Abstract
Objective:
We aimed at determining the relationship of the gut microbiota and short chain fatty acids with obesity and fat partitioning and at testing potential differences in the ability of gut microbiota to ferment equal amounts of carbohydrates (CHO) between lean and obese youth.
Research Design and Methods:
We analyzed the gut microbiota of 84 youth in whom body fat distribution was measured by fast-magnetic resonance imaging, de novo lipogenesis (DNL) quantitated using deuterated water, and the capability of gut flora to ferment CHO was assessed by 13C-fructose treatment in vitro.
Results:
A significant association was found between the Firmicutes to Bacteroidetes ratio, and the abundance of Bacteroidetes and Actinobacteria with body mass index, visceral and SC fat (all P < .05). Plasma acetate, propionate, and butyrate were associated with body mass index and visceral and SC fat (all P < .05) and with hepatic DNL (P = .01, P = .09, P = .04, respectively). Moreover, the rate of CHO fermentation from the gut flora was higher in obese than in lean subjects (P = .018).
Conclusions:
These data demonstrate that obese youth show a different gut flora composition than lean and that short chain fatty acids are associated with body fat partitioning and DNL. Also, the gut microbiota of obese youth have a higher capability than the gut flora of lean to oxidize CHO.
We studied the association between gut microbiota and childhood obesity and observed that gut microbiota derived SCFA predispose to early onset obesity by enhancing hepatic de novo lipogenesis.
The prevalence of childhood obesity has progressively increased in the past four decades (1, 2). A clear understanding of the pathogenesis of obesity in children and adolescents is lacking and, more important, the underlying mechanisms contributing to the pathogenesis of the obese phenotype (irreversible in most cases) remain unexplored. Recent evidence for a role of the gut microbiota in the development of human obesity has raised several questions. The human gut microbiota is a complex community of 100 trillion archaeal and bacterial cells distributed over more than 1000 species (3, 4). Seminal work by Gordon et al provided strong evidence that the intestinal microbiota may mediate diet-induced obesity by increasing energy harvest from the diet (5–7). Microbiota dysbiosis may lead to the development of obesity through several mechanisms, including the production of bioactive metabolites (4, 8), in particular, the short chain fatty acids (SCFA) (acetate, propionate and butyrate) (9). Among the most abundant microbiota-derived metabolites, SCFA can be produced by gut microbiota through the fermentation of dietary carbohydrates (CHO) (10). Although the exact mechanisms by which SCFA contribute to pathologies associated with obesity remain unclear, one hypothesis is that they serve as substrates for hepatic de novo lipogenesis (DNL) and result in increased capacity for energy harvest from food (11). To date, many questions regarding the association between the composition of the gut microbiota and human adiposity remain unanswered. For instance, it is unclear whether the relations of microbial species observed in some populations of overweight adults apply to pediatric obesity and also it is unknown how the composition of the gut microbiota relate to fat partitioning in humans. Moreover, mechanisms linking gut microbiota to human adiposity remain to be determined.
The purpose of the present study was to test the following hypotheses: 1) that a pattern of gut microbiota composition is associated with pediatric obesity and body fat partitioning; 2) that circulating plasma concentrations of microbiota-derived SCFA would vary as a function of adiposity, fat partitioning, and would predict changes in body mass index (BMI) over time in children and adolescents; 3) that increasing serum levels of SCFA might be linked to increased hepatic DNL; and 4) that gut flora of obese youth might have a greater capability to ferment CHO than the gut flora of lean individuals. We tested these hypotheses by studying 84 children and adolescents in whom the composition of gut microbiota was assessed by sequencing the V4 region of the 16S ribosomal RNA gene, plasma fasting SCFA were measured using gas chromatography/mass spectrometry and total body fat, abdominal and liver fat were evaluated by fast-magnetic resonance imaging. Furthermore, in a subset of children and adolescents we used deuterated water to evaluate hepatic DNL and the capability of gut flora to ferment CHO was determined in vitro by measuring the oxidation of 13C-fructose. Last, changes in BMI over a period of 2.2 ± 1.7 years were measured to determine if baseline levels in SCFA in this young cohort would predict further weight gain and thus provide a mechanistic link between body weight gain longitudinally and the obese phenotype in youth.
Materials and Methods
Study cohort
As indicated in Table 1, we studied 84 children and adolescents, 15 nonobese (BMI below 85th percentile), 7 overweight (BMI between 85th and 95th percentile), 27 obese (BMI between 95th and 99th percentile), and 35 severely obese (BMI above 99th percentile), all of them underwent the assessment of body fat partitioning by fast-magnetic resonance imaging and the assessment of fasting plasma SCFA by gas chromatography mass spectrometry and a 3-day food record. The BMI of 72 children and adolescents (mean age 12.4 ± 2.9 years; mean BMI 30.4 ± 7.0) was reevaluated after 2.2 ± 1.7 years (range 0.5–6.8 years). To be eligible for this study, patients recruited from the Yale Pediatric Obesity Clinic were not to be on medications known to affect liver function or alter glucose or lipid metabolism. The study was approved by the Yale University Human Investigation Committee. Written parental informed consent and written child assent were obtained from all participants. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
Table 1.
