Abstract
Context:
Jansen's metaphyseal chondrodysplasia (JMC) is a rare skeletal dysplasia characterized by abnormal endochondral bone formation and typically severe hypercalcemia despite normal/low levels of PTH. Five different heterozygous activating PTH/PTHrP receptor (PTH1R) mutations that change one of three different amino acid residues are known to cause JMC.
Objectives:
Establishing the diagnosis of JMC during infancy or early childhood can be challenging, especially in the absence of family history and/or overt hypercalcemia. We therefore sought to provide radiographic findings supporting this diagnosis early in life.
Patients and Methods:
Three patients, a mother and her two sons, had radiographic evidence for JMC. However, obvious hypercalcemia and suppressed PTH levels were encountered only in both affected children. Sanger sequencing and endonuclease (SphI) digestion of PCR-amplified genomic DNA were performed to search for the H223R-PTH1R mutation.
Results:
The heterozygous H223R mutation was identified in all three affected individuals. Surprisingly, however, the now 38-year-old mother was never overtly hypercalcemic and was therefore not diagnosed until her sons were found to be affected by JMC at the ages of 28 months and 40 days, respectively. The presented radiographic findings at different ages will help diagnose other infants/toddlers suspected of having JMC.
Conclusion:
The H223R mutation is typically associated with profound hypercalcemia despite low/normal PTH levels. However, the findings presented herein show that overt hypercalcemia is not always encountered in JMC, even if caused by this relatively frequent mutation, which is similar to observations with other PTH1R mutations that show less constitutive activity.
Jansen Metaphyseal Chondrodysplasia is caused by heterozygous, constitutively active PTH1Rs. We now show that typical skeletal abnormalities can occur in the absence of overt hypercalcemia.
Jansen's metaphyseal chondrodysplasia (JMC) (1) is a rare skeletal dysplasia characterized by progressive growth plate abnormalities and sclerosis of some bones that is, in most cases, associated with major changes in mineral ion homeostasis (2–6). JMC is usually diagnosed during childhood, based on a combination of radiographic and biochemical abnormalities, but some patients with less severe disease are not diagnosed until adulthood. Most cases appear to be caused by de novo mutations, and only few parent-to-child transmissions of an established disease-causing PTHrP receptor (PTH1R) mutation have been reported (6–8).
The limbs of affected individuals typically show progressive postnatal changes that are caused by an abnormal regulation of chondrocyte growth and differentiation, eventually leading to short and bowed legs (4, 9–11). Radiographic features observed during infancy include diffuse demineralization, rickets-like metaphyseal changes, and erosion of the bone cortex. Cupping and fraying of the metaphyses, simulating a rachitic appearance, becomes apparent during childhood. These findings are associated with failure of the long bones to grow normally resulting in severely reduced adult height. Sclerosis of the base of the skull is typically recognized by late childhood and is evident in adults but has also been documented during infancy (12, 13). Only a few JMC cases have been diagnosed as neonates or young infants. Longitudinal postnatal disease progression of the skeletal and laboratory JMC features is therefore limited.
JMC is usually associated with severe, early-onset hypercalcemia, despite the absence of elevated levels of PTH or PTHrP (for review, see reference 5). PTH is, in addition to 1,25-dihydroxyvitamin D (1,25[OH]2D), the most important hormonal regulator of systemic calcium homeostasis (14), whereas PTHrP slows maturation of proliferating growth plate chondrocytes into hypertrophic cells, thereby delaying skeletal mineralization and allowing normal bone growth (15). The actions of PTH and PTHrP are both mediated through a shared G protein-coupled receptor, the PTH/PTH1R, which is abundantly expressed in the kidney and bone and at particularly high levels in growth plate chondrocytes. Mice lacking PTHrP or the PTH1R show a profound acceleration of chondrocyte maturation and thus the premature mineralization of the entire skeleton (16–18). Developmental abnormalities equivalent to those of PTH1R-null mice are observed in patients affected by a disorder referred to as Blomstrand lethal chondrodysplasia, a rare disease caused by inactivating homozygous or compound heterozygous PTH1R mutations (5).
