Abstract
Context:
Normal weight polycystic ovary syndrome (PCOS) women may have altered adipose structure-function underlying metabolic dysfunction.
Objective:
This study examines whether adipose structure-functional changes exist in normal weight PCOS women and correlate with hyperandrogenism and/or hyperinsulinemia.
Design:
This is a prospective cohort study.
Setting:
The setting was an academic medical center.
Patients:
Six normal weight PCOS women and 14 age- and body mass index-matched normoandrogenic ovulatory (NL) women were included.
Intervention(s):
All women underwent circulating hormone and metabolic measurements; frequently sampled intravenous glucose tolerance testing; total body dual-energy x-ray absorptiometry; abdominal magnetic resonance imaging; and SC abdominal fat biopsy.
Main Outcome Measure(s):
Circulating hormones and metabolites, body fat and its distribution, and adipocyte size were compared between PCOS and NL women, and were correlated with each other in all women.
Results:
Circulating LH and androgen levels were significantly greater in PCOS than NL women, as were fasting insulin levels, pancreatic β-cell responsiveness to glucose, and total abdominal fat mass. Intra-abdominal fat mass also was significantly increased in PCOS women and was positively correlated with circulating androgen, fasting insulin, triglyceride, and non-high-density lipoprotein cholesterol levels in all women. SC abdominal fat mass was not significantly increased in PCOS women, but contained a greater proportion of small SC abdominal adipocytes that positively correlated with serum androgen levels in all women.
Conclusion:
Hyperandrogenism in normal weight PCOS women is associated with preferential intra-abdominal fat deposition and an increased population of small SC abdominal adipocytes that could constrain SC adipose storage and promote metabolic dysfunction.
We found that normal weight PCOS women exhibit preferential intra-abdominal fat storage and have an increased number of small subcutaneous abdominal adipocytes that could impair insulin action.
Polycystic ovary syndrome (PCOS) is a complex, heterogeneous endocrinopathy of women characterized by clinical or biochemical hyperandrogenism, oligo-anovulation, and polycystic ovarian morphology (1). Affecting at least 6–10% of reproductive-aged women, 60–95% of all PCOS women also have insulin resistance (2–7) that is exaggerated by increased total and abdominal adiposity (8, 9), although neither insulin resistance nor excess adiposity are part of the diagnostic criteria (1). In PCOS women who are insulin-resistant, hyperinsulinemia in the presence of excess adiposity (10, 11) interacts with hyperandrogenism to worsen the PCOS phenotype (9, 12). Insulin resistance in PCOS women, however, cannot be fully explained by total body or abdominal adiposity (1, 13–15), and has been variably attributed to other factors, such as defective insulin-mediated glucose and lipid metabolism, perturbed insulin receptor/postreceptor signal transduction, altered adipokine secretion, and abnormal steroid metabolism (1, 3, 16–18).
In humans, SC abdominal adipose normally stores lipid as protection against insulin resistance, while intra-abdominal adipose has the opposite effect (19). Moreover, in overweight PCOS women, SC abdominal adipose has enlarged adipocytes, along with decreased insulin-mediated glucose utilization, reduced glucose transporter type 4 (GLUT-4) expression and lipolytic catecholamine resistance from diminished protein levels of β2-adrenergic receptor, hormone-sensitive lipase, and protein kinase A regulatory-IIβ component (3, 14, 17, 20). These findings imply an androgen-related disorder of abnormal SC abdominal adipocyte size and/or function. If so, impaired capacity of SC abdominal adipose to store fat could enhance free fatty acid (FFA) uptake in nonadipose cells and intra-abdominal fat, promoting oxidative/endoplasmic reticulum stress linked with insulin resistance and inflammation through a process called lipotoxicity (10, 14, 19, 21–23).
Lipotoxicity from ectopic lipid deposition, rather than excess total body fat per se (22, 23), therefore, could explain why insulin resistance occurs in some (4, 10, 24), but not all (11, 25, 26), normal weight PCOS women, based upon a balance between caloric intake and energy utilization. In other words, a reduced capacity of SC abdominal adipose to store fat in normal weight PCOS women could resemble respective adipose of overweight/moderately obese individuals with insulin resistance, in whom increased numbers of dysfunctional small and enlarged adipocytes accompany decreased adiponectin, peroxisome proliferator-activated receptor, and GLUT-4 gene expression (27, 28). As sonographic evidence of this phenomenon, decreased SC abdominal thickness in normal weight PCOS women is associated with increased intra-abdominal fat thickness and metabolic dysfunction (29).
The present study examines whether hyperandrogenism in normal weight PCOS women is associated with altered abdominal adipose structure-function compared to that of age- and body mass index (BMI)-matched normoandrogenic ovulatory (NL) women. Our data demonstrate that hyperandrogenism in normal weight PCOS women is associated with increased total abdominal fat mass due to preferential deposition of intra-abdominal fat compared to age- and BMI-matched NL women of similar body fat content. This preferential intra-abdominal fat distribution in normal weight PCOS women is accompanied by an increased population of small SC abdominal adipocytes that could constrain the ability of SC adipose to safely store fat and promote lipotoxicity.