Clinical Features of the Study Population
| Lean (n = 15) | Overweight (n = 7) | Obese (n = 27) | Severely Obese (n = 35) | P Value | |
|---|---|---|---|---|---|
| Age (y) | 15.42 ± 3.01 | 14.92 ± 1.93 | 12.55 ± 2.81 | 12.85 ± 2.94 | .006 |
| Gender (M/F) % | 47/53 | 43/57 | 52/48 | 54/46 | .928 |
| Race (C/AA/H/AI) % | 64/7/29/0 | 29/28/43/0 | 19/26/48/7 | 34/20/43/3/0 | .392 |
| BMI (kg/m2) | 21.43 ± 2.38 | 25.88 ± 1.38 | 29.54 ± 4.33 | 38.25 ± 5.46 | <.001 |
| Body fat (%) | 20.62 ± 5.69 | 31.07 ± 5.59 | 41.31 ± 7.16 | 48.48 ± 9.11 | <.001 |
| Lipid profile | |||||
| Cholesterol (mg/dl) | 141.46 ± 28.76 | 141.28 ± 26.71 | 151.31 ± 31.54 | 151.38 ± 23.66 | .418 |
| HDL (mg/dl) | 50 ± 13.22 | 47.14 ± 12.96 | 45.31 ± 9.17 | 43.53 ± 9.49 | .477 |
| LDL (mg/dl) | 77.08 ± 26.62 | 78.14 ± 19.7 | 86.46 ± 27.74 | 88.41 ± 18.56 | .339 |
| TG (mg/dl) | 71.61 ± 21.22 | 79 ± 27.98 | 97.42 ± 47.05 | 107.5 ± 90.21 | .2 |
| Liver function | |||||
| ALT (U/liter) | 14.5 ± 4.63 | 13.57 ± 5.22 | 26.41 ± 21.72 | 31.89 ± 25.74 | .023 |
| AST (U/liter) | 21.14 ± 2.56 | 22.43 ± 9.53 | 24.32 ± 13.08 | 27.64 ± 18.96 | .522 |
| Glucose metabolism | |||||
| Fasting glucose (mg/dl) | 86.96 ± 8.2 | 89.16 ± 4.71 | 92.02 ± 6.68 | 93.5 ± 12.26 | .124 |
| Fasting insulin (mcU/ml) | 13.29 ± 4.28 | 26.25 ± 13.08 | 29.69 ± 16.61 | 45.5 ± 29.74 | <.001 |
| 2-h glucose (mg/dl) | 96.35 ± 23.31 | 107.86 ± 13.59 | 122.69 ± 29.35 | 128.06 ± 31.27 | .003 |
| HOMA | 2.86 ± 0.97 | 5.59 ± 2.69 | 6.86 ± 4.35 | 11.09 ± 9.13 | .001 |
| Body fat composition | |||||
| Visceral (cm2) | 20.17 ± 11.18 | 36.60 ± 18.12 | 57.44 ± 23.79 | 79.31 ± 30.74 | <.001 |
| SC (cm2) | 153.79 ± 87.07 | 313.90 ± 12.87 | 434.86 ± 164.21 | 648.19 ± 214.20 | <.001 |
| Hepatic fat content (%) | 1.26 ± 1.81 | 0.466 ± 1.09 | 9.16 ± 11.36 | 13.00 ± 14.33 | .003 |
Abbreviations: AA, African Americans; AI, Asian Indian; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, Caucasian; H, Hispanic; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein; TG, triglyceride.
A general linear model was used to assess the differences among the groups (n = 84). Statistically significant P values are indicated in bold.
The imaging studies, the method to assess hepatic de novo lipogenesis, the biochemical analyses and the methods for DNA extraction, PCR amplification, quantitative PCR, sequencing of taxonomic marker, and assessment of CHO oxidation by gut flora are described in the Supplemental Material.
Sequence data processing
Sequences were processed in Mothur v. 1.36.1 following the MiSeq SOP (12). After demultiplexing and quality checking steps, the sequences were clustered at 97% similarity. Alpha and beta diversity statistics were calculated by subsampling to 10 000 reads per sample. Permanova was run on Bray Curtis dissimilarities of the 3% operational taxonomic units (OTUs) using the vegan package in R 3.2.0. A subsampled species matrix was of the most abundant OTUs (minimum 10% of the community of at least one sample) used to generate the heatmap. The hierarchical clustering of samples using average linkage in the heatmap was based on the entire community, not just the most abundant OTUs, using Bray-Curtis dissimilarity. The abundant OTUs were also clustered using average linkage.
Statistics
Distribution of continuous variables was examined for skewness and when appropriate data were statistically log-transformed to approximate univariate normality before association analyses, for the hepatic fat content and the gut microbiota (phylum and genus) an inverse normal transformation was used. A χ2 test was used to assess differences in prevalence. A general linear model was used to assess the studied associations and age, gender, and ethnicity were used as covariates. A Bonferroni correction for multiple comparisons was applied when appropriate. The correlations between hepatic lipogenesis and SCFA and fat depots were assessed by using a Spearman correlation. Differences in 13CO2 production between lean and obese were assessed by using a Mann-Whitney test. Data are presented as mean and SD.
Results
Body composition and metabolic phenotypes.
