Abstract
Polar and charged amino acids (AAs) are heavily expressed in non-collagenous proteins (NCPs), and are involved in hydroxyapatite (HA) mineralization in bone. Here, we review what is known on the effect of single AAs on HA precipitation. Negatively charged AAs, such as aspartic acid, glutamic acid (Glu) and phosphoserine are largely expressed in NCPs and play a critical role in controlling HA nucleation and growth. Positively charged ones such as arginine (Arg) or lysine (Lys) are heavily involved in HA nucleation within extracellular matrix proteins such as collagen. Glu, Arg and Lys intake can also increase bone mineral density by stimulating growth hormone production. In vitro studies suggest that the role of AAs in controlling HA precipitation is affected by their mobility. While dissolved AAs are able to inhibit HA precipitation and growth by chelating Ca2+ and PO43− ions or binding to nuclei of calcium phosphate and preventing their further growth, AAs bound to surfaces can promote HA precipitation by attracting Ca2+ and PO43− ions and increasing the local supersaturation. Overall, the effect of AAs on HA precipitation is worth being investigated more, especially under conditions closer to the physiological ones, where the presence of other factors such as collagen, mineralization inhibitors, and cells heavily influences HA precipitation. A deeper understanding of the role of AAs in HA mineralization will increase our fundamental knowledge related to bone formation, and could lead to new therapies to improve bone regeneration in damaged tissues or cure pathological diseases caused by excessive mineralization in tissues such as cartilage, blood vessels and cardiac valves.
Keywords: hydroxyapatite, biomineralization, amino acids
1. Introduction
Biomineralization is the process by which living organisms produce minerals. Biominerals have complex hierarchical structures, often coupled with exceptional properties, such as high mechanical, electrical or magnetic properties, which are achieved under the direct control of biomolecules [1–5]. The most well-known and maybe most complex example of biomineralization occurs during the formation of bone, an organic–inorganic hybrid material made of collagen, non-collagenous proteins (NCPs), and carbonated hydroxyapatite (CHA) (Ca10 − x(PO4)6 −x(CO3)x(OH)2 −x) crystals [1]. Collagen fibres provide a framework known as extracellular matrix (ECM), where CHA nucleates and grows. Even though the ECM determines the ultimate structure and orientation of hydroxyapatite (HA, Ca5(PO4)3OH) crystals, it does not have the capacity to initiate HA mineralization despite the fact that body fluids are supersaturated with respect to HA. HA nucleation is mainly initiated by a set of negatively charged phosphorylated NCPs associated with the ECM. These proteins attract Ca2+ and PO43− ions through their charged amino acid (AA) domains and increase the local supersaturation to a level sufficient to form nuclei of a critical size, which can develop into HA crystals [1,2]. Another set of NCPs have the ability to inhibit undesirable formation of HA in tissues such as cartilage and arteries, continuously exposed to body fluids. These inhibitory proteins are either associated with the ECM of tissues or found in the plasma and limit the formation of HA by binding to the surface of nascent mineral nuclei, thus restricting their further growth [3].
While striving to understand the process of biomineralization, researchers have investigated the effect of smaller biomolecules such as AAs and peptides (short polymers of AAs) on HA mineralization. AAs are the building blocks of proteins, and negatively charged AAs such as aspartic acid (Asp), glutamic acid (Glu) and phosphoserine (PSer) are highly abundant in the acidic domain of NCP (e.g. osteopontin, bone sialoprotein, dentin matrix protein 1 and dentin phosphophoryn) involved in HA mineralization in bone and dentine. In addition, recent studies have shown both positively and negatively charged AAs are present in the hole zone of collagen, where HA nucleates intrafibrillarly [4]. The presence of these AAs in these areas seems to be crucial for interacting with both Ca2+ and PO43− ions necessary for HA precipitation [4,5].
In vitro studies have shown that, similar to proteins, charged AAs are able to either inhibit or induce HA mineralization [6–8]. AAs are also effective at modifying the morphology and crystalline structure of HA owing to the electrostatic and stereochemical effects of their charged residues, especially carboxylate and phosphate groups [7,9–11]. Compared with proteins or peptides that have long molecular chains and can often fold into three-dimensional conformations [12–14], AAs are much smaller (7 nm on average) and have short side chains; thus, a single AA cannot interact with more than one HA facet at the same time [15], differently from what happens with proteins and peptides. However, AAs are much less expensive and more stable, which makes them cost-effective candidates for clinical applications. In vitro studies show that promoting AAs are useful for improving bone regeneration in damaged tissues [10], whereas inhibitory AAs are potential candidates for treating pathological diseases caused by an excessive mineralization of HA in tissues such as cartilages [16], blood vessels and cardiac valves [17].
Despite the potential of AAs in controlling HA precipitation, the mechanism by which the AAs interact with Ca2+ and PO43− ions or with HA crystals to induce or inhibit mineralization is still controversial—several papers report contradictory results on the promoting or inhibitory effect of specific AAs [6,7,18–22]. While there are many review papers focused on the effect of larger biomolecules such as proteins on HA biomineralization, a thorough review of current knowledge on the effect of single AAs on HA mineralization is still missing.
This paper aims at filling this gap by reviewing all in vitro evidence aimed at explaining the role of single AAs, either dissolved in solution or bound to surfaces, in HA precipitation. The review also compares experimental and computational results, and briefly discusses what is known about the role of individual AAs in mineralization in vivo. In the conclusion section, we point out what is still missing to complete our understanding of the role of AAs in HA precipitation; and we mention a few examples showing how this knowledge could lead to new strategies to cure mineralization-related disorders.
2. In vitro studies of the effect of individual amino acids on hydroxyapatite mineralization
In vitro studies show that charged AAs have very different effects on HA crystallization if they are dissolved [6,18–28] or bound to a surface [8]. AAs dissolved in a solution can either chelate the Ca2+ and PO43− ions in solution or cover the surface of nascent HA nuclei, thus inhibiting its further growth [6,18–28]. On the other hand, AAs bound to surfaces are able to promote HA crystallization by attracting Ca2+ and PO43− ions, thus creating a local supersaturated environment that promotes heterogeneous nucleation of HA [8]. We first describe the effect of dissolved AAs on HA mineralization, and then move to that of AAs bound to surfaces.
2.1. Amino acids dissolved in solution
2.1.1. Effect of amino acids on hydroxyapatite nucleation and growth
Tables 1 and 2 summarize the studies on HA precipitation in the presence of AAs dissolved in solution. Most of these studies focus on the effect of AAs on HA growth rather than its nucleation. In addition, these experiments are usually conducted under non-physiological conditions. As is evident from table 2, almost all AAs are able to inhibit both HA particle growth and crystal growth, irrespective of their electrical charges. However, charged AAs, such as Glu, Asp, arginine (Arg) and lysine (Lys), show a significantly stronger effect than non-charged ones [6,7,23]. This is related to the presence of stronger interactions between charged AAs and Ca2+ and PO43− ions, as well as with the surface of HA particles already formed in solution.
Table 1.
Different methods used to synthesize HA in the presence of dissolved AAs. In the pH column, ‘fix’ means that the pH was kept constant using a buffer or acid and base titration; in the AA, Ca and P concentration columns, ‘(—)’ means that the concentrations were not mentioned in the work cited.