Because the PTH1R mediates the actions of PTH and PTHrP to thereby regulate calcium homeostasis and chondrocyte differentiation, respectively, the discovery of gain-of-function PTH1R mutations leading to constitutive receptor activation provided a reasonable explanation for the changes in bone formation and mineral ion homeostasis that are observed in JMC (7, 19–22). Most patients affected by this disease carry the H223R mutation in the PTH1R. The substitution of arginine for histidine at position 223 was shown to result in a higher degree of agonist-independent cAMP formation than any other possible amino acid substitution at this position (23). Consistent with these findings, patients with this specific PTH1R mutation appear to have the most pronounced hypercalcemia, particularly when compared with JMC patients with the T410R or the I458K mutation in this G protein-coupled receptor (7, 8, 19–22, 24).
Herein we report a now 38-year-old woman affected by JMC, who carries the H223R mutation in the PTH1R. Although followed up clinically for years, overt hypercalcemia was never observed, which hindered establishing the clinical diagnosis of JMC and the search for her underlying genetic defect. Indeed, a search for the disease-causing PTH1R mutation was not pursued until her two sons revealed radiographic and biochemical abnormalities characteristic of the disease.
Case reports
All subjects underwent biochemical and genetic evaluation, and clinical data and imaging findings were collected from hospital records. The study was approved by the Institutional Review Board of Massachusetts General Hospital. Consent for performing genetic testing to search for the H223R-PTH1R mutation was obtained from the adult female patient, who also provided consent for her two sons.
Patient 1
A 28-month-old male child was brought for evaluation of progressive bowing of both legs. The boy was the first of two children born to a nonconsanguineous couple; an Indian mother and a Caucasian father of Northern European ancestry. He was born full term with a birth weight of 3500 g. The child did not start walking until the age of 19 months. At that point, the parents noticed bowing of both legs, which worsened progressively. Milestones for speech and cognitive development were normal. The first tooth erupted at 6 months.
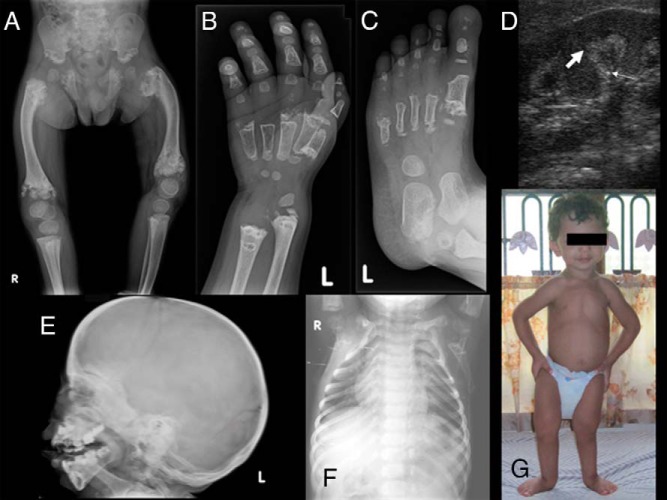
At 28 months his weight was 11 kg (fifth percentile) and his height was 80 cm (less than the fifth percentile); the head circumference was 50 cm (75th percentile). The upper-to-lower segment ratio was 1.35:1, which is consistent with disproportionate short stature. He presented with a broad forehead, proptosis, widely spread eyes, high arched narrow palate, broad nasal tip, microretrognathia, prominence of the lateral aspects of the jaws, and small conical teeth with enamel hypoplasia. He also had rhizomelic shortening of the upper and lower limbs, a prominent squatting stance, waddling gait, bilateral bowing of the femur, a narrow thorax, sloping shoulders and bilateral wrist widening, and bilateral pesplanovalgus. The anterior fontanel remained open (Figure 1).
Figure 1.