As indicated in Table 1, 15 subjects were nonobese, 7 were overweight (BMI between 85th and 95th percentile), 27 were obese (BMI between 95th and 99th percentile), and 35 were severely obese (BMI above the 99th percentile) (Table 1). There were no significant differences between the groups in terms of gender and race, but the lean subjects were slightly older than the obese patients (P < .001). As expected, the obese groups displayed significantly higher alanine aminotransferase (P = .023), fasting insulin (P < .001), 2-hour glucose (P = .003), and homeostasis model assessment (P = .001) and greater quantity of visceral and SC abdominal fat as well as an increased intrahepatic fat content compared to the lean group (Table 1). According to the three day food record, lean and obese did not differ in terms of intake of percent CHO, lipids, and proteins in the diet (all P > .05).
The composition of human gut microbiota associated with the degree of obesity and the distribution across body fat depots.
The mean bacterial load was 6.3 × 105 ± 3.1 × 105 copies of 16S/ng of DNA (the values ranged from 6.2 × 104 to 2.8 × 106), but there was no association between the total bacterial load and the body composition (Table 2). The distribution of abundant OTUs according to degree of adiposity is shown in Figure 1. We found that the Firmicutes to Bacteroidetes ratio (F/B), and the relative abundance of Actinobacteria were positively associated with BMI (P = .0016, P = .01), whereas Bacteroidetes were inversely associated with BMI (P = .0003) (Supplemental Figures 1 and 2). Moreover, F/B, Bacteroidetes, and Actinobacteria were associated with visceral fat (P = .075, P = .031, and P = .039, respectively) and SC fat (P = .032, P = .012, and P = .053, respectively), and hepatic fat content (P = .002, P = .003, and P = .078, respectively) independent of age, gender, and ethnicity (Table 2).
Table 2.
Association Between Body Fat Composition Phyla and Genera
| Total Body Fat (%) | Visceral Fat (cm2) | SC Fat (cm2) | Hepatic Fat Content (%) | |
|---|---|---|---|---|
| Phylum | ||||
| Total bacterial load (copies of 16S/ng of DNA) | r = −0.044, r2 = 0.002, P = .695 | r = −0.091, r2 = 0.008, P = .413 | r = −0.207, r2 = 0.046, P = .09 | r = −0.030, r2 = 0.0009, P = .787 |
| Firmicutes/Bacteroidetes | r = 0.187, r2 = 0.035, P = .042 | r = 0.208, r2 = 0.043, P = .075 | r = 0.251, r2 = 0.063, P = .032 | r = 0.339, r2 = 0.115, P = .002 |
| Bacteroidetes (%) | r = 0.209, r2 = 0.044, P = .027 | r = 0.250, r2 = 0.062, P = .031 | r = 0.288, r2 = 0.083, P = .012 | r = 0.330, r2 = 0.109, P = .003 |
| Actinobacteria (%) | r = 0.150, r2 = 0.022, P = .131 | r = 0.250, r2 = 0.063, P = .039 | r = 0.241, r2 = 0.058, P = .053 | r = 0.194, r2 = 0.038, P = .078 |
| GENUS | ||||
| Actinomyces (%) | r = 0.391, r2 = 0.153, P < .001 | r = 0.415, r2 = 0.172, P < .001 | r = 0.528, r2 = 0.279, P < .001 | r = 0.299, r2 = 0.090, P = .002 |
| Odoribacter (%) | r = 0.439, r2 = 0.193, P < .001 | r = 0.332, r2 = 0.110, P = .003 | r = 0.436, r2 = 0.190, P < .001 | r = 0.267, r2 = 0.071, P = .011 |
| Oscillospira (%) | r = 0.279, r2 = 0.078, P = .003 | r = 0.310, r2 = 0.096, P = .01 | r = 0.262, r2 = 0.069, P = .012 | r = 0.349, r2 = 0.122, P = .002 |
| Bifidobacterium (%) | r = 0.247, r2 = 0.061, P = .039 | r = 0.262, r2 = 0.069, P = .024 | r = 0.275, r2 = 0.076, P = .043 | r = 0.163, r2 = 0.026, P = .121 |
| Streptococcus (%) | r = 0.384, r2 = 0.148, P = .001 | r = 0.227, r2 = 0.052, P = .028 | r = 0.310, r2 = 0.096, P = .022 | r = 0.269, r2 = 0.072, P = .006 |
| Bacteroides (%) | r = 0.411, r2 = 0.169, P < .001 | r = 0.462, r2 = 0.213, P < .001 | r = 0.436, r2 = 0.190, P < .001 | r = 0.379, r2 = 0.144, P < .001 |
| Faecalibacterium (%) | r = 0.447, r2 = 0.200, P < .001 | r = 0.395, r2 = 0.156, P < .001 | r = 0.365, r2 = 0.133, P = .001 | r = 0.243, r2 = 0.059, P = .032 |
| Blautia (%) | r = 0.356, r2 = 0.127, P = .001 | r = 0.305, r2 = 0.093, P = .008 | r = 0.358, r2 = 0.129, P = .001 | r = 0.283, r2 = 0.080, P = .015 |
A general linear model was used to assess the studied associations and age, gender and ethnicity were used as covariates. The r was calculated by using a Pearson correlation. Eighty-four subjects were analyzed. Statistically significant P values are indicated in bold.