| year | Ca source (conc. (mM)) | P source (conc. (mM)) | AAs (conc. (mM)) | method | pH | temp. (°C) | time (h) | references |
|---|---|---|---|---|---|---|---|---|
| 2015 | CaCl2 (25) | (NH4)2HPO4 (15) | PSer, Glu (20–200) | conventional | 9.5 (fix) | 37 | 72 | Wang et al. [29] |
| 2012, 2015 | CaCl2 (3.1) | NaH2PO4 (1.9) | Arg, Glu (10) | conventional | 7.4 (fix) | 37 | 48 | Tavafoghi et al. [30,31] |
| 2011 | CaCl2 (0.5) | KH2PO4 (0.3) | Asp, Glu (0.2) | const. comp. | 8.45 | 25 | 48–96 | Chu et al. [32] |
| 2009 | (CH3COO)2Ca (75) | H3PO4 (50) | Ala, Arg, Asp (150) | conventional | 10 (fix) | RT | 24 | Palazzo et al. [7] |
| 2007 | Ca(NO3)2 · 4H2O (−) | orthophosphoric acid (−) | Gly, Ala, Ser, Lys, Asn (−) | conventional | 9 | 80 | 18 | Jack et al. [6] |
| 2007 | CaCl2 (0.25–0.5) | KH2PO4 (0.17–0.33) | Leu (0–1.52) | const. comp. | 7.4 (fix) | 37 | — | Dalas et al. [18] |
| 2007 | CaCl2 (3.2) | Na2HPO4 (0.96) | Gly, Glu (40) | conventional | 9.5 (fix) | 37 | 720 | Pan et al. [11] |
| 2007 | CaCl2 (2) | Na2HPO4 (1.2) | Gly, Glu (11) | conventional | 9 (fix) | 37 | 24 | Tao et al. [33] |
| 2006 | Ca(NO3)2 (33.4–38) | (NH4)2HPO4 (20) | Gly, Asp, Glu, Lys (4) | conventional | 7.5 | 37 | 24 | Rosseeva et al. [34] |
| 2006 | Ca(NO3)2 · 4H2O (540) | (NH4)2HPO4 (320) | Asp, Glu (50–200) | conventional | 90 | 5 | Boanini et al. [35] | |
| 2004 | Ca(NO3)2 (1000) | (NH4)3PO4 (333.33) | Gly, Ala, Val, Asn, Ser, Lys, Arg, Asp (166.67) | conventional | 9 (fix) | 80 | 16 | Gonzalez et al. [23] |
| 2002 | Ca(CH3COO)2(100) | (NH4)2HPO4 (60) | Gly, Ala, Pro, Hyp, Ser, Val, Thr, Met, Asp, Glu, Arg, His (500) | conventional | 7.4 | 60 | — | Matsumoto et al. [24] |
| 2002 | CaCl2, Ca(NO3)2 (400) | (NH4)2HPO4 (400) | Asp, Glu, Ser (20) | conventional | 7.4 | 25 | approximately 120 | Aiden-Abmann et al. [36] |
| 2001 | CaCl2 (0.25–0.5) | KH2PO4 (0.17–0.33) | Gly, Cys, Gln (0–0.66) | const. comp. | 7.4 (fix) | 37 | — | Koutsopoulos et al. [21] |
| 2001 | CaCl2 (250) | Na2HPO4 (150) | Ser (1–10) | conventional | 7.4 (fix) | 37 | 3 | Spanos et al. [37] |
| 2000 | CaCl2 (0.25–0.5) | KH2PO4 (0.17–0.33) | Ala, Phe, Pro, Met (0–2.25) | const. comp. | 7.4 (fix) | 37 | — | Koutsopolos et al. [19] |
| 2000 | CaCl2 (−) | KH2PO4 (−) | Glu, Asp (−) | const. comp. | 7.4 (fix) | 37 | — | Koutsopolos and Dalas [22] |
| 2000 | CaCl2(−) | KH2PO5 (−) | Ser, Tyr, Hyp (−) | const. comp. | 7.4 (fix) | 37 | 0.5–12 | Koutsopolos and Dalas [20] |
Table 2.
Particle and crystal size of HA synthesized in the presence of AAs dissolved in solution.
| amino acids | HA particle size |
HA crystallite size |
references | ||
|---|---|---|---|---|---|
| width (nm) | length (nm) | D002 (nm) | D310(nm) | ||
| none | — | — | 29 ± 3 | 27 ± 5 | [30] |
| 15 ± 3 | 25 ± 5 | 23 ± 5 | 9 ± 3 | [7] | |
| 28 ± 1 | 258 ± 6 | — | — | [6] | |
| 20 | 80 | 36 | 8 | [23] | |
| apolar | width (nm) | length (nm) | D002 (nm) | D310(nm) | references |
| Gly | 29 ± 1 | 162 ± 1 | — | — | [6] |
| 8 | 80 | 16 | 5.5 | [23] | |
| Ala | 15 ± 3 | 25 ± 5 | 18 ± 2 | 6 ± 2 | [7] |
| 30 ± 1 | 96 ± 2 | — | — | [6] | |
| 8 | 60 | 18.5 | 6.5 | [23] | |
| Val | 5 | 50 | — | — | [23] |
| uncharged polar | width (nm) | length (nm) | D002 (nm) | D310(nm) | references |
| Asn | 8 | 50 | 15 | 5 | [23] |
| Ser | 29 ± 1 | 118 ± 3 | — | — | [6] |
| 5 | 25 | 14 | 4.5 | [23] | |
| charged polar | width (nm) | length (nm) | D002 (nm) | D310(nm) | references |
| Arg | 6 ± 2 | 70 ± 5 | 19 ± 2 | 6 ± 2 | [7] |
| 8 | 80 | 21 | 7 | [23] | |
| — | — | 29 ± 3 | 22 ± 2 | [30] | |
| Glu | — | — | 26 ± 5 | 18 ± 2 | [30] |
| Arg + Glu | — | — | 28 ± 1 | 17 ± 1 | [30] |
| Asp | 6 ± 2 | 70 ± 5 | 15 ± 3 | 15 ± 2 | [7] |
| 20 ± 1 | 57 ± 1 | — | — | [6] | |
| 3.5 | 150 | 10 | 3.5 | [23] | |
| Lys | 32 ± 1 | 123 ± 2 | — | — | [6] |
Our group investigated the effect of differently charged AAs on both HA nucleation and growth at physiological conditions. We showed that a positively charged AA, Arg, had a strong inhibitory effect on HA nucleation, whereas a negatively charged AA, Glu, was more effective in inhibiting HA crystal growth. We attributed these differences to the higher stability constant of complexes formed between Arg and Ca2+ and PO43− ions at the nucleation stage, but the stronger bonding of Glu to HA crystal faces during the growth stage. If both Glu and Arg were combined in the same solution, we noted an overall lower effect on HA nucleation, although the combination could still inhibit HA crystal growth. We related this to the strong complexes formed between Arg and Glu, which interfere with their ability to interact with the HA precursor ions [30]. In another study, we showed that if the Ca- and P-containing precursor solutions are aged in the presence of AAs, the Ca/AA and P/AA complexes initially formed in the solutions can grow into large aggregates. These aggregates can interact with each other once the precursor solutions are mixed, and regulate HA precipitation following a different pathway from that observed in the non-aged solutions, thus resulting in a slower precipitation rate [31].
In contrast with our findings relative to the inhibitory effect of charged AAs on HA nucleation, Boanini et al. [35] showed that the presence of Asp or Glu in reaction solution did not inhibit HA nucleation. This can be explained by the high concentration of Ca and P precursor solutions (table 1) in their experiment, which results in fast HA precipitation that AAs can with more difficulty inhibit. In fact, in agreement with our results, both Glu and Arg inhibited HA crystal growth, with Glu showing a stronger effect. Spanos et al. [37] showed that a non-charged AA, serine (Ser), was also able to inhibit HA crystal growth by adsorbing to HA surface and blocking the HA active growth sites. They suggested a Langmuir type of adsorption based on electrostatic attractions between negative sites on HA surface (i.e. phosphate or hydroxyl ions) and the positively charged amino group of Ser.
AA/HA surface interactions have been extensively studied [6,15,18–23,38,39]. In general, AAs show an increase in adsorption to HA surfaces based on the charge of their side groups: apolar (alanine (Ala)) < positively charged (Lys) < polar (Ser) < negatively charged (Asp) [23]. Koutsopoulos & Dalas conducted a number of studies on the affinity of various AAs to HA surfaces [18–22]. They showed that a negatively charged AA, Asp, has the highest affinity for HA (table 3). However, Jack et al. measured a stronger affinity to HA for a positively charged AA, Lys, and attributed this to the decrease in repulsion between the net negative charges of HA surface and any negatively charged segments on the AA side chains [6]. Despite their differences, both these sets of results imply a stronger effect of charged (positive or negative) AAs, such as Arg and Asp on inhibiting HA precipitation. Among the non-charged AAs, tyrosine (Tyr) and phenylalanine (Phe) show the largest affinity constant and strongest inhibiting effect on HA particle growth, respectively [19,20]. This was attributed to the presence of an aromatic ring on Tyr and Phe side groups. This aromatic ring can lie on the HA surface, possibly acting as an electron donor and creating a weak bond with the HA surface.
Table 3.
Affinity constants for various amino acid inhibitors of HA crystal growth [18].
| inhibitor | Kaff × 102 l mol−1 |
|---|---|
| alanine | 2.86 |
| phenylalanine | 24.39 |
| proline | 5.74 |
| methionine | 6.21 |
| lysine | 8.77 |
| aspartic acid | 41.66 |
| glutamic acid | 30.21 |
| serine | 9.01 |
| tyrosine | 30.30 |
| 4-hydroxyproline | 7.46 |
| cysteine | 6.64 |
| glutamine | 34.72 |
| glycine | 17.14 |
| leucine | 20.26 |
In addition to affinity constants, geometrical factors must be taken into account to determine the inhibitory effect of AAs on HA crystal growth. Adsorbed AAs can rotate freely around an axis perpendicular to the crystal surface, and thus the effective volume of adsorption can be described by a cone. For example, for Asp and Glu, the projection of the cone onto HA surface is a circle of radius 3.32 and 5.23 Å, respectively [15], which, considering the average HA crystallite size of 40 × 22 nm, indicates a single molecule of these AAs has low coverage ability. However, these results imply that the adsorbed Glu molecules cover a larger part of the HA crystal surface compared with Asp, and thus are more effective in inhibiting HA growth. Asp, on the other hand, has a higher affinity for the HA surface with maximum adsorption sites of 53 compared with 26 for Glu [15]. Therefore, it can be hypothesized that these two phenomena act simultaneously and result in a comparable inhibitory effect of the two acidic AAs against HA crystal growth. Overall, the maximum possible coverages calculated for different AAs were relatively low compared with the specific surface area of the HA powder. This indicated the weaker adsorption of AAs on HA surfaces as compared with proteins that can form multiple bonds through their side chains and structural conformations [15].
