Abstract
Context:
Patients with pheochromocytoma (pheo) show presence of multilocular adipocytes that express uncoupling protein 1 within periadrenal (pADR) and omental (OME) fat depots. It has been hypothesized that this is due to adrenergic stimulation by catecholamines produced by the pheo tumors.
Objective:
To characterize the prevalence and respiratory activity of brown-like adipocytes within pADR, OME, and SC fat depots in human adult pheo patients.
Design:
This was an observational cohort study.
Setting:
The study took place in a university hospital.
Patients:
We studied 46 patients who underwent surgery for benign adrenal tumors (21 pheos and 25 controls with adrenocortical adenomas).
Main outcome measure:
We characterized adipocyte browning in pADR, SC, and OME fat depots for histological and immunohistological features, mitochondrial respiration rate, and gene expression. We also determined circulating levels of catecholamines and other browning-related hormones.
Results:
Eleven of 21 pheo pADR adipose samples, but only one of 25 pADR samples from control patients exhibited multilocular adipocytes. The pADR browning phenotype was associated with higher plasma catecholamines and raised uncoupling protein 1. Mitochondria from multilocular pADR fat of pheo patients exhibited increased rates of coupled and uncoupled respiration. Global gene expression analysis in pADR fat revealed enrichment in β-oxidation genes in pheo patients with multilocular adipocytes. No SC or OME fat depots exhibited aspects of browning.
Conclusion:
Browning of the pADR depot occurred in half of pheo patients and was associated with increased catecholamines and mitochondrial activity. No browning was detected in other fat depots, suggesting that other factors are required to promote browning in these depots.
Browning of the periadrenal fat occurred in half of pheochromocytoma patients and was associated with increased catecholamines and mitochondrial activity. No browning was detected in other fat depots.
Evidence for the presence of functional brown adipose tissue (BAT) in humans (1–4) rekindled research into the proposal made more than 30 years earlier that the high fat-oxidizing, energy-expending capacity of BAT might be exploited to treat obesity (5). The hallmark property of BAT that promotes energy expenditure is the expression of uncoupling protein (UCP)1, a mitochondrial transporter that creates proton leaks across the inner mitochondrial membrane, leading to the dissipation of energy as heat (6). Positron emission tomography-computed tomography imaging revealed that fat depots in the supraclavicular region exhibit high [18F]-fluorodeoxyglucose uptake, suggesting high metabolic activity (2–4) that was increased upon cold exposure (4). Potential strategies to increase energy expenditure include activation of established BAT depots or induction of brown adipocyte progenitors within white adipose tissue (WAT) depots using pharmacological or environmental stimuli (7–9). Thus far, there is limited evidence regarding whether humans have the potential for appreciable induction of metabolically active brown adipocytes within WAT.
Studies in mice have revealed that “classical” brown adipocytes from the interscapular adipose depot are derived from a cell lineage that is distinct from white adipocytes. Brown adipocytes derive from a Myf5+ lineage, whereas brown-like adipocytes that are induced within WAT (referred to as “beige” or “brite” cells) originate preferentially from Myf5– progenitor cells (10). Brown and beige adipocytes have distinct molecular and developmental characteristics, but the mitochondrial and regulatory differences are not fully understood. In humans, fat depots that exhibit a brown/beige gene expression signature include the supraclavicular, paravertebral, perirenal, and epicardial fat depots. These fat depots appear to be a mixture of brown and/or beige adipocytes embedded within WAT (7, 11–15). The identification of specific markers for brown vs beige adipocytes has been difficult due to a lack of pure brown or beige fat samples, as well as possible differences between humans and mice.
Pheochromocytoma (pheo) is a catecholamine-secreting neoplasm arising primarily from the adrenal medulla or within paragangliomas (16). Multilocular adipocytes with UCP1 expression have been detected in pheo patients in perirenal fat (17–20) and omental (OME) fat (21, 22), but not in SC adipose tissue (19, 22). These studies have been constrained by very small samples sizes. In addition, the browning in perirenal and OME fat does not seem to occur in all pheo patients, and may be as low as 50–60% (18, 21). At present, it is unknown whether the brown-like adipocytes that occur in WAT of some pheo patients exhibit metabolic changes that are characteristic of brown adipocytes, such as increased mitochondrial function and uncoupled respiration.
In the present study, we have characterized multiple WAT depots from pheo subjects and control subjects with noncatecholamine secreting adrenal tumors 1) to determine the prevalence of browning in different anatomical WAT depots and 2) to determine whether pheo-associated WAT browning is associated with increased mitochondrial respiration activity.
Materials and Methods
Human subjects
The study protocol was reviewed and approved by the University of California Los Angeles Medical Institutional Review Board. Each patient provided written consent after the study goals, side effects, and tissue sampling procedure were explained in detail. Patients in the experimental group had sporadic unilateral benign adrenal pheos (ie, no family history of a pheo). Pheo patients were routinely prepared preoperatively with a 2- to 4-week course of the long-acting α-adrenergic receptor blocking drug phenoxybenzamine supplemented, when needed, with β-blockers. Patients in the control group had aldosterone–or cortisol-secreting, or nonfunctioning neoplasms. Exclusion criteria were 1) paragangliomas, 2) malignant tumor, 3) type 2 diabetes treated with a thiazolidinedione, or 4) untreated hyper- or hypothyroidism.