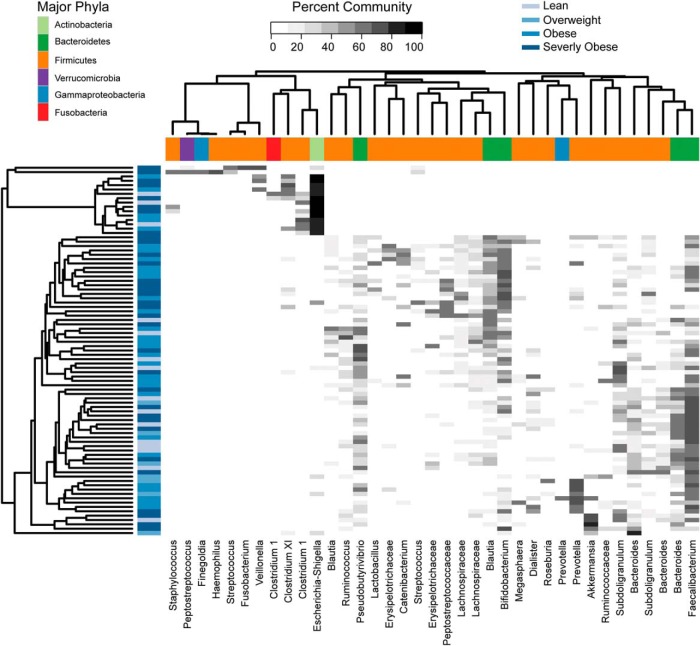
Figure 1.
Distribution of abundant OTUs among samples. This heatmap shows the abundance (shades of gray) of common OTU with samples as rows and OTU as columns. The samples were clustered based on the entire community, not just the OTU shown in this figure. The blue bars on the sides of the heatmap indicate the BMI category of the subject, whereas the colored blocks along the top indicate the phyla that the OTU belongs to. Eighty-four subjects were included in the analysis.
Among the examined genera, 21 showed a nominal association with BMI (Supplemental Table 1) and 8 (Actinomyces, Bacteroides, Odoribacter, Faecalibacterium, Oscillospira, Bifidobacterium, Streptococcus, and Blautia) were still statistically significant after correcting for multiple comparisons (P value lower than 9.8 × 10−4). All eight genera showed a statistically significant association with visceral, SC fat, and intrahepatic fat content independent of age, gender, and ethnicity (Table 2). In particular, Actinomyces, Bifidobacterium, Streptococcus, and Blautia showed a positive correlation with obesity and body fat depots, whereas Odoribacter, Oscillospira, Bacteroides, and Faecalibacterium were inversely correlated with adiposity. To assess whether any microbial community might be specific for the nonobese or for any phenotype within the spectrum of obesity, we divided the population into four groups based on the BMI percentile (nonobese <85th, overweight 85–95th, obese 95th–99th, severely obese >99th percentile). The microbial communities were significantly different among the groups with the BMI categories explaining about 7% of the total variability in the bacterial community independent of age, gender and ethnicity (P = .02).
Relationship between gut microbiota composition and SCFA.
F/B and Bacteroidetes were associated with acetate levels (P = .001) and there was a trend toward an association between F/B and Bacteroidetes with propionate levels (P = .09) (Table 3). Among the examined genera associated with BMI, Actinomyces, Odoribacter, Bacteroides, and Faecalibacterium showed a statistically significant association with all SCFA plasma concentrations as well, whereas Oscillospira, Streptococcus, and Blautia were associated only with acetate levels (all P < .05) independent of age, gender, and ethnicity (Table 3).
Table 3.
Association Between SCFA Concentrations and Phyla and Genera
| Acetate (uM/liter) | Propionate (uM/liter) | Butyrate (uM/liter) | |
|---|---|---|---|
| Phylum | |||
| Firmicutes/Bacteroidetes | r = 0.419, r2 = 0.176, P = .001 | r = 0.215, r2 = 0.046, P = .090 | r = 0.182, r2 = 0.033, P = .134 |
| Bacteroidetes (%) | r = 0.395, r2 = 0.156, P = .001 | r = 0.215, r2 = 0.046, P = .090 | r = 0.173, r2 = 0.030, P = .157 |
| Actinobacteria (%) | r = 0.235, r2 = 0.055, P = .137 | r = 0.216, r2 = 0.047, P = .220 | r = 0.170, r2 = 0.029, P = .310 |
| Genus | |||
| Actinomyces (%) | r = 0.546, r2 = 0.298, P < .001 | r = 0.308, r2 = 0.095, P = .018 | r = 0.271, r2 = 0.073, P = .037 |
| Odoribacter (%) | r = 0.518, r2 = 0.268, P < .001 | r = 0.381, r2 = 0.145, P = .004 | r = 0.370, r2 = 0.137, P = .005 |
| Oscillospira (%) | r = 0.283, r2 = 0.080, P = 0.03 | r = 0.104, r2 = 0.011, P = 0.582 | r = 0.087, r2 = −0.008, P = 0.569 |
| Bifidobacterium (%) | r = 0.187, r2 = 0.035, P = .217 | r = 0.183, r2 = 0.034, P = .317 | r = 0.146, r2 = 0.021, P = .474 |
| Streptococcus (%) | r = 0.458, r2 = 0.210, P < .001 | r = 0.277, r2 = 0.077, P = .065 | r = 0.269, r2 = 0.073, P = .068 |