In summary, AAs dissolved in solution are able to inhibit HA precipitation and growth, however to a lower extent than larger biomolecules, such as proteins and peptides. Polar AAs with negatively or positively charged groups generally show the strongest inhibitory effect on HA precipitation. Yet, the inhibitory effect of non-polar AAs is not negligible either.
The inhibitory effect of AAs on HA nucleation and growth results from a few key factors.
1. The electrostatic interactions or the stability of complexes formed between the AAs and Ca2+ and PO43− ions present in the precipitation solution [6,19–22,30,31]. For example, our group showed that Arg has a stronger inhibitory effect than Glu on HA nucleation because it can form stronger complexes with Ca2+ and PO43− ions [30], and that these complexes, if left undisturbed, can grow into larger aggregates that can delay HA precipitation more than single AAs [31].
- 2. The affinity of AAs to HA particle surfaces [6,19,30]. This factor itself depends on
- a. Electrostatic interactions between AAs and HA particles. This includes both the electrostatic interaction between the net charges of AAs and HA surface as well as the interaction between specific segments of the AAs and HA particles. For example, although AAs usually interact through their COOH groups with calcium ions present on HA, a positively charged AA such as Lys shows a high affinity with the HA probably because overall it is less repelled by the negatively charged HA surface [6].
- b. Possibility of forming chemical bonds between specific segments of AAs and HA surfaces. For example, among the polar AAs, Tyr shows the highest affinity to HA because it has an aromatic ring that can act as a π-electron donor and form a stronger bond with the HA surface [20].
3. Surface coverage ability of AAs. This mainly depends on geometrical factors; for example, Glu can cover a larger fraction of HA surface than Asp [15].
2.1.2. Effect of amino acids on hydroxyapatite phase transformation and morphology
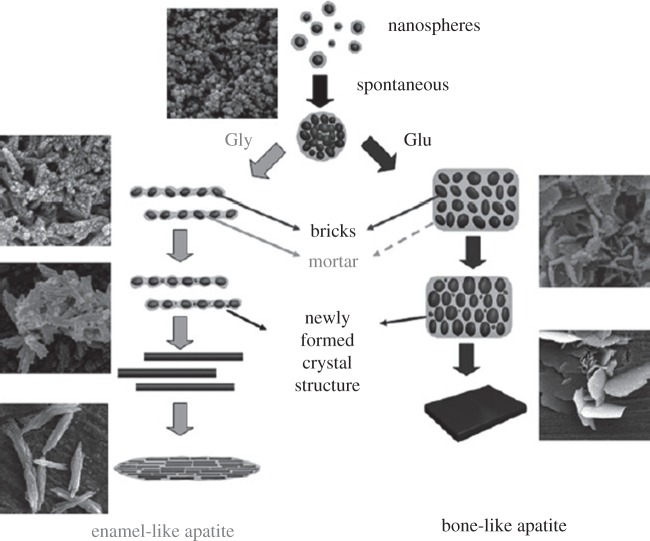
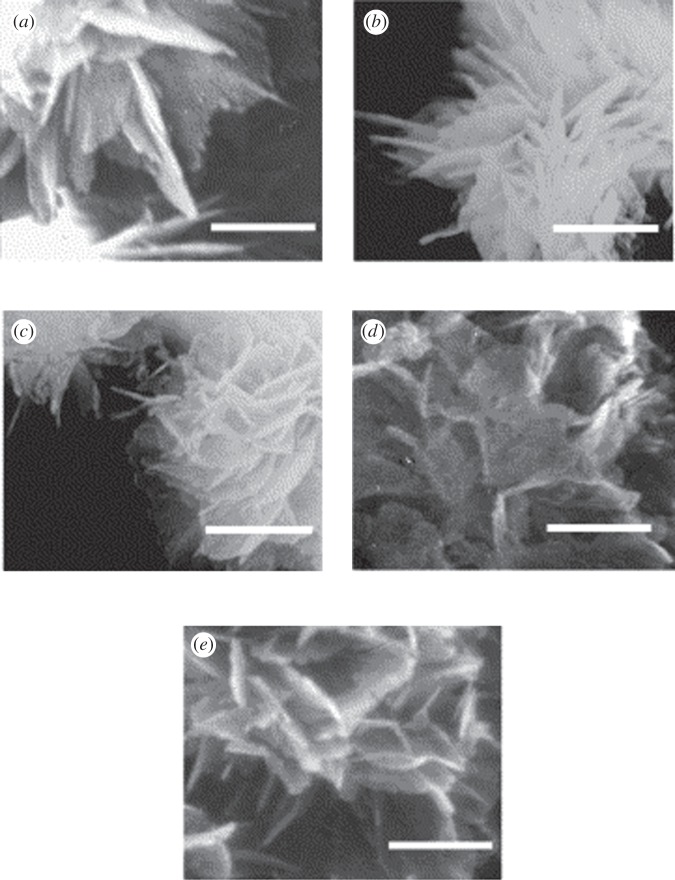
A few studies investigate the effect of AAs on HA morphology or transformation [24,32,40]. Tao et al. showed that the addition of glycine (Gly) in a solution where HA is nucleating can result in the formation of one-dimensional rod-like crystals, whereas two-dimensional plate-like crystals are obtained in the presence of Glu (figure 1). They suggested a brick and mortar model where an amorphous phase, amorphous calcium phosphate (ACP), acts as mortar and cements the bricks of rod-like or plate-like HA crystals to make enamel-like or bone-like HA, respectively [40]. Matsumoto et al. [24] found flake-like particles consisting of nano-sized platelets for HA precipitation in the presence of AAs, such as Gly, Ser, Asp and Glu (figure 2). The flake-like morphology was also observed on the control sample; however, the platelets on this sample were larger and thicker than those observed in the presence of AAs. These AAs reduced the degree of the crystallinity of the HA precipitate by stabilizing ACP [24]. The flake-like HA morphology was also reported by Eiden-Abmann et al. [36] in the presence of AAs, such as Asp, Glu and Ser.
Figure 1.
Different pathways for HA precipitation in a bricks (HA) and mortar (ACP) configuration in presence of Glu and Gly. Reproduced from [40] with permission from the Royal Society of Chemistry.
Figure 2.
Scanning electron microscopy images of the HA crystals synthesized without any AAs (a) or in the presence of Gly (b), Ser (c), Asp (d) and Glu (d). Scale bar, 300 nm. Reproduced from [24] with permission from Elsevier.
Chu et al. [32] showed that the acidic AAs, Asp and Glu, can improve brushite to HA phase transformation by reducing the interfacial energy barrier between brushite (dicalcium phosphate dihydrate, DCPD) and HA (figure 3). Because HA is a basic calcium phosphate, its formation from acidic phases such as brushite is not usually favoured. Chu et al. attributed this to the higher solid/liquid interfacial energy of HA than brushite, which would make the brushite to HA transformation thermodynamically unfavourable. This was even more significant in Chu's study, because HA grew in the form of small crystallites with a high surface area, whereas the initial brushite seeds were much larger. However, Chu et al. showed that Glu and Asp, if added to the reaction solution, adsorbed on the brushite surface and increased its interfacial energy to a value closer to that of HA, thus favouring HA nucleation on brushite surface.
Figure 3.
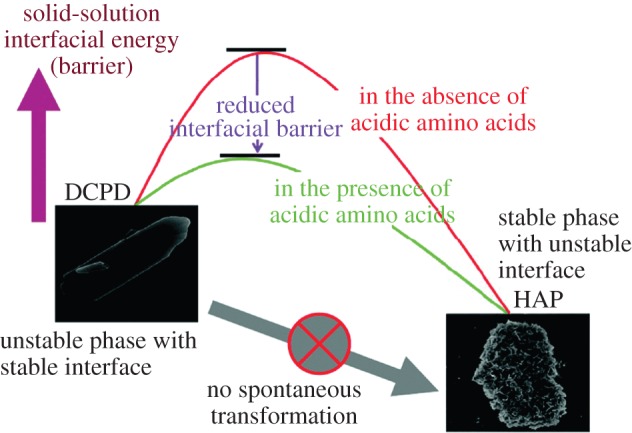
Schematic shows the reduced interfacial energy barrier between brushite (DCPD) and HA in the presence of acidic AAs. Reproduced from [32] with permission from the American Chemical Society. (Online version in colour.)
