Abstract
Context:
High-plasma very low-density lipoprotein (VLDL) triglyceride (TG) concentration and alterations in VLDL-TG metabolism are associated with cardiometabolic disease.
Objective:
This study sought to evaluate the interrelationships among factors purported to regulate VLDL-TG metabolism in a large cohort of men and women with a wide range in body adiposity and fat distribution but without diabetes.
Subjects and Design:
We assessed body composition and fat distribution, plasma insulin concentration, free fatty acid availability, and basal VLDL-TG and VLDL-apoB-100 (VLDL particle number) kinetics in 233 lean, overweight, and obese men and women.
Results:
We found that: 1) plasma VLDL-TG concentration is determined primarily by VLDL-TG secretion rate (SR) in men and by VLDL-TG clearance rate in women; 2) there is a dissociation between VLDL-TG and VLDL-apoB-100 SRs, and VLDL-apoB-100 SR only explains ∼30% of the variance in VLDL-TG SR; 3) ∼50% of people with obesity have high plasma VLDL-TG concentration due to both an increased VLDL-TG SR and a decreased rate of VLDL-TG plasma clearance, and they have lower plasma high-density lipoprotein-cholesterol concentration and more intra-abdominal and liver fat than those with normal VLDL-TG concentration; and 4) fat-free mass, liver fat content and the rate of free fatty acid release into plasma are independent predictors (with a sex × race interaction) of VLDL-TG SR.
Conclusions:
The regulation of plasma VLDL-TG concentration is complex and influenced by multiple metabolic factors. Many people with obesity have normal plasma VLDL-TG concentrations and kinetics, whereas those with high plasma VLDL-TG concentrations have increased VLDL-TG SR and other markers of cardiometabolic disease risk.
Fat-free mass, intra-hepatic triglyceride content and fatty acid rate of appearance in plasma were identified as statistically independent predictors (with a sex by race interaction) of VLDL-TG secretion rate.
High plasma triglyceride (TG) concentration is a component of the dyslipidemia associated with obesity and diabetes, and an important risk factor for cardiovascular disease (1–5). During postabsorptive conditions, very low-density lipoprotein (VLDL) particles produced by the liver are the major carrier of TG in plasma (6). Each VLDL particle is composed primarily of TGs, a single molecule of apolipoprotein B-100 (apoB-100), and some cholesterol, phospholipids, and small, exchangeable lipoproteins (6). The secretion of VLDL provides a mechanism for exporting water-insoluble lipids from the liver as water soluble particles via the bloodstream to peripheral tissues. The subsequent removal of TG from VLDL, by lipoprotein lipase in tissue capillary beds or transfer to high-density lipoprotein (HDL) via neutral lipid exchange, converts nascent TG-rich VLDL to small, dense VLDL remnants and intermediate-density and low-density lipoproteins. An imbalance between hepatic TG synthesis and hepatic TG export via VLDL contributes to the development of nonalcoholic fatty liver disease (NAFLD) and an imbalance between hepatic VLDL-TG secretion and VLDL-TG plasma clearance can lead to increased plasma VLDL-TG concentration. Accordingly, an understanding of the factors involved in the regulation of VLDL-TG kinetics and the relationship between VLDL-TG kinetics and its plasma concentration has important physiological and clinical implications.
Data from different studies have shown that increased circulating insulin, plasma free fatty acids (FFAs), fat-free mass (FFM), total body and intra-abdominal fat, intrahepatic triglyceride (IHTG) content, and a person's race and sex influence VLDL-TG concentration and kinetics (7–15). However, the inter-relationship among these factors and their independent contribution in determining VLDL-TG metabolism are not known. The purpose of the present study was to provide a comprehensive evaluation of the inter-relationships among body composition, fat distribution, plasma insulin, and FFA availability and VLDL-apoB-100 and VLDL-TG kinetics and plasma concentrations in a large cohort of lean, overweight, and obese men and women.
Materials and Methods
Subjects
A total of 233 men (n = 89) and women (n = 144), age 18–55 years, with a body mass index (BMI) of 19–45 kg/m2 participated in this study. In most, but not all subjects, the data or blood samples used for analysis in the present study were obtained as part of their baseline evaluation in other studies (8, 11, 13, 16–24). Subjects with diabetes or severe hypertriglyceridemia (plasma TG ≥ 400 mg/dL) were excluded to avoid the potential confounding effects of differences in glycemic control and genetic extremes of hypertriglyceridemia on our outcome measures. Pregnant, lactating, and postmenopausal women and persons who were taking medications or dietary supplements known to affect lipid metabolism, or smoked tobacco products were also excluded. All subjects were weight stable and sedentary for at least 2 months before the study. Written informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Studies Committee of Washington University School of Medicine in St. Louis, MO.
Experimental procedures
Body composition
Total fat mass (FM) and FFM were determined by using dual-energy x-ray absorptiometry, intra-abdominal and sc abdominal adipose tissue masses were assessed by using magnetic resonance imaging, and IHTG content was assessed by using magnetic resonance spectroscopy.
Substrate kinetics
Subjects were admitted to the Clinical Research Unit in the late afternoon, consumed a standard meal at approximately 1900 hours, and then fasted (except for water) and rested in bed until completion of the tracer infusion study the next day. At approximately 0530 hours the following morning a catheter was inserted into a forearm vein to administer tracers, and a second catheter was inserted into a vein in the contralateral hand, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples. At approximately 0700 hours, after baseline blood samples were obtained, a bolus of [1,1,2,3,3-2H5]glycerol dissolved in 0.9% NaCl solution was administered through the catheter in the forearm vein and constant infusions of [2,2-2H2] or [U-13C]palmitate, dissolved in 25% human albumin solution, and [5,5,5-2H3]leucine, dissolved in 0.9% NaCl solution, were started and maintained for 12 hours. Blood samples were collected at 5, 15, 30, 60, 90, and 120 minutes and then every hour for 10 hours to determine glycerol and palmitate tracer-to-tracee ratios (TTRs) in plasma and VLDL-TG, and leucine TTR in plasma and VLDL-apoB-100. In some of our studies, an assessment of VLDL-TG kinetics was performed without a concomitant assessment of VLDL-apoB-100 kinetics, so VLDL-apoB-100 kinetics are only available in 201 of our 233 subjects (72 of 89 men and 129 of 144 women). We did not control for menstrual cycle phase because we have shown that menstrual cycle phase does not affect basal VLDL-TG and VLDL-apoB-100 kinetics (25).