| Bacteroides (%) | r = 0.456, r2 = 0.208, P < .001 | r = 0.372, r2 = 0.139, P = .009 | r = 0.345, r2 = 0.119, P = .016 |
| Faecalibacterium (%) | r = 0.371, r2 = 0.138, P = .001 | r = 0.372, r2 = 0.138, P = .002 | r = 0.350, r2 = 0.123, P = .003 |
| Blautia (%) | r = 0.388, r2 = 0.151, P = .001 | r = 0.198, r2 = 0.039, P = .098 | r = 0.210, r2 = 0.044, P = .078 |
| Total body fat and fat depots | |||
| Total body fat (%) | r = 0.283, r2 = 0.080, P = .004 | r = 0.534, r2 = 0.285, P < .001 | r = 0.555, r2 = 0.308, P < .001 |
| Visceral fat (cm2) | r = 0.329, r2 = 0.108, P = .002 | r = 0.496, r2 = 0.246, P < .001 | r = 0.455, r2 = 0.207, P < .001 |
| SC fat (cm2) | r = 0.397, r2 = 0.157, P = .001 | r = 0.571, r2 = 0.326, P < .001 | r = 0.539, r2 = 0.290, P < .001 |
| Hepatic fat content (%) | r = 0.031, r2 = 0.001, P = .611 | r = 0.200, r2 = 0.040, P = .075 | r = 0.261, r2 = 0.068, P = .019 |
A general linear model was used to assess the studied associations and age, gender, and ethnicity were used as covariates. The r was calculated by using a Pearson correlation. Eighty-four subjects were analyzed. Statistically significant P values are indicated in bold.
Plasma concentrations of SCFA are associated with degree of adiposity and fat depots.
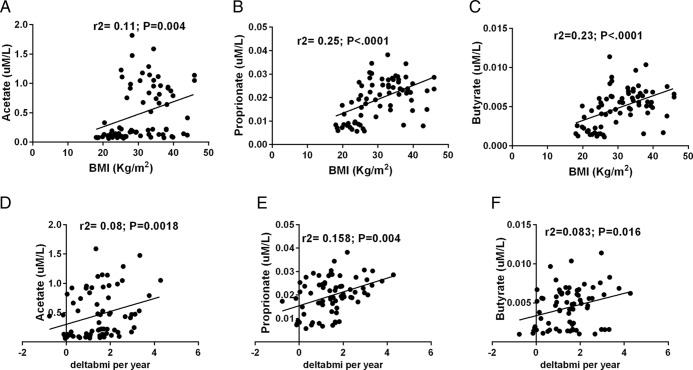
As shown in Figure 2, the fasting plasma concentrations of SCFA were associated with the degree of adiposity (acetate r2 = 0.11, P = .004; propionate r2 = 0.25, P < .001; butyrate r2 = 0.23, P < .001) independent of age, gender, and ethnicity. Moreover, all three plasma SCFAs were positively associated with percent total body fat, visceral fat, and SC fat (all P < .05) (Table 3). Among the SCFA, only butyrate was found to be significantly associated with hepatic fat (P = .019), whereas we observed a trend for an association between propionate and hepatic fat (P = .075) (Table 3).
Figure 2.
Association of plasma concentrations of SCFA with baseline BMI and changes of BMI over time. Association between SCFA and BMI for (A) acetate (r2 = 0.11, P = .004) (B) propionate (r2 = 0.25, P < .001), and (C) butyrate (r2 = 0.23, P < .001) independent of age, gender, and ethnicity. Changes of BMI per year are positively correlated with plasma levels of (D) acetate (r2 = 0.08, P = .0018), (E) propionate (r2 = 0.158, P = .004), and (F) butyrate (r2 = 0.083, P = .016), independent of age, gender, follow-up time, ethnicity, and baseline BMI. The SCFA are expressed in uM/L and changes of BMI over time are expressed as delta BMI/year. A general linear model was used to assess the studied associations and age, gender and ethnicity were used as covariates; 84 subjects were analyzed at baseline and 72 subjects were analyzed at follow-up.
SCFA are associated with changes in adiposity over time.
The relationship between SCFA and trajectories of adiposity over time was evaluated in 72 children and adolescents (mean age 12.4 ± 2.9 years; mean BMI 30.4 ± 7.0; mean follow-up time 2.2 ± 1.7 years). Of particular note, we observed that plasma levels of acetate (P = .018), propionate (P = .004), and butyrate (P = .016) positively predicted changes in adiposity (measured as delta BMI per year) independent of baseline BMI, age, gender, and ethnicity (Figure 2). Moreover, in multiple regression analyses, including as independent variables the total SCFA, age, ethnicity, gender, follow-up time, and baseline BMI, the concentration of total SCFA was associated to delta BMI changes per year (P = .017) along with age (P = .041) and explained about 7% of the variance of changes in adiposity.
SCFA levels are linked to hepatic DNL in obese adolescents.