2.1.3. Poly amino acids and peptides
To better understand the role of the AAs present in the proteins involved in HA mineralization, a number of studies have been conducted on poly-AAs and peptides with residues that are abundant in body proteins [41–44]. Poly-Glu and poly-Asp are among the strongest inhibitors of HA growth when dissolved in solution, but they can act as HA nucleators when adsorbed on a surface Phosphorylated residues are responsible for the inhibitory effect of statherin-like pentapeptides dissolved in solution [45]. This can explain the role of statherin as an inhibitory protein in the saliva [25]. However, cross-linked poly-Glu can induce the heterogeneous nucleation of HA even when present in simulated body fluid. In general, all these studies elucidate the important role of negatively charged AAs, such as PSer, Glu and Asp, on the regulation of HA mineralization.
2.2. Amino acids bound to surfaces
Template-directed precipitation is defined as precipitation in the presence of functionalized surfaces [46–49]. Functional groups with electrical charges are able to promote calcium phosphate precipitation [50–54]. This is attributed to the ability of these functional groups to attract Ca2+ and PO43− ions, thus increasing the degree of local supersaturation with respect to calcium phosphate precipitation at regions close to the surfaces [50,53].
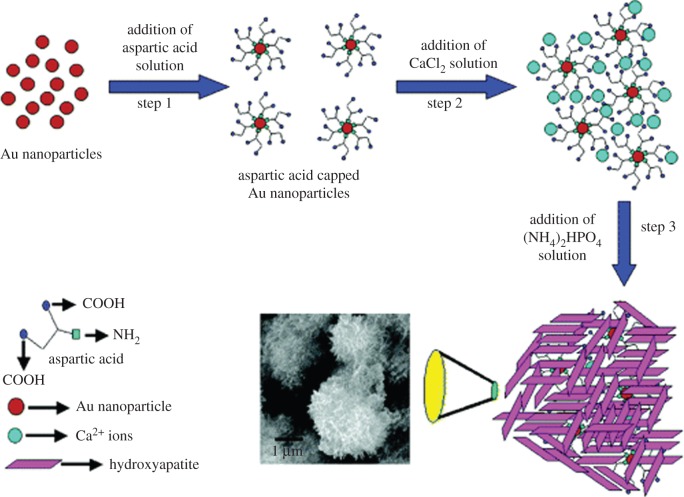
While there have been many studies investigating the inhibitory effect of AAs dissolved in solution on HA mineralization [6,18–28], the effect of AAs bound to surfaces has been subject of just a few studies [8,55,56]. Rautaray et al. [8] investigated HA precipitation in the presence of Asp-capped gold nanoparticles (figure 4). They showed that HA precipitation was promoted in the presence of Asp owing to the interaction between COOH groups in Asp and the Ca2+ ions. Using isothermal titration calorimetry (ITC), they showed the interaction between Ca2+ and Asp was favoured thermodynamically. In addition to their role in mineralization, charged AAs such as Asp, Glu and Arg bound to HA surface were also shown to promote protein absorption and osteoblast proliferation [57–59]. Other researchers have focused on the effect of surface functional groups with different electrical charges on HA precipitation. Zhu et al. [50] prepared self-assembled monolayers (SAMs) of silanes with NH2 and OH functional groups on silicon to investigate the effect of positively versus negatively charged surfaces on HA precipitation. They showed that HA precipitation was faster in the presence of the negatively charged OH-SAM than the positively charged NH2-SAM. Similar results were obtained on gold substrates. Tanahashi et al. investigated HA formation on gold modified with SAMs of alkanethiols terminated with neutral, negative and positive groups, such as CH3, PO4H2, COOH, CONH2, OH and NH2 [53]. They showed that negatively charged functional groups were the most potent enhancers of HA precipitation (PO4H2 first and then COOH), whereas SAMs terminated with positively charged groups such as CONH2 and NH2 induced much less HA precipitation. Liu et al. explored in more detail the effect of negatively charged groups, such as OH, PO4H2 and COOH bound to titanium foil [52], and showed that poorly crystalline calcium phosphate was precipitated in the presence of COOH and PO4H2 functional groups. However, consistent with the study by Zhu et al. [53], PO4H2 exhibited a stronger nucleating ability than COOH. The control titanium sample and the OH-modified sample showed less calcium phosphate deposition than the COOH- and PO4H2-modified samples [52].
Figure 4.
Schematic shows the steps involved in the precipitation of HA in the presence of Asp-capped gold nanoparticles. Reproduced from [8] with permission from the American Chemical Society. (Online version in colour.)
While most of the results so far indicate that negatively charged functional groups are more effective than positive or neutral groups in inducing heterogeneous nucleation of HA [50,53], there are some studies showing different results: Zhang et al. [60] investigated HA precipitation in the presence of PO4, COOH and NH2 functional groups present on Langmuir monolayers made of dipalmitoylphosphatidylcholine, arachidic acid and octadecylamine, respectively. They showed that all PO4, COOH and NH2 functional groups promoted the nucleation of calcium phosphate to very similar extents [60]. However, the Ca/P ratio for the calcium phosphates formed in the presence of PO4 was similar to that of HA (1.67), whereas COOH (1.49) and NH2(1.60) showed lower Ca/P ratios indicating the presence of ACP precursor. In addition, our group showed that while both Arg and Glu bound to graphene oxide (GO) surface increased HA precipitation rate, Arg was more effective than Glu, thus resulting in larger amounts of HA precipitates forming on GO surface [61]. We explained these results based on the fact that Arg can form more stable complexes with Ca2+ and PO43− ions than Glu. In addition, the presence of both carboxyl and amino groups in Arg can favour HA precipitation by electrostatically attracting both Ca2+ and PO43− ions. These results mirror recent evidence showing that indeed, positively charged AAs are involved in the intrafibrillar mineralization of collagen. Landis and Silver showed that the presence of positive AAs, such as Lys, Glu and Arg, in the vicinity of the hole zone of collagen fibrils creates a pocket that can accommodate and retain calcium and phosphate ions, thus inducing HA nucleation in these regions [4,5]
3. In silico studies of the effect of amino acid on hydroxyapatite mineralization
A molecular understanding of the interactions between AAs and HA is crucial to grasp the details of biomineralization. However, the molecular level details of biomineralization are difficult to investigate by experimental techniques exclusively. In the last few decades, sophisticated experimental techniques have been successfully coupled with computational techniques to model the mineralization of HA in the presence of AAs [11,26,62,63].
Molecular dynamic (MD) simulations can investigate organic–inorganic interfaces at the atomic level; therefore, it can provide us useful information relative to the adsorption energy and adsorption sites of AAs to HA. Zhang et al. [26] used MD simulations to investigate the adsorption of the different types of AAs on the (100) face of HA. Their results indicate that the AAs occupy the vacant Ca and P sites of the growing HA (100) surfaces mainly through their amino and carboxylate groups, thus inhibiting HA growth along (100) direction. They showed that small and charged AAs, such as Gly, Glu and Asp, have a higher adsorption on HA, which was consistent with the experimental findings by Koutsapolous et al. [20,22,64]. Pan et al. [11] investigated the specific adsorption sites of Gly and Glu on the (100) and (001) faces of HA crystals at the atomic level using MD simulations. Consistent with the results by Zhang et al., they showed that both Gly and Glu can bind to HA (001) and (100) faces through their amino groups. These groups occupy the vacant calcium sites, whereas carboxylate groups replace the phosphate or OH sites. However, they showed the adsorption of Glu was higher on the (001) face, whereas Gly did not have a preferential adsorption on a specific face. Xu et al. [65] used MD simulations to investigate adsorption of AAs on HA faces. They showed both PSer and Glu adsorbed more on the (100) than on the (001) face. This would explain the plate-like morphology with a greater extension of the (100) faces that was also observed in experimental findings from the same group [29]. These results were consistent with the MD simulation results by Pan et al. [11]. However, Almora-Barrios et al. used ab initio density functional theory (DFT)-based calculations, which can model systems at the atomic level without use of force fields, and showed that non-charged AAs, such as Gly, proline (Pro) and hydroxyproline (Hyp) (the main constituent AAs of the collagen matrix), showed a preferential adsorption to the (100) faces of HA rather than to the (001) faces. This was due to the interaction between the AA carboxylate groups and the calcium ions on HA; Hyp showed the strongest adsorption [62]. The preferential adsorption of AAs to (100) faces could explain why the HA crystals are elongated in the c-direction, as observed in bone.