Radiographs of patient 1 showing metaphyseal expansion and calcification of upper and lower ends of femur with bowing and ragged acetabular roof (A), wide separation of epiphyseal center from metaphysis at the distal ends of the metacarpals and proximal phalanges with cupping and fraying of distal radius and ulna (B), similar changes in feet (C), thick arrows depicting medullary nephrocalcinosis (D), sclerosis of the base and skull vault with antegonial notching of mandible (E), a bell-shaped thorax (F), and a photograph of the index case showing microretrognathia, squatting stance, narrow thorax, and sloping shoulders (G).
Bone age was delayed at 18 months and notable radiographic findings included widening, cupping, and fraying of the metaphysis of the distal radius, ulna, and metacarpals. Calcified areas extended from the irregular distal metaphysis of the radius and ulna to the diaphysis. There was wide separation of the well-formed epiphyseal centers from the metaphyses at the distal ends of the metacarpals and proximal phalanges, and discrete calcifications were visible for metacarpals in this wide zone of nonossified tissue. The changes encountered in both feet were similar to those of the hands. Furthermore, there was widening and fragmentation in the proximal portion of the first metatarsal and in the distal portions in other metatarsals. The tarsal bones were not affected, but the calcaneus showed fragmentation at the posterosuperior aspect. The proximal phalanges of the big toes were oval shaped and the distal phalanges in all other toes were hypoplastic. There was considerable metaphyseal expansion, fragmentation, and calcification of the upper and lower ends of the femur with bowing. The epiphyses of both knees were normal. The basilar portions of the iliac bones and ischia were irregular with ragged acetabular roofs. A chest X-ray showed narrowing of the upper chest leading to a bell-shaped thorax deformity. Cupping was noticeable at the anterior ends of the ribs. The vertebrae were normal. Radiography of the skull showed marked sclerosis of the base and vault and underdevelopment of the paranasal sinuses and mastoid cells. There was marked hyperplasia of the mandible and prominent concavity of the lower border of the horizontal ramus of the mandible (also called antegonial notching).
Patient 2
The now 38-year-old mother of patient 1 had been born to nonconsanguineous healthy parents. She had been noted to have severe short stature since early childhood; her two siblings are healthy.
Patient 2 could not walk independently until 2.5 years of age; by then she had developed bowing of the legs and a waddling gait. Bowing of the forearms were noticed by 5 years of age. She underwent multiple corrective osteotomies on both tibiae and her left femur. Her maximal adult height was 126 cm. She had absolute macrocephaly, with a head circumference of 58 cm (+2.5 SD), severe microretrognathia, prominent lateral aspects of the jaws and supraorbital ridges, very narrow shoulders, small clavicles, prominent curvature of both forearms with hyperextendible fingers, brachydactyly, physically disabled feet, and small toes. Her hearing assessment was normal.
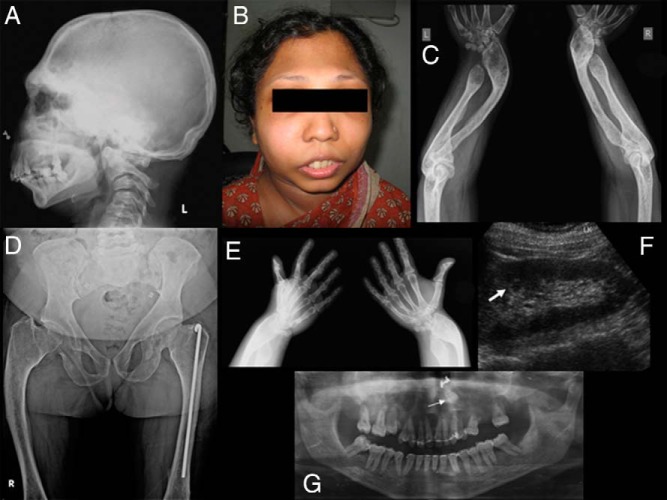
Radiological evaluation showed short physically disabled radius and ulna with bulbous radiolucent distal ends (Figure 2). The metacarpals and phalanges were short with normal carpals. Both humeri had a normal appearance. She had undergone osteotomies at the lower third of both tibiae. The metatarsals and phalanges of both feet were undermodeled with normal tarsal bones. The chest was bell shaped with broad short clavicles and a normal appearing spine. Radiographic studies of the pelvis showed severe shortening of the femoral neck with varus deformity and flattening of the femoral heads. The joint spaces of both hip joints were narrowed with more involvement of the left hip. The deformity of her left femur was corrected by insertion of a Rush nail and the right femur was bent especially in the upper half.