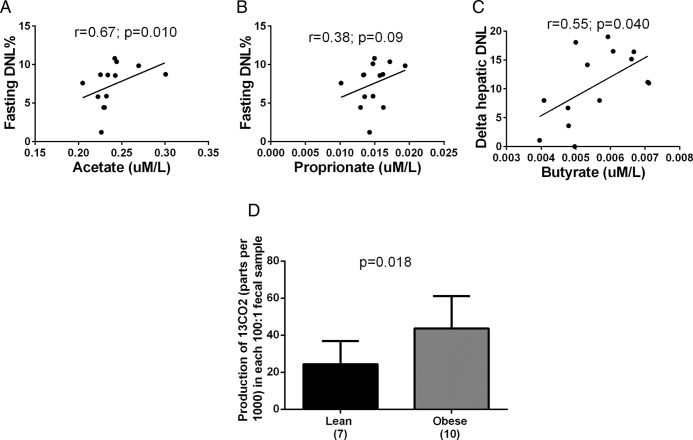
To assess whether plasma SCFA concentrations might be related to the rates of DNL in youth, we measured DNL in 14 adolescents during the fasting state and after a CHO load consisting of 75 g of glucose and 25 g of fructose (13). Plasma levels of acetate were associated with fasting hepatic DNL (r = 0.67; P = .01) and a trend was observed for propionate (r = 0.38; P = .09), whereas levels of plasma butyrate were associated with a delta increase in hepatic DNL (r = 0.55; P = .04) after the CHO load (Figure 3).
Figure 3.
Relationship between SCFA and hepatic de novo lipogenesis and differences in gut flora capability to ferment carbohydrates between lean and obese. Plasma concentrations of (A) acetate were associated with fasting hepatic DNL (P = .01) and (B) the same trend was observed for propionate (P = .09), whereas (C) concentrations of butyrate were associated with delta increase of hepatic DNL (P = .04) after a carbohydrates load (75 g of glucose and 25 g of fructose). The DNL is expressed in percent and the SCFA are expressed in uM/L. A Spearman correlation was used to analyze the data (n = 14). (D) The gut flora of obese individuals have a higher ability to ferment carbohydrates (express as 13CO2 derived from the 13C fructose) than the gut flora of lean subjects (P = .018). The production of 13CO2 is expressed as parts per thousand in each 100:1 fecal sample. A Mann-Whitney test was used to analyze the data (n = 17).
Differences in carbohydrate fermentation between obese and lean children and adolescents.
Using a novel in vitro assay, we measured 13C-fructose oxidation in the stools obtained from 7 lean subjects and 10 obese children. These two groups were similar for sex and age, whereas the mean BMI was 21.0 ± 2.3 kg/m2 for the lean subjects and 34.3 ± 6.0 kg/m2 for the obese subjects. The mean concentration of 13CO2 produced by 13C-fructose fermentation was found to be significantly higher in the obese group compared to the lean subjects (P = .018) (Figure 3).
Discussion
This study shows for the first time that, in children and adolescents, the composition of the gut microbiota is associated with the degree of obesity, body fat partitioning, and importantly with ectopic fat accumulation (namely intrahepatic fat) as well as to weight gain over a 2-year period. Our data suggest that the obese phenotype is characterized more by the abundance of several distinct communities than by the presence of one specific species. In fact, although our analysis did not find one specific microbial species or community type exclusively represented in the obese group, we identified several distinct communities associated with being obese. In particular, hierarchical clustering revealed six different clades. The most distinct group was dominated by Escherichia/Shigella and Clostridium sequences corresponding to clade 1. This clade consists of 11 subjects with only one lean subject. Clade 2 in clustering analysis is dominated by sequences belonging to Prevotella and it is found exclusively in the obese group being more abundant in subjects with a BMI greater than 95% (P < .008). although several studies consider Prevotella to be associated with non-Western diets and more dominant in subjects consuming diets with a high fiber content (14–16), one very small study consisting of nine subjects reported H2-producing Prevotellacaea to be enriched in obese subjects (17). Clade 3 was dominated by Faecalibacterium and clade 5 by Faecalibacterium and Roseburia. Both of these clades contained samples from lean and obese subjects. In a few studies, Faecalibacterium OTUs have been reported to be less abundant in obese subjects (18, 19), whereas Roseburia has been reported to be more abundant in obese subjects (19, 20). Clade 4 was dominated by Bifidobacterium and Blautia and only one lean subject was among the 16 donors. In contrast, two previous studies reported Bifidobacterium spp to be present at lower numbers in obese subjects; however, in one study the change was very small and in the other, only a very few subjects were positive for Bifidobacteria (21, 22). Clade 6 is dominated by Akkermansia, which has been shown to be able to degrade host-produced mucus and has been shown in mice to help in preventing obesity (23).
We also observed that the gut bacteria of obese youth had a higher capability to ferment an equal amount of fructose than the microbes present in the gut of lean subjects and that obesity-related gut microbiota communities correlated significantly with plasma SCFA concentrations, which is a consistent with findings from animal studies (11, 24). Furthermore, we show for the first time in humans that plasma levels of the SCFA—acetate, propionate, and butyrate—are associated with body fat partitioning, and hepatic lipogenesis.