Corno et al. [63] investigated in more detail the interaction of differently charged AAs, Gly, Glu and Lys, with HA surfaces, again using DFT calculations. They showed that Gly interacted with the (001) surface in its zwitterionic state, with the carboxylate group interacting electrostatically with the calcium ions and the amino group forming hydrogen bond with the oxygen atoms. However, Gly had a higher affinity for the (010) HA surface, where it was adsorbed as an anion as a result of spontaneous proton transfer towards the surface. Lys interacted very similarly with both the (001) and the (010) surfaces: the amino group of its side chain bound to the closest calcium ions. However, based on a MD simulation study by Lou et al. [66], multiple interactions would be involved between Lys and HA (100) surface. The adsorption mainly includes Ca–O ionic bonding, followed by phosphate-O and Lys–HA van der Waals or hydrogen interactions. The behaviour of Glu was more dependent on the surface: on the (001) surface, the carboxylate group of the side chain interacted with the closest calcium ions and formed H-bond with the surface P = O groups. On the (010) surface, however, the carboxylate groups of Glu could only form a relatively weak H-bond with the P–O moieties present on the (010) surface. In summary, the affinity of the studied AAs for the (001) surface followed the trend Gly < Lys < Glu, and for (010) surface, Gly < Glu < Lys.
Addison et al. used RosettaSurface Monte Carlo-based simulations to simulate the adsorption of the VTKHLNQISQSY peptide to HA; the affinity of this peptide to apatite-based materials was previously discovered by phage display [67]. They showed that the peptide/apatite adsorption was primarily determined by the composition and net charge of the AAs rather than their order in the peptide sequence. In fact, the phosphorylated Ser residues were mainly responsible for the adsorption of the peptide to HA.
Overall, consistent with experimental data, computational techniques showed that charged AAs, such as Glu and phosphorylated Ser, have the highest affinity for HA surfaces.
4. Effect of amino acids on mineralization in vivo
A crucial question that arises after reviewing the in vitro evidence related to the effect of AAs on HA mineralization is, are these results relevant to what actually happens in the human body? Charged AAs are highly expressed in NCPs and in the hole zones of collagen fibrils, and in this sense, studies involving single AAs can be considered simple models of the action of these proteins; but are single AAs per se at all involved in bone mineralization, in vivo?
The average concentration of AAs in human blood at fasting ranges between 6.3 and 7.4 mg m−3 [68]. However, body fluids passing though different organs contain various amounts of AAs depending on the activity of such organs. For example, Ishikawa et al. [69] reported that the overall concentrations of AAs in chicken cartilage (18.3–55.3 mM) and muscle (44.3–114.7 mM) were generally higher than in serum (21.4 mM) and blood plasma (6.3 mM). More specifically, they showed that the concentrations of charged AAs, such as Arg, Asp and Glu, which are strongly involved in mineralization, were 0.44, 3.76 and 15.37 mM in chicken hypertophic cartilage extracellular fluid (ECF), whereas it was 0.37, 0.06 and 0.16 mM, respectively, in blood plasma [69]. They also revealed that increasing the concentration of eight AAs (Asp, Glu, Ser, Gly, Ala, Pro, taurine (Tau), and asparagine (Asn)) in the culture medium to the level present in the native cartilage ECF remarkably stimulated the formation of alkaline phosphatase (AP) and AP-rich matrix vesicles (MV) by chondrocytes [70]. Among these AAs, Pro and Gly showed the weakest effect on AP production while the combination of only two of the AAs, Ala and Glu, produced stimulatory effect on MV formation comparable to that seen in the presence of the eight AAs. Glu can form a-ketoglutarate, a tricarboxylic acid cycle intermediate product of the mitochondrial enzyme glutamate dehydrogenase, and Ala can be directly transminated to pyruvate, another important mitochondrial metabolite. Therefore, the effect of Ala and Glu on MV formation can be explained by the formation of mitochondrial metabolites that can interact with calcium ions, accumulate them and finally release them. This is believed to be the key step in both MV formation and initiation of mineralization [71]. In summary, this study revealed that the level of specific AAs in the cartilage ECF is not only critical for the growth of the chondrocytes, but also it is important for the formation of MV [70]. However, the local environment of cells in vivo varies significantly from cell to cell; therefore, this finding cannot be generalized to all cell types, and further studies are required to determine the composition of media in which the response of various cell types can be achieved [70].
AA intake can also increase bone mineral density (BMD) by increasing insulin-like growth factor 1 (IGF-1), which is involved in osteoblastic activity and the synthesis of collagen and muscle proteins [72]. Six AAs, namely Glu, Arg, Ala, Pro, Lys and leucine (Leu), were shown to especially stimulate growth hormone and insulin secretion, thus increasing BMD [72–74]. In another study, AA supplements including Arg, Leu, Lys, Phe, isoleucine (Ile), valine (Val), methionine (Met), threonine (Thr), histidine (His) and tryptophan (Trp) increased bone strength through modifications of BMD, trabecular bone structure and cortical bone thickness possibly by the same IGF-1-mediated process [75]. Hauschka et al. [76] suggested a key role for γ-carboxyglutamic acid in interacting with calcium ions and regulating Ca-containing mineral precipitation in the body.
While most results seem to imply that AAs increase BMD, a few researchers proposed that dietary AAs are a source of metabolic acid that can lower the pH of urine and increase urinary calcium removal, which could potentially decrease BMD [72]. The relation between protein intake and bone health also depends on the protein source, animal versus vegetable. Proteins from animal sources are richer in acidic AAs and could thus be more responsible for calcium excretion and osteoporosis [72]. In addition, foods or supplements with sulfur-containing AAs can increase endogenous sulfuric acid production, and therefore calcium excretion [77]. In general, it is suggested that increase in BMD is associated with the intake of non-sulfur-containing essential AAs [78].
5. Conclusion and perspective
Polar and charged AAs are the main components of NCPs, and are heavily involved in HA mineralization in the body. However, the details of the interactions between AAs and HA crystal or the precursor ions are still the subject of debate. Overall, in vitro studies agree that the role of AAs in controlling HA precipitation is affected by their mobility: while AAs dissolved in solutions similar to body fluids are able to inhibit HA precipitation and growth by chelating Ca2+ and PO43− ions or binding to nuclei of calcium phosphate and preventing their further growth, AAs bound to surfaces promote HA precipitation by attracting Ca2+ and PO43− ions and increasing the local supersaturation.
Most of the in vitro studies highlighted a crucial role in controlling HA nucleation and growth for negatively charged AAs, such as Glu, Asp and PSer; this is in agreement with the fact that such AAs are highly expressed in NCPs involved in bone mineralization regulation. The dominant role of negatively charged AAs was also highlighted by in silico studies, where Glu and PSer showed the highest affinity for HA surfaces among the charged AAs. However, recent reports showed the importance of positively charged AAs on HA intrafibrillar nucleation within collagen [4,5]; and in agreement with this, both our group and a few others showed a strong inhibitory and promoting effect on in vitro HA nucleation and growth for positively charged AAs such as Arg and Lys.
Interestingly, these results are somewhat similar to that found in relation to another biomineral, calcite. Similar to HA, calcite crystal surfaces have high affinity for AAs and larger biomolecules, especially negatively charged ones [79,80]. Most studies indicate that the peptides or proteins bind to calcite through the interaction between negatively charged AA residues and calcium ions present on calcite surfaces [79–81]. However, a recent study highlighted the important role of positively charged sequences on calcite/peptide interactions: Masica et al. [82] showed that calcite interacted more strongly with the positively charged AA sequence of a chemically synthesized peptide than the negatively charged sequences.
To further elucidate the role of AAs in HA precipitation, the interactions between Ca2+ or PO43− ions and AAs should be better understood. A few studies have attempted to investigate the interaction between AAs and ions dissolved in solution [83–86]; however, none of these studies focus on AAs bound to surfaces, which could mimic the most realistic situation of AAs found on collagen, for example. ITC could be used for this purpose, because it allows quantifying energies of interactions between molecules in solution or between solutions and solids. This technique was used in the past to investigate the heat of adsorption of statherins on HA surfaces [87], but no one so far has used it to analyse the interactions between ions and AAs, either in solution or bound to surfaces. Comparing the energies involved in these interactions would allow exploring differences in interactions between immobilized and free AAs, as well as the influence of surface AA concentration, disposition and arrangement on their interaction with ions. In general, surfaces with controlled AA density and organization could be used as models to understand the role of different domains of larger biomolecules such as NCPs in regulating HA precipitation.
Most of the studies on HA/AA interactions performed so far were still far from in vivo conditions, where many other agents, such as collagen, mineralization inhibitors and cells are involved in HA precipitation. The lack of such a complex apparatus could explain why in vitro dissolved AAs inhibit HA precipitation, whereas in vivo soluble AA intake promotes collagen formation and overall seems to increase BMD. In addition, the interaction of mineralization inhibitors present in vivo with the AAs is likely to be complex and worth being studied. Experiments performed in the presence of AAs in conditions closer to the physiological ones should thus be the subject of future work.