Figure 2.
Radiographs of patient 2 showing massive sclerosis of the base of skull, calvarium, supraorbital ridges, and complete absence of all paranasal sinuses with antegonial notching of mandible (A), a photograph showing microretrognathia, prominent supraorbital ridges (B), a short physically disabled radius and ulna with bulbous distal ends (C), a bowed femur with coxa vara and flattened femoral heads and short neck (D), short metacarpals and phalanges (E), nephrocalcinosis (F), and an orthopantomogram showing retained left upper canine (G).
The skull showed massive sclerosis of the base, calvarium, and supraorbital ridges. There was a total absence of all paranasal sinuses and mastoids, and the mandible was very short with antegonial notching. An orthopantomogram showed retention of the upper canines and the right retained canine had already been extracted.
The patient indicated having no problems during both pregnancies and breast milk production was sufficient; however, both infants had difficulties breast-feeding, presumably because of their microretrognathia.
Patient 3
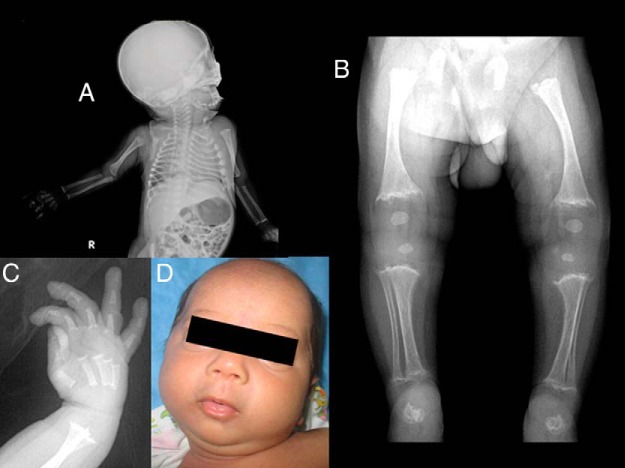
This younger sibling of patient 1 and second son of patient 2 was born full term with a birth weight of 2600 g. When first examined at 40 days of age, his weight was 3850 g (fifth percentile) with a length of 53 cm (less than the fifth percentile), and a head circumference of 37 cm (10th percentile). He had wide anterior and posterior fontanels, hypertelorism, proptosis, a high arched palate, and microretrognathia.
Radiography showed rachitiform-like changes at the ends of all tubular bones, with cupping and fraying of the metaphyses with wide growth plates; the changes were most prominent in the lower limbs. Both femurs showed bowing. Metaphyseal cupping was evident in the metacarpals and proximal phalanges as well. Radiographs of the chest revealed prominent changes in the anterior ends of the ribs resembling rickets. The epiphyses of the knee were rounded and small. The calcaneus was irregular and fragmented. The base of the skull was dense, but the calvarium was normal. The pelvis and spine were normal (Figure 3).
Figure 3.
Infantogram of patient 3 showing rachitiform changes in the anterior ends of ribs and dense base of skull (A), cupping and fraying of metaphysis at knee and ankle with bowing of femur (B), metaphyseal cupping in the metacarpals and proximal phalanges (C), and a photograph showing microretrognathia (D).
Genetic and laboratory findings
Laboratory results (Table 1) for patient 1 at 24/12 and 6 years of age revealed mild hypercalcemia, with normal serum phosphate levels, and undetectable or reduced PTH levels. At both ages, total alkaline phosphatase activity was elevated considerably, as was the urinary calcium to creatinine ratio. His younger brother (patient 3) had a normal serum calcium level when first tested at the age of 40 days, whereas serum phosphate was age appropriate and PTH at the lower end of the normal range. By 4 years of age, he had become mildly hypercalcemic, phosphate remained within normal limits, and PTH was now well below the normal range. Total alkaline phosphatase activity was elevated at both ages and the urinary calcium to creatinine ratio was elevated.