Sample analyses
VLDL was isolated from plasma by ultracentrifugation (6, 21). VLDL-TG concentration was determined by using a colorimetric enzymatic kit (SIGMA Chemicals) and VLDL-apoB-100 concentration was determined by using a turbidimetric immunoassay (Wako Pure Chemical Industries). Plasma FFA concentrations were quantified by gas chromatography. Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT plus, Yellow Spring Instrument Co). Plasma insulin concentration was measured by using a RIA (Linco Research). Plasma free glycerol, palmitate, and leucine TTRs, the TTRs of glycerol and palmitate in VLDL-TG, and the TTR of leucine in VLDL-apoB-100 were determined by using gas chromatography–mass spectrometry.
Calculations
Palmitate rate of appearance (Ra) in plasma was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR value between 60 and 240 minutes of the VLDL kinetics study; total FFA Ra was derived by dividing palmitate Ra by the proportional contribution of palmitate to total plasma FFA concentration (26).
The fractional turnover rate (FTR; pools h−1) of VLDL-TG was determined by fitting the glycerol TTR time-course in plasma and in VLDL-TG to a compartmental model. The rate of VLDL-TG secretion was calculated as the product of VLDL-TG FTR and VLDL-TG concentration in plasma and expressed as total VLDL-TG secretion rate (SR, in μmol · min−1), which represents the total amount of VLDL-TG secreted by the liver or as VLDL-TG SR per unit of plasma (in μmol · l plasma−1 · min−1), which represents the relationship between VLDL-TG secretion and its distribution space (21).
The contribution of systemic plasma FFA to VLDL-TG was calculated by fitting the palmitate TTR in plasma and VLDL-TG to a compartmental model (21). This approach permits partitioning the fatty acids within VLDL-TG into those derived from “systemic” and “nonsystemic” fatty acid pools. The systemic source is composed of FFA present in the systemic circulation that are taken up by the liver and directly incorporated into VLDL-TG. The nonsystemic source is composed of fatty acids derived from: 1) lipolysis of IHTG, 2) lipolysis of ip TG, 3) intrahepatic lipolysis of TG from lipoproteins that are taken up by the liver, and 4) hepatic de novo lipogenesis. The proportion of FFA Ra that was incorporated into VLDL-TG was calculated by dividing the rate of secretion of plasma FFA incorporated into VLDL-TG (3 × VLDL-TG SR × fraction of VLDL-TG derived from systemic FFA) by whole-body FFA Ra. The mean residence time (MRT) of VLDL-TG was calculated as 1/FTR and refers to the average time that VLDL-TG circulate in the VLDL fraction of blood (ie, until TG is lost from the VLDL fraction via all possible routes, including lipoprotein lipase-mediated hydrolysis, transfer to HDL via neutral lipid exchange, tissue uptake of intact particles, or conversion to higher-density particles (ie, intermediate and low-density lipoproteins).
The FTR of VLDL-apoB-100 was calculated by fitting the TTR time-courses of free leucine in plasma and leucine in VLDL-apoB-100 to a compartmental model (21). The rate of VLDL-apoB-100 secretion and the MRT of VLDL-apoB-100, which provide indices of the SR and the MRT of VLDL particles in the bloodstream, were calculated by using the plasma VLDL-apoB-100 concentration and VLDL-apoB-100 FTR values as described above for VLDL-TG kinetics. The VLDL-apoB-100 MRT refers to the loss of the VLDL particle from the VLDL density range either through conversion to higher-density particles or irreversible loss from plasma (eg, direct uptake of the particle by tissues). A molecular mass of 512 723 g/mol for apoB-100 was used for unit conversions.
Hepatic insulin resistance with respect to VLDL-TG secretion was calculated as the product of plasma insulin concentration and the secretion rate of VLDL-TG (VLDL-TG secretion insulin resistance index [VLDL-TG secretion IRI]). This calculation is based on the same principle used to develop a hepatic IRI (27).
Statistical analysis
Simple linear regression analysis was used to determine the bivariate relationship between selected variables. The method developed by R.A. Fisher was used to compare the correlation coefficients for bivariate relationships in men and women. Student t test was used to compare mean outcome values and regression slopes between men and women, obese subjects with normal and elevated VLDL-TG concentration, women with VLDL-TG FTR above and below the 90th percentile, and subjects with a high and low VLDL-TG-to-apoB-100 SR ratio, respectively. The χ2 test was used to compare race and sex distributions of these study subpopulations. A general linear model with sex and race as fixed factors and age, FFM, percent body fat, intra-abdominal adipose tissue volume, IHTG content, FFA Ra, and plasma insulin concentration as independent variables was used to determine the independent contribution of these factors to VLDL-TG SR. Values in the text and table are presented as mean ± SEM or medians with quartiles (25th and 75th percentiles) in parentheses.
Results
Subject characteristics
Age, body composition, and metabolic characteristics of the study subjects are shown in Supplemental Table 1. Both male and female groups contained subjects who were lean, overweight, and obese with large ranges in FFM, total FM, regional fat distribution, and plasma insulin and lipid concentrations. Thirty-five percent of our subjects had high plasma TG concentrations (150–395 mg/dL).