A deeper understanding of the role of AAs on HA mineralization will not only help increase our fundamental knowledge related to bone formation; it could also lead to new therapies to cure mineralization-related disorders. For example, Asp, Glu and Phe were shown to reduce calcification of porcine and human valves up to 32% and 28%, respectively [17], and Glu seems to be able to inhibit chondral calcification [16]. Smaller molecules with functionalities similar to AAs are also able to inhibit tissue calcification: phosphocitrate and N-sulfo-2-amino tricarballylate administered either by daily injection or local delivery could inhibit calcification of bovine pericardium [88].
Finally, understanding the effect of single AAs could lead to rationally designed oligopeptides to tune mineralization. This approach is likely to control mineralization more effectively, because the multivalency introduced by joining differently charged AAs can provide several sites at which attachment to calcium and phosphate ions can occur cooperatively. Peptides rationally designed to bind to HA crystals could then be linked to drugs and allow for their specific targeting to bone mineral; for example, ENB-0400, the only drug effective against hypophosphatasia, a rare genetic disease that leads to soft bones and 50% mortality of affected patients, is based on tissue non-specific AP enzyme fused to a patented bone-targeting peptide [89]. A similar strategy could be used to promote HA mineralization in other instances where bone or teeth need to be regenerated, or vice versa, to target diseases caused by pathological mineralization of HA in tissues such as cartilage, ligaments, blood vessels or cardiac valves.
Authors' contributions
As the first author of the paper, M.T., wrote the manuscript, gathered data from the literature, and created the tables and figures. M.C. guided the whole process and extensively reviewed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge funding from the Natural Science and Engineering Research Council of Canada (NSERC), and the Canada Research Chair Foundation.
References
- 1.Song J, Malathong V, Bertozzi CR. 2005. Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J. Am. Chem. Soc. 127, 3366–3372. ( 10.1021/ja043776z) [DOI] [PubMed] [Google Scholar]
- 2.George A, Veis A. 2008. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 108, 4670–4693. ( 10.1021/cr0782729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal AK. 2007. The role of noncollagenous proteins in mineralization. Curr. Opin. Orthopaed. 18, 449–453. ( 10.1097/BCO.0b013e32825e1d84) [DOI] [Google Scholar]
- 4.Landis W, Jacquet R. 2013. Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif. Tissue Int. 93, 329–337. ( 10.1007/s00223-013-9725-7) [DOI] [PubMed] [Google Scholar]
- 5.Silver FH, Landis WJ. 2011. Deposition of apatite in mineralizing vertebrate extracellular matrices: a model of possible nucleation sites on type I collagen. Connect. Tissue Res. 52, 242–254. ( 10.3109/03008207.2010.551567) [DOI] [PubMed] [Google Scholar]
- 6.Jack KS, Vizcarra TG, Trau M. 2007. Characterization and surface properties of amino-acid-modified carbonate-containing hydroxyapatite particles. Langmuir 23, 12 233–12 242. ( 10.1021/la701848c) [DOI] [PubMed] [Google Scholar]
- 7.Palazzo B, Walsh D, Iafisco M, Foresti E, Bertinetti L, Martra G, Bianchi CL, Cappelletti G, Roveri N. 2009. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals. Acta Biomater. 5, 1241–1252. ( 10.1016/j.actbio.2008.10.024) [DOI] [PubMed] [Google Scholar]
- 8.Rautaray D, Mandal S, Sastry M. 2005. Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir 21, 5185–5191. ( 10.1021/la048541f) [DOI] [PubMed] [Google Scholar]
- 9.Almora-Barrios N, de Leeuw NH. 2010. A density functional theory study of the interaction of collagen peptides with hydroxyapatite surfaces. Langmuir 26, 14 535–14 542. ( 10.1021/la101151e) [DOI] [PubMed] [Google Scholar]
- 10.Sugino A, Miyazaki T, Ohtsuki C. 2008. Apatite-forming ability of polyglutamic acid hydrogels in a body-simulating environment. J. Mater. Sci. 19, 2269–2274. ( 10.1007/s10856-007-3327-8) [DOI] [PubMed] [Google Scholar]
- 11.Pan H, Tao J, Xu X, Tang R. 2007. Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level. Langmuir 23, 8972–8981. ( 10.1021/la700567r) [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi A, Ohtsuki C, Kamitakahara M, Ogata S, Miyazaki T, Tanihara M. 2008. Biomimetic deposition of hydroxyapatite on a synthetic polypeptide with beta sheet structure in a solution mimicking body fluid. J. Mater. Sci. Mater. Med. 19, 387–393. ( 10.1007/s10856-007-3179-2) [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi A, Ohtsuki C, Miyazaki T, Kamitakahara M, Ogata S-I, Yamazaki M, Furutani Y, Kinoshita H, Tanihara M. 2005. Heterogeneous nucleation of hydroxyapatite on protein: structural effect of silk sericin. J. R. Soc. Interface 2, 373–378. ( 10.1098/rsif.2005.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfield NJ, Fasman GD. 1969. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8, 4108–4116. ( 10.1021/bi00838a031) [DOI] [PubMed] [Google Scholar]
- 15.Kresak M, Moreno EC, Zahradnik RT, Hay DI. 1977. Adsorption of amino acids onto hydroxyapatite. J. Colloid Interface Sci. 59, 283–292. ( 10.1016/0021-9797(77)90010-8) [DOI] [Google Scholar]
- 16.Wang L, Hinoi E, Takemori A, Nakamichi N, Yoneda Y. 2006. Glutamate inhibits chondral mineralization through apoptotic cell death mediated by retrograde operation of the cystine/glutamate antiporter. J. Biol. Chem. 281, 24 553–24 565. ( 10.1074/jbc.M600939200) [DOI] [PubMed] [Google Scholar]
- 17.Koutsopoulos S, Kontogeorgou A, Dalas E, Petroheilos J. 1998. Calcification of porcine and human cardiac valves: testing of various inhibitors for antimineralization. J. Mater. Sci. Mater. Med. 9, 421–424. ( 10.1023/A:1013291715326) [DOI] [PubMed] [Google Scholar]
- 18.Dalas E, Malkaj P, Vasileiou Z, Kanellopoulou DG. 2008. The effect of Leucine on the crystal growth of calcium phosphate. J. Mater. Sci. Mater. Med. 19, 277–282. ( 10.1007/s10856-006-0050-9) [DOI] [PubMed] [Google Scholar]
- 19.Koutsopoulos S, Dalas E. 2000. Inhibition of hydroxyapatite formation in aqueous solutions by amino acids with hydrophobic side groups. Langmuir 16, 6739–6744. ( 10.1021/la000057z) [DOI] [Google Scholar]
- 20.Koutsopoulos S, Dalas E. 2000. Hydroxyapatite crystallization in the presence of serine, tyrosine and hydroxyproline amino acids with polar side groups. J. Crystal Growth 216, 443–449. ( 10.1016/S0022-0248(00)00415-2) [DOI] [Google Scholar]
- 21.Koutsopoulos S, Dalas E. 2001. Hydroxyapatite crystallization in the presence of amino acids with uncharged polar side groups: glycine, cysteine, cystine, and glutamine. Langmuir 17, 1074–1079. ( 10.1021/la000820p) [DOI] [Google Scholar]
- 22.Koutsopoulos S, Dalas E. 2000. The effect of acidic amino acids on hydroxyapatite crystallization. J. Crystal Growth 217, 410–415. ( 10.1016/S0022-0248(00)00502-9) [DOI] [Google Scholar]
- 23.Gonzalez-McQuire R, Chane-Ching J-Y, Vignaud E, Lebugle A, Mann S. 2004. Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 14, 2277–2281. ( 10.1039/b400317a) [DOI] [Google Scholar]
- 24.Matsumoto T, Okazaki M, Inoue M, Hamada Y, Taira M, Takahashi J. 2002. Crystallinity and solubility characteristics of hydroxyapatite adsorbed amino acid. Biomaterials 23, 2241–2247. ( 10.1016/S0142-9612(01)00358-1) [DOI] [PubMed] [Google Scholar]
