Abstract
Automatic identification of the necrotic zone boundary is important in the assessment of treatments on in vitro tumour spheroids. This has been difficult especially when the difference in cell density between the necrotic and viable zones of a tumour spheroid is small. To help overcome this problem, we developed novel one-dimensional pair-correlation functions (PCFs) to provide quantitative estimates of the radial distance of the necrotic zone boundary from the centre of a tumour spheroid. We validate our approach on synthetic tumour spheroids in which the position of the necrotic zone boundary is known a priori. It is then applied to nine real tumour spheroids imaged with light sheet-based fluorescence microscopy. PCF estimates of the necrotic zone boundary are compared with those of a human expert and an existing standard computational method.
Keywords: tumour spheroid, necrotic zone, pair-correlation function
1. Introduction
Tumour spheroids are in vitro cell aggregates grown from a smaller number of cells initially placed in a non-adhesive environment [1–3]. They provide a way to study cancer cell behaviours and interactions in a well-controlled environment, while mimicking the in vivo arrangement of cells more closely than monolayer cultures. Importantly, tumour spheroids are used in drug testing assays [1,4], and assessments of the effectiveness of anti-tumour treatments often rely on quantitative measures of the cell distribution within the spheroid [5,6].
In a sufficiently large spheroid, only the cells in the outermost cell layers can receive enough oxygen and other nutrients to proliferate. This region is termed the proliferative zone, while deeper inside the spheroid, quiescent and necrotic zones are formed [3]. Cells in the quiescent zone remain viable but do not proliferate, while the innermost cells die due to an accumulation of toxic waste products and a lack of oxygen and nutrient supply, forming the necrotic zone [7]. The schematic diagram of figure 1 illustrates the necrotic, quiescent and proliferative zones within a tumour spheroid. Identifying and quantifying these three regions is important in the analysis of comparative assays on tumour spheroids [8–10] and mathematical models of the tumour growth process [11–14].
Figure 1.
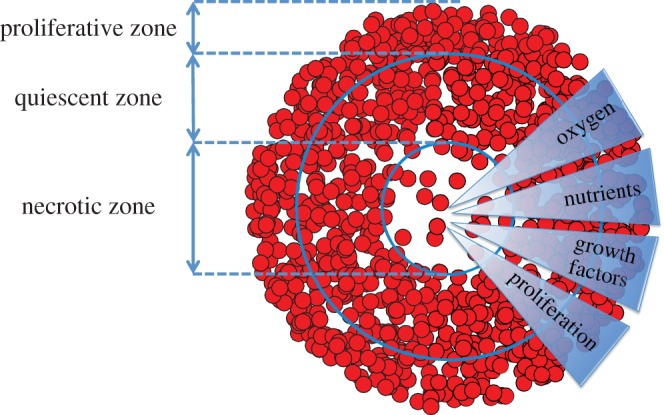
Schematic of the necrotic, quiescent and proliferative zones within a tumour spheroid. In the necrotic zone, cell death occurs due to an accumulation of toxic waste products and a lack of oxygen and nutrient supply [7]. The quiescent zone is a region of viable and non-proliferative cells. In the proliferative zone, cells receive enough oxygen and nutrients to proliferate [3]. (Online version in colour.)
In this paper, we analyse the in vitro distribution of cells in nine (homotypic) tumour spheroids. Using light sheet-based fluorescence microscopy in combination with optical clearing, high-quality three-dimensional images are generated [15]. Subsequently, the images are processed with a three-dimensional segmentation method to obtain a point cloud representing the cell distribution. The cell distribution gives a point pattern that is subsequently analysed, with the aim of being able to provide an estimate of the position of the necrotic zone boundary, i.e. the distance from the spheroid centre to where the necrotic zone transitions into the quiescent zone.
Standard density-based spatial clustering and data clustering methods (DBSCAN and k-means) are implemented to identify the boundary of the necrotic zone [16–18]. However, we find that using such existing methods can fail, or produce unreliable results when the difference in the cell density between the quiescent zone and necrotic zone is small. Therefore, we offer an alternative, statistically based, approach by developing a one-dimensional pair-correlation function (PCF) to identify the necrotic zone boundary in tumour spheroids.
The PCF is a second-order summary statistic commonly used for analysing point patterns in cell biology [19–24]. Typically, PCFs describe the relative frequency of Euclidean distances between pairs of data points, indicating the extent of deviations from complete spatial randomness (CSR) [25–29]. The PCF for a stationary and isotropic point process is defined as
| 1.1 |
;where r is usually the Euclidean distance between points, 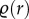 is the second-order product density (frequency of points separated by a distance r) and λ is the intensity of the point process [27]. When points are distributed uniformly at random (i.e. CSR)
is the second-order product density (frequency of points separated by a distance r) and λ is the intensity of the point process [27]. When points are distributed uniformly at random (i.e. CSR) 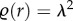 and thus g(r) = 1 at all distances. Consequently, aggregation and segregation length-scales correspond to g(r) > 1 and g(r) < 1, respectively. Therefore, we can quantify spatial features by estimating the PCF for point patterns [30–33].
and thus g(r) = 1 at all distances. Consequently, aggregation and segregation length-scales correspond to g(r) > 1 and g(r) < 1, respectively. Therefore, we can quantify spatial features by estimating the PCF for point patterns [30–33].
However, in some situations the Euclidean distance between points is not the most appropriate distance to study. For example, in a scratch assay used to assess wound healing in vitro, the cells move into the wounded region as a front, which is approximately a straight line. Binder & Simpson [34] used a one-dimensional PCF to quantify the spatial patterning of the cells in the Cartesian direction perpendicular to the front in both experimental images and simulations. They also showed that there was no spatial structure in the Cartesian direction parallel to the front. In other situations, the Euclidean distance between points may not be the quantity of interest. For example, Binder et al. [35] analysed the angular separation of filaments emanating from two-dimensional images of yeast colonies with a one-dimensional angular PCF. Similarly, in attempting to identify the necrotic zone boundary in tumour spheroids, we are concerned primarily with variations of cell density in one particular direction (the radial direction).
This then motivates us to formulate a projected one-dimensional PCF to analyse three-dimensional spatial point patterns with respect to the directions (radial, polar and azimuthal) of the spherical coordinates. In the derivation of this PCF, the (usual) conditions of stationarity and isotropy are relaxed because the projected point processes are in general non-stationary and anisotropic. We examine the accuracy of our method by estimating the PCF for simulated CSR and regular spatial patterns in the spherical coordinate system. In addition, we generate synthetic datasets of cell distributions in tumour spheroids, and demonstrate that the PCF can accurately identify the necrotic zone boundary. This helps with the interpretation and analysis of the PCF results for nine experimental datasets, which provide a ‘proof of concept’ for the usefulness of our approach.
2. Mathematical methods
We derive a projected one-dimensional (non-periodic) PCF to analyse three-dimensional spatial point patterns described in spherical coordinates, 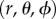 . The aim is to use a sample of N data points to estimate the PCF of the underlying point process. The sample, or point pattern, is a finite subset of three-dimensional space that we can define by
. The aim is to use a sample of N data points to estimate the PCF of the underlying point process. The sample, or point pattern, is a finite subset of three-dimensional space that we can define by
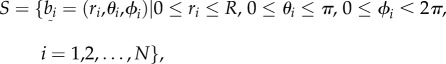 |
;where 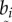 is the position vector of the ith sample point, and
is the position vector of the ith sample point, and 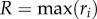 for
for 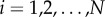 . Without loss of generality, we consider the projection, Q, of S onto the interval [0, L], to obtain the projected point pattern
. Without loss of generality, we consider the projection, Q, of S onto the interval [0, L], to obtain the projected point pattern
;where 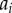 is the projected position of the ith sample point. Note that for the radial, polar and azimuthal projections, we have
is the projected position of the ith sample point. Note that for the radial, polar and azimuthal projections, we have  and L = R,
and L = R, 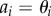 and
and 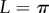 , and
, and 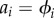 and
and 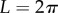 , respectively. The approach now taken in deriving the PCF is based on normalizing the proportion of pairs of projected data points,
, respectively. The approach now taken in deriving the PCF is based on normalizing the proportion of pairs of projected data points, 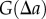 , that are separated by a distance
, that are separated by a distance 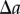 in
in 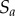 . The normalization is with respect to the probability of observing such pairs in the projection of the Poisson process,
. The normalization is with respect to the probability of observing such pairs in the projection of the Poisson process, 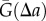 . Owing to the discrete nature of points,
. Owing to the discrete nature of points, 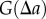 has to be estimated using the average over an interval (numerator of
has to be estimated using the average over an interval (numerator of 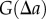 in equation (2.1)). The quantities
in equation (2.1)). The quantities 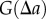 and
and 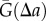 are analogous to the numerator and denominator in equation (1.1).
are analogous to the numerator and denominator in equation (1.1).