Relationship between VLDL-TG concentration and adiposity
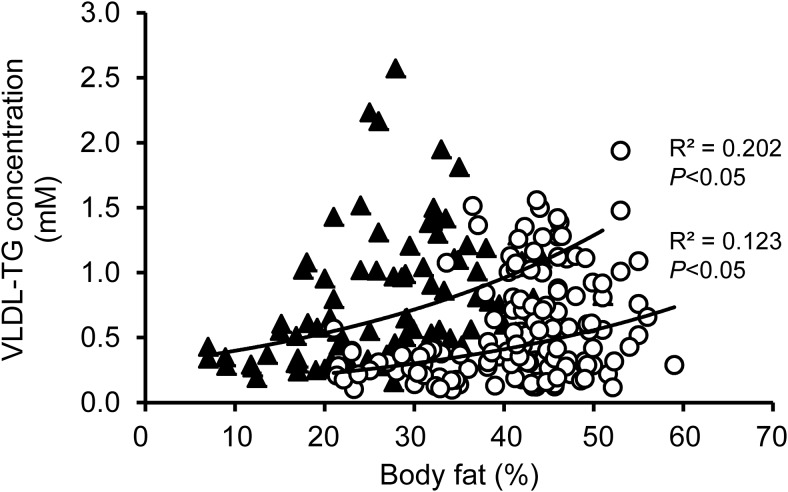
Plasma VLDL-TG concentration increased with increasing adiposity in both men and women but the relationships between VLDL-TG concentration and percent body fat (Figure 1) and between VLDL-TG concentration and BMI (data not shown) were weak. In general, women had lower plasma VLDL-TG concentrations than men at any given percent body fat value and the variability in plasma VLDL-TG concentration was greater at any given percent body fat value in obese than very lean subjects. Fifty percent of men and women who were obese (BMI ≥ 30.0 kg/m2) had plasma VLDL-TG concentrations that were below the upper threshold value (0.60mM) observed in lean (BMI 18.5–24.9 kg/m2) men and women (Table 1). Compared with the group that had high plasma VLDL-TG concentrations, the low VLDL-TG group had a higher percentage of subjects who were African American, lower IHTG content and intra-abdominal adipose tissue volume, and channeled a smaller proportion of FFA Ra toward VLDL-TG secretion. The lower plasma VLDL-TG concentration was due to both a decrease in VLDL-TG SR and an increase in plasma VLDL-TG clearance, and was associated with greater hepatic insulin sensitivity with respect to VLDL-TG metabolism (lower VLDL-TG secretion IRI).
Figure 1.
Relationship between adiposity and VLDL-TG concentration in men (black triangles) and women (open circles).
Table 1.
Characteristics and VLDL Kinetics of Subjects with Obesity Who Had Low and High VLDL-TG Concentrations
| Characteristic | VLDL-TG Concentration ≤ 0.60mm (n = 76) | VLDL-TG Concentration > 0.60mm (n = 79) |
|---|---|---|
| VLDL-TG concentration, mm | 0.37 ± 0.02 | 1.09 ± 0.04 |
| Sex, n (%) | ||
| Men | 17 (22%) | 33 (42%) |
| Women | 59 (78%) | 46 (58%) |
| Race, n (%) | ||
| Caucasians | 48 (63%) | 70 (88%) |
| African American | 26 (34%) | 7 (9%)a |
| Other | 2 (3%) | 2 (3%) |
| Age, y | 38 ± 1 | 39 ± 1 |
| Body mass index, kg/m2 | 36.1 ± 0.5 | 36.3 ± 0.4 |
| Body fat, % body weight | 43 ± 1 | 41 ± 1 |
| Intrahepatic TG content, % | 10 ± 1 | 18 ± 1a |
| Intra-abdominal adipose tissue volume, cm3 | 1381 ± 82 | 1964 ± 137a |
| Insulin, mU/L | 14 ± 1 | 17 ± 1 |
| HDL-cholesterol concentration, mg/dL | 46 ± 1 | 40 ± 1a |
| Free fatty acid concentration, μM | 449 ± 15 | 444 ± 17 |
| Free fatty acid Ra, μmol/min | 355 ± 13 | 392 ± 13 |
| VLDL-TG secretion rate, μmol/min | 12.9 ± 0.8 | 21.5 ± 0.9a |
| VLDL-TG FTR, pools/h | 0.70 ± 0.04 | 0.37 ± 0.02a |
| VLDL-TG MRT, min | 102 ± 5 | 184 ± 7a |
| VLDL-TG secretion IRI | 211 ± 26 | 356 ± 31a |
| FFA Ra secreted as VLDL-TG, % | 6.4 ± 0.3 | 8.7 ± 0.5a |
| VLDL-TG:VLDLapoB-100 secretion rate ratio | 10 348 (7732; 13 726) | 12 442 (9726; 17 579) |
Data are means ± sem or medians (quartiles). Values in bold indicate significant difference between groups.
Values significantly different between groups after Bonferroni correction for multiple comparisons; P < .05.
Relationship between VLDL-TG and VLDL-apoB-100 SRs and VLDL-TG kinetics and plasma VLDL-TG concentration
There was a statistically significant but weak relationship between VLDL-TG and VLDL-apoB-100 (VLDL particle number) SRs (R2 = 0.276; Supplemental Figure 1). The characteristics and VLDL kinetics of subjects who had high (upper tertile: ≥13 908) and low (lower tertile: ≤8978) VLDL-TG-to-VLDL-apoB-100 SR ratios are shown in Supplemental Table 2. This ratio provides an index of the average TG content of nascent VLDL particles. Intrahepatic TG content, VLDL-TG SR, and plasma VLDL-TG concentration were approximately double and FFA Ra and plasma FFA concentration were approximately 25% greater in the group of subjects who had a high than those who had a low VLDL-TG-to-VLDL-apoB-100 SR ratio, whereas VLDL-apoB-100 SR was approximately 30% lower in those with a high VLDL-TG-to-VLDL-apoB-100 SR ratio. Age, BMI, percent body fat, intra-abdominal adipose tissue volume, plasma insulin concentration, and the proportion of subjects who were women or African Americans were not different in the groups with high and low VLDL-TG-to-VLDL-apoB-100 SR ratios.