Materials and Methods
Study participants
Six PCOS women and 14 BMI- and age-matched NL women were recruited. All women were in good health, non-Hispanic Caucasian, normal weight (BMI, 18.5–25 kg/m2), and ranged in age from 18 to 35 years to avoid confounding differences by BMI, age, and race (1). All study subjects were recruited from the general community through advertising on college campuses and in local community businesses. One PCOS women also had previously attended an outpatient clinic for symptoms related to androgen excess. Subjects underwent: 1) blood sampling for steroid measurements by liquid chromatography-tandem mass spectrometry (LC-MS/MS), hormone levels and lipid analysis; 2) a frequently sampled IV glucose tolerance (FSIGT) test; 3) total body dual-energy x-ray absorptiometry (DXA) for body fat assessment; 4) abdominal magnetic resonance imaging (MRI) for SC abdominal and intra-abdominal fat quantification; and 5) abdominal SC fat biopsy for adipocyte size. All subjects completed the study, except for one NL woman who did not return for her total body DXA scan.
All NL women had regular menstrual cycles at 21- to 35-day intervals, and a luteal phase progesterone (P4) level, without evidence of hirsutism, acne, or alopecia (1). Subjects with PCOS were diagnosed by 1990 National Institutes of Health (NIH) criteria, including 1) clinical and/or biochemical hyperandrogenism, 2) oligo-amenorrhea (ie, intermenstrual intervals >35 days or 3 months of amenorrhea), and 3) excluding other endocrinopathies, to avoid phenotypic heterogeneity of broader Rotterdam criteria (1). Hirsutism was defined by the modified Ferriman-Gallwey method, with hirsutism defined as a modified Ferriman-Gallwey score above 6 (30). Biochemical hyperandrogenism was defined as an elevated serum total or free testosterone (T) >2 SD above the normal ranges of the NL group and confirmed in PCOS women on two separate occasions by mean total or free T levels.
Exclusion criteria were thyroid dysfunction, hyperprolactinemia, late-onset congenital adrenal hyperplasia (screening serum 17-hydroxyprogesterone >2 ng/ml), ovarian cyst(s) larger than 2 cm; present/history of smoking (<1 year), cancer, alcohol abuse, drug addiction, depression, or posttraumatic stress; diabetes; uncontrolled hypertension (≥165/100); clinically significant hepatic or renal disease; or other major medical illness. Additional exclusion criteria were recent (<3 months) use of diabetes medications, antidepressants, β-adrenergic blocking agents, weight loss medications, androgens, anabolic steroids, or hormonal agents (ie, oral contraceptives/insulin sensitizers).
Anthropometric analysis
The waist-to-hip circumference ratio was calculated from the following circumferences measured with the subject in the standing position during normal respiration: waist (ie, girth at the upper border of the iliac crests); hip (ie, largest girth over the greater trochanters) (31).
Blood sampling
All blood sampling was performed during the follicular phase (days 5–10 of the menstrual cycle) in NL women and during a period of documented oligo-anovulation, as determined by serum P4 levels, in PCOS women. Fasting blood samples were used to measure LH, FSH, total and free T, dihydrotestosterone (DHT), androstenedione (A4), dehydroepiandrosterone sulfate (DHEAS), estrone (E1), estradiol (E2), SHBG, glucagon, total and high molecular weight (HMW) adiponectin, C-reactive protein (CRP), lipid profile (total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglycerides [TG], and FFAs). Blood sampling for glucose and insulin also was performed under fasting conditions before FSIGT test, as stated later (32).
Hormone and metabolite assays
Serum levels of DHEAS, A4, total T, DHT, and E1 were measured by LC-MS/MS (Quest Diagnostics Nichols Institute). The intra-assay coefficients of variation (CVs) were: DHEAS, 5.4%; A4, 3.8%; total T, 10.7%; DHT, 15%; and E1, 3%. The interassay CVs were: DHEAS, 5.6%; A4, 6.5%; total T, 13.4%; DHT, 10%; and E1, 7%. Free T was calculated from the concentrations of total T, SHBG, and albumin. The intra- and interassay CVs for free T were 5.0% and 7.8%, respectively.
Serum FFAs were measured by quantitative spectrophotometry (ARUP Laboratories). The intra- and interassay CVs for FFAs were 1.9% and 1.7%, respectively. Serum determinations of LH, FSH, insulin, and E2 by electrochemiluminescence; glucose by a hexokinase method; CRP by nephelometry; and fasting lipids by spectrophotometry were performed at the University of California Los Angeles Center for Pathology Research Services. The intra-assays CVs were: LH, 2.8%; FSH, 2.8%; insulin, 0.6%; E2, 7.0%; glucose, 1.1%; CRP, 4.3%; total cholesterol, 0.9%; LDL, 1.2%; HDL 1.1%; and TG, 1.0%. The interassays CVs were: LH, 2.6%; FSH, 2.6%; insulin, 2.1%; E2, 3.6%; glucose, 1.0%; CRP, 6.5%; total cholesterol, 0.9%; LDL, 1.3%; HDL, 1.1%; and TG, 1.0%.
Serum determinations of total and HMW adiponectin by ELISA; and glucagon by RIA were measured at the Endocrine Technologies Support Core Lab, Oregon National Primate Research Center. The intra-assay CVs were: total adiponectin, 3.7%; HMW adiponectin, 2.9%; and glucagon, 2.3%. The interassay CVs were: total adiponectin, 6.5%; HMW adiponectin, 11.1%; and glucagon, 3.6%.