To evaluate 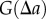 , we introduce the bandwidth h, and obtain the expression
, we introduce the bandwidth h, and obtain the expression
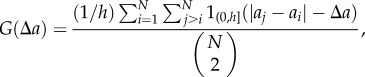 |
2.1 |
;where
;Note that the denominator in equation (2.1) accounts for the total number of all possible combinations of pairs of data points.
To evaluate 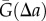 , we first consider the homogeneous Poisson process, P, which is synonymous with CSR. Depending on the form of the projection operator, Q, the projection of the Poisson process,
, we first consider the homogeneous Poisson process, P, which is synonymous with CSR. Depending on the form of the projection operator, Q, the projection of the Poisson process, 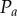 , can be non-stationary and anisotropic (e.g. radial projection). However, as P is the Poisson process, the probability density function
, can be non-stationary and anisotropic (e.g. radial projection). However, as P is the Poisson process, the probability density function 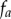 for the projected points of
for the projected points of 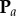 is known. The projected points,
is known. The projected points, 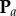 , can be considered as samples drawn from a random variable A with probability density function
, can be considered as samples drawn from a random variable A with probability density function 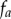 .
.
In order to find the probability of having a pair of points that are separated by a distance 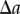 in
in 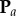 , we can use the density at a location
, we can use the density at a location 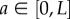 , and the cumulative distribution of the points in the intervals
, and the cumulative distribution of the points in the intervals 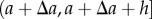 and
and 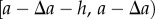 . Integrating over the interval [0, L] then gives
. Integrating over the interval [0, L] then gives
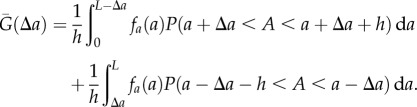 |
2.2 |
;As the probability of finding a pair of points a distance 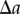 apart does not depend on the order of counting the possible pairs (left-wise or right-wise), it can be shown that the two integrals in equation (2.2) are equal. When written in terms of the probability density function, equation (2.2) becomes
apart does not depend on the order of counting the possible pairs (left-wise or right-wise), it can be shown that the two integrals in equation (2.2) are equal. When written in terms of the probability density function, equation (2.2) becomes
| 2.3 |
;Using equations (2.1) and (2.3), the estimate of the non-periodic PCF is then given by
| 2.4 |
;where the probability density function, 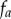 , for each of the three projections is
, for each of the three projections is
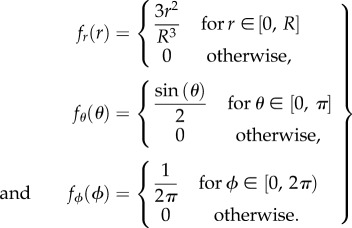 |
2.5 |
;
2.1. Complete spatial randomness and regular spatial patterns
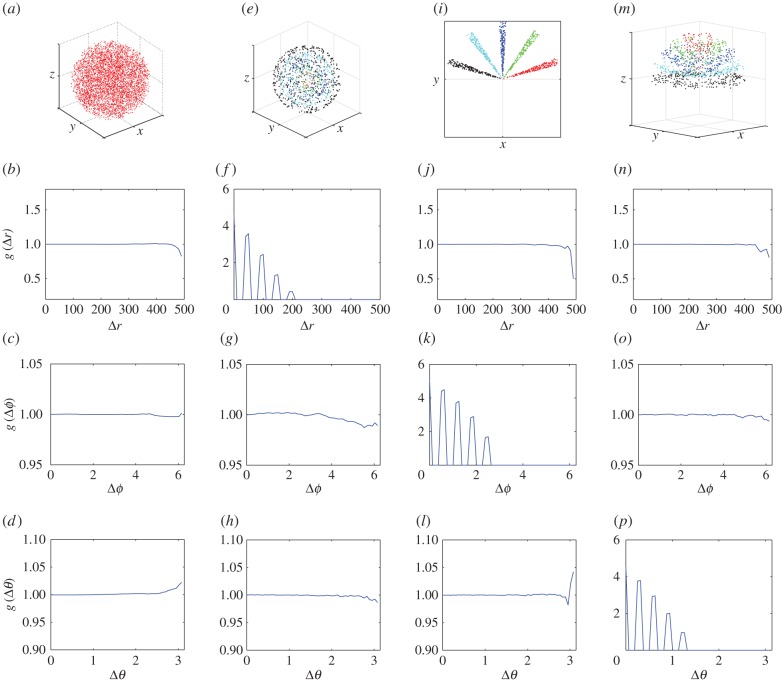
The method is validated by evaluating the non-periodic PCF, equation (2.4), for simulated CSR and regular spatial patterns within a sphere of radius R. The point patterns are shown in the panels of the top row of figure 2. From left to right they are: (i) CSR pattern, (ii) segregated clusters of points in spherical shells, (iii) segregated clusters of points that are locally aggregated around prescribed angles of ϕ, and (iv) segregated clusters of points in conical shells. The three panels directly below each test pattern in figure 2 correspond to the PCF evaluation of the radial, azimuthal and polar projections from 1000 simulations.
Figure 2.
CSR and regular point patterns, h = L/50. (a) CSR pattern, (e) segregated clusters of points in spherical shells, (i) segregated clusters of points that are locally aggregated around prescribed angles of 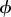 , and (m) segregated clusters of points in conical shells. In the CSR pattern, N = 5000 and R = 500. In all of the regular patterns, N = 1000. The centres of clusters are equally spaced with distance (e) R/10, (i)
, and (m) segregated clusters of points in conical shells. In the CSR pattern, N = 5000 and R = 500. In all of the regular patterns, N = 1000. The centres of clusters are equally spaced with distance (e) R/10, (i) 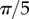 , (m)
, (m) 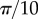 , and the points are distributed uniformly in each cluster in an interval of size (e) R/100, (i)
, and the points are distributed uniformly in each cluster in an interval of size (e) R/100, (i) 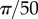 , (m)
, (m) 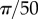 . The remaining rows are for the averaged non-periodic PCF from 1000 simulated patterns. (b,f,j,n) Radial projection. (c,g,k,o) Azimuthal projection. (d,h,l,p) Polar projection. (Online version in colour.)
. The remaining rows are for the averaged non-periodic PCF from 1000 simulated patterns. (b,f,j,n) Radial projection. (c,g,k,o) Azimuthal projection. (d,h,l,p) Polar projection. (Online version in colour.)
At short and intermediate length-scales the results for the simulated CSR pattern, in the first column of figure 2, indicate that there is no spatial structure (in any direction) as 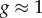 . However, we see that there is significant deviation from unity in the PCF signals at large distances. This deviation at large distances appears to be inconsistent with our formulation of the PCF, as we might expect the signal to be close to unity at all distances. The explanation for this disparity is the division of small numbers in equation (2.4), where the observed frequency and expected frequency of pairs of points at large distances are both small. The results show that the non-periodic PCF is a reliable predictor of CSR at short and intermediate length-scales.