- 25.Tsortos A, Nancollas GH. 2002. The role of polycarboxylic acids in calcium phosphate mineralization. J. Colloid Interface Sci. 250, 159–167. ( 10.1006/jcis.2002.8323) [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z-S, Pan H-H, Tang R-K. 2008. Molecular dynamics simulations of the adsorption of amino acids on the hydroxyapatite {100}-water interface. Front. Mater. Sci. China 2, 239–245. ( 10.1007/s11706-008-0046-0) [DOI] [Google Scholar]
- 27.Huang S-P, Zhou K-C, Li Z-Y. 2007. Inhibition mechanism of aspartic acid on crystal growth of hydroxyapatite. Trans. Nonferrous Metals Soc. China 17, 612–616. ( 10.1016/S1003-6326(07)60143-5) [DOI] [Google Scholar]
- 28.Wong ATC, Czernuszka JT. 1995. Transformation behaviour of calcium phosphate 2. Effects of various phosphorylated amino acids. Colloids Surf. A 103, 23–36. ( 10.1016/0927-7757(95)03216-Z) [DOI] [Google Scholar]
- 29.Wang Z, Xu Z, Zhao W, Sahai N. 2015. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 3, 9157–9167. ( 10.1039/C5TB01036E) [DOI] [PubMed] [Google Scholar]
- 30.Jahromi MT, Yao G, Cerruti M. 2012. The importance of amino acid interactions in the crystallization of hydroxyapatite. J. R. Soc. Interface 10, 20120906 ( 10.1098/rsif.2012.0906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahromi MT, Cerruti M. 2015. Amino acid/ion aggregate formation and their role in hydroxyapatite precipitation. Crystal Growth Des. 15, 1096–1104. ( 10.1021/cg501369q) [DOI] [Google Scholar]
- 32.Chu X, Jiang W, Zhang Z, Yan Y, Pan H, Xu X, Tang R. 2010. Unique roles of acidic amino acids in phase transformation of calcium phosphates. J. Phys. Chem. B 115, 1151–1157. ( 10.1021/jp106863q) [DOI] [PubMed] [Google Scholar]
- 33.Tao J, Pan H, Zeng Y, Xu X, Tang R. 2007. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 111, 13 410–13 418. ( 10.1021/jp0732918) [DOI] [PubMed] [Google Scholar]
- 34.Rosseeva EV, Golovanova OA, Frank-Kamenetskaya OV. 2007. The influence of amino acids on the formation of nanocrystalline hydroxyapatite. Glass Phys. Chem. 33, 283–286. ( 10.1134/S1087659607030170) [DOI] [Google Scholar]
- 35.Boanini E, Fini M, Gazzano M, Bigi A. 2006. Hydroxyapatite nanocrystals modified with acidic amino acids. Eur. J. Inorg. Chem. 2006, 4821–4826. ( 10.1002/ejic.200600423) [DOI] [Google Scholar]
- 36.Eiden-Aßmann S, Viertelhaus M, Heiß A, Hoetzer KA, Felsche J. 2002. The influence of amino acids on the biomineralization of hydroxyapatite in gelatin. J. Inorg. Biochem. 91, 481–486. ( 10.1016/S0162-0134(02)00481-6) [DOI] [PubMed] [Google Scholar]
- 37.Spanos N, Klepetsanis PG, Koutsoukos PG. 2001. Model studies on the interaction of amino acids with biominerals: the effect of l-serine at the hydroxyapatite–water interface. J. Colloid Interface Sci. 236, 260–265. ( 10.1006/jcis.2000.7396) [DOI] [PubMed] [Google Scholar]
- 38.El Rhilassi A, Mourabet M, El Boujaady H, Bennani-Ziatni M, Hamri RE, Taitai A. 2012. Adsorption and release of amino acids mixture onto apatitic calcium phosphates analogous to bone mineral. Appl. Surf. Sci. 259, 376–384. ( 10.1016/j.apsusc.2012.07.055) [DOI] [Google Scholar]
- 39.El Rhilassi A, Mourabet M, Bennani-Ziatni M, El Hamri R, Taitai A. In press Interaction of some essential amino acids with synthesized poorly crystalline hydroxyapatite. J. Saudi Chem. Soc. ( 10.1016/j.jscs.2013.05.003) [DOI] [Google Scholar]
- 40.Cai Y, Tang R. 2008. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 18, 3775–3787. ( 10.1039/b805407j) [DOI] [Google Scholar]
- 41.Hartgerink JD, Beniash E, Stupp SI. 2001. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1688. ( 10.1126/science.1063187) [DOI] [PubMed] [Google Scholar]
- 42.Long JR, Dindot JL, Zebroski H, Kiihne S, Clark RH, Campbell AA, Stayton PS, Drobny GP. 1998. A peptide that inhibits hydroxyapatite growth is in an extended conformation on the crystal surface. Proc. Natl Acad. Sci. USA 95, 12 083–12 087. ( 10.1073/pnas.95.21.12083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy MD, Stanley SK, Amis EJ, Becker ML. 2008. Identification of a highly specific hydroxyapatite-binding peptide using phage display. Adv. Mater. 20, 1830–1836. ( 10.1002/adma.200702322) [DOI] [Google Scholar]
- 44.Moreno EC, Kresak M, Hay DI. 1978. Adsorption of two human parotid salivary macromolecules on hydroxy-, fluorhydroxy- and fluorapatites. Arch. Oral. Biol. 23, 525–533. ( 10.1016/0003-9969(78)90266-2) [DOI] [PubMed] [Google Scholar]
- 45.Wikiel K, Burke EM, Perich JW, Reynolds EC, Nancollas GH. 1994. Hydroxyapatite mineralization and demineralization in the presence of synthetic phosphorylated pentapeptides. Arch. Oral Biol. 39, 715–721. ( 10.1016/0003-9969(94)90099-X) [DOI] [PubMed] [Google Scholar]
- 46.Aizenberg J, Black AJ, Whitesides GM. 1999. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver. J. Am. Chem. Soc. 121, 4500–4509. ( 10.1021/ja984254k) [DOI] [Google Scholar]
- 47.Toworfe GK, Bhattacharyya S, Composto RJ, Adams CS, Shapiro IM, Ducheyne P. 2009. Effect of functional end groups of silane self-assembled monolayer surfaces on apatite formation, fibronectin adsorption and osteoblast cell function. J. Tissue Eng. Regener. Med. 3, 26–36. ( 10.1002/term.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavalli S, Popescu DC, Tellers EE, Vos MRJ, Pichon BP, Overhand M, Rapaport H, Sommerdijk NAJM, Kros A. 2006. Self-organizing β-sheet lipopeptide monolayers as template for the mineralization of CaCO3. Angew. Chem. Int. Ed. 45, 739–744. ( 10.1002/anie.200501654) [DOI] [PubMed] [Google Scholar]
- 49.Hu Y-Y, et al. 2009. Self-assembled calcium phosphate nanocomposites using block copolypeptide templates. Soft Matter 5, 4311–4320. ( 10.1039/b904440j) [DOI] [Google Scholar]
- 50.Zhu P, Masuda Y, Koumoto K. 2004. The effect of surface charge on hydroxyapatite nucleation. Biomaterials 25, 3915–3921. ( 10.1016/j.biomaterials.2003.10.022) [DOI] [PubMed] [Google Scholar]
- 51.Li H, Huang W, Zhang Y, Zhong M. 2007. Biomimetic synthesis of enamel-like hydroxyapatite on self-assembled monolayers. Mater. Sci. Eng. C 27, 756–761. ( 10.1016/j.msec.2006.08.002) [DOI] [Google Scholar]
- 52.Liu Q, Ding J, Mante FK, Wunder SL, Baran GR. 2002. The role of surface functional groups in calcium phosphate nucleation on titanium foil: a self-assembled monolayer technique. Biomaterials 23, 3103–3111. ( 10.1016/S0142-9612(02)00050-9) [DOI] [PubMed] [Google Scholar]
- 53.Tanahashi M, Matsuda T. 1997. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 34, 305–315. ( 10.1002/(SICI)1097-4636(19970305)34:3%3C305::AID-JBM5%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 54.Miyazaki T, et al. 2003. Apatite deposition on polyamide films containing carboxyl group in a biomimetic solution. J. Mater. Sci. Mater. Med. 14, 569–574. ( 10.1023/A:1024000821368) [DOI] [PubMed] [Google Scholar]
- 55.Liu H, et al. 2012. Simultaneous reduction and surface functionalization of graphene oxide for hydroxyapatite mineralization. J. Phys. Chem. C 116, 3334–3341. ( 10.1021/jp2102226) [DOI] [Google Scholar]
- 56.Zhao J, Zhang Z, Yu Z, He Z, Yang S, Jiang H. 2014. Nucleation and characterization of hydroxyapatite on thioglycolic acid-capped reduced graphene oxide/silver nanoparticles in simplified simulated body fluid. Appl. Surf. Sci. 289, 89–96. ( 10.1016/j.apsusc.2013.10.106) [DOI] [Google Scholar]
- 57.Lee W-H, Loo C-Y, Chrzanowski W, Rohanizadeh R. 2015. Osteoblast response to the surface of amino acid-functionalized hydroxyapatite. J. Biomed. Mater. Res. A 103, 2150–2160. ( 10.1002/jbm.a.35353) [DOI] [PubMed] [Google Scholar]