Altogether, these data support the novel concept that obese children and adolescents have a higher relative abundance of bacteria capable of fermenting carbohydrates than lean children do. This results in an increased rate of SCFA biosynthesis, which ultimately provides an extra source of energy for the host that ends up being stored as lipids or glucose. The extra energy derived from the SCFA has been estimated to be at least 10% of the overall energy intake in adults on a westernized diet (25). In fact, SCFA not metabolized in the colonocyte reach the liver via the portal system, in which the molar fraction of the SCFA has been estimated to be 69:23:8 for acetate, propionate, and butyrate, respectively (26). Once in the liver, the acetate and butyrate can be converted in acetyl-CoA and propionate to propionyl-CoA, which serve as substrates for the tricarboxylic acid cycle (27) whose products are substrates for gluconeogenesis and DNL. Although carbons derived from acetate and butyrate metabolism seem to be directed mainly into the tricarboxylic acid cycle and subsequently to DNL (28), propionate has been suggested to be a preferential a substrate for gluconeogenesis and it might serve as a substrate for DNL because of the production of glucose obtained during its conversion (28). Moreover, the effect of SCFA on metabolism may be receptor mediated because acetate and propionate, and to a lesser extent butyrate, are ligands for G-protein coupled receptors, Gpr41 and Gpr43 (29–31). In fact, animal studies have shown that knock-out mice for Gpr41 receptor are significantly leaner than their wild-type littermates (32), despite similar energy intake. This evidence suggests that some of the effects of the gut flora on weight might be dependent on this receptor and suggests an integrated coupling of human and microbiota metabolism.
On the other hand, it should be noted that some studies have found that SCFA have an antiobesity role, which is in contrast to our findings (31–34). This hypothesis is based on animal studies showing that SCFA regulates the balance between fatty acid synthesis and oxidation, favoring the latter and inhibiting the former (33–36). Moreover, more recently Chambers et al (37) have shown that a 24-week targeted delivery of propionate ester supplementation, like butyrate supplementation (36), causes a reduction of body weight and an amelioration of several metabolic features consequent to the release of PYY and GLP-1 (37). However, the SCFA levels given to supplement the diet in rodent studies may result in pharmacologic effects compared with the lower levels that are produced during microbial fermentation. Nonetheless, it could also be that an increase in SCFA in obese subjects might be the consequence, more than the cause, of an increased energy intake, and might represent a mechanism of defense against energy accumulation. If this were the case, the high concentration of SCFA that we observed would suggest that obese subjects become resistant to the effect of SCFA on appetite and energy expenditure over time (38) and that in obese individuals the effect of SCFA might be completely diverted toward energy accumulation.
Despite that, evidence from studies in humans clearly show that SCFA may represent an important source of energy in humans. In fact, studies in subjects with short bowel syndrome and in preterm infants have shown that if the colon is intact, clinically significant dietary energy can be salvaged from carbohydrate not digested in the small bowel (39, 40) (41). Therefore, while the SCFA effect on human metabolism may vary according to the targeted pathways/tissues, being also beneficial in some cases (42, 43), SCFA synthesis and absorption per se represent a powerful source of extra energy intake in humans.
It could be argued that SCFA are the final product of the fiber digestion and that high fiber intake is unlikely in obese individuals and it is associated with weight loss (44). Despite this evidence, it seems that the effect of fibers on body weight is not unequivocal. This is probably because not all the dietary fibers can be digested by the gut flora and converted in SCFA. In fact, while some cellulose-containing fibers are not fermentable and do impair energy intake, human studies have shown that dietary fermentable fibers decrease metabolizable energy when added to a low fat diet, but increase metabolizable energy when added to a high fat diet (45). This data have been corroborated by animal studies showing that mice fed soluble fiber and high-fat diet gain weight and experience a high colonic fermentation with high production of SCFA (46). Therefore, the overall effect of fibers on body weight may ultimately depend on the nature of the fiber (fermentable or not fermentable) as well as on the interaction with other macronutrients that may limit the ability of some fibers to reduce colonic salvage of energy causing ultimately an increased production of SCFA. Moreover, although most of the oligo- and mono-saccharides, such as fructose, are absorbed in the small intestine, our ability to absorb them is limited. In particular, it has been estimated that 50% of the American population cannot absorb more than 25 g of fructose at once. Therefore, if we think that 12 ounces of a regular soda contain 32–36 g of fructose and that an average American boy consume about 75 g of fructose per day (47), it might be reasonable to think that, nowadays, some of the substrates for SCFA synthesis may come from sweet beverages and other oligo- and mono-saccharide rich food (milkshakes, ice cream, soft drinks, sugars, candy, cakes etc.).
We acknowledge that this study has some limitations. For example, in vivo physiologic studies assessing the fluxes of SCFA and a closer examination of the relationship between SCFA synthetic rates and lipid and glucose metabolism are needed in children across the spectrum of adiposity. Another limitation of the present study is the lack of measurement of fecal SCFA. Although previous studies focused on the association between fecal SCFA and adiposity (22), we think that plasma SCFA are more biologically relevant. Moreover, it has been shown that most circulating SCFA (about 85%) derives from the bacterial oxidation of carbohydrates in the intestine (48). On the other hand, to our best knowledge this is the first pediatric study assessing the association between gut microbiota and fat partitioning and it is the first study in humans showing an association between SCFA and hepatic DNL.