Fat biopsies were removed after tumor resection under stable intraoperative hemodynamic conditions. Depending on the surgical approach taken to perform adrenalectomy, approximately 1 g was resected from superficial SC fat in the anterior upper abdomen or in the posterior abdominal wall below the 12th rib. A total of 1–3 g was taken from retroperitoneal fat adjacent to the adrenal tumor. When the operative approach was ip, 1–3 g OME fat was also collected. Fat samples were placed on ice and processed within 30 minutes of collection. After cleaning, fat samples were cut into pieces and used fresh for bioenergetics experiments, fixed in formalin for histology, or snap frozen in liquid nitrogen and stored at –80° C.
Blood collection and analyses
Fasting blood was collected between 7 am and 10 am in EDTA tubes from patients just before entering the operating room. Plasma aliquots were sent to Quest Diagnostics to measure fractionated catecholamines by HPLC or stored at –80 C. Plasma glucose concentrations were measured using a colorimetric glucose assay kit (GAGO-20, Sigma-Aldrich). Atrial natriuretic peptide (ANP) (EIA-ANP, RayBiotech, Inc.), B-type natriuretic peptide (BNP) (ELH-BNP, RayBiotech, Inc.), and cortisol (ADI-900-071, Enzo) were measured according to the manufacturer's instructions. For cortisol determination, steroid displacement reagent was used to displace steroid binding to proteins.
Histology
Hematoxylin and eosin staining was performed on 7-μm sections from each fat depot. Several sections from different regions of each biopsy were evaluated. The presence of unilocular and multilocular adipocytes in each section was assessed visually by bright-field microscopy by two to three independent observers.
Immunohistochemistry
The presence of UCP1 protein in tissue samples was assessed by immunohistochemistry with a UCP1 antibody (#662045, Calbiochem) at 1:500 dilution. Staining specificity was confirmed on slides where primary antibody was omitted. Sections were counterstained with hematoxylin.
Gene expression analysis
RNA was extracted from frozen tissue samples using TRIzol (Life Technologies). For real-time quantitative PCR analysis (RT-qPCR), 1 μg of RNA was reverse transcribed using iScript (Bio-Rad). A standard curve was constructed from pooled cDNA samples to take account the efficiency of primers and to obtain the standard quality values. Target gene standard quality values were normalized to B2M and 36B4, which did not differ significantly between the groups. Primer sequences are listed in Supplemental Table 1.
For global gene expression, RNA from periadrenal (pADR) fat depots was arrayed on an Illumina HT-12 v4.0 bead chip at the University of California, Los Angeles Neuroscience Genomics Core. Analysis was performed with GenomeStudio V2011.1 using quantile normalization, background subtraction, and a present call P < .05. Differentially regulated genes were defined as having more than 2-fold difference compared to control. Lists of genes that were significantly up- or down-regulated were subjected to functional enrichment using DAVID annotation tools and the “single protein of protein information resource” (SP_PIR) category (23). Venn analysis and heat map representations were obtained with GenePattern (genepattern.broadinstitute.org).
Protein analysis by Western blotting
Western blots were performed essentially as published previously (14) with minor changes. Briefly, 8 μg mitochondrial protein extracts were separated by SDS-PAGE and transferred to a nitrocellulose membrane. After blocking in 5% milk and 0.2% Tween 20 in Tris-buffered saline, anti-UCP1 antibody (1:1000 dilution, #662045, Calbiochem) was incubated overnight at 4° C in 3% milk and 0.2% Tween 20 in Tris-buffered saline. An anticytochrome c antibody (136F3, Cell Signaling Technology) was used in 5% BSA and also incubated overnight. A goat antirabbit secondary antibody (sc-2030, Santa Cruz Biotechnology, Inc.) was used at 1:20,000 dilution for 1 hour at room temperature. Immunoreactive bands were developed with ECL Prime (RPN2232, Amersham) and visualized with a Bio-Rad Gel-Doc imager.
Mitochondrial bioenergetics
Mitochondria were isolated from fresh tissues and immediately used in an XF24 Analyzer (Seahorse Bioscience) as previously described (24). Briefly, mitochondrial protein yield was determined by Bradford assay and 50 μg pADR or 100 μg SC or OME mitochondria were seeded per well by centrifugation. For the coupling assay, basal oxygen consumption rate (OCR) was measured in the presence of 10 mM succinate and 2 μM rotenone, and after sequential addition of 4 mM adenosine 5'-diphosphate (Complex V substrate), 2.5 μg/ml oligomycin (Complex V inhibitor), 4 μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (mitochondrial uncoupler) and 4 μM antimycin A (Complex III inhibitor). For electron flow assays, basal OCR was measured in presence of 10 mM pyruvate (Complex I substrate), 2 mM malate and 4 μM FCCP, and after sequential addition of 2 μM rotenone (Complex I inhibitor), 10 mM succinate (Complex II substrate), 4 μM antimycin A (Complex III inhibitor), and 1 mM N,N,N',N'-tetramethyl-p-phenylenediamine containing 10 mM ascorbate (Complex IV substrate). OCR was normalized per microgram of mitochondrial protein.