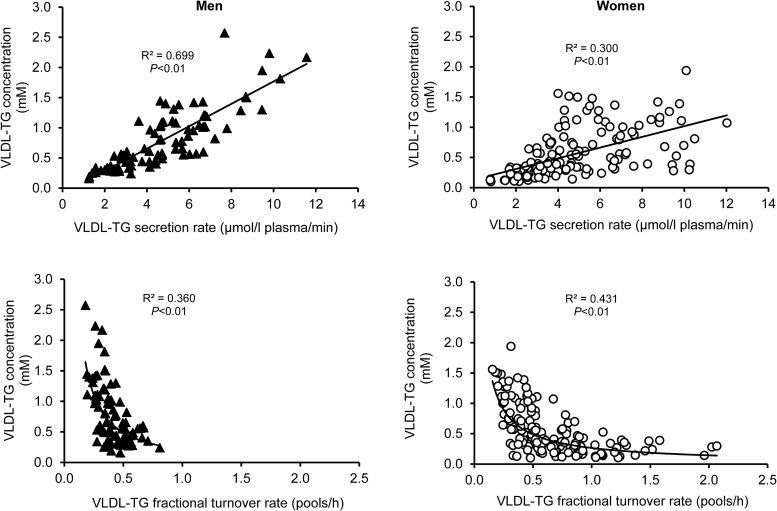
Plasma VLDL-TG concentration was directly related to VLDL-TG SR in both men and women, but the relationship was significantly stronger (P < .001) in men than in women; VLDL-TG SR accounted for 70% of the variance in VLDL-TG concentration in men, but only 30% of the variance in women (Figure 2). In addition, the slope of the association between VLDL-TG SR and concentration was significantly steeper in men than in women (P < .01); every μmol/L plasma/min increase in VLDL-TG SR was associated with a 0.19 ± 0.013 mmol/L increase in VLDL-TG concentration in men and a 0.089 ± 0.011 mmol/L increase in women.
Figure 2.
Relationships between VLDL-TG secretion rate and VLDL-TG concentration (top) and between VLDL-TG fractional turnover rate and VLDL-TG concentration (bottom) in men (black triangles, left panels) and women (open circles, right panels).
The range of values for VLDL-TG FTR in men was small and differences in VLDL-TG FTR did not distinguish men with low and high VLDL-TG concentrations (Figure 2). In women, there was an inverse, curvilinear relationship between VLDL-TG concentration and VLDL-TG FTR due to very rapid FTR in a subset of subjects (Figure 2). The 90th percentile FTR value in all 233 subjects was 0.93 pools/h, which was greater than the highest value in men and was exceeded in 23 women (characteristics of these women are shown in Table 2). The group of women with high VLDL-TG FTR values contained proportionally more women who were African American but their age, BMI, percent body fat, body fat distribution, and plasma insulin concentration were not different from the group of women with lower VLDL-TG FTR. Plasma HDL-cholesterol concentration was greater, and plasma VLDL-TG concentration was much lower in women with high VLDL-TG FTR than in the other women, even though VLDL-TG SR was not different between groups. The ratio of VLDL-TG to VLDL-apoB-100 SRs was greater and the ratio of VLDL-TG MRT to VLDL-apoB-100 MRT (an index of intravascular VLDL particle delipidation via lipoprotein lipase or neutral lipid exchange with HDL) was lower in these women than in other women.
Table 2.
Characteristics and VLDL Kinetics of Women with VLDL-TG FTR Above and Below the 90th Percentile
| Characteristic | VLDL-TG FTR ≤90th Percentile (n = 121) | VLDL-TG FTR >90th Percentile (n = 23) |
|---|---|---|
| VLDL-TG FTR, pools/h | 0.51 (0.37; 0.70) | 1.24 (1.10; 1.47) |
| VLDL-TG MRT, min | 117 (86; 160) | 48 (41; 55) |
| Race, n (%) | ||
| Caucasians | 95 (78%) | 12 (52%) |
| African American | 20 (17%) | 9 (39%)a |
| Asian | 4 (3%) | 2 (9%) |
| Other | 2 (2%) | 0 (0%) |
| Age, y | 39 (30; 45) | 37 (26; 46) |
| BMI, kg/m2 | 34 (31; 38) | 30 (22; 36) |
| Body fat, % body weight | 43 (39; 47) | 40 (29; 45) |
| Intrahepatic TG content, % | 5.8 (2.7; 18.1) | 3.1 (1.3; 13.1) |
| Intra-abdominal adipose tissue volume, cm3 | 1299 (721; 1790) | 887 (566; 1388) |
| Subcutaneous abdominal adipose tissue volume, cm3 | 3419 (2562; 4529) | 3228 (2147; 4192) |
| FFA Ra, μmol/min | 314 (260; 436) | 313 (241; 369) |
| Insulin, mU/L | 11.5 (7.0; 16.1) | 8.3 (4.8; 18.1) |
| HDL-cholesterol, mg/dL | 45 (39; 54) | 53 (46; 68)a |
| VLDL-TG concentration, mm | 0.49 (0.29; 0.86) | 0.24 (0.15; 0.33)a |
| VLDL-TG secretion rate, μmol/min | 11.4 (7.8; 17.0) | 14.2 (7.6; 20.0) |
| VLDL-TG:VLDLapoB-100 secretion rate ratio | 10 539 (8261; 14 524) | 15 015 (10 878; 23 118)a |
| VLDL-TG:VLDL-apoB MRT ratio | 0.61 (0.50; 0.78) | 0.34 (0.29; 0.47)a |
Data are medians and quartiles in parentheses. Values in bold indicate significant difference between groups.
Values significantly different between groups after Bonferroni correction for multiple comparisons; P < .05.
Relationships between body composition, plasma insulin concentration, and systemic FFA availability and VLDL-TG SR
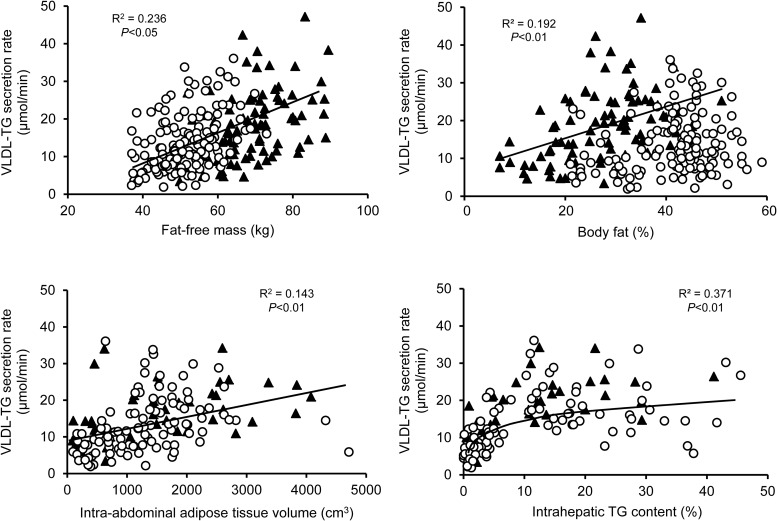
VLDL-TG SR was weakly related to percent body fat in men but there was no statistically significant relationship between VLDL-TG SR and percent body fat in women (Figure 3). There was a direct but weak linear relationship between intra-abdominal adipose tissue volume and total VLDL-TG SR (Figure 3) and the SR of VLDL-TG derived from nonsystemic fatty acid pools (R2 = 0.186; data not shown). There was a curvilinear relationship between IHTG content and total VLDL-TG SR (Figures 3) and the SR of VLDL-TG derived from nonsystemic fatty acid pools (R2 = 0.320; data not shown), manifested by a steep linear increase in VLDL-TG SR up to approximately 6% IHTG content, and a subsequent plateau in VLDL-TG SR despite increasing IHTG content.
Figure 3.
Relationships between FFM (top, left), adiposity (top, right), intra-abdominal adipose tissue volume (bottom, left), and intra-hepatic TG content (bottom, right) and VLDL-TG secretion rate in men (black triangles) and women (open circles).
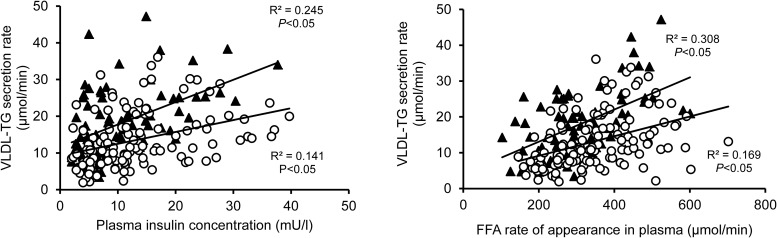
VLDL-TG SR was directly related to plasma insulin concentration in both men and women but the relationship was weak and at any given plasma insulin concentration, women secreted fewer moles of VLDL-TG than men (Figure 4).
Figure 4.
Relationships between plasma insulin concentration (left) and free fatty acid rate of appearance in plasma (right) and VLDL-triglyceride secretion rates in men (black triangles) and women (open circles). FFA: free fatty acid; TG: triglyceride.
Although approximately two thirds (60 ± 1%) of fatty acids in VLDL-TG were derived from systemic plasma FFA, the number of plasma FFA incorporated into VLDL-TG represents only a small percentage (8.2 ± 0.4%) of total FFA released from adipose tissue TGs into the systemic circulation. There was a significant but weak relationship between FFA Ra and VLDL-TG SR in both men and women (Figure 4). At any given value for FFA Ra, women secreted fewer moles of VLDL-TG than men. No significant relationship between FFA concentration and VLDL-TG SR was detected (data not shown).
Multiple regression analysis to identify independent predictors of VLDL-TG SR
A general linear model with sex and race as fixed factors and age, FFM, percent body fat, intra-abdominal adipose tissue volume, IHTG content, FFA Ra, and plasma insulin concentration as independent variables identified a sex × race interaction and FFM, IHTG content, and FFA Ra as independent predictors of VLDL-TG SR and accounted for 50.9% of the variance in VLDL-TG SR (Supplemental Table 3). After adjusting for age, FFM, percent body fat, intra-abdominal adipose tissue volume, IHTG content, FFA Ra, and plasma insulin concentration, the VLDL-TG SR was approximately 15% lower in African American than in Caucasian women (13.0 ± 1.5 vs 15.1 ± 1.1 μmol/min) and approximately 55% lower in African American than in Caucasian men (7.7 ± 3.1 vs 17.1 ± 1.9 μmol/min).
Discussion
We evaluated the inter-relationships among a series of factors purported to be involved in regulating VLDL-TG metabolism and the relationships among VLDL-TG and VLDL-apoB-100 kinetics and VLDL-TG kinetics and plasma concentrations in a large cohort of men and women who had a wide range in body adiposity and plasma VLDL-TG concentration. Stable isotopically-labeled glycerol, leucine, and palmitate tracers were infused to measure hepatic VLDL-TG and VLDL-apoB-100 secretion and plasma clearance rates, and the rate of FFA release into plasma from lipolysis of adipose tissue TGs. The most significant findings from our study are 1) mean plasma VLDL-TG concentration and the range in their values are greater in men and women who are overweight and obese than those who are lean; however, approximately 50% of men and women who are obese have plasma VLDL-TG concentrations that are below the maximum value observed in lean subjects (≤0.60mM) due to both a reduced VLDL-TG SR and increased VLDL-TG plasma clearance rate compared with those who are obese with elevated VLDL-TG concentration; 2) women have lower plasma VLDL-TG concentrations than men at any given degree of adiposity; 3) plasma VLDL-TG concentration is directly related to VLDL-TG SR but VLDL-TG SR is a much stronger determinant of VLDL-TG concentration in men than in women; 4) a subset of women, but no men, had very high rates of VLDL-TG clearance that result in very low plasma VLDL-TG concentrations; 5) VLDL-TG SR is not simply a reflection of the number of VLDL particles that are secreted (VLDL-apoB-100 SR), and VLDL-apoB-100 SR only explains approximately 30% of the variance in VLDL-TG SR; 6) approximately 8% of FFA released into the circulation from lipolysis of adipose tissue TGs are incorporated into VLDL-TG; 7) FFA Ra in plasma is an independent predictor of VLDL-TG SR, but women secrete fewer moles of VLDL-TG than do men at any given FFA Ra value; 8) FFM and IHTG content are also independent predictors of VLDL-TG SR; 9) total body adiposity and intra-abdominal adipose tissue volume are not important independent regulators of VLDL-TG SR; and 10) African Americans secrete less VLDL-TG than Caucasians, even when differences in body composition, body fat distribution, and plasma insulin and FFA availability are taken into account. These findings provide important insights into how race, sex, and body composition influence VLDL-TG kinetics and how metabolic dynamics of VLDL and FFA metabolism influence plasma VLDL-TG concentration.
Although mean plasma VLDL-TG concentration was higher in our obese than lean subjects, approximately half of our subjects with obesity had plasma VLDL-TG concentrations that were below the maximum value observed in the lean group (≤0.60mM). These normal VLDL-TG values were due to both a lower rate of secretion and a higher rate of clearance, compared with obese subjects who had high plasma VLDL-TG concentrations. The obese subjects with normal VLDL-TG concentration also had a lower rate of secretion and a higher rate of plasma clearance of VLDL-apoB-100, a lower percentage of FFA Ra incorporated into VLDL-TG, higher plasma HDL-cholesterol concentration, less intra-abdominal adipose tissue and IHTG, and a lower VLDL-TG secretion IRI than obese subjects with high VLDL-TG concentration. These data explain the metabolic mechanisms responsible for normal plasma VLDL-TG in people with obesity and demonstrate that normal plasma VLDL-TG is associated with other markers of metabolic health and hepatic insulin sensitivity with respect to VLDL metabolism.
The sex differences in VLDL-TG metabolism observed in our subjects are consistent with and expand the results from previous studies conducted in fewer subjects, men alone, or women alone (28). Men had higher plasma VLDL-TG concentrations and higher VLDL-TG SRs than women at any given degree of adiposity. Although hepatic VLDL-TG SR was an important determinant of plasma VLDL-TG concentration in both men and women, VLDL-TG secretion explained 70% of VLDL-TG concentration in plasma in men but only approximately 30% in women. In contrast, plasma VLDL-TG clearance rate was a more important predictor of plasma VLDL-TG concentration and spanned a wider range in women than in men (0.15–2.1 vs 0.18–0.81 pools/h, respectively). In fact, we identified a subgroup of women who had markedly high rates of VLDL-TG plasma clearance that resulted in very low plasma VLDL-TG concentrations, even though their VLDL-TG SRs were within the normal range. Sixteen percent of our women, but none of our men, had VLDL-TG clearance rates that exceeded the 90th percentile value of the entire population. This group had higher plasma HDL-cholesterol concentrations and contained more than twice as many women who were African Americans than the remaining group of women (39 vs 17%), whereas mean age, BMI, and percent body fat body composition were not different between the two groups. The exact mechanisms responsible for greater VLDL-TG clearance in women than men are not clear but could be related to differences in sex hormones and adipose tissue and muscle lipoprotein lipase activity between men and women. Lipoprotein lipase activity is greater in women than in men (29–32) and estradiol reduces plasma VLDL-TG concentration due to accelerated VLDL-TG plasma clearance, whereas progesterone and T do not alter VLDL-TG kinetics (33, 34). In addition, our data suggest that the dissociation between hepatic VLDL-apoB-100 (VLDL particle) and VLDL-TG SRs influences the intravascular metabolism of VLDL. Women with high rates of VLDL-TG clearance secrete larger, TG-richer VLDL particles (determined by a high ratio of VLDL-TG to VLDL-apoB-100 SRs) than women with lower rates of clearance. Data from studies conducted in cell systems have demonstrated that lipoprotein lipase–mediated lipolysis of TGs in large, TG-rich lipoproteins is more efficient than lipolysis of TGs in small, TG-poor particles (35, 36). The faster clearance of VLDL-TG from plasma also helps explain the higher plasma HDL-cholesterol concentration observed in these women, because more rapid clearance limits the time for VLDL-TG-and HDL-cholesterol exchange.
The differences in VLDL-TG metabolism between African Americans and Caucasians observed in our subjects suggest that a reduced VLDL-TG SR is responsible for the low VLDL-TG concentration commonly observed in African Americans; moreover, our data suggest that the lower VLDL-TG SR in African Americans than in Caucasians is not due to differences in body composition, fat distribution, plasma insulin concentration, or FFA availability (13–15) but is likely an inherent racial effect on VLDL metabolism.
We found that FFA Ra was an independent, albeit small, predictor of VLDL-TG SR. Although more than 80% of plasma FFA delivered to the liver via the portal vein and hepatic artery are derived from lipolysis of sc adipose tissue TGs (37, 38), only a small portion (8%) of FFA released into the systemic circulation was incorporated into VLDL-TG. Nonetheless, systemic FFAs are the major source of fatty acids for VLDL-TG production [present study and others (11, 17, 18, 39)], and VLDL-TG SR is responsive to experimentally induced increases or decreases in plasma FFA concentration (10). In addition, we found the relationship between FFA Ra and VLDL-TG SR was different in men and women. Women secreted fewer moles of VLDL-TG than men at any given FFA Ra value, presumably due to preferential channeling of plasma FFA to ketone bodies and oxidation in women compared with men (40, 41). These results suggest that plasma FFAs themselves are involved in regulating VLDL-TG secretion, but the use of plasma FFAs for VLDL-TG production is tightly regulated by the liver and is different in men and women.
NAFLD results from an imbalance between hepatic TG synthesis and secretion (23), and is associated with increased plasma TG concentrations (42). We found a direct but curvilinear relationship between IHTG content and total VLDL-TG SR and the secretion of VLDL-TG derived from “nonsystemic fatty acids” (presumably derived from lipolysis of intrahepatic and intra-abdominal TG and de novo lipogenesis). These data are consistent with our previous observation made in a much smaller cohort of only people with obesity (24), and suggest that IHTG itself contributes to an increased secretion of VLDL-TG in people with NAFLD. However, the liver seems to have a limited capacity for TG export, which plateaus when IHTG content exceeds approximately 6%, and thereby contributes to the development of NAFLD.
Absolute FFM (in kg) was an important predictor of VLDL-TG SR, and accounted for approximately 25% of the variance in VLDL-TG secretion. In contrast, percent body fat and intra-abdominal adipose tissue volume were not independent predictors of VLDL-TG SR. Therefore, the direct linear relationship we observed between FFM and VLDL-TG SR helps explain some of the difference in VLDL-TG SR between lean and obese subjects and between men and women because people with obesity have more FFM than lean people and men have more FFM than women. The relationship between FFM and VLDL-TG SR is consistent with results from a previous study that identified resting energy expenditure as a predictor of VLDL-TG secretion in lean and obese men and women (43), because FFM is also an important determinant of resting energy expenditure (44, 45).
The large number of subjects, who had wide ranges in body composition, fat distribution, and plasma insulin and lipid concentrations, permitted a comprehensive analysis of the many factors purported to regulate hepatic VLDL-TG metabolism. Although we identified several key factors associated with VLDL kinetics, our correlative analyses do not permit establishing cause-and-effect relationships. Additional studies that target these specific factors are needed to determine whether these factors are simply associated with or directly regulate the secretion and clearance of VLDL-TG and VLDL-apoB-100, and the cellular mechanisms responsible for these relationships.
Our comprehensive analysis of many of the metabolic factors purported to regulate hepatic VLDL-TG metabolism underscore the complexity of this system. Although we found FFM, percent body fat, intra-abdominal adipose tissue volume, IHTG content, FFA Ra, and plasma insulin concentration each correlated with VLDL-TG kinetics, and the specific relationships were often affected by a person's race and sex, only FFM, IHTG content and FFA Ra were identified as statistically independent predictors (with a sex × race interaction) of VLDL-TG SR. However, these findings do not necessarily mean that the other associated factors (ie, total adiposity and intra-abdominal adipose tissue volume) do not contribute to the hepatic production and secretion of VLDL-TG, because many of these factors track together which could have masked their individual contributions. Nevertheless, the data demonstrate that the regulation of plasma VLDL-TG differ between men and women, and that normal plasma VLDL-TG is a marker of hepatic metabolic health in people with obesity. In addition, our data suggest that substrate availability for VLDL-TG synthesis is a major driver for VLDL-TG secretion, particularly FFA derived from lipolysis of sc and intrahepatic TGs, and local intrahepatic factors that are affected by a person's sex and race ultimately determine the metabolic fate of intrahepatic fatty acids.
Acknowledgments
We thank the staff of the Center for Human Nutrition for help with subject recruitment and technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
This work was supported by National Institutes of Health grants DK 37948, AT 0110, AR 49869, HD 57796, DK 94483, DK 56341 (Washington University School of Medicine Nutrition and Obesity Research Center), DK 020579 (Washington University School of Medicine Diabetes Research Center), GM 103422 (Washington University School of Medicine Biomedical Mass Spectrometry Resource), UL1 TR000448 (Washington University School of Medicine Clinical Translational Science Award), T32 DK07296, and DK007120 (Diabetes Research Postdoctoral Training Program) and grants from the International Life Sciences Foundation, the American Heart Association (0365436Z and 0510015Z), and the Pershing Square Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- apoB-100
- apolipoprotein B-100
- BMI
- body mass index
- FFA
- free fatty acid
- FFM
- fat-free mass
- FM
- fat mass
- FTR
- fractional turnover rate
- HDL
- high-density lipoprotein
- IHTG
- intrahepatic triglyceride
- IRI
- insulin resistance index
- MRT
- mean residence time
- NAFLD
- nonalcoholic fatty liver disease
- Ra
- rate of appearance
- SR
- secretion rate
- TG
- triglyceride
- TTR
- tracer-to-tracee ratio
- VLDL
- very low-density lipoprotein.
References
- 1. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 2. Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol. 2000;86:943–949. [DOI] [PubMed] [Google Scholar]
- 3. Eckel RH. What is it about very low density lipoproteins (VLDL) and cardiovascular disease in patients with type 2 diabetes mellitus: Is it the triglycerides or the cholesterol? Atherosclerosis. 2014;237:138–139. [DOI] [PubMed] [Google Scholar]
- 4. Sarwar N, Sandhu MS, Ricketts SL, et al. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Int Med. 2003;139:802–809. [DOI] [PubMed] [Google Scholar]
- 6. Converse CA, Skinner ER, eds. Lipoprotein analysis: A practical approach. New York: Oxford University Press; 1992. [Google Scholar]
- 7. Gormsen LC, Nellemann B, Sørensen LP, Jensen MD, Christiansen JS, Nielsen S. Impact of body composition on very-low-density lipoprotein-triglycerides kinetics. Am J Physiol Endocrinol Metab. 2009;296:E165–E173. [DOI] [PubMed] [Google Scholar]
- 8. Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579. [DOI] [PubMed] [Google Scholar]
- 9. Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. [DOI] [PubMed] [Google Scholar]
- 10. Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Søndergaard E, Nellemann B, Sørensen LP, Christensen B, Gormsen LC, Nielsen S. Lean body mass, not FFA, predicts VLDL-TG secretion rate in healthy men. Obesity. 2015;23:1379–1385. [DOI] [PubMed] [Google Scholar]
- 13. Miller BV, 3rd, Patterson BW, Okunade A, Klein S. Fatty acid and very low density lipoprotein metabolism in obese African American and Caucasian women with type 2 diabetes. J Lipid Res. 2012;53:2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard BV, Zech L, Davis M, et al. Studied of very low density lipoprotein triglyceride metabolism in an obese population with low plasma lipids: Lack of influence of body weight or plasma insulin. J Lipid Res. 1980;21:1032–1041. [PubMed] [Google Scholar]
- 15. Sumner AE, Furtado JD, Courville AB, et al. ApoC-III and visceral adipose tissue contribute to paradoxically normal triglyceride levels in insulin-resistant African-American women. Nutr Metab. 2013;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mittendorfer B, Patterson BW, Klein S, Sidossis LS. VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: Effects of sex and obesity. Am J Physiol Endocrinol Metab. 2003;284:E708–E715. [DOI] [PubMed] [Google Scholar]
- 17. Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–E362. [DOI] [PubMed] [Google Scholar]
- 18. Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. [DOI] [PubMed] [Google Scholar]
- 19. Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. [DOI] [PubMed] [Google Scholar]
- 20. Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith GI, Magkos F, Reeds DN, Okunade AL, Patterson BW, Mittendorfer B. One day of mixed meal overfeeding reduces hepatic insulin sensitivity and increases VLDL particle but not VLDL-triglyceride secretion in overweight and obese men. J Clin Endocrinol Metab. 2013;98:3454–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fabbrini E, Mohammed BS, Korenblat KM, et al. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fabbrini E, Tiemann Luecking C, et al. Physiological Mechanisms of Weight Gain-Induced Steatosis in People With Obesity. Gastroenterology. 2016;150:79–81 e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on basal very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Am J Physiol Endocrinol Metab. 2006;291:E1243–E1249. [DOI] [PubMed] [Google Scholar]
- 26. Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. [DOI] [PubMed] [Google Scholar]
- 27. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 28. Magkos F, Mittendorfer B. Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am. 2009;36:245–265, vii [DOI] [PubMed] [Google Scholar]
- 29. Pedersen SB, Jønler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;78:1354–1359. [DOI] [PubMed] [Google Scholar]
- 30. Taskinen MR, Kuusi T. High density lipoproteins in postprandial lipemia. Relation to sex and lipoprotein lipase activity. Atherosclerosis. 1986;59:121–130. [DOI] [PubMed] [Google Scholar]
- 31. Nikkilä EA, Taskinen MR, Rehunen S, Härkönen M. Lipoprotein lipase activity in adipose tissue and skeletal muscle of runners: Relation to serum lipoproteins. Metabolism. 1978;27:1661–1667. [DOI] [PubMed] [Google Scholar]
- 32. Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: Relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–2060. [DOI] [PubMed] [Google Scholar]
- 33. Smith GI, Reeds DN, Okunade AL, Patterson BW, Mittendorfer B. Systemic delivery of estradiol, but not testosterone or progesterone, alters very low density lipoprotein-triglyceride kinetics in postmenopausal women. J Clin Endocrinol Metab. 2014;99:E1306–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang X, Smith GI, Patterson BW, et al. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am J Physiol Endocrinol Metab. 2012;302:E740–E746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher RM, Coppack SW, Humphreys SM, Gibbons GF, Frayn KN. Human triacylglycerol-rich lipoprotein subfractions as substrates for lipoprotein lipase. Clin Chim Acta. 1995;236:7–17. [DOI] [PubMed] [Google Scholar]
- 36. Breckenridge WC. The catabolism of very low density lipoproteins. Can J Biochem Cell Biol. 1985;63:890–897. [DOI] [PubMed] [Google Scholar]
- 37. Klein S. The case of visceral fat: Argument for the defense. J Clin Invest. 2004;113:1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. [DOI] [PubMed] [Google Scholar]
- 40. Marinou K, Adiels M, Hodson L, Frayn KN, Karpe F, Fielding BA. Young women partition fatty acids towards ketone body production rather than VLDL-TAG synthesis, compared with young men. Br J Nutr. 2011;105:857–865. [DOI] [PubMed] [Google Scholar]
- 41. Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab. 2002;283:E58–E65. [DOI] [PubMed] [Google Scholar]
- 42. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. [DOI] [PubMed] [Google Scholar]
- 43. Gormsen LC, Jensen MD, Schmitz O, Møller N, Christiansen JS, Nielsen S. Energy expenditure, insulin, and VLDL-triglyceride production in humans. J Lipid Res. 2006;47:2325–2332. [DOI] [PubMed] [Google Scholar]
- 44. Wolfe RR, Durkot MJ. Role of very low density lipoproteins in the energy metabolism of the rat. J Lipid Res. 1985;26:210–217. [PubMed] [Google Scholar]
- 45. Schrauwen P, Wagenmakers AJ, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Increase in fat oxidation on a high-fat diet is accompanied by an increase in triglyceride-derived fatty acid oxidation. Diabetes. 2000;49:640–646. [DOI] [PubMed] [Google Scholar]