Table 1.
Laboratory Results of Patients
| Patient 2 (Mother) | Patient 1 | Patient 3 | ||||
|---|---|---|---|---|---|---|
| Age | 32 y | 38 y | 24/12 y | 6 y | 40 d | 4 y |
| Length/height, cm | 126 | 80 | 97.5 | 80 | ||
| Weight, kg | n.d. | 11 | 18.1 | 12.4 | ||
| Total calcium, mg/dL | 8.7 (8.4–10.2) | 9.1 (8.8–10.5) | 11.1 (8.8–10.8) | 11.2 (8.8–10.5) | 9.0 (8.8–10.8) | 11.2 (8.8–10.5) |
| Ionized calcium, mmol/L | n.d. | 1.25 (1.14–1.29) | n.d. | 1.42 (1.14–1.29) | n.d. | 1.43 (1.14–1.29) |
| Phosphate, mg/dL | 2.8 (2.7–4.7) | 3.3 (2.7–4.7) | 4.6 (3.8–6.5) | 4.4 (4.1–5.4) | 4.8 (3.8–6.5) | 4.3 (4.1–5.4) |
| Alkaline phosphatase, IU/L | 268 (50–130) | n.d. | 701 (145–420) | 507 (50–350) | 837 (145–420) | 464 (50–350) |
| PTH, pg/mL | 70.0 (15–50) | 40.0 (15–65) | <1.0 (7–53) | 6.0 (15–65) | 12.6 (10–55) | 5.0 (15–65) |
| uCa to creatinine ratio, mg/mg | 0.20 (<0.2) | 0.57 (<0.2) | 0.50 (<0.2) | 1.60 (<0.2) | 0.20 (<0.8) | 0.60 (<0.2) |
| Nephrocalcinosis | Yes | Yes | Yes | |||
| Heterozygous H223R mutation | Yes | Yes | Yes | |||
Abbreviations: n.d., not determined; uCa, urinary calcium. Reference ranges in parentheses.
An increased total serum calcium was never documented for the mother (patient 2), even during childhood (calcium 9.3 and phosphate 4.9 at the age of 8.5 y). During her most recent evaluation at age 38 years, her ionized blood calcium was at the upper end of the reference range and her urinary calcium to creatinine ratio was significantly increased (0.57; normal: < 0.2); the 1,25[OH]2D level was elevated (94.9 pg/mL) with a low-normal 25-vitamin D level (20.6 ng/mL). When measured at the ages of 32 and 38 years, her PTH level was either slightly elevated or well within the normal range.
An abdominal ultrasound revealed nephrocalcinosis for all three patients. PCR-amplified genomic DNA from peripheral blood cells was Sanger sequenced and digested with the endonuclease SphI, as described (7, 19, 25); both analyses revealed that all three patients were heterozygous for the H223R-PTH1R mutation.
Discussion
In 1934, Murk Jansen described the first case of JMC (1), a rare, autosomal dominant disorder characterized by short-limbed short stature, which was later shown to develop postnatally due to delayed chondrocyte differentiation. JMC is typically associated with severe and, generally, asymptomatic hypercalcemia and hypophosphatemia, with low-normal or undetectable serum PTH levels. Five different activating PTH1R mutations affecting one of three different amino acid residues have been identified thus far in affected patients, which all cause constitutive, ligand-independent receptor activation (7, 8, 19–22, 24). The mutant PTH1Rs thus activate the cAMP/protein kinase A signaling pathway in the kidney, bone, and growth plates in the absence of PTH or PTHrP, thereby causing an abnormal regulation of both mineral ion homeostasis and endochondral bone formation. Hence, most JMC patients develop biochemical features suggestive of hyperparathyroidism, including elevated serum calcium, low serum phosphorus, and elevated alkaline phosphatase activity, but without elevated PTH or PTHrP levels.