. However, we see that there is significant deviation from unity in the PCF signals at large distances. This deviation at large distances appears to be inconsistent with our formulation of the PCF, as we might expect the signal to be close to unity at all distances. The explanation for this disparity is the division of small numbers in equation (2.4), where the observed frequency and expected frequency of pairs of points at large distances are both small. The results show that the non-periodic PCF is a reliable predictor of CSR at short and intermediate length-scales.
We now consider the regular spatial patterns. They were chosen such that for each spatial pattern there is only spatial structure expected in one of the three corresponding projected patterns. For example, we discuss the results for the points distributed in spherical shells, in the second column of figure 2. As expected, the PCF indicates that there is no spatial structure for the azimuthal and polar projections (figure 2g,h). For the radial projection (figure 2f), we see a series of five decreasing peaks for increasing values of 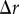 in the PCF. The highest peak at
in the PCF. The highest peak at 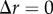 corresponds to pairs of points within each of the five spherical shells (e.g. pairs of red points, pairs of green points, etc.). The smallest peak at
corresponds to pairs of points within each of the five spherical shells (e.g. pairs of red points, pairs of green points, etc.). The smallest peak at 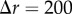 corresponds to a pair of points with one point belonging to the innermost spherical shell (red) and the other to the outermost spherical shell (black). Further information about the spatial patterning can be easily obtained from the signal (e.g. the distance between the peaks in the signal is a measure of distance between the spherical shells). A similar discussion holds for the two sets of results in the last two columns of figure 2. This demonstrates the application of the PCF to quantify spatial structures in point patterns.
corresponds to a pair of points with one point belonging to the innermost spherical shell (red) and the other to the outermost spherical shell (black). Further information about the spatial patterning can be easily obtained from the signal (e.g. the distance between the peaks in the signal is a measure of distance between the spherical shells). A similar discussion holds for the two sets of results in the last two columns of figure 2. This demonstrates the application of the PCF to quantify spatial structures in point patterns.
2.2. Periodic pair-correlation function
In our analysis of the simulated CSR point pattern of figure 2, we observed that the non-periodic PCF, equation (2.4), had significant deviations from unity at large distances—due to the low frequency of occurrence of large separations. This can lead to the incorrect assessment that a point pattern has spatial structure at large distances. This problem of the division of small numbers at large distances can be overcome by defining a periodic PCF.
Following the work of Agnew et al. [19] (and others), we re-define the distance between two points in equation (2.1) as
| 2.6 |
;with the consequence that
;Using equations (2.1) and (2.6), the proportion of pairs of data points, 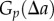 , separated by a distance
, separated by a distance 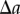 is given by
is given by
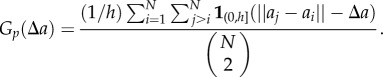 |
2.7 |
;This implies that the number of pairs of points separated by a distance 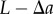 will be added to the number of pairs of points separated by a distance
will be added to the number of pairs of points separated by a distance 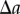 (for
(for 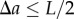 ). Therefore, we also need to re-formulate the normalization term for the periodic PCF, which yields
). Therefore, we also need to re-formulate the normalization term for the periodic PCF, which yields
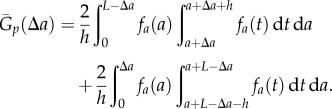 |
2.8 |
;
Equations (2.7) and (2.8) then give the periodic PCF
| 2.9 |
;When evaluating the periodic PCF, equations (2.9), for the simulated CSR pattern in figure 2, we observed a reduction in the deviations from unity at large distances (results not shown).
For the azimuthal projection, the periodic distance that separates two points has a clear physical interpretation. It is simply the acute angle that separates the two points 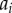 and
and 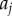 (
(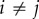 ). However, the physical interpretation of the distance at which departures from unity occur in the periodic PCF for the radial and polar projections are, in isolation, unclear. This is because the periodic PCF cannot distinguish the distances
). However, the physical interpretation of the distance at which departures from unity occur in the periodic PCF for the radial and polar projections are, in isolation, unclear. This is because the periodic PCF cannot distinguish the distances 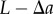 and
and 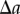 . Therefore, we evaluate both the non-periodic PCF and periodic PCF for synthetic (§3) and experimental datasets of cell distributions within a tumour spheroid (§4).
. Therefore, we evaluate both the non-periodic PCF and periodic PCF for synthetic (§3) and experimental datasets of cell distributions within a tumour spheroid (§4).
3. Synthetic tumour spheroids
We first analyse synthetic tumour spheroid datasets, to help with interpreting the PCFs for the nine experimental datasets. A spatial model is used to generate point patterns with two zones of uniform density. For each synthetic dataset, a total of N points are distributed within a sphere of radius R. The points are distributed under the assumption that there are two zones of uniform cell density, partitioned by a radial distance, r = B, representing the necrotic zone boundary. The inner zone, r < B, is the necrotic zone of the synthetic tumour. The outer zone, B < r < R, corresponds to a viable zone (i.e. the quiescent and proliferative zones together). To ensure that the viable zone has a larger cell density than the necrotic zone, we distribute uniformly at random  points throughout the entire spatial domain, along with additional
points throughout the entire spatial domain, along with additional 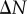 points in the viable zone only. There is no spatial structure in the azimuthal and polar projections of the synthetic datasets. Therefore, we analyse the radial projection of the synthetic datasets with the non-periodic and periodic PCF.
points in the viable zone only. There is no spatial structure in the azimuthal and polar projections of the synthetic datasets. Therefore, we analyse the radial projection of the synthetic datasets with the non-periodic and periodic PCF.
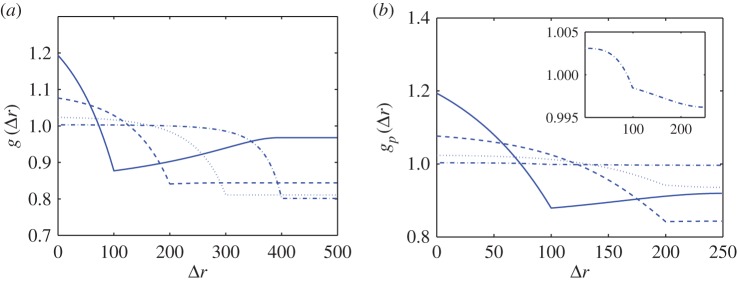
As the point process is known for the synthetic tumour spheroids, we can derive analytic expressions for the non-periodic and periodic PCF, see electronic supplementary material, appendix A. Results for four values of the necrotic boundary are shown in figure 3. We observe that the distance at which a slope discontinuity occurs in the non-periodic PCF (figure 3a), 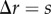 , uniquely identifies the width of the viable zone, W = s. This means that the necrotic boundary,
, uniquely identifies the width of the viable zone, W = s. This means that the necrotic boundary, 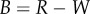 , can be identified from the non-periodic PCF. But the slope discontinuity in the periodic PCF (at
, can be identified from the non-periodic PCF. But the slope discontinuity in the periodic PCF (at 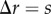 in figure 3b) identifies either the width of the viable zone, W = s, or the necrotic boundary, B = s, with the non-uniqueness being due to periodicity, as discussed in §2. Therefore, we cannot uniquely determine the necrotic zone boundary from the distance at which the slope discontinuity occurs in the periodic PCF.
in figure 3b) identifies either the width of the viable zone, W = s, or the necrotic boundary, B = s, with the non-uniqueness being due to periodicity, as discussed in §2. Therefore, we cannot uniquely determine the necrotic zone boundary from the distance at which the slope discontinuity occurs in the periodic PCF.
Figure 3.
Analytic PCFs for synthetic tumour spheroids, N = 5000, 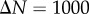 , R = 500 and h = R/50. Solid curves, B = 400. Dashed curves, B = 300. Dotted curves, B = 200. Dashed-dotted curves, B = 100. (a) Non-period PCF. (b) Periodic PCF. The inner graph in (b) is for B = 100 and it illustrates the small variations of
, R = 500 and h = R/50. Solid curves, B = 400. Dashed curves, B = 300. Dotted curves, B = 200. Dashed-dotted curves, B = 100. (a) Non-period PCF. (b) Periodic PCF. The inner graph in (b) is for B = 100 and it illustrates the small variations of 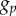 in this case. (Online version in colour.)
in this case. (Online version in colour.)
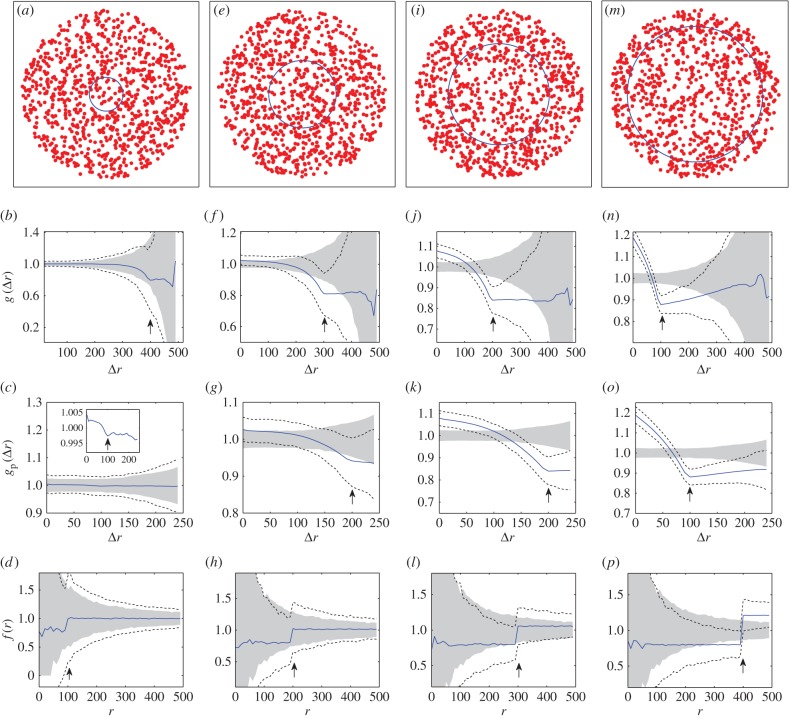
We now examine estimates of the non-periodic and periodic PCF from samples of 1000 synthetic tumour spheroids. The central slice from synthetic tumour spheroids for four values of the necrotic boundary are shown in the first row of figure 4, where the difference in density between the necrotic and viable zones is visually undetectable (i.e. visually indistinguishable from CSR spatial patterning). In the panels of the second and third row, directly below each test pattern, are the corresponding average non-periodic and periodic PCFs (solid curves). The upper and lower broken curves are the 95% confidence intervals, which are the 97.5 and 2.5 percentiles of the 1000 simulations. The arrows identify critical points in the estimates of the PCFs, similar to that seen for the points of slope discontinuity in the continuous PCFs (figure 3).
Figure 4.
Synthetic tumour spheroid point patterns, N = 5000, 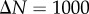 , R = 500 and h = R/50. Columns: left to right B = {100, 200, 300, 400}. (a,e,i,m) Central slice of synthetic tumour spheroid. The remaining rows are for statistics from 1000 realizations. (b,f,j,n) Average non-periodic PCF, solid curves. (c,g,k,o) Average periodic PCF, solid curves. (d,h,l,p) Average normalized density, solid curves. The upper and lower broken curves are for the 97.5 and 2.5 percentiles, and the arrows are for the known distances of the critical points. The shaded region is for the 97.5 and 2.5 percentiles of 1000 CSR point patterns. (Online version in colour.)
, R = 500 and h = R/50. Columns: left to right B = {100, 200, 300, 400}. (a,e,i,m) Central slice of synthetic tumour spheroid. The remaining rows are for statistics from 1000 realizations. (b,f,j,n) Average non-periodic PCF, solid curves. (c,g,k,o) Average periodic PCF, solid curves. (d,h,l,p) Average normalized density, solid curves. The upper and lower broken curves are for the 97.5 and 2.5 percentiles, and the arrows are for the known distances of the critical points. The shaded region is for the 97.5 and 2.5 percentiles of 1000 CSR point patterns. (Online version in colour.)
At short to moderate distances, we find a comparable amount of (small) variability in both the non-periodic and periodic PCFs (broken curves, second and third rows, figure 4). However, at moderate to large distances there is a much greater variability in the non-periodic PCF than that of the periodic PCF. This implies that we have greater confidence inferring salient features of the periodic PCF at large distances. It is therefore advantageous to examine both PCFs together when assessing the spatial pattern of tumours.
To assess the PCFs of the synthetic tumour spheroids, it is useful to consider statistical significance of the results, i.e. our confidence in distinguishing the results from the CSR state (null case). This is done by presenting the 2.5 and 97.5 percentiles of the PCFs of simulated CSR point patterns (the grey regions in the second and third rows of figure 4). In each case, 1000 CSR point patterns are generated with the same number of points as the synthetic spheroid. Then, a point pattern's PCF signal found within the grey region could be interpreted as not distinctive from the CSR point process. Therefore, this makes it difficult to estimate the critical points of the PCFs with just one point pattern (or a small sample size) for B = 100 in figure 4b,c, where the broken curves essentially bound the grey CSR regions. By contrast, the two broken curves at the critical points in figure 4j,k,n,o both lie below the grey CSR region. This indicates that it is possible to provide an estimate of the necrotic boundary from just one point pattern in the cases when B = 300 and B = 400. We note that this contrast is mainly due to the difference in density between the necrotic and viable zones, rather than the increase in the necrotic boundary (see electronic supplementary material, appendix B, for a fixed value of B = 200 and varying 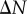 ).
).
The statistical significance of the PCF results is further examined by comparison to those for the normalized density [35]. This first-order statistic is derived by considering the proportion of points at a distance r from the origin
| 3.1 |
;
Equation (3.1) is normalized by the radial projection of a Poisson process
;to obtain the normalized density
| 3.2 |
;
In the bottom row of figure 4, three of the four critical points lie within the grey CSR region and the broken curves essentially bound the grey CSR region. This illustrates that it is difficult to provide an estimate of the necrotic zone boundary from just one point pattern (or small sample size) with the normalized density, demonstrating a greater confidence in the statistical significance of the second-order PCF method.
4. Real tumour spheroids
We have shown that the PCF method can provide a reliable estimate of the (known) radial distance of the necrotic zone boundary, B, from the centre of a synthetic tumour spheroid. However, in practice, there are two important distinctions to consider when examining real tumour spheroids.
The first distinction is that real tumour spheroids can be ellipsoidal in shape, for example, see figure 5. Therefore, we consider an ellipsoidal spatial domain centred at the origin of a Cartesian coordinate system and aligned with the axes,
;where X, Y, Z are the lengths of the three semi-principle axes. We now project, or map the points to the interval [0, 1] via
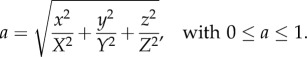 |
4.1 |
;The previous PCF analysis for the radial distance of spherical synthetic tumours holds for this non-dimensional problem, with r = a and R = 1 in equation (2.5). Therefore, we can estimate the dimensionless necrotic zone boundary, 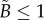 , for this projection of the data points. The inverse mapping then provides estimates for the lengths of the three semi-principle axes of the ellipsoidal necrotic zone boundary
, for this projection of the data points. The inverse mapping then provides estimates for the lengths of the three semi-principle axes of the ellipsoidal necrotic zone boundary
| 4.2 |
;The second distinction is that the necrotic zone boundary in a real tumour spheroid is unknown, but it can be estimated by a human expert. Without prior knowledge of the PCF estimates, human expert estimates for the necrotic zone boundary in nine tumour spheroids were obtained (table 2). In addition to this, we automate the data collection process and subsequent evaluation of the PCF estimates of the necrotic zone boundary for each tumour (see §§4.1 and 4.2). Together, this allows for an unbiased comparison between the human expert and PCF estimates of the necrotic zone boundary for each tumour (tables 2 and 3).
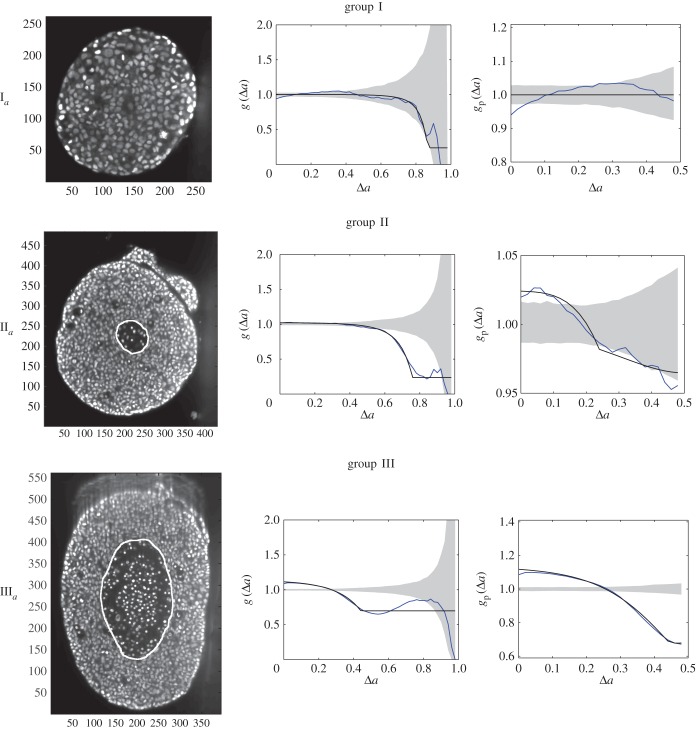
Figure 5.
Central image slice and corresponding point pattern analysis for one tumour spheroid from each of the three groups. The rows top to bottom correspond to 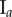 ,
, 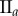 and
and 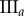 , respectively. The first column is the central slice of the tumour spheroids. The unit of the length in the images is a micrometre. The white curves superimposed on the central image slices outline the necrotic zone identified by a human assessor. The second and third columns are the non-periodic PCF and periodic PCF respectively, h = 0.02. The blue curves are for the PCF statistics. The black curves are the analytic PCFs best fit to the statistics (blue curves), using a nonlinear least-squares method. The shaded region is for the 97.5 and 2.5 percentiles of 1000 CSR point patterns. (Online version in colour.)
, respectively. The first column is the central slice of the tumour spheroids. The unit of the length in the images is a micrometre. The white curves superimposed on the central image slices outline the necrotic zone identified by a human assessor. The second and third columns are the non-periodic PCF and periodic PCF respectively, h = 0.02. The blue curves are for the PCF statistics. The black curves are the analytic PCFs best fit to the statistics (blue curves), using a nonlinear least-squares method. The shaded region is for the 97.5 and 2.5 percentiles of 1000 CSR point patterns. (Online version in colour.)
Table 2.
Necrotic zone boundary estimates in micrometres for the nine tumour spheroids. The italicized PCF estimates are for fitted PCFs (and therefore critical points) that reside within the 97.5 and 2.5 percentiles of 1000 CSR point patterns (e.g. see first row of results for spheroid 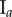 in figure 5).
in figure 5).
| group I |
group II |
group III |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 |
 |
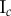 |
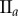 |
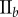 |
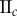 |
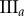 |
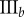 |
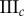 |
||
| human | 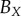 |
— | — | — | 36 | 36 | 34 | 81 | 83 | 93 |
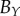 |
— | — | — | 43 | 40 | 53 | 138 | 90 | 128 | |
| non-periodic PCF | 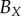 |
12 | 6 | 23 | 42 | 46 | 34 | 94 | 92 | 96 |
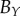 |
15 | 6 | 30 | 46 | 47 | 46 | 140 | 94 | 132 | |
| periodic PCF | 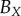 |
— | — | 52 | 42 | 49 | 30 | 94 | 101 | 95 |
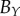 |
— | — | 69 | 46 | 50 | 42 | 139 | 104 | 131 | |
| DBSCAN | 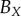 |
84 | — | — | 43 | 47 | 33 | 77 | 74 | 80 |
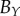 |
103 | — | — | 47 | 48 | 45 | 114 | 77 | 110 | |
Table 3.
Percentage difference in computed estimates relative to the human estimates, for group II and III tumour spheroids. The smallest (absolute) percentage differences are italicized. The rightmost column shows the average (absolute) percentage difference for each method.
| group II |
group III |
|||||||
|---|---|---|---|---|---|---|---|---|
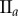 (%) (%) |
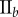 (%) (%) |
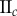 (%) (%) |
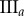 (%) (%) |
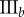 (%) (%) |
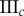 (%) (%) |
average (%) | ||
| non-periodic PCF | 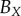 |
15.9 | 26.9 | 0.1 | 16.5 | 10.7 | 3.2 | 12.2 |
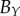 |
7.6 | 18.2 | −12.5 | 1.4 | 4.7 | 3.5 | 8 | |
| periodic PCF | 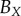 |
15.9 | 35.2 | −11.8 | 16.5 | 21.6 | 2.1 | 17.2 |
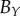 |
7.6 | 25.8 | −20.1 | 0.6 | 15.9 | 2.7 | 12.1 | |
| DBSCAN | 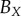 |
18.1 | 30.7 | −4.2 | −4.1 | −10.6 | −13.8 | 13.6 |
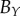 |
10.0 | 21.2 | −14.1 | −17.1 | −14.7 | −13.4 | 15.1 | |
4.1. Experiments and data collection
Nine homotypic tumour spheroids were formed from the breast cancer cell line T47D by the liquid overlay method [36]. An initial number, 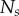 , of seed cells (table 1) together with medium were placed in a convex well, which is coated with a non-adhesive layer. Mature spheroids were obtained after being cultured for 12 days in the incubator, each with
, of seed cells (table 1) together with medium were placed in a convex well, which is coated with a non-adhesive layer. Mature spheroids were obtained after being cultured for 12 days in the incubator, each with 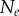 number of cells (table 1). The spheroids were then removed from the wells, fixed, stained with Draq 5 to label the nuclei, and optically cleared. Imaging of the spheroids was performed with a digital scanned laser light-sheet fluorescence microscope [37]. They were categorized, by visual inspection, into three groups: (i) spheroids with no visible necrotic core, (ii) spheroids with a small necrotic core and, (iii) spheroids with a large necrotic core. A central slice of the image stack of one tumour spheroid from each of the three groups is shown in figure 5, with central slices from the remaining six tumour spheroids presented in electronic supplementary material, figure S2 (appendix C).
number of cells (table 1). The spheroids were then removed from the wells, fixed, stained with Draq 5 to label the nuclei, and optically cleared. Imaging of the spheroids was performed with a digital scanned laser light-sheet fluorescence microscope [37]. They were categorized, by visual inspection, into three groups: (i) spheroids with no visible necrotic core, (ii) spheroids with a small necrotic core and, (iii) spheroids with a large necrotic core. A central slice of the image stack of one tumour spheroid from each of the three groups is shown in figure 5, with central slices from the remaining six tumour spheroids presented in electronic supplementary material, figure S2 (appendix C).
Table 1.
Data for nine tumour spheroids. Group I: no visible necrotic core. Group II: small necrotic core. Group III: large necrotic core. X, Y, Z are the lengths of the three semi-principle axes in micrometres for each ellipsoidal spatial domain. 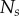 is the number of initially seeded cells for each tumour spheroid.
is the number of initially seeded cells for each tumour spheroid. 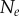 is the total number of cells in the raw dataset for each mature tumour spheroid. N is the number of cells in each data subset (i.e. ellipsoidal spatial domain) used in the spatial analysis.
is the total number of cells in the raw dataset for each mature tumour spheroid. N is the number of cells in each data subset (i.e. ellipsoidal spatial domain) used in the spatial analysis.
| group I |
group II |
group III |
|||||||
|---|---|---|---|---|---|---|---|---|---|
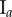 |
 |
 |
 |
 |
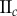 |
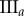 |
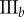 |
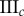 |
|
| X | 93 | 102 | 114 | 173 | 173 | 145 | 169 | 190 | 169 |
| Y | 115 | 107 | 149 | 190 | 176 | 201 | 250 | 196 | 233 |
| Z | 110 | 88 | 133 | 145 | 159 | 178 | 218 | 165 | 180 |
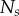 |
500 | 1000 | 2000 | 5000 | 5000 | 5000 | 10 000 | 10 000 | 10 000 |
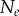 |
4597 | 3983 | 10 334 | 25 806 | 23 739 | 25 943 | 36 732 | 32 916 | 35 200 |
| N | 3607 | 3127 | 7900 | 16 356 | 15 593 | 17 864 | 30 555 | 21 226 | 24 729 |
The point patterns or raw datasets are the positions of the nuclei of the cells. Detecting the cell nuclei in the three-dimensional images of the spheroids is achieved by applying a custom multi-scale Laplacian of Gaussian (LoG) detection algorithm [38]. Further details of the detection method can be found in the electronic supplementary material, appendix D.
Subsets of the raw data points are obtained by removing data points associated with the irregularities of the surface of the tumour spheroids. For each data subset, a spatial domain is defined by an ellipsoid centred at the origin with the three semi-principal axes aligned with the Cartesian axes. This is done systematically. (i) Find the smallest convex set of points (i.e. the convex hull) that contains all the raw data points [39]. (ii) Fit an ellipsoid to the convex hull, using a linear least-squares algorithm [40], to obtain initial estimates of the lengths of the three semi-principal axes, 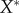 ,
, 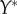 and
and 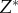 , with
, with 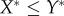 . (iii) The origin is chosen as the centre of mass in each dataset. The Matlab function #pca is used to find three orthogonal directions (principle components) in which each dataset has the largest variances. The point pattern is then rotated so that the three principle axes of each dataset coincide with the Cartesian coordinate system [41]. (iv) The data points are projected onto the interval [0, 1] using equation (4.1), with
. (iii) The origin is chosen as the centre of mass in each dataset. The Matlab function #pca is used to find three orthogonal directions (principle components) in which each dataset has the largest variances. The point pattern is then rotated so that the three principle axes of each dataset coincide with the Cartesian coordinate system [41]. (iv) The data points are projected onto the interval [0, 1] using equation (4.1), with 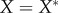 ,
, 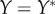 and
and 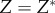 . The global maximum of F(a), given by equation (3.1), provides an estimate of the non-dimensional distance,
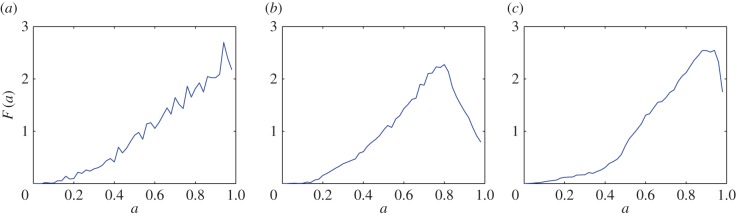
. The global maximum of F(a), given by equation (3.1), provides an estimate of the non-dimensional distance, 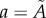 , associated with the surface irregularities of each tumour spheroid (figure 6). (v) Data points with
, associated with the surface irregularities of each tumour spheroid (figure 6). (v) Data points with 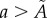 are removed from each raw dataset and the inverse map, equation (4.2), provides the lengths of the three semi-principal axes,
are removed from each raw dataset and the inverse map, equation (4.2), provides the lengths of the three semi-principal axes, 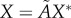 ,
, 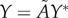 and
and 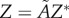 , (with
, (with 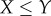 ) for the ellipsoidal spatial domains (table 1).
) for the ellipsoidal spatial domains (table 1).
Figure 6.
Removal of data points associated with the irregularities of the surface for three tumour spheroids. Points with 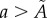 are removed from the raw datasets. (a) Tumour spheroid
are removed from the raw datasets. (a) Tumour spheroid 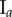 ,
, 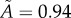 . (b) Tumour spheroid
. (b) Tumour spheroid 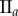 ,
, 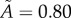 . (c) Tumour spheroid
. (c) Tumour spheroid 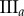 ,
, 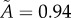 . (Online version in colour.)
. (Online version in colour.)
The subsets of N data points for each of the nine tumours (see electronic supplementary material, appendix E) are subsequently analysed with the PCF methods.
4.2. Spatial analysis
The nine subsets of data points, with ellipsoidal spatial domains, are mapped to the interval [0, 1] using equation (4.1). The non-periodic and periodic PCFs (blue curves) are shown in figure 5, and electronic supplementary material figure S2 (appendix C). The PCFs for the real tumour spheroids can be examined in a similar way to that of the synthetic tumour spheroids, as discussed in §3. However, unlike the synthetic tumour spheroids, the non-dimensional necrotic zone boundary, 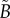 , is unknown in the real tumour spheroids. Additionally, it is difficult to provide an estimate of the necrotic zone boundary by visual examination of the PCFs alone. To overcome this difficulty, and to automate the PCF estimates of necrotic zone boundary, we fit the analytical PCFs (see electronic supplementary material, appendix A) to the statistical PCFs. A nonlinear least-squares method with two parameters
, is unknown in the real tumour spheroids. Additionally, it is difficult to provide an estimate of the necrotic zone boundary by visual examination of the PCFs alone. To overcome this difficulty, and to automate the PCF estimates of necrotic zone boundary, we fit the analytical PCFs (see electronic supplementary material, appendix A) to the statistical PCFs. A nonlinear least-squares method with two parameters 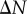 and
and 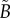 is used to find the best fit (e.g. see black curves in figure 5). The point at which there is slope discontinuity in the fitted PCF is taken as the critical point used in determining the estimates for the necrotic zone boundary,
is used to find the best fit (e.g. see black curves in figure 5). The point at which there is slope discontinuity in the fitted PCF is taken as the critical point used in determining the estimates for the necrotic zone boundary, 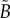 . Equation (4.2) gives estimates for the lengths,
. Equation (4.2) gives estimates for the lengths, 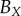 ,
, 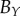 and
and 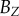 , of the three semi-principle axes of the ellipsoidal necrotic zone boundary in each tumour spheroid. The two lengths
, of the three semi-principle axes of the ellipsoidal necrotic zone boundary in each tumour spheroid. The two lengths 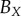 and
and 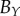 (with
(with 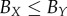 ) for each tumour spheroid are recorded in the second and third row of table 2.
) for each tumour spheroid are recorded in the second and third row of table 2.
The italicized PCF estimates in table 2 are for fitted PCFs (and therefore critical points) that reside within the CSR shaded regions (e.g. see black curves for spheroid 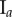 in figure 5). This means that we can reject these estimates as they may be considered as not distinctive from the CSR point process. This is the case for all three group I tumour spheroids with no visible necrotic cores, and because the group I tumour spheroids are the smallest in size it is reasonable to infer that the innermost cells still have sufficient nutrient and oxygen to remain viable [42].
in figure 5). This means that we can reject these estimates as they may be considered as not distinctive from the CSR point process. This is the case for all three group I tumour spheroids with no visible necrotic cores, and because the group I tumour spheroids are the smallest in size it is reasonable to infer that the innermost cells still have sufficient nutrient and oxygen to remain viable [42].
We compare the non-periodic and periodic PCF estimates for 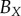 and
and 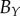 with those of a human. The human estimates are based on visual examination of a central image slice of each tumour, where white curves are superimposed onto the images to identify the necrotic core boundary in each tumour spheroid (e.g. see central image slices in figure 5). Fitting an ellipse to the white curves in each image then provides human estimates for
with those of a human. The human estimates are based on visual examination of a central image slice of each tumour, where white curves are superimposed onto the images to identify the necrotic core boundary in each tumour spheroid (e.g. see central image slices in figure 5). Fitting an ellipse to the white curves in each image then provides human estimates for 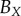 and
and 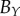 , which are shown in the first row of table 2.
, which are shown in the first row of table 2.
Recorded in table 3 (first and second rows) are the percentage difference in the PCF estimates relative to the human estimates for the group II and III tumour spheroids, with visible necrotic cores. The averaged results (rightmost column) show that the non-periodic PCF estimates have the smallest (absolute) percentage difference, when compared with those of the human. A similar result is found when calculating the overall mean squared error (MSE) for each PCF method; non-periodic PCF MSE = 45 and periodic PCF MSE = 96. We believe that the main difference between the PCF and human estimates can be attributed to the fact that the human estimates are based on a single two-dimensional central slice of each spheroid, whereas the PCF estimates are based on the three-dimensional point pattern data of each spheroid. Other contributing factors in the percentage difference are likely to include the processing method of the raw data and the spatial model used for the estimation of the critical point in the PCFs. We also note that there appears to be a positive bias in the estimates (21 out of 24 in table 3), and further investigation of this is left to future research.
To conclude the analysis, we implement an existing method commonly used to evaluate spatial clustering, using the three-dimensional point pattern data of each spheroid. The density-based spatial clustering of applications with noise (DBSCAN) algorithm classifies points in high-density regions (e.g. viable zone) as a cluster [16]. Points that are in low-density regions (e.g. necrotic zone) are classified as outliers. A subset of outliers for each tumour spheroid is used to calculate the DBSCAN estimates shown in the bottom row of table 2 (see electronic supplementary material, appendix F). The difference in the DBSCAN and human estimates is comparable to the difference in the PCF and human estimates (see bottom row of table 3), with an overall MSE = 138. The results demonstrate that the PCF method is a potentially useful alternative to existing standard clustering methods in providing estimates for the necrotic zone boundary in tumour spheroids.
5. Discussion
We have derived one-dimensional non-periodic and periodic PCFs for the radial, azimuthal and polar projections of a point pattern. This is different from the usual Euclidean distance based PCF [25–29]. Analysis of spatial structure with one-dimensional PCFs is useful in situations where the Euclidean distance is not the main quantity of interest, as is the case in identifying the necrotic zone boundary in tumour spheroids.
We analysed synthetic tumour spheroids (i.e. simulated datasets) with our PCFs to illustrate the methodology for identifying the necrotic zone boundary. It was found that a critical point (at which there is a slope discontinuity) in the non-periodic PCFs corresponds to the width of the viable zone in synthetic tumour spheroids, and this allows us to identify the necrotic zone boundary. However, for small sample sizes, when the non-periodic PCF is used, the critical point can be obscured by noise (at large distances). Using the periodic PCF, we reduced the noise in the PCFs, but with the drawback that the critical point corresponds to either the width of the viable zone, or the necrotic zone boundary itself. However, by examining both the non-periodic and periodic PCFs, we are able to provide a more statistically significant (i.e. it lies farther from CSR) estimate of the necrotic zone boundary than that obtained using the normalized cell density.
The PCF method was modified to provide estimates for the lengths of the three semi-principle axes of an ellipsoidal shaped necrotic zone boundary, and then applied to three-dimensional point patterns of nine experimental tumour spheroids. The PCF estimates were compared with those of a human and the DBSCAN method [16]. The primary difference in the computed and human estimates was attributed to the human estimates being based only on a two-dimensional slice (image) of each tumour spheroid. The average percentage differences of the PCF and DBSCAN method were comparable (see rightmost column in table 3), and this demonstrates that the PCF method potentially has merit as an alternative to the existing DBSCAN method.
The focus of this study has been to provide estimates for the radial distance(s) of the necrotic core boundary from the centre of a tumour spheroid. These estimates provide a simple metric to classify and categorize tumour spheroids, which has potential application to high-throughput comparative assays [8–10]. For example, the PCF method could be used to investigate population-level variability in the size of the necrotic zone by using a larger sample of mature tumour spheroids from the same cell line, grown from the same number of seeded cells. This would allow one to study differences in nutrient consumption between cell types, or the effects of different cell culture methods, or drug treatments. The automatic evaluation of PCF estimates has clear advantage over manual human estimates in the assessment of such high-throughput comparative assays.
The PCF method also has two main advantages over the DBSCAN method. The first being that the DBSCAN method is semi-automatic, requiring human input for each tumour spheroid analysed. The second advantage is that only the PCF method provides reliable estimates for point pattern data that is visually indistinguishable from the CSR point process (see electronic supplementary material, appendix F).
Although this work is concerned with homotypic spheroids there are approaches that aim at more complex spheroids, including different cell types and heterogeneities in the microenvironment. Our data analysis can be readily applied to datasets from such complex spheroids. Furthermore, adjusting the segementation method would allow for the extraction of cell position information from histological stains and enable the analysis of sections of cancer patient tumours. Therefore, the PCF method could potentially have a role in diagnostic testing and personalized cancer treatment.
More generally, and in addition to the estimates for the necrotic zone boundary, it is important to understand that the PCFs can provide multi-scale spatial information on tumour spheroids (e.g. figure 2). Previous studies have shown that the PCF is close to a sufficient summary statistic, essentially capturing all the spatial information in a given point pattern [34,43]. Therefore, our PCF method could be implemented in combination with inferencing algorithms such as approximate Bayesian computation [43], which require close to sufficient summary statistics, to parametrize tumour growth models for specific cell types and culture conditions [11–14].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Competing interests
We declare we have no competing interests.
Funding
The work of S.D. and J.E.F.G. was supported by an Australian Research Council Discovery Early Career Researcher Award (DE130100031) to J.E.F.G. S.D. also acknowledges a University of Adelaide Full Fees Scholarship. B.J.B. was supported by a National Health and Medical Research Council Project grant no. (APP1069757) and Australian Research Council Discovery Project Grant (DP160102644). N.G.B. was supported by the Australian Research Council Centre of Excellence for Mathematical and Statistical Frontiers (CE140100049). S.C.F., C.M., A.S. and E.H.K.S. are supported by the Deutsche Forschungsgemeinschaft (DFG) and the Cluster of Excellence for Macromolecular Complexes (CEF-MC II, EXC115).
Authors' contributions
The experiments were conceived and designed by C.M. and E.H.K.S. The experiments were performed by C.M. and the data analysis performed by A.S. The PCF method was conceived and designed by S.D., B.J.B., J.E.F.G. and N.G.B., with input from S.C.F. The mathematical derivations, numerical computations and interpretation of results were carried out by S.D. under the guidance of B.J.B., J.E.F.G., N.G.B. and S.C.F. The manuscript was written by S.D., B.J.B., J.E.F.G., S.C.F. and N.G.B. All authors edited the manuscript and approved submission.
References
- 1.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. 2010. Multicellular tumor spheroids: an underestimated tool is catching up again. J. Biotechnol. 148, 3–15. ( 10.1016/j.jbiotec.2010.01.012) [DOI] [PubMed] [Google Scholar]
- 2.Mueller-Klieser W. 1987. Multicellular spheroids. J. Cancer Res. Clin. Oncol. 113, 101–122. ( 10.1007/BF00391431) [DOI] [PubMed] [Google Scholar]
- 3.Sutherland RM. 1988. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240, 177–184. ( 10.1126/science.2451290) [DOI] [PubMed] [Google Scholar]
- 4.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. 2010. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31, 8494–8506. ( 10.1016/j.biomaterials.2010.07.064) [DOI] [PubMed] [Google Scholar]
- 5.Blacher S, et al. 2014. Cell invasion in the spheroid sprouting assay: a spatial organisation analysis adaptable to cell behaviour. PLoS ONE 9, e97019 ( 10.1371/journal.pone.0097019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinci M, et al. 2012. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 10, 29 ( 10.1186/1741-7007-10-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dairkee SH, Deng G, Stampfer MR, Waldman FM, Smith HS. 1995. Selective cell culture of primary breast carcinoma. Cancer Res. 55, 2516–2519. [PubMed] [Google Scholar]
- 8.Deisboeck T, Berens M, Kansal A, Torquato S, Stemmer-Rachamimov A, Chiocca E. 2001. Pattern of self-organization in tumour systems: complex growth dynamics in a novel brain tumour spheroid model. Cell Prolif. 34, 115–134. ( 10.1046/j.1365-2184.2001.00202.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufau I, et al. 2012. Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer. BMC Cancer 12, 15 ( 10.1186/1471-2407-12-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Fischer S, Schuman E, Stelzer E. 2015. Lateral assembly of N-cadherin drives tissue integrity by stabilizing adherens junctions. J. R. Soc. Interface 12, 20141055 ( 10.1098/rsif.2014.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araujo R, McElwain D. 2004. A history of the study of solid tumour growth: the contribution of mathematical modelling. Bull. Math. Biol. 66, 1039–1091. ( 10.1016/j.bulm.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 12.Casciari JJ, Sotirchos SV, Sutherland RM. 1992. Mathematical modelling of microenvironment and growth in EMT6/Ro multicellular tumour spheroids. Cell Prolif. 25, 1–22. ( 10.1111/j.1365-2184.1992.tb01433.x) [DOI] [PubMed] [Google Scholar]
- 13.Grimes DR, Kelly C, Bloch K, Partridge M. 2014. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J. R. Soc. Interface 11, 20131124 ( 10.1098/rsif.2013.1124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein AM, Demuth T, Mobley D, Berens M, Sander LM. 2007. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys. J. 92, 356–365. ( 10.1529/biophysj.106.093468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stelzer EHK. 2015. Light-sheet fluorescence microscopy for quantitative biology. Nat. Methods 12, 23–26. ( 10.1038/nmeth.3219) [DOI] [PubMed] [Google Scholar]
- 16.Ester M, Kriegel HP, Sander J, Xu X.1996. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proc. of the Second International Conf. on Knowledge Discovery and Data Mining (KDD), Portland, Oregon, vol. 96, pp. 226–231. See http://www.aaai.org/Papers/KDD/1996/KDD96-037.pdf .
- 17.Hartigan JA, Wong MA. 1979. Algorithm AS 136: A K-means clustering algorithm. Appl. Stat. 28, 100–108. ( 10.2307/2346830) [DOI] [Google Scholar]
- 18.Jain AK, Dubes RC. 1988. Algorithms for clustering data. Upper Saddle River, NJ: Prentice-Hall, Inc. [Google Scholar]
- 19.Agnew DJG, Green JEF, Brown TM, Simpson MJ, Binder BJ. 2014. Distinguishing between mechanisms of cell aggregation using pair-correlation functions. J. Theor. Biol. 352, 022705. [DOI] [PubMed] [Google Scholar]
- 20.Binny RN, Plank MJ, James A. 2015. Spatial moment dynamics for collective cell movement incorporating a neighbour-dependent directional bias. J. R. Soc. Interface 12, 20150228 ( 10.1098/rsif.2015.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fozard JA, Kirkham GR, Buttery LD, King JR, Jensen OE, Byrne HM. 2011. Techniques for analysing pattern formation in populations of stem cells and their progeny. BMC Bioinform. 12, 396 ( 10.1186/1471-2105-12-396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattfeldt T, Gottfried HW, Frey H, Vogel U. 1993. Second-order stereology of prostatic adenocarcinoma and normal prostatic tissue. Acta Stereol. 12, 203–208. [Google Scholar]
- 23.Riedel IH, Kruse K, Howard J. 2005. A self-organized vortex array of hydrodynamically entrained sperm cells. Science 309, 300–303. ( 10.1126/science.1110329) [DOI] [PubMed] [Google Scholar]
- 24.Treloar KK, Simpson MJ, Binder BJ, McElwain DS, Baker RE. 2014. Assessing the role of spatial correlations during collective cell spreading. Sci. Rep. 4, 5713 ( 10.1038/srep05713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler D, Percus JK. 1988. Introduction to modern statistical mechanics. Phys. Today 41, 114 ( 10.1063/1.2811680) [DOI] [Google Scholar]
- 26.Dieckmann U, Law R, Metz JA. 2000. The geometry of ecological interactions: simplifying spatial complexity, vol. 1 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Illian J, Penttinen A, Stoyan H, Stoyan D. 2008. Statistical analysis and modelling of spatial point patterns, vol. 70 Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 28.Jones BJ, Martínez VJ, Saar E, Trimble V. 2005. Scaling laws in the distribution of galaxies. Rev. Mod. Phys. 76, 1211 ( 10.1103/RevModPhys.76.1211) [DOI] [Google Scholar]
- 29.Martínez VJ, Saar E. 2010. Statistics of the galaxy distribution. Boca Raton, FL: CRC Press. See; http://www.crcnetbase.com/doi/book/10.1201/9781420036169. [Google Scholar]
- 30.Ripley BD. 1976. The second-order analysis of stationary point processes. J. appl. Probability 13, 255–266. See https://www.cambridge.org/core/journals/journal-of-applied-probability/article/the-second-order-analysis-of-stationary-point-processes/63ECBD08B25F19C844C8FEC5B88C5266. [Google Scholar]
- 31.Ohser J, Mücklich F. 2000. Statistical analysis of microstructures in materials science. Chichester, UK: Wiley; See http://au.wiley.com/WileyCDA/WileyTitle/productCd-0471974862.html. [Google Scholar]
- 32.Stoyan D, Stoyan H. 1996. Estimating pair correlation functions of planar cluster processes. Biom. J. 38, 259–271. ( 10.1002/bimj.4710380302) [DOI] [Google Scholar]
- 33.Stoyan D, Stoyan H. 2000. Improving ratio estimators of second order point process characteristics. Scand. J. Stat. 27, 641–656 ( 10.1111/1467-9469.00213) [DOI] [Google Scholar]
- 34.Binder BJ, Simpson MJ. 2013. Quantifying spatial structure in experimental observations and agent-based simulations using pair-correlation functions. Phys. Rev. E 88, 022705 ( 10.1103/PhysRevE.88.022705) [DOI] [PubMed] [Google Scholar]
- 35.Binder BJ, Sundstrom JF, Gardner JM, Jiranek V, Oliver SG. 2015. Quantifying two-dimensional filamentous and invasive growth spatial patterns in yeast colonies. PLoS Comput. Biol. 11, e1004070 ( 10.1371/journal.pcbi.1004070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlsson J, Yuhas JM. 1984. Liquid-overlay culture of cellular spheroids. In Spheroids in cancer research: methods and perspectives (eds Acker H, Carlsson J, Durand R, Sutherland RM), pp. 1–23. Berlin, Germany: Springer; See 10.1007/978-3-642-82340-4_1. [DOI] [PubMed] [Google Scholar]
- 37.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. 2008. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069. ( 10.1126/science.1162493) [DOI] [PubMed] [Google Scholar]
- 38.Lowe DG. 2004. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 60, 91–110. ( 10.1023/B:VISI.0000029664.99615.94) [DOI] [Google Scholar]
- 39.De Berg M, Van Kreveld M, Overmars M, Schwarzkopf OC. 2000. Computational geometry. Berlin, Germany: Springer. [Google Scholar]
- 40.Rosin PL. 1993. A note on the least squares fitting of ellipses. Pattern Recognit. Lett. 14, 799–808. ( 10.1016/0167-8655(93)90062-I) [DOI] [Google Scholar]
- 41.Jolliffe I. 2014. Principal component analysis. Hoboken, NJ: John Wiley & Sons, Ltd. [Google Scholar]
- 42.Greenspan HP. 1972. Models for the growth of a solid tumor by diffusion. Stud. Appl. Math. 51, 317–340. ( 10.1002/sapm1972514317) [DOI] [Google Scholar]
- 43.Johnston ST, Simpson MJ, McElwain DS, Binder BJ, Ross JV. 2014. Interpreting scratch assays using pair density dynamics and approximate Bayesian computation. Open Biol. 4, 140097 ( 10.1098/rsob.140097) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.