natl Acad. Sci. USA 112, 4245–4250. ( 10.1073/pnas.1420932112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ojha L, Wilhelm MB, Murchie SL, McEwen AS, Wray JJ, Hanley J, Massé M, Chojnacki M. 2015. Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 8, 829–832. ( 10.1038/ngeo2546) [DOI] [Google Scholar]
- 72.Formisano V, Atreya S, Encrenaz T, Ignatiev N, Giuranna M. 2004. Detection of methane in the atmosphere of Mars. Science 306, 1758–1761. ( 10.1126/science.1101732) [DOI] [PubMed] [Google Scholar]
- 73.Webster CR, et al. 2015. Mars methane detection and variability at Gale crater. Science 347, 415–417. ( 10.1126/science.1261713) [DOI] [PubMed] [Google Scholar]
- 74.Atreya SK, Mahaffy PR, Wong A-S. 2007. Methane and related trace species on Mars: origin, loss, implications for life, and habitability. Planet. Space Sci. 55, 358–369. ( 10.1016/j.pss.2006.02.005) [DOI] [Google Scholar]
- 75.Lefevre F, Forget F. 2009. Observed variations of methane on Mars unexplained by known atmospheric chemistry and physics. Nature 460, 720–723. ( 10.1038/nature08228) [DOI] [PubMed] [Google Scholar]
- 76.Cottin H, Gazeau MC, Raulin F. 1999. Cometary organic chemistry: a review from observations, numerical and experimental simulations. Planet. Space Sci. 47, 1141–1162. ( 10.1016/S0032-0633(99)00024-0) [DOI] [Google Scholar]