Members of the family presented herein, the mother and her two sons, are heterozygous for the H223R-PTH1R mutation and consequently exhibited in early life skeletal abnormalities consistent with JMC. Both affected children showed extensive metaphyseal cupping and fraying with normal epiphyses, ie, radiographic findings suggestive of rickets. Sclerosis of the base of the skull, which was evident even during infancy in both affected siblings, is not typically encountered in patients with vitamin D-deficient rickets or hyperparathyroidism and thus helped establish the diagnosis of JMC.
It is now becoming apparent that JMC is not always associated with severe hypercalcemia because previously reported patients, who are heterozygous for the T410R- or the I458R-PTH1R mutation, were found to have relatively normal blood calcium levels (8, 24). Until the present report, all cases with JMC due to the H223R mutation were found to exhibit elevated blood calcium levels (7, 19–22). However, these patients were all older when tested, and it is conceivable that mineral ion changes are not readily detectable early in life. In fact, patient 3 had normal blood calcium when first tested at the age of 40 days, which is reminiscent of the early postnatal findings in a JMC patient with the I458R mutation (24). Other blood and urine chemistries were also normal at that time, with the exception of an elevated blood alkaline phosphatase activity, a marker of bone turnover.
The finding that blood calcium and phosphate levels can be within the normal range, in at least some patients with JMC, could be an indication that the expression levels of constitutively active PTH1Rs in target tissues may be variable, dependent on the patient's age and/or type of the mutant allele, or it may be influenced by some other as-yet-unidentified regulatory mechanism(s). Most PTH1R mutants are expressed at lower levels than the wild-type receptor when tested in vitro (23). It is therefore conceivable that considerable differences may occur in the downstream responses in some tissues. For example, in the proximal renal tubule, relatively normal expression of the mutant PTH1R could increase 1,25(OH)2D synthesis and/or reduce expression of the two sodium-dependent phosphate transporters, NPT2a and NPT2c, therefore contributing to an elevation in serum calcium and a reduction in serum phosphate level. Furthermore, if the mutant PTH1R is only poorly expressed in craniofacial bones, the resulting haploinsufficiency may have contributed in patient 2 to primary tooth eruption failure, ie, a developmental defect similar to that reported for numerous, heterozygous inactivating PTH1R mutations (26–28). It appears likely that patient 2 had normal breast development, which involves PTHrP-dependent signaling through the PTH1R for ductal branching (29, 30). In fact, she had sufficient milk production after both pregnancies, making it plausible that branching morphogenesis was normal either because of postzygotic mosaicism for the H223R mutant allele or because of a much reduced expression of the mutant PTH1R in breast tissue and thus predominant PTH1R expression from the normal allele.
It remains uncertain as to why patient 2 was never documented to have abnormalities in mineral ion homeostasis. When tested at the age of 32 years, her total serum calcium level was found to be at the lower end of the reference range, with a slightly elevated PTH level. A repeat measurement at the age of 38 years showed that serum levels of total calcium, phosphate, and PTH were within normal limits. However, blood-ionized calcium was at the upper end of the reference range, and the urinary calcium to creatinine ratio and 1,25(OH)2D level were elevated. These latter laboratory findings suggest that the filtered calcium load may be elevated, at least intermittently, which might help explain her nephrocalcinosis.
The lack of overt hypercalcemia in the affected mother is particularly puzzling because the H223R mutation has typically been associated with severe hypercalcemia (7, 19–22). In addition to possible variations in the tissue-specific expression of the PTH1R mutant, it is conceivable that another variant gene product limited the development of hypercalcemia in patient 2. Furthermore, a mosaic de novo PTH1R mutation may have led predominantly to growth plate abnormalities with relatively minor effects on the bone/renal systems involved in systemic calcium and phosphate homeostasis. In either case, our current findings raise the question as to whether there are more patients with an activating H223R-PTH1R mutation who developed radiographic findings that are consistent with JMC yet lack frank hypercalcemia, just like cases caused by the T410R and the I458R mutations (8, 24). JMC due to the H223R mutation may thus occur more frequently than previously thought.
Conclusions
The radiographic spectrum of Jansen's metaphyseal chondrodysplasia varies with age, as shown in the presented family in which the mother and her two children carry the same heterozygous H223R-PTH1R mutation that had been previously identified in several other JMC cases. Sclerosis of the skull base, an unusual finding in other skeletal dysplasias along with rickets-like metaphyseal changes, helped establish the diagnosis during infancy. However, the H223R mutation did not cause overt hypercalcemia in the affected mother and has been a confounding factor in establishing the diagnosis.
Acknowledgments
This work was supported by the National Institutes of Health Grant RO1 DK46718-20 (to H.J.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- JMC
- Jansen's metaphyseal chondrodysplasia
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- PTH1R
- PTH/PTHrP receptor.
References
- 1. Jansen M. Über atypische chondrodystrophie (achondroplasie) und über eine noch nicht beschriebene angeborene wachstumsstörung des knochensystems: metaphysäre dysostosis. Zeitschr Orthop Chir. 1934;61:253–286. [Google Scholar]
- 2. Gram PB, Fleming JL, Frame B, Fine G. Metaphyseal chondrodysplasia of Jansen. J Bone Joint Surg. 1959;41A:951–959. [PubMed] [Google Scholar]
- 3. Jaffe HL. Certain other anomalies of skeletal development. In: Jaffe HL, ed. Metabolic, Degenerative, and Inflammatory Diseases of Bones and Joints. Philadelphia: Lea and Feibiger; 1972:222–226. [Google Scholar]
- 4. Frame B, Poznanski AK. Conditions that may be confused with rickets. In: DeLuca HF, Anast CS, eds. Pediatric Diseases Related to Calcium. New York: Elsevier; 1980:269–289. [Google Scholar]
- 5. Silve C, Jüppner H. Genetic disorders caused by mutations in the PTH/PTHrP receptor and down-stream effector molecules. In: Bilezikian J, ed. The Parathyroids: Basic and Clinical Concepts. San Diego: Academic Press; 2015:587–605. [Google Scholar]
- 6. Charrow J, Poznanski AK. The Jansen type of metaphyseal chondrodysplasia: conformation of dominant inheritance and review of radiographic manifestations in the newborn and adult. J Med Genet. 1984;18:321–327. [DOI] [PubMed] [Google Scholar]
- 7. Schipani E, Langman CB, Parfitt AM, et al. Constitutively activated receptors for parathyroid hormone and parathyroid hormone-related peptide in Jansen's metaphyseal chondrodysplasia. N Engl J Med. 1996;335:708–714. [DOI] [PubMed] [Google Scholar]
- 8. Bastepe M, Raas-Rothschild A, Silver J, Weissman I, Jüppner H, Gillis D. A form of Jansen's metaphyseal chondrodysplasia with limited metabolic and skeletal abnormalities is caused by a novel activating PTH/PTHrP receptor mutation. J Clin Endocrinol Metab. 2004;89:3595–3600. [DOI] [PubMed] [Google Scholar]
- 9. Rao DS, Frame B, Reynolds WA, Parfitt AM. Hypercalcemia in metaphyseal chondrodysplasia of Jansen (MCD): an enigma. In: Norman AW, Schaefer K, von Herrath D, Grigoleit HG, Coburn JW, DeLuca HF, Mawer EB, Suda T, eds. Vitamin D, Basic Research and Its Clinical Application. Berlin: Walter de Gruyter; 1979:1173–1176. [Google Scholar]
- 10. Silverthorn KG, Houston CS, Duncan BP. Murk Jansen's metaphyseal chondrodysplasia with long-term followup. Pediatr Radiol. 1983;17:119–123. [DOI] [PubMed] [Google Scholar]
- 11. Kruse K, Schütz C. Calcium metabolism in the Jansen type of metaphyseal dysplasia. Eur J Pediatr. 1993;152:912–915. [DOI] [PubMed] [Google Scholar]
- 12. Holthusen W, Holt JF, Stoeckenius M. The skull in metaphyseal chondrodysplasia type Jansen. Pediat Radiol. 1975;3:137–144. [DOI] [PubMed] [Google Scholar]