In conclusion, this study provides evidence that 1) the composition of the gut flora is associated with fat depots in children and adolescents, and the gut flora of obese children has a higher ability to ferment carbohydrates than that of lean subjects; 2) there is an increase of plasma SCFA levels across the spectrum of adiposity; and 3) SCFA are associated with hepatic DNL. Altogether these data indicate that for similar amounts of dietary energy, the gut microbiota of obese youth may drive a higher accumulation of energy than that of lean adolescents through an elevated production of colonic SCFA, which in turn could enhance hepatic DNL causing lipid accumulation in all the fat depots.
Acknowledgments
The authors thank the patients and their families as well as the Yale Center for Genome Analyses and Yale Center for Clinical Investigation (YCCI) and Hospital Research Unit personnel. N.S. thanks Andrew Goodman, Microbial Sciences Institute at Yale University, for sharing the protocol of DNA extraction from the stools and Sonia Caprio and Varman Samuel at Yale University for insightful suggestions. The authors have declared that no conflict of interest exists.
This work was supported by the American Heart Association through the 13SDG14640038, the 11CRP5620013, and the 16IRG27390002 awards (to N.S.), by the Yale Center for Clinical Investigation (2012 YCCI scholar award and YCCI Just in Time Grant), and by the Allen Foundation Award (to N.S.); by DK045735 to the Yale Diabetes Research Center; and by the Clinical and Translational Science Awards Grant UL1-RR- 024139 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. N.S. is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analyses.
Author Contributions: N.S. conceived the study, M.G. was responsible for samples collection and preliminary data analyses, M.G. and N.S. wrote the first draft of the manuscript, E.J.P. measured de novo lipogenesis, B.P. and M.S. helped recruit the patients, E.A.M. ran the quantitative PCR and uploaded the metagenomic data to the Sequence Read Archive (SRA), D.A.W. measured in vitro fermentation of carbohydrates, G.C. measured plasma levels of short chain fatty acids, and J.G. and K.M. sequenced the 16S RNA gene and provided the bioinformatics interpretation. All the authors have read and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CHO
- carbohydrate
- DNL
- de novo lipogenesis
- F/B
- Firmicutes to Bacteroidetes ratio
- OTU
- operational taxonomic unit
- SCFA
- short chain fatty acid.
References
- 1. Boelsen-Robinson T, Gearon E, Peeters A. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:1659–1660. [DOI] [PubMed] [Google Scholar]
- 2. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. [DOI] [PubMed] [Google Scholar]
- 3. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. [DOI] [PubMed] [Google Scholar]
- 5. Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. [DOI] [PubMed] [Google Scholar]
- 8. Kuwahara A Contributions of colonic short-chain Fatty Acid receptors in energy homeostasis. Front Endocrinol. 2014;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003;62:67–72. [DOI] [PubMed] [Google Scholar]
- 10. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 11. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 12. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microb. 2013;79:5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santoro N, Caprio S, Pierpont B, Name MV, Savoye M, Parks EJ. 2015 Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J Clin Endocrinol Metab. 2015;100:E1125–E1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnorr SL, Candela M, Rampelli S, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21:E607–E615. [DOI] [PubMed] [Google Scholar]
- 20. Tims S, Derom C, Jonkers DM, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathogen. 2012;53:100–108. [DOI] [PubMed] [Google Scholar]
- 22. Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. [DOI] [PubMed] [Google Scholar]
- 23. Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. [DOI] [PubMed] [Google Scholar]
- 25. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obesity Rev. 2013;14:950–959. [DOI] [PubMed] [Google Scholar]
- 26. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solinas G, Boren J, Dulloo AG. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol Metab. 2015;4:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. [DOI] [PubMed] [Google Scholar]
- 30. Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. [DOI] [PubMed] [Google Scholar]
- 31. Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, Hiemori M, Tsuji H. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007;71:1236–1243. [DOI] [PubMed] [Google Scholar]
- 34. Zydowo MM, Smolenski RT, Swierczynski J. Acetate-induced changes of adenine nucleotide levels in rat liver. Metab Clin Exp. 1993;42:644–648. [DOI] [PubMed] [Google Scholar]
- 35. Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. [DOI] [PubMed] [Google Scholar]
- 36. Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chambers ES, Viardot A, Psichas A, et al. 2014 Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328–336. [DOI] [PubMed] [Google Scholar]
- 39. Nordgaard I, Hansen BS, Mortensen PB. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am J Clin Nutr. 1996;64:222–231. [DOI] [PubMed] [Google Scholar]
- 40. Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet. 1994;343:373–376. [DOI] [PubMed] [Google Scholar]
- 41. Kien CL, Kepner J, Grotjohn K, Ault K, McClead RE. Stable isotope model for estimating colonic acetate production in premature infants. Gastroenterology. 1992;102:1458–1466. [DOI] [PubMed] [Google Scholar]
- 42. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature reviews. Endocrinology. 2015;11:577–591. [DOI] [PubMed] [Google Scholar]
- 43. Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obesity. 2015;39:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baer DJ, Rumpler WV, Miles CW, Fahey GC., Jr Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. The Journal of nutrition 1997;127:579–586. [DOI] [PubMed] [Google Scholar]
- 46. Isken F, Klaus S, Osterhoff M, Pfeiffer AF, Weickert MO. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J Nutr Biochem. 2010;21:278–284. [DOI] [PubMed] [Google Scholar]
- 47. Latulippe ME, Skoog SM. Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit Rev Food Sci Nutr. 2011;51:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]