Statistics
Statistical analyses were performed with GraphPad Prism. Normal distribution of samples was tested to select parametric or nonparametric tests as indicated in the figure legends. Two-tailed Student's t test or one-way ANOVA for multiple comparisons was used to determine P values. Pearson's coefficient correlation (r) and P values were calculated for the linear correlations. All results are expressed as mean ± SEM or mean ± SD for subject characteristics. Statistical significance was defined as P < .05.
Results
Forty-six patients with benign adrenal tumors were enrolled in the study; clinical characteristics are presented in Table 1. Of these, 21 tumors were confirmed to be pheos on histopathology and 25 were adrenal cortical adenomas, which served as controls. The controls included 12 aldosterone-secreting adenomas, two cortisol-secreting adenomas, and 11 nonfunctioning tumors. The mean age, plasma glucose, and free fatty acid levels were not different between the pheo and control groups. Body mass index was lower in the pheo group compared to controls (P = .048).
Table 1.
Characteristics of Subjects Used in the Study
| Control Subjects | All Pheo | PheoUni | PheoMulti | |
|---|---|---|---|---|
| Age (y) | 53.4 ± 8.2 | 51.2 ± 13.4 | 47.1 ± 13.6 | 54.9 ± 12.7 |
| Male/female (n) | 15/10 | 9/12 | 4/6 | 5/6 |
| BMI (kg/m2) | 30.3 ± 7.1 | 26.6 ± 4.5a | 27.7 ± 5.6 | 25.7 ± 3.4 |
| Glucose (mg/dl) | 88.9 ± 35.5 | 78.6 ± 12.8 | 84.1 ± 15.7 | 74.1 ± 7.9 |
| FFA (mmol/liter) | 0.73 ± 0.40 | 0.54 ± 0.30 | 0.47 ± 0.15 | 0.58 ± 0.34 |
| ANP (pg/ml) | 33.0 ± 10.2 | 32.2 ± 4.2 | 31.9 + 8.3 | 32.4 ± 1.1 |
| BNP (pg/ml) | 116 ± 81 | 134 ± 88 | 177 ± 91 | 102.3 ± 77 |
| Cortisol (μg/dl) | 11.5 ± 3.1 | 9.2 ± 3.5 | 9.3 ± 4.9 | 9.0 ± 2.0 |
Abbreviations: ANP, atrial natriuretic peptide; BMI, body mass index; BNP, B-type natriuretic peptide; FFA, free fatty acid; pheo, pheochromocytoma; pheoMulti, having some multilocular adipocytes; pheouni, having exclusively unilocular adipocytes.
Data are expressed as mean ± sd.
P < .05 vs control.
Histology was performed for pADR and SC fat samples from all patients (21 pheos, 25 controls). OME fat was collected only from individuals having ip surgery, leading to availability of OME samples from only five control and four pheo patients. All sections examined from OME and SC depots contained adipocytes with unilocular morphology. In pADR samples, multilocular adipocytes characteristic of brown/beige adipose tissue were present in 52.4% (11/21) of pheo samples, but in only 4% (1/25) of the controls (P < .001, Fisher's exact test). Typically, the multilocular adipocytes in pADR fat occurred in pockets that were dispersed throughout the white adipocytes (Figure 1 and Supplemental Figure 1). Based on histology, we classified pheo subjects for subsequent analysis as either pheoUni (having exclusively unilocular adipocytes) or pheoMulti (having some multilocular adipocytes).
Figure 1.
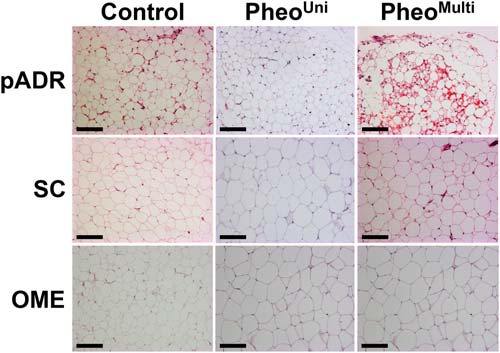
Histomorphology of white adipose tissue depots. Representative images of hematoxylin and eosin stained sections from pADR, SC, and OME fat of control and pheo patients. Multilocular adipocytes were present exclusively in pADR fat, and observed in 4% and 52.4% of control and pheo patients, respectively (10× magnification. Black bar represents 100 μm).
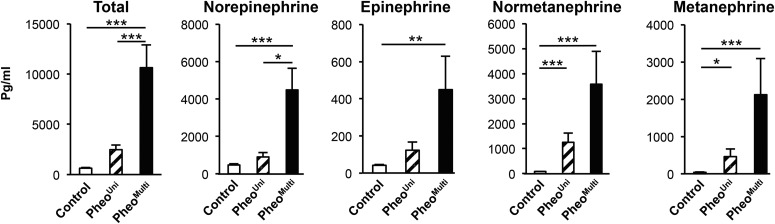
The control, pheoUni, and pheoMulti groups did not differ in age, body mass index, or plasma glucose and free fatty acid levels (Table 1). ANP and BNP as well as cortisol levels have been associated with browning in WAT depots (25–27). Plasma levels of these hormones did not differ among the three groups of patients (Table 1), suggesting no influence on browning in the pheoMulti group. In addition, use of β-blockers was equally divided between pheoUni and pheoMulti (5/10 and 5/11 patients, respectively) thus ruling out an inhibitory effect of the β-adrenergic blockers on adipocyte browning. We hypothesized that differences in catecholamine levels released by the adrenal tumors may influence the development of multilocular adipocytes. The pheoMulti group had higher total and individual plasma catecholamines (norepinephrine, epinephrine, normetanephrine, and metanephrine) than the control group (Figure 2). Notably, the pheoMulti group had higher total catecholamine and norepinephrine levels than the pheoUni group. The pheoUni group also showed significantly higher normetanephrine and metanephrine levels than controls, but the levels were not as high as in the pheoMulti group. Thus, the pheoMulti patients had higher levels than control subjects for all catecholamines measured, and were distinguished from the pheoUni subjects by higher total catecholamine levels.