- 13. Kozlowski K, Campbell J, Azouz M, Sprague P. Metaphyseal chondrodysplasia, type Jansen. Australas Radiol. 1999;43:544–547. [DOI] [PubMed] [Google Scholar]
- 14. Gardella TJ, Jüppner H, Brown EM, Kronenberg HM, Potts JT., Jr Parathyroid hormone and parathyroid hormone receptor type 1 in the regulation of calcium and phosphate homeostasis and bone metabolism. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 7th ed Philadelphia: W. B. Saunders Co; 2016:969–990. [Google Scholar]
- 15. Maes C, Kronenberg HM. Bone development and remodeling. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 7th ed Philadelphia: W. B. Saunders Co; 2016:1038–1062. [Google Scholar]
- 16. Karaplis AC, Luz A, Glowacki J, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. [DOI] [PubMed] [Google Scholar]
- 17. Lanske B, Karaplis AC, Lee K, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. [DOI] [PubMed] [Google Scholar]
- 18. Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg H. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schipani E, Kruse K, Jüppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. [DOI] [PubMed] [Google Scholar]
- 20. Minagawa M, Arakawa K, Minamitani K, Yasuda T, Niimi H. Jansen-type metaphyseal chondrodysplasia: analysis of PTH/PTH-related protein receptor messenger RNA by the reverse transcription-polymerase chain method. Endocr J. 1997;44:493–499. [DOI] [PubMed] [Google Scholar]
- 21. Brown WW, Jüppner H, Langman CB, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onuchic L, Ferraz-de-Souza B, Mendonca BB, Correa PH, Martin RM. Potential effects of alendronate on fibroblast growth factor 23 levels and effective control of hypercalciuria in an adult with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2012;97:1098–1103. [DOI] [PubMed] [Google Scholar]
- 23. Schipani E, Jensen GS, Pincus J, Nissenson RA, Gardella TJ, Jüppner H. Constitutive activation of the cAMP signaling pathway by parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptors mutated at the two loci for Jansen's metaphyseal chondrodysplasia. Mol Endocrinol. 1997;11:851–858. [DOI] [PubMed] [Google Scholar]
- 24. Savoldi G, Izzi C, Signorelli M, et al. Prenatal presentation and postnatal evolution of a patient with Jansen metaphyseal dysplasia with a novel missense mutation in PTH1R. Am J Med Genet. A. 2013;161:2614–2619. [DOI] [PubMed] [Google Scholar]
- 25. Schipani E, Langman CB, Hunzelman J, et al. A novel PTH/PTHrP receptor mutation in Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 1999;84:3052–3057. [DOI] [PubMed] [Google Scholar]
- 26. Yamaguchi T, Hosomichi K, Narita A, et al. Exome resequencing combined with linkage analysis identifies novel PTH1R variants in primary failure of tooth eruption in Japanese. J Bone Miner Res. 2011;26:1655–1661. [DOI] [PubMed] [Google Scholar]
- 27. Frazier-Bowers SA, Simmons D, Wright JT, Proffit WR, Ackerman JL. Primary failure of eruption and PTH1R: the importance of a genetic diagnosis for orthodontic treatment planning. Am J Orthod Dentofacial Orthop. 2010;137:160 e161–e167; discussion e160–e161. [DOI] [PubMed] [Google Scholar]
- 28. Roth H, Fritsche LG, Meier C, et al. Expanding the spectrum of PTH1R mutations in patients with primary failure of tooth eruption. Clin Oral Investig. 2014;18(2):377–384. [DOI] [PubMed] [Google Scholar]
- 29. Foley J, Dann P, Hong J, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:513–525. [DOI] [PubMed] [Google Scholar]
- 30. Wysolmerski JJ, Cormier S, Philbrick W, et al. Absence of functional type 1 PTH/PTHrP receptors in humans is associated with abnormal breast development and tooth impactation. J Clin Endocrinol Metab. 2001;86:1788–1794. [DOI] [PubMed] [Google Scholar]