FSIGT
After an overnight fast, glucose in 50% concentration (0.3 g/kg) and regular human insulin (0.03 U/kg) were injected through an IV line at 0 and 20 minutes, respectively (32). Blood was collected at –20, –15, –5, 0, 2, 4, 8, 19, 22, 30, 40, 50, 70, 90, and 180 minutes for glucose and insulin determinations. Mathematical modeling of serial plasma glucose and insulin determinations by the modified minimal model of Bergman (32) was used to define: insulin sensitivity index (Si, ie, the action of insulin to accelerate glucose uptake and suppress glucose production), glucose effectiveness index (Sg, ie, the combined effect of glucose to enhance glucose uptake and suppress endogenous glucose production at fasting insulin levels), the acute response to glucose (AIRg, ie, the pancreatic β-cell response to glucose infusion) and the disposition index (DI: β-cell compensation index; product of Si and AIRg).
Total body DXA
Whole body scans were measured with a Hologic QDR Discovery A densitometer (Hologic, Inc.) according to the manufacturer's protocol, using fast-array modem standard regions of interest, and Apex software, versions 4.0.2 and 4.5.3.1. After standard regions of interest for the whole body scan were set, Apex automatically defined android and gynoid regions according to set parameters: the android region extended between the superior aspect of the pelvis and the first lumbar vertebra, the gynoid region spanned from the head of the femur to midthigh.
Abdominal MRI
Abdominal MRI scans were performed on a 3T Prisma (Siemens) MRI scanner in a feet-first supine position, using radio frequency coil elements of a surface matrix array combined with a spinal matrix array coil in the scanner bed. After initial localizer scans, multislice MRI scans were recorded using the standard T1-weighted spin-echo imaging with respiratory compensation using the following parameters: repetition time/echo time = 113/1.89 msec, field of view = 400 mm, excitation flip angle = 70°, parallel acceleration factor (Generalized Autocalibrating Partially Parallel Acquisitions) = 2, bandwidth = 500 Hz/pixel, acquisition matrix = 320 × 320, averages = 1, and slice thickness = 10 mm. A total of 25–30 slices were acquired between vertebral levels T12 and L5.
A commercially available image analysis software package, Slice-O-Matic (version 4.2, Tomovision) was used to quantify SC and intra-abdominal adipose. A single trained observer performed the image analysis. All raw fat only images were loaded into Slice-O-Matic and sorted automatically into anatomical order. To increase estimation accuracy, tagging was initiated at the image containing the upper margin of the liver and continued down to the L5–S1 image. For consistency and maximum contrast, Slice-O-Matic brightness settings were set at zero for the lowest signal intensity level and at 1000 for the highest signal intensity level for the gray-scale images. “Region Growing/Painting” mode was used to tag both SC and intra-abdominal adipose with the upper threshold set to the maximum possible setting and the lower threshold set to a value within the range of 150–250 depending on the pixel intensity of the SC and intra-abdominal adipose in that image series. Same-region growing procedures were used to tag each preceding slices. The resulting SC and intra-abdominal adipose tags were then manually inspected and edited, identifying any image artifacts and missed adipose tissue tagging. The SC and intra-abdominal adipose areas (cm2) were computed automatically from the respective tissue regions in each slice by summing the given tissues' pixels and multiplying by the pixel surface area. Finally, adipose volume (cm3) for each slice was calculated by multiplying the tissue area (cm2) by slice thickness, with adipose volume converted to the average density of TG (0.9 g/ml).
SC abdominal fat biopsy
A SC abdominal fat biopsy was performed during the follicular phase following confirmation of a serum P4 level below 3.0 ng/ml. With the patient in the supine position, a small region of the lower lateral abdomen was cleansed with betadine and anesthetized with 1% lidocaine containing a 1:100,000 dilution of epinephrine. A 1.0 cm incision was made using sterile technique and approximately 1.0 g of SC abdominal adipose was removed using sharp dissection and hemostatic techniques. Two small pieces of SC fat (∼100 mg) were placed into sterile cryovials, which were immediately placed into liquid nitrogen and transported to a –80 C freezer. Following removal of the fat tissue, the abdominal incision was closed with a 4-Vicryl subcuticular stitch and covered with a protective bandage.
SC abdominal adipocyte size
For adipocyte sizing, frozen SC abdominal fat was embedded in optimal cutting temperature medium, cut into 10- to 20-μm sections, and stained with hematoxylin and eosin following standard protocols. Images were digitally scanned using Aperio ScanScope at 20× magnification. Four sections per sample were sliced with approximately 250 cells per section analyzed via manual cell circling. Adipocyte area was determined using Aperio ImageScope Software.
Statistical analysis
Student's t test was used to compare circulating hormone and metabolite levels, body fat and its distribution, Sg, Si, AIRg, DI, and adipocytes size between PCOS and NL women. Measures with skewed distributions with outlying values (such as LH, TG, and AIRg) were log transformed before analysis (33). Pearson correlation coefficients were computed to assess associations between body fat (android, SC abdominal and intra-abdominal fat) and relevant reproductive and metabolic parameters. Partial correlation coefficients (adjusted for fasting insulin levels or BMI) were also computed to see if the associations remained significant after adjustment for fasting insulin levels or BMI. Adipocyte size was transformed using the natural log transformation, with a log value less than 7.0 used to identify a subpopulation of small adipocytes, as previously described (28). The empirical cumulative distribution function was calculated and plotted for each group (PCOS vs NL women) and was constructed by finding the probability that the adipocyte size was less than or equal to the observed value. The Kolmogorov-Smirnov test was used to compare SC abdominal adipocyte size distribution between PCOS and NL women.