Figure 2.
Plasma catecholamine levels in control and pheo patients. PheoMulti patients show the highest catecholamine levels. *P < .05, **P < .01, ***P < .001. Data analyzed by Kruskal-Wallis multiple comparison test, except for total catecholamine levels where a one-way ANOVA multiple comparison test was used (n = 10–25).
We examined UCP1 mRNA and protein levels in adipose tissue from pADR, SC, and OME depots. UCP1 mRNA abundance was significantly higher in pADR fat from the pheoMulti group compared to the control (9-fold) and the pheoUni (24-fold) groups (Figure 3A). By contrast, UCP1 mRNA levels in SC or OME depots were low and not different between the three patient groups. Immunohistochemistry localized UCP1 protein exclusively to multilocular adipocytes within the pADR fat samples (Figure 3B). No UCP1 staining was observed in pADR fat from control or pheoUni groups, or in OME or SC fat depots from any group. By Western blot analysis, we detected UCP1 protein in pADR fat from individuals in the pheoMulti group, but not in the pheoUni or control groups (Figure 3C). No UCP1 protein was detected in SC or OME fat from any group (data not shown).
Figure 3.
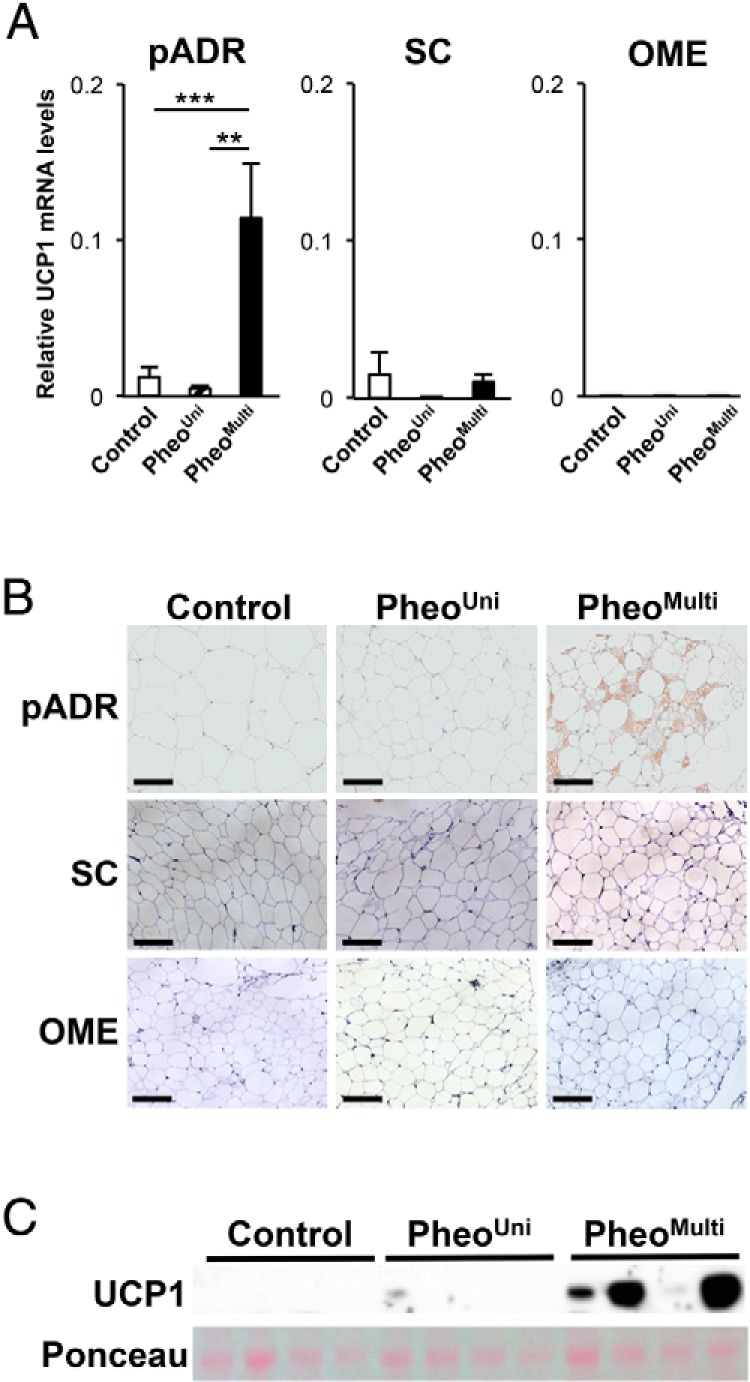
Detection of UCP1 in pADR fat. A, UCP1 mRNA levels in pADR (n = 8–20), SC (n = 6–20), and OME (n = 2–5) fat. **P < .01, ***P < .001 using a one-way ANOVA multiple comparison test. B, Immunohistochemistry using an antibody against UCP1. Positive staining was present only in the multilocular adipocytes from pADR fat (10× magnification. Black bar represents 100 μm). C, UCP1 protein in pADR fat detected by Western blot. Ponceau red stain of total protein is shown for normalization.
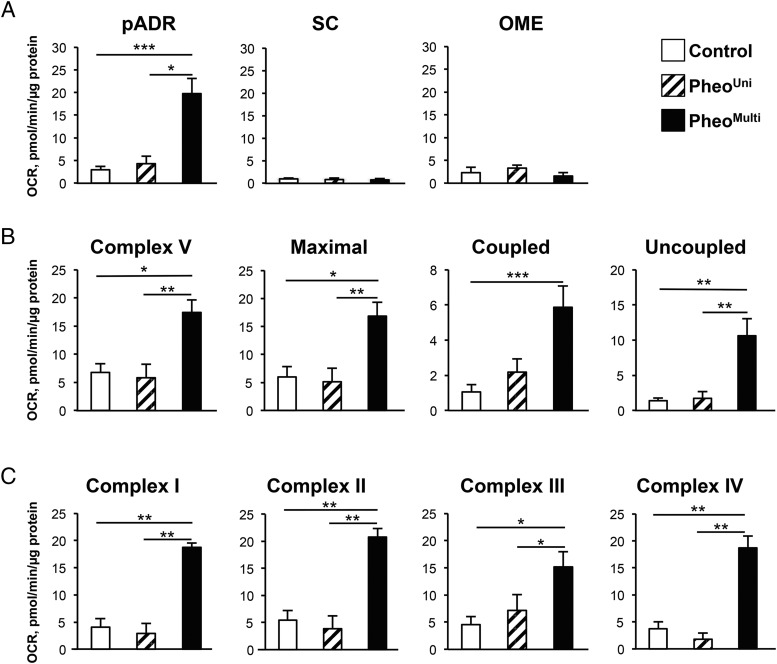
The presence of multilocular adipocytes expressing UCP1 in pADR fat from pheo patients has been reported previously (17–20), but it has not been determined whether these adipocytes exhibit enhanced mitochondrial function. To evaluate, we isolated mitochondria from the pADR, SC, and OME fat depots of control, pheoUni, and pheoMulti groups and measured total respiration and activity of specific mitochondrial respiratory chain complexes. First, using a coupling assay, basal oxygen consumption rate (OCR) was 7-fold higher in mitochondria from pADR fat of pheoMulti subjects than from the other groups (Figure 4A), whereas in SC and OME fat depots it was similar in all groups. Complex V and maximal respiration rates were also raised in the pheoMulti group (Figure 4B) as was coupled and uncoupled respiration rates. Finally, we assessed the activity of the respiratory chain complexes I–IV by performing an electron flow assay. We detected increased OCR for all four complexes in pheoMulti mitochondria compared to both control and pheoUni groups, while pheoUni and control groups did not differ from one another (Figure 4C). Overall, these results demonstrate that mitochondria from the pADR fat of pheoMulti patients have higher electron transport chain (ETC) activity and capacity.
Figure 4.
Mitochondrial respiration is increased in pheoMulti pADR fat. A, Complex II-driven oxygen consumption rate (OCR) in isolated mitochondria from pADR (n = 8–12), SC (n = 5–10), and OME (n = 2–3) fat depots. B, Mitochondrial respiration parameters from a coupling assay. Complex V and maximal respiration were obtained after sequentially injections of adenosine 5'-diphosphate and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, respectively. Coupled respiration was the oligomycin-sensitive OCR while uncoupled was the OCR difference between oligomycin and antimycin A injections (n = 8–12). C, Different electron transport chain complexes respiration. Complex I, II, and IV respirations were measured after the sequential injection of pyruvate, succinate, and ascorbate/N,N,N',N'-tetramethyl-p-phenylenediamine, respectively. Complex III respiration corresponded to the antimycin A-sensitive respiration (n = 5–12). *P < .05, **P < .01, ***P < .001. Data analyzed by Kruskal-Wallis multiple comparison test.
To evaluate the relationship between UCP1 and mitochondrial uncoupling, we assessed the Pearson's correlation between the two traits in all pheo patients. There was a significant positive correlation between UCP1 mRNA levels and uncoupled respiration rate (r = 0.536; P < .05). Correlations between total catecholamines and mitochondrial ETC respiratory chain complex I (r = 0.743; P < .01), II (r = 0.802; P < .01), III (r = 0.806; P < .01), and IV (r = 0.762; P < .01) were each significant, suggesting an association between plasma catecholamines and mitochondrial activity.
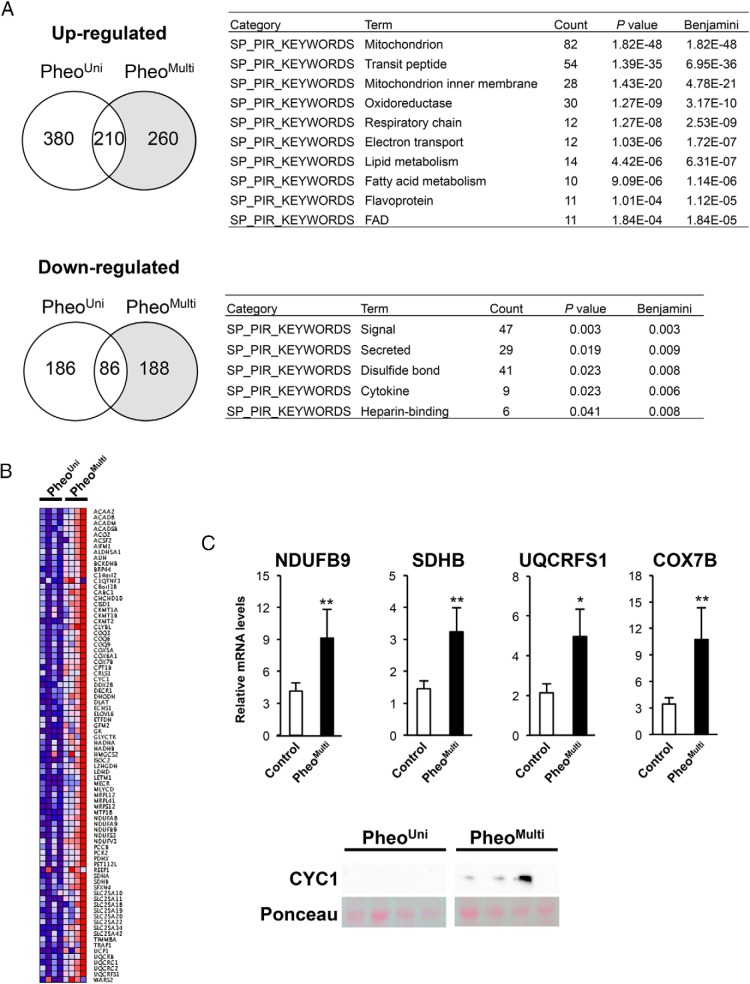
To provide an unbiased assessment of transcriptional changes that lead to enhanced mitochondrial activation in pADR fat of pheoMulti samples, we performed gene expression profiling. We analyzed pADR adipose tissue mRNA from control, pheoUni, and pheoMulti samples (n = 4 patients from each group) by microarray hybridization. Compared to controls, pheoMulti samples showed 2-fold up-regulation of 470 genes and down-regulation of 274 genes (P < .05); pheoUni samples showed up-regulation of 590 and down-regulation of 272 genes (Figure 5A). Most relevant to the observed differences in mitochondrial activation between pheoMulti and pheoUni groups, 260 genes were uniquely up-regulated, and 188 genes uniquely down-regulated, in pheoMulti fat (Figure 5A, shaded region).
Figure 5.
Gene expression profiling of pADR fat from control, pheoUni and pheoMulti subjects by microarray analysis. (A) Left, Venn diagrams illustrating the genes up- and down-regulated in pADR fat from pheoUni and pheoMulti compared to control subjects. Right, functional enrichment analysis of genes that are uniquely up- or down-regulated in the pheoMulti group, using DAVID analysis with SP_PIR categories. The number of genes for each functional category (Count), enrichment P values, and multiple testing correction (Benjamini < 0.0001 and < 0.05 for up- and down-regulated genes, respectively) are presented. (B) Heat map representation of gene expression levels for genes in the top SP_PIR category (“mitochondrion”) genes that are up-regulated in the pheoMulti group. Genes are presented in alphabetical order. (C) Validation of five electron transport genes up-regulated in pADR pheoMulti by RT-qPCR or Western blot. *P < .05, **P < .01 using a one-way ANOVA multiple comparison test (n = 7–20).
We performed functional annotation of the genes that were specifically altered in the pheoMulti group (shaded regions in Figure 5A) using the DAVID functional annotation tool (23). The genes that were specifically up-regulated in pheoMulti pADR fat were enriched in mitochondrion- and oxidation reduction-related categories (Figure 5A). The genes that were uniquely down-regulated in pheoMulti pADR fat were enriched in categories that include signaling, secreted proteins, and cytokines (Figure 5B). The heat map in Figure 5B displays the expression pattern of the 82 up-regulated genes present in the top enrichment category, mitochondrion (P < 1.82E-48). Genes in this category were associated with the tricarboxylic acid cycle (ACO2, L2HGDH, DLAT, PDHX), β-oxidation (ACAA2, ACADM, CPT1B, HADHA, HADHB), and respiration (BRP44, CABC1, ETFDH, SFXN4, UCP1). Notably, several genes were components of the electron transport chain complex I (NDUFA8, NDUFA9, NDUFS3, NDUFV3), complex II (SDHA and SDHB), complex III (CYC1, COQ3, COQ6, COQ9), coenzyme Q complex (UQCRB, UQCRC1, UQRC2, UQCRFS1), and complex IV (COX5A, COX6A1, COX7B). We validated expression levels of several genes and proteins by RT-qPCR or Western blot. ETC-related gene expression levels were significantly higher in the pheoMulti compared to the control group (Figure 5C). These gene expression differences, together with increased mitochondrial activity, suggest that mitochondria in pheoMulti pADR adipose tissue are altered to promote higher β-oxidation and respiration.
The expression of specific gene markers has been proposed to distinguish brown adipocytes from beige adipocytes (7, 15, 28–31). We assessed representative brown and beige gene expression markers in pADR fat in our control, pheoUni, and pheoMulti samples using the microarray data or by RT-qPCR (Supplemental Table 2). Of 17 genes assessed in pADR, only PAT2 exhibited higher expression in pheoMulti compared to the other groups. These data suggest that the multilocular adipocytes in pADR fat do not exhibit a typical classical brown or beige adipose tissue gene expression signature.
Discussion
In the present study, we analyzed the effects of increased catecholamine levels present in pheo patients on browning of adipose tissue depots located adjacent to and distant from the pheo tumors. In our sample, which represents the largest series of pheo patients analyzed for effects on browning reported to date, we identified multilocular adipocytes that express UCP1 in approximately half of the pheo subjects. Adipocytes with brown character were detected in pADR fat, but not in SC or OME adipose tissue depots, which are anatomically distant from the pheo tumor. The subset of pheo patients that exhibited multilocular adipocytes containing UCP1 had higher plasma catecholamine levels than pheo patients that did not exhibit multilocular pADR fat and control subjects with noncatecholamine-secreting adrenal tumors. We demonstrate, for the first time, that the browning phenotype occurring in pADR fat of pheo subjects is associated with elevated mitochondrial respiration, characterized by increased activity of all ETC complexes, as well as increased uncoupled respiration. The increased mitochondrial respiration was associated with elevated expression of a panel of genes involved in mitochondrial energy metabolism.
Pheochromocytoma is a catecholamine-secreting tumor, but there is variation among patients in the levels of circulating catecholamines and in the time between tumor formation and removal, which may be several years (16). Our detection of browning in pADR fat of 11/21 of pheo patients is consistent with a previous study where 62% of pheo cases (5/8) had multilocular adipocytes (18). Notably, we found that the induction of browning did not extend to SC or OME fat depots despite the fact that the catecholamines secreted by the adrenal tumors enter the systemic circulation. In mice, the SC fat depot is susceptible to browning, but may have less capacity to undergo remodeling in humans (9). For example, in a previous study of eight pheo patients, multilocular adipocytes were visible in the pADR fat but not the SC depot (19). Similarly, no browning was detected in abdominal fat after 8 hours of cold exposure in overweight human adults under conditions that activated BAT glucose metabolism and increased energy expenditure (32). Healthy human volunteers exposed to 10 days of cold also did not show SC browning despite elevated plasma catecholamines (33). In contrast, using major burn trauma as a model for adrenergic stress, multilocular adipocytes expressing UCP1 in SC fat were observed after only 3 weeks (34, 35). Increased UCP1 expression (3-fold) was also observed in SC fat during winter (36). There are some reports of browning in OME fat in a portion of pheo patients (21, 22) but plasma catecholamines were not reported, making it difficult to compare to the current study. Variations in browning in pheo patients could be due to catecholamines, genetic, and/or environmental factors that differ among individuals (eg, seasonal temperature at the time samples were obtained).
The induction of browning in pheo subjects appears to be adipose depot-dependent, with pADR, and to a lesser extent OME fat depots, more prone to adipose tissue remodeling than SC fat. These findings could have important repercussions on the use of thermogenic agonists to modulate obesity since most human fat is stored in SC depots (37). Differences in vascularization, innervation, or intrinsic properties of adipocyte precursor cells could contribute to differences in the capacity for browning among adipose depots together with mitochondrial capacity to increase respiratory activity, a prominent feature of pADR fat of pheo patients that exhibited browning. We also cannot rule out the possibility that elevated local catecholamine levels adjacent to the tumor play a role in the browning of pADR fat in pheo patients.
Molecular markers for classical brown adipocytes vs beige adipocytes have been identified in mice, and have also been used to characterize human brown/beige cells (7, 11–13, 15, 30, 38). By measuring several of these mRNA markers we did not observe a clear classical brown or beige signature in pADR of pheoMulti patients. The use of these gene expression markers to distinguish brown and beige adipocytes remains controversial and inconclusive. We cannot rule out that the heterogeneity of the pADR tissue, containing regions of typical white adipocytes neighboring the pockets of multilocular adipocytes, may prevent definitive gene expression profiles to be determined. Additionally, it is possible that beige adipocyte markers are fat depot-specific—that is, pADR, OME, and epicardial fat may not induce the same subset of genes during browning, leading to distinct molecular signatures (39, 40). Regardless of the gene expression markers present, our studies of mitochondrial activity definitively demonstrate that pADR fat from pheoMulti patients exhibits a key functional characteristic of brown and beige adipocytes in having enhanced total and uncoupled respiratory activity and up-regulation of genes directly associated with mitochondrial activity. Future studies may reveal whether these genes are also up-regulated in white adipose tissue in other conditions that promote browning.
In conclusion, the phenotypic browning in pADR fat of pheo patients is accompanied by metabolic alterations in mitochondrial activity and related gene expression changes, which could influence fuel utilization and energy expenditure. The induction of browning in pADR from a subset of pheo patients is positively correlated with plasma catecholamines, but additional factors may also contribute. SC and OME fat may not undergo browning in response to chronic adrenergic stress per se. Further analyses of the differential gene expression profile and mitochondrial activity in pADR compared to SC and OME fat may shed light on the regulation of browning in pheo pADR adipose tissue, and potential differences between the capacity of human pADR and SC adipose tissues to undergo browning.
Acknowledgments
The authors thank Jennifer Isorena for technical assistance and all the study participants for their contribution.
This work was supported by the Fondation Leducq 12CVD04 (L.V., K.R.), National Institutes of Health P01 HL28481 (K.R.), the National Center for Research Resources Grant S10RR026744 (K.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANP
- atrial natriuretic peptide
- BAT
- brown adipose tissue
- BNP
- B-type natriuretic peptide
- ETC
- electron transport chain
- OCR
- oxygen consumption rate
- OME
- omental
- pADR
- periadrenal
- pheo
- pheochromocytoma
- pheoMulti
- having some multilocular adipocytes
- pheouni
- having exclusively unilocular adipocytes
- RT-qPCR
- real-time quantitative PCR analysis
- SP_PIR
- single protein of protein information resource
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue.
References
- 1. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. [DOI] [PubMed] [Google Scholar]
- 2. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. [DOI] [PubMed] [Google Scholar]
- 4. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. [DOI] [PubMed] [Google Scholar]
- 5. Himms-Hagen J. Brown adipose tissue thermogenesis and obesity. Prog Lipid Res. 1989;28:67–115. [DOI] [PubMed] [Google Scholar]
- 6. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 7. Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocyte. J Biol Chem. 2010;285:7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15:405–424. [DOI] [PubMed] [Google Scholar]
- 10. Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. [DOI] [PubMed] [Google Scholar]
- 12. Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sacks HS, Fain JN, Bahouth SW, et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98:E1448–E1455. [DOI] [PubMed] [Google Scholar]
- 15. Ussar S, Lee KY, Dankel SN, et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med. 2014;6:247ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plouin P-F, Gimenez-Roqueplo A-P. Pheochromocytomas and secreting paragangliomas. Orphanet J Rare Dis. 2006;1:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hondares E, Gallego-Escuredo JM, Flachs P, et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism. 2014;63:312–317. [DOI] [PubMed] [Google Scholar]
- 18. Betz MJ, Slawik M, Lidell ME, et al. Presence of brown adipocytes in retroperitoneal fat from patients with benign adrenal tumors: relationship with outdoor temperature. J Clin Endocrinol Metab. 2013;98:4097–4104. [DOI] [PubMed] [Google Scholar]
- 19. Di Franco A, Guasti D, Mazzanti B, et al. Dissecting the origin of inducible brown fat in adult humans through a novel adipose stem cell model from adipose tissue surrounding pheochromocytoma. J Clin Endocrinol Metab. 2014;99:E1903–E1912. [DOI] [PubMed] [Google Scholar]
- 20. Nagano G, Ohno H, Oki K, et al. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with PRDM16-EHMT1 complex expression. PLoS One. 2015;10:e0122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frontini A, Vitali A, Perugini J, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013;1831:950–959. [DOI] [PubMed] [Google Scholar]
- 22. Søndergaard E, Gormsen LC, Christensen MH, et al. Chronic adrenergic stimulation induces brown adipose tissue differentiation in visceral adipose tissue. Diabet Med. 2015;32:e4–e8. [DOI] [PubMed] [Google Scholar]
- 23. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 24. Rogers GW, Brand MD, Petrosyan S, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 2011;6:e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bordicchia M, Liu D, Amri E-Z, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol. 1995;268:R183–R191. [DOI] [PubMed] [Google Scholar]
- 27. Stepniakowski K, Januszewicz A, Lapiński M, et al. Plasma atrial natriuretic peptide (ANP) concentration in patients with pheochromocytoma. Blood Press. 1992;1:157–161. [DOI] [PubMed] [Google Scholar]
- 28. Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J Physiol Endocrinol Metab. 2012;302:E19–E31. [DOI] [PubMed] [Google Scholar]
- 29. Svensson P-A, Jernås M, Sjöholm K, et al. Gene expression in human brown adipose tissue. Int J Mol Med. 2011;27:227–232. [DOI] [PubMed] [Google Scholar]
- 30. Shinoda K, Luijten IHN, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Jong JMA, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308:E1085–E1105. [DOI] [PubMed] [Google Scholar]
- 32. Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Lans AAJJ, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sidossis LS, Porter C, Saraf MK, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patsouris D, Qi P, Abdullahi A, et al. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 2015;13:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kern PA, Finlin BS, Zhu B, et al. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. J Clin Endocrinol Metab. 2014;99:E2772–E2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leibel RL, Edens NK, Fried SK. Physiologic basis for the control of body fat distribution in humans. Annu Rev Nutr. 1989;9:417–443. [DOI] [PubMed] [Google Scholar]
- 38. Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. [DOI] [PubMed] [Google Scholar]
- 39. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]