- 58.Lee WH, Loo CY, Zavgorodniy AV, Rohanizadeh R. 2013. High protein adsorptive capacity of amino acid-functionalized hydroxyapatite. J. Biomed. Mater. Res. A 101, 873–883. ( 10.1002/jbm.a.34383) [DOI] [PubMed] [Google Scholar]
- 59.Lee WH, Loo CY, Van KL, Zavgorodniy AV, Rohanizadeh R. 2012. Modulating protein adsorption onto hydroxyapatite particles using different amino acid treatments. J. R. Soc. Interface 9, 918–927. ( 10.1098/rsif.2011.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang LJ, et al. 2004. Mineralization mechanism of calcium phosphates under three kinds of Langmuir monolayers. Langmuir 20, 2243–2249. ( 10.1021/la035381j) [DOI] [PubMed] [Google Scholar]
- 61.Tavafoghi M, Brodusch N, Gauvin R, Cerruti M. 2016. Hydroxyapatite formation on graphene oxide modified with amino acids: arginine versus glutamic acid. J. R. Soc. Interface 13, 20150986 ( 10.1098/rsif.2015.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almora-Barrios N, Austen KF, de Leeuw NH. 2009. Density functional theory study of the binding of glycine, proline, and hydroxyproline to the hydroxyapatite (0001) and (011̄0) surfaces. Langmuir 25, 5018–5025. ( 10.1021/la803842g) [DOI] [PubMed] [Google Scholar]
- 63.Corno M, Rimola A, Bolis V, Ugliengo P. 2010. Hydroxyapatite as a key biomaterial: quantum-mechanical simulation of its surfaces in interaction with biomolecules. Phys. Chem. Chem. Phys. 12, 6309–6329. ( 10.1039/c002146f) [DOI] [PubMed] [Google Scholar]
- 64.Koutsopoulos S, Dalas E. 2000. The crystallization of hydroxyapatite in the presence of lysine. J. Colloid Interface Sci. 231, 207–212. ( 10.1006/jcis.2000.7144) [DOI] [PubMed] [Google Scholar]
- 65.Xu Z, Yang Y, Wang Z, Mkhonto D, Shang C, Liu Z-P, Cui Q, Sahai N. 2014. Small molecule-mediated control of hydroxyapatite growth: free energy calculations benchmarked to density functional theory. J. Comput. Chem. 35, 70–81. ( 10.1002/jcc.23474) [DOI] [PubMed] [Google Scholar]
- 66.Lou Z, Zeng Q, Chu X, Yang F, He D, Yang M, Xiang M, Zhang X, Fan H. 2012. First-principles study of the adsorption of lysine on hydroxyapatite (1 0 0) surface. Appl. Surf. Sci. 258, 4911–4916. ( 10.1016/j.apsusc.2012.01.116) [DOI] [Google Scholar]
- 67.Addison WN, Miller SJ, Ramaswamy J, Mansouri A, Kohn DH, McKee MD. 2010. Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences. Biomaterials 31, 9422–9430. ( 10.1016/j.biomaterials.2010.08.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolton C, Wright GP. 1937. The absorption of amino acids and their distribution in the body fluids. J. Physiol. 89, 269–286. ( 10.1113/jphysiol.1937.sp003477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishikawa Y, Chin JE, Hubbard HL, Wuthier RE. 1985. Utilization and formation of amino acids by chicken epiphyseal chondrocytes: comparative studies with cultured cells and native cartilage tissue. J. Cell Physiol. 123, 79–88. ( 10.1002/jcp.1041230113) [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa Y, Chin JE, Schalk EM, Wuthier RE. 1986. Effect of amino acid levels on matrix vesicle formation by epiphyseal growth plate chondrocytes in primary culture. J. Cell Physiol. 126, 399–406. ( 10.1002/jcp.1041260310) [DOI] [PubMed] [Google Scholar]
- 71.Wuthier RE. 1982. A review of the primary mechanism of endochondral calcification with special emphasis on the role of cells, mitochondria and matrix vesicles. Clin. Orthop. Relat. Res. 169, 219–242. [PubMed] [Google Scholar]
- 72.Jennings A, MacGregor A, Spector T, Cassidy A. 2016. Amino acid intakes are associated with bone mineral density and prevalence of low bone mass in women: evidence from discordant monozygotic twins. J. Bone Miner. Res. 31, 326–335. ( 10.1002/jbmr.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Visser JJ, Hoekman K. 1994. Arginine supplementation in the prevention and treatment of osteoporosis. Med. Hypotheses 43, 339–342. ( 10.1016/0306-9877(94)90113-9) [DOI] [PubMed] [Google Scholar]
- 74.Torricelli P, Fini M, Giavaresi G, Giardino R. 2003. Human osteopenic bone-derived osteoblasts: essential amino acids treatment effects. Artif. Cells Blood Substit. Immobil. Biotechnol. 31, 35–46. ( 10.1081/BIO-120018002) [DOI] [PubMed] [Google Scholar]
- 75.Ammann P, Laib A, Bonjour JP, Meyer JM, Ruegsegger P, Rizzoli R. 2002. Dietary essential amino acid supplements increase bone strength by influencing bone mass and bone microarchitecture in ovariectomized adult rats fed an isocaloric low-protein diet. J. Bone Miner. Res. 17, 1264–1272. ( 10.1359/jbmr.2002.17.7.1264) [DOI] [PubMed] [Google Scholar]
- 76.Hauschka PV, Lian JB, Gallop PM. 1975. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc. Natl Acad. Sci. USA 72, 3925–3929. ( 10.1073/pnas.72.10.3925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM. 2005. Amino acid supplementation alters bone metabolism during simulated weightlessness. J. Appl. Physiol. (1985) 99, 134–140. ( 10.1152/japplphysiol.01406.2004) [DOI] [PubMed] [Google Scholar]
- 78.Hackney KJ, English KL. 2014. Protein and essential amino acids to protect musculoskeletal health during spaceflight: evidence of a paradox? Life: Open Access J. 4, 295–317. ( 10.3390/life4030295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albeck S, Aizenberg J, Addadi L, Weiner S. 1993. Interactions of various skeletal intracrystalline components with calcite crystals. J. Am. Chem. Soc. 115, 11 691–11 697. ( 10.1021/ja00078a005) [DOI] [Google Scholar]
- 80.Nelea V, Chien Y-C, Paquette J, McKee MD. 2014. Effects of full-length phosphorylated osteopontin and constituent acidic peptides and amino acids on calcite dissolution. Crystal Growth Des. 14, 979–987. ( 10.1021/cg4012394) [DOI] [Google Scholar]
- 81.Ukrainczyk M, Gredičak M, Jerić I, Kralj D. 2014. Interactions of scalenohedral calcite crystals with acidic amino acid derivatives of salicylic acid. Crystal Growth Des. 14, 4335–4346. ( 10.1021/cg500396x) [DOI] [Google Scholar]
- 82.Masica DL, Schrier SB, Specht EA, Gray JJ. 2010. De novo design of peptide-calcite biomineralization systems. J. Am. Chem. Soc. 132, 12 252–12 262. ( 10.1021/ja1001086) [DOI] [PubMed] [Google Scholar]
- 83.Clarke ER, Martell AE. 1970. Metal chelates of arginine and related ligands. J. Inorg. Nucl. Chem. 32, 911–926. ( 10.1016/0022-1902(70)80070-7) [DOI] [Google Scholar]
- 84.Daniele PG, Foti C, Gianguzza A, Prenesti E, Sammartano S. 2008. Weak alkali and alkaline earth metal complexes of low molecular weight ligands in aqueous solution. Coordin. Chem. Rev. 252, 1093–1107. ( 10.1016/j.ccr.2007.08.005) [DOI] [Google Scholar]
- 85.Lumb RF, Martell AE. 1953. Metal chelating tendencies of glutamic and aspartic acids. J. Phys. Chem. 57, 690–693. ( 10.1021/j150508a021) [DOI] [Google Scholar]
- 86.Ho Y-P, Yang M-W, Chen L-T, Yang Y-C. 2007. Relative calcium-binding strengths of amino acids determined using the kinetic method. Rapid Commun. Mass Spectrom. 21, 1083–1089. ( 10.1002/rcm.2927) [DOI] [PubMed] [Google Scholar]
- 87.Goobes R, Goobes G, Campbell CT, Stayton PS. 2006. Thermodynamics of statherin adsorption onto hydroxyapatite. Biochemistry 45, 5576–5586. ( 10.1021/bi052321z) [DOI] [PubMed] [Google Scholar]
- 88.Tsao JW, Schoen FJ, Shankar R, Sallis JD, Levy RJ. 1988. Retardation of calcification of bovine pericardium used in bioprosthetic heart valves by phosphocitrate and a synthetic analogue. Biomaterials 9, 393–397. ( 10.1016/0142-9612(88)90002-6) [DOI] [PubMed] [Google Scholar]
- 89.Millan JL, et al. 2008. Enzyme replacement therapy for murine hypophosphatasia. J. Bone Miner. Res. 23, 777–787. ( 10.1359/jbmr.071213) [DOI] [PMC free article] [PubMed] [Google Scholar]