Results
Subject characteristics
All studies were performed in accordance with the Declaration of Helsinki and approved by the University of California Los Angeles Institutional Review Board, with informed consent obtained from each subject before study participation. Of 658 subjects who initially contacted us regarding the study, 590 individuals did not fulfill study criteria by telephone interview because of age (N = 5), BMI (N = 35), health issues (N = 13), medication use (N = 34), race (N = 68), smoking (N = 21), multiple issues (N = 43), disinterest (N = 326), or loss to follow-up (N = 45). The remaining 68 women (30 normal weight NL: 38 PCOS) who fulfilled study criteria were invited for a screening visit, which consisted of a history and physical examination, serum hormone determinations, and transvaginal ovarian sonography. Of individuals who completed a screening visit, 14 NL and six PCOS subjects entered the study, whereas the remaining 48 individuals did not because of abnormal physical findings or laboratory data (N = 28), health issues (N = 4), undisclosed medication use (N = 1), or disinterest in further participation (N = 15). All subjects who entered the study completed testing except for one NL female who did not undergo DXA scanning for personal reasons. Age, BMI, and waist and hip measurements were similar between the PCOS and NL women (Table 1).
Table 1.
Patient Characteristics and Serum Hormonal Levels in NL vs PCOS Women
| Patient Characteristics | NL (n = 14) | PCOS (n = 6) | P Value |
|---|---|---|---|
| Age (y) | 26.9 ± 1.4 | 25.3 ± 1.8 | .4 |
| BMI (kg/m2) | 21.7 ± 0.5 | 21.8 ± 0.8 | .9 |
| Waist (cm) | 75.9 ± 1.2 | 74.8 ± 2.2 | .6 |
| Hip (cm) | 89.6 ± 1.6 | 86.9 ± 2.0 | .4 |
| Log LH (mIU/ml) | 0.9 ± 0.05 | 1.2 ± 0.09 | .01 |
| FSH (mIU/ml) | 6.5 ± 0.6 | 5.9 ± 0.3 | .4 |
| E1 (pg/ml) | 65.2 ± 7.7 | 85.2 ± 12.9 | .2 |
| E2 (pg/ml) | 83.9 ± 21.8 | 62.5 ± 9.3 | .4 |
| Total T (ng/dl) | 29.9 ± 2.3 | 66.9 ± 7.9 | .004 |
| Free T (pg/ml) | 2.4 ± 0.3 | 5.9 ± 0.5 | <.0001 |
| A4 (ng/dl) | 115.4 ± 10.2 | 231.5 ± 35.7 | .02 |
| DHT (ng/dl) | 8.9 ± 0.6 | 14.0 ± 3.0 | .15 |
| DHEAS (ug/dl) | 188.5 ± 29.3 | 190.2 ± 20.2 | 1.0 |
| Fasting glucose (mg/dl) | 84.2 ± 1.5 | 85.6 ± 2.0 | .6 |
| Fasting insulin (μU/ml) | 4.1 ± 0.3 | 6.0 ± 0.7 | .01 |
| FSIGT | NL (n = 14) | PCOS (n = 6) | P Value |
|---|---|---|---|
| Si (×10−4/min/μU/ml) | 7.2 ± 1.5 | 5.5 ± 1.2 | .5 |
| Log AIRg (μU/ml) | 2.4 ± 0.05 | 2.6 ± 0.08 | .04 |
| DI (Si × AIRg)/100 | 22.7 ± 4.3 | 21.5 ± 3.3 | .9 |
| Sg (×10/min) | 0.02 ± 0.003 | 0.02 ± 0.002 | .6 |
Abbreviations: A4, androstenedione; AIRg, pancreatic β-cell response to glucose infusion; BMI, body mass index; DHEAS, dehydroepiandrosterone sulfate; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; FSIGT, frequently sampled IV glucose tolerance; NL, normoandrogenic ovulatory; PCOS, polycystic ovary syndrome; Sg, glucose effectiveness index.
Data are mean ± sem.
Fasting hormone and metabolic characteristics
Serum log LH, total and free T, as well as A4 concentrations were greater in PCOS than NL women (Table 1). Serum FSH, E1, E2, DHT, and DHEAS levels were comparable between the two female types (Table 1).
There were no female type differences in fasting serum levels of FFAs, SHBG, total adiponectin, HMW adiponectin, CRP, or glucagon (Supplemental Table 1). In contrast, fasting serum concentrations of log TG and HDL-C tended to be higher and lower, respectively, in PCOS than NL women, despite similar levels of total cholesterol, LDL-C and non-HDL-C levels between female groups.
FSIGT
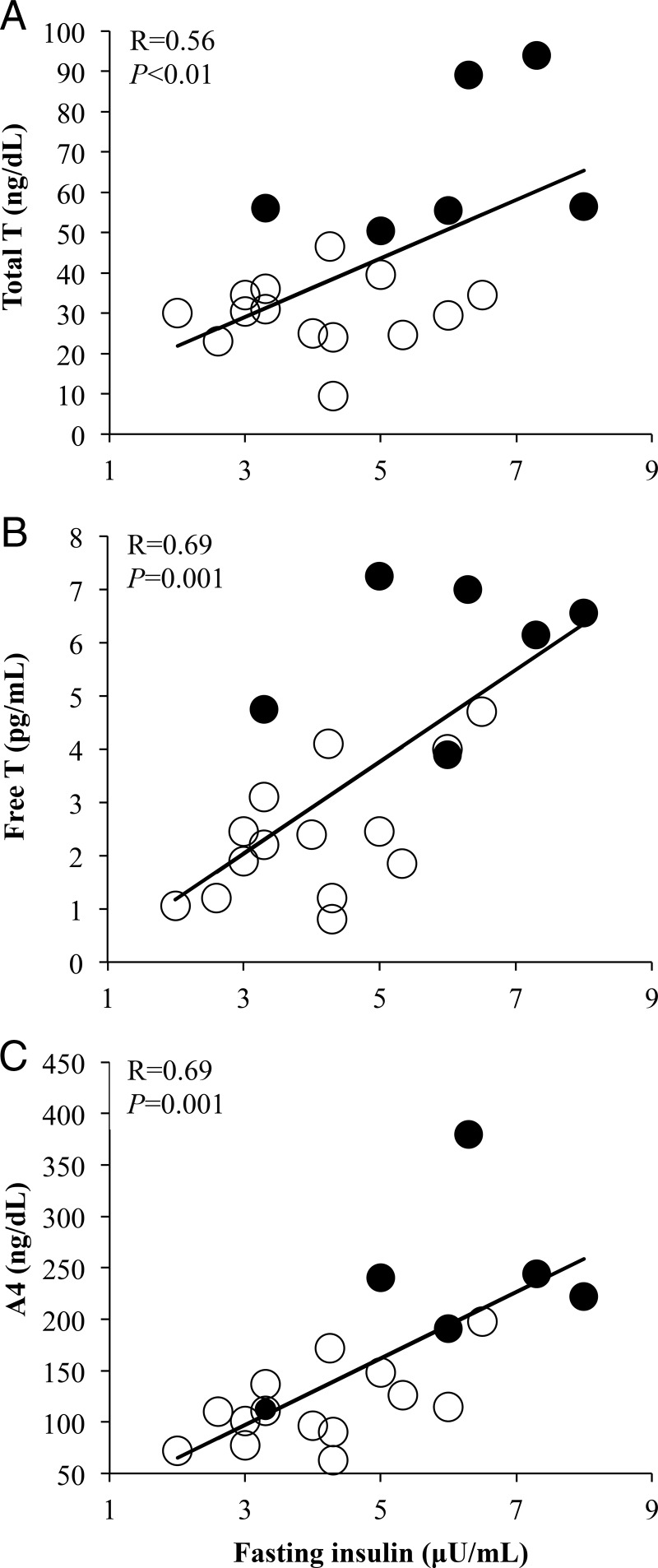
Immediately before FSIGT testing, fasting insulin levels were significantly greater in PCOS than NL women despite similar fasting glucose levels between the two female groups (Table 1). Fasting serum insulin levels in all women combined positively correlated with serum androgen levels (total T: R = 0.56, P < .01; free T: R = 0.69, P = .001; A4: R = 0.69, P = .001) (Figure 1).
Figure 1.
Positive correlations of fasting serum insulin levels with (A) total testosterone (T), (B) free T, and (C) androstenedione (A4) in normal weight polycystic ovary syndrome (PCOS) women (N = 6) and normoandrogenic ovulatory (NL) (N = 14) women combined. Filled circles, PCOS women; open circles, NL women.
During FSIGT testing, log AIRg was significantly greater in PCOS than NL women (Table 1). Insulin sensitivity in PCOS women was reduced to levels within the lower-normal range, whereas DI and Sg were similar between PCOS and NL women (Table 1).
Radiologic analysis
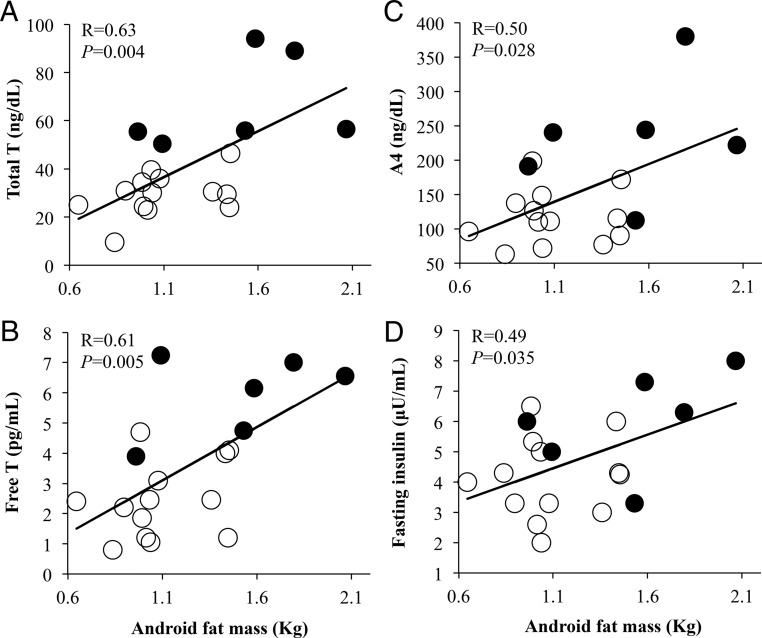
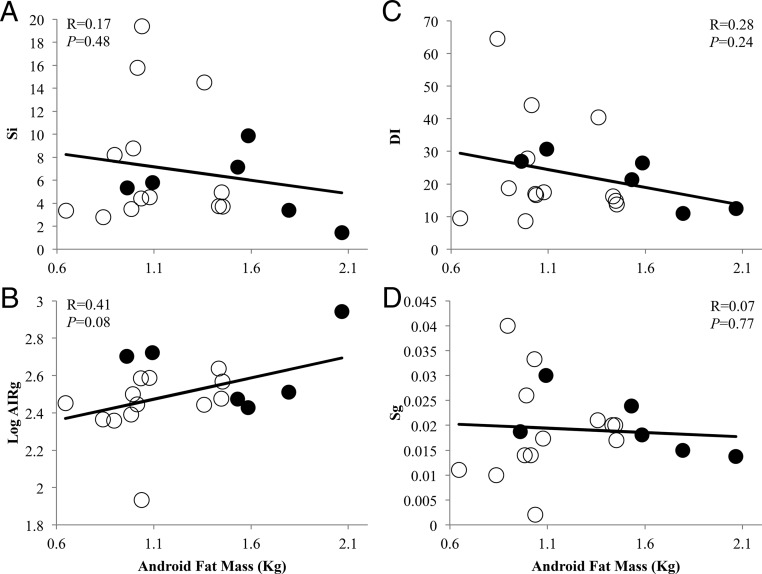
DXA confirmed similar amounts of total body mass between PCOS women (total body fat mass, 21.2 ± 1.7 kg; lean body mass, 39.8 ± 2.2 kg; percent total body fat, 34.0 ± 1.3%) and NL women (total body fat mass, 19.8 ± 0.9 kg, P = .5; lean body mass, 40.4 ± 1.3 kg, P = .8; percent total body fat, 31.5 ± 0.8%, P = .1) (Table 2). Nevertheless, android fat mass and percent android fat relative to total body fat were greater in PCOS women (android fat, 1.5 ± 0.2 kg; percent android fat, 7.1 ± 0.5%) than NL women (android fat, 1.1 ± 0.1 kg, P = .02; percent android fat, 5.5 ± 0.2%, P = .02) (Table 2). In all women combined, android fat mass positively correlated with circulating levels of total T (R = 0.63, P = .004), free T (R = 0.61, P = .005), A4 (R = 0.50, P = .028), and fasting insulin (R = 0.49, P = .035) (Figure 2A-D), as did the percent android fat mass relative to total body fat with circulating levels of total T (R = 0.69, P = .001), free T (R = 0.76, P = .0002), A4 (R = 0.71, P = .0007), and fasting insulin (R = 0.50, P = .028). Moreover, adjusting for fasting insulin levels, android fat mass remained positively correlated with circulating total T levels, although decreased in magnitude (total T: R = 0.50, P = .036; free T: R = 0.44, P = .068; A4: R = 0.27, P = .28), as did the percent android fat mass relative to total body fat with circulating levels of total T (R = 0.57, P = .013), free T (R = 0.66, P = .003), and A4 (R = 0.58, P = .011). In contrast, android fat mass did not significantly correlate with Si, log AIRg, DI, or Sg (Figure 3A-D).
Table 2.
Body Fat Characteristics of NL vs PCOS Women
| Total Body DXA | NL (n = 13) | PCOS (n = 6) | P Value |
|---|---|---|---|
| Total body fat (kg) | 19.8 ± 0.9 | 21.2 ± 1.7 | .5 |
| Total body fat (%) | 31.5 ± 0.8 | 34.0 ± 1.3 | .1 |
| Total body lean mass (kg) | 40.4 ± 1.3 | 39.8 ± 2.2 | .8 |
| Android fat (kg) | 1.1 ± 0.1 | 1.5 ± 0.2 | .02 |
| Android fat (%) | 5.5 ± 0.2 | 7.1 ± 0.5 | .02 |
| Gynoid fat (kg) | 4.3 ± 0.2 | 4.4 ± 0.3 | .9 |
| Gynoid fat (%) | 21.9 ± 0.4 | 20.8 ± 0.4 | .08 |
| Abdominal MRI | NL (n = 14) | PCOS (n = 6) | P Value |
|---|---|---|---|
| SC abdominal fat (kg) | 4.4 ± 0.2 | 5.0 ± 0.4 | .2 |
| Intra-abdominal fat (kg) | 1.8 ± 0.1 | 2.4 ± 0.3 | .03 |
Abbreviations: DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging; NL, normoandrogenic ovulatory; PCOS, polycystic ovary syndrome.
Data are mean ± sem.
Figure 2.
Positive correlations of android fat mass with circulating levels of (A) total T, (B) free T, (C) A4, and (D) fasting insulin in normal weight polycystic ovary syndrome (PCOS) women (N = 6) and normoandrogenic ovulatory (NL) (N = 13) women combined. Filled circles, PCOS women; open circles, NL women.
Figure 3.
Relationships of android fat mass with (A) insulin sensitivity index (Si), (B) log pancreatic β-cell response to glucose infusion (AIRg), (C) β-cell compensation index (DI), and (D) glucose effectiveness index (Sg) in normal weight polycystic ovary syndrome (PCOS) women (N = 6) and normoandrogenic ovulatory (NL) (N = 13) women combined undergoing a frequently sampled IV glucose tolerance test. Filled circles, PCOS women; open circles, NL women.
Gynoid fat mass and percent gynoid fat relative to total body fat were similar between PCOS women (gynoid fat, 4.4 ± 0.3 kg; percent gynoid fat, 20.8 ± 0.4%) and NL women (gynoid fat, 4.3 ± 0.2 kg, P = .9; percent gynoid fat, 21.9 ± 0.4%, P = .08) (Table 2). Neither gynoid fat mass nor percent gynoid fat relative to total body fat was related to circulating androgen or insulin levels (data not shown).
Abdominal MRI scans showed that SC abdominal fat mass was not statistically different between PCOS (5.0 ± 0.4 kg) and NL (4.4 ± 0.2 kg, P = .2) women. The amount of intra-abdominal fat in PCOS women (2.4 ± 0.3 kg), however, was greater than that of NL women (1.8 ± 0.1 kg, P = .03) (Table 2).
Clinical correlates with abdominal fat distribution
SC abdominal fat mass positively correlated with BMI in all women combined (R = 0.73, P < .001) so that all data were adjusted for BMI. Accordingly, SC abdominal fat mass positively correlated with several other fat depots: total body fat (R = 0.85, P < .001), percent total body fat (R = 0.66, P = .003), android fat mass (R = 0.78, P < .001), percent android fat mass relative to total body fat (R = 0.50, P = .04), and gynoid fat mass (R = 0.69, P = .001) (Table 3). SC abdominal fat mass, however, was unrelated to waist circumference or serum androgen, fasting insulin, and lipid levels (log TG, non-HDL-cholesterol, total cholesterol) (Table 3).
Table 3.
Abdominal Fat Correlates With Serum Androgenic and Metabolic Levels
| SC Abdominal Adipose (kg) |
Intra-abdominal Adipose (kg) |
|||
|---|---|---|---|---|
| Pearson Correlation Adjusted for BMI | P Value | Pearson Correlation Adjusted for BMI | P Value | |
| BMI (kg/m2) | — | — | — | — |
| Waist (cm) | 0.33 | .18 | 0.52 | .03 |
| Total T (ng/dl) | 0.33 | .19 | 0.51 | .03 |
| Free T (pg/ml) | 0.43 | .07 | 0.52 | .03 |
| A4 (ng/dl) | 0.14 | .57 | 0.57 | .02 |
| Fasting insulin (μU/ml) | 0.27 | .29 | 0.56 | .02 |
| Log TG (mg/dl) | 0.20 | .42 | 0.51 | .03 |
| Non-HDL-C (mg/dl) | 0.26 | .29 | 0.59 | .01 |
| Total cholesterol (mg/dl) | 0.19 | .44 | 0.50 | .03 |
| Total body fat (kg) | 0.85 | <.001 | 0.37 | .13 |
| % Total body fat | 0.66 | .003 | 0.23 | .36 |
| Android fat mass (kg) | 0.78 | <.001 | 0.69 | .002 |
| % Android fat/total body fat | 0.50 | .04 | 0.63 | .005 |
| Gynoid fat mass (kg) | 0.69 | .001 | 0.03 | .91 |
Abbreviations: A4, androstenedione; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; TG, triglycerides. Boldface P values indicate significant correlations.
Intra-abdominal fat mass positively correlated with android fat mass (R = 0.69, P = .002) and percent android fat mass relative to total body fat (R = 0.63, P = .005), but was unrelated to total body fat (R = 0.37, P = .13), percent total body fat (R = 0.23, P = .36), or gynoid fat mass (R = 0.03, P = .91) (Table 3). Intra-abdominal fat mass also positively correlated with circulating levels of androgens (total T: R = 0.51, P = .03; free T: R = 0.52, P = .03; A4: R = 0.57, P = .02), fasting insulin (R = 0.56, P = .02), log TG (R = 0.51, P = .03), non-HDL-C (R = 0.59, P = .01), and total cholesterol (R = 0.50, P = .03).
SC abdominal adipocyte size
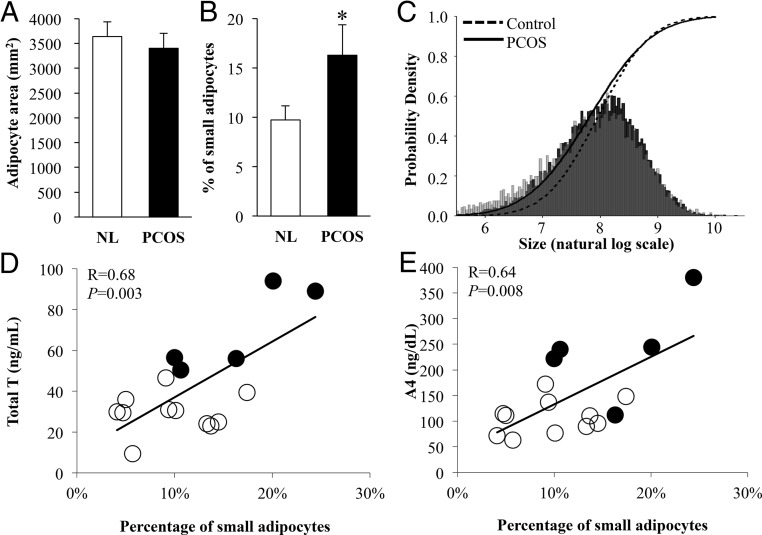
The mean SC abdominal adipocyte size was similar between PCOS and NL women (Figure 4A, NL: 3500 ± 329; PCOS: 3402 ± 288 μm2, P = .85). Analysis of cell size distribution, however, showed a significant shift toward smaller SC abdominal adipocytes in PCOS compared with NL women (Figure 4B-C; P < .05, P < .001, respectively). Moreover, in all women combined, the percentage of small adipocytes positively correlated with serum T (R = 0.68, P = .003) and A4 (R = 0.64, P = .008) levels (Figure 4D-E), but did not correlate with circulating concentrations of total and HMW adiponectin, fasting insulin, lipids, or FFAs (data not shown).
Figure 4.
Characteristics of SC abdominal adipocytes in normal weight polycystic ovary syndrome (PCOS) (N = 5) compared to normoandrogenic ovulatory (NL) (N = 11) women by (A) mean adipocyte area (P = .85), (B) percent small adipocytes (log value less than 7.0) (P < .05), and (C) adipocyte size distribution (P < .001). SC abdominal adipocyte size distribution shows a leftward shift toward a greater population of small adipocytes in normal weight PCOS women compared to age- and body mass index-matched NL women. Light gray, adipocyte size distribution of PCOS women alone; black, adipocyte size distribution of NL women alone; medium gray, adipocyte size distributions of both PCOS and NL women. The percentage of small adipocytes positively correlates with (D) total T and (E) androstenedione (A4) levels in normal weight PCOS (N = 5) and NL (N = 11) women combined. Filled circles, PCOS women; open circles, NL women. *P < .05
Discussion
Many women with PCOS defined by 1990 NIH criteria exhibit a unique form of insulin resistance that is exaggerated by increased total and abdominal adiposity (34, 35). This interaction between PCOS-related hyperandrogenism and adiposity-dependent hyperinsulinemia worsens the PCOS phenotype (8, 9, 11, 15, 36), and predisposes PCOS women to metabolic syndrome and type 2 diabetes as risk factors for cardiovascular disease (1, 3, 7, 36). Normal weight PCOS women with hyperandrogenism, however, may (4, 10, 24) or may not (11, 25, 26) exhibit insulin resistance, and may variably show defective insulin-mediated glucose and lipid metabolism, perturbed insulin receptor/postreceptor signal transduction, altered adipokine secretion, and abnormal steroid metabolism (1, 3, 16–18, 37). The present study demonstrates that normal weight PCOS women exhibit increased fasting insulin levels and greater pancreatic β-cell responses (ie, AIRg) to reduced Si within the lower-normal range than age- and BMI-matched NL women of similar total body fat. Unaccompanied by changes in the relative proportion of circulating insulin to C-peptide, these findings in normal weight PCOS women imply an underlying pancreatic β-cell compensation for prevailing modest insulin resistance in peripheral target tissues with normal hepatic insulin clearance (3, 11).
Insulin resistance in normal weight PCOS women has been variably linked with increased abdominal adiposity, with some (38), but not all (39, 40), normal weight PCOS women having a greater amount of upper body fat than age- and BMI-matched normal women. The use of total body DXA in our study confirmed that abdominal (ie, android) fat mass and its percent of total body fat were greater in normal weight PCOS than NL women, a difference that was not previously detected by less precise anthropometric measurements (41). Gluteo-femoral (ie, gynoid) fat mass and its percent of total body fat, however, were similar between the two female groups. Moreover, the amount and percent of android, but not gynoid, fat mass in all women combined positively correlated with fasting serum insulin and androgen levels. Use of total body DXA, hyperinsulinemia-euglycemic clamp techniques, and LC-MS/MS has improved understanding of the complex interrelationships among increased abdominal body fat, hyperinsulinemia, and hyperandrogenism in PCOS women (42), clarifying previous inconclusive results (9). Using these sophisticated techniques, PCOS women have been shown to exhibit increased abdominal body fat underlying hyperinsulinemia from insulin resistance over a wide BMI range, which could exaggerate hyperandrogenism (42). Our findings support this concept, and further show that the association of preferential android fat mass accumulation with circulating androgens is not explained by a function of insulin alone. Although the causal relationship between hyperinsulinemia and hyperandrogenism in normal weight PCOS women is beyond the scope of this study, previous studies have shown that administration of diazoxide for 8 days lowers A4 (43), whereas flutamide therapy for 3–4 months partially reverses insulin resistance in similar individuals (44).
Importantly, increased intra-abdominal fat mass is a risk factor for insulin resistance, independent of gender and BMI (19). Using abdominal MRI, intra-abdominal fat mass was greater in our normal weight hyperandrogenic PCOS women by NIH criteria than normal women. Our findings differ from those of a previous study, in which normal weight nonhyperandrogenic PCOS women by Rotterdam criteria did not have increased intra-abdominal fat (45). Adjusting for BMI, moreover, intra-abdominal fat deposition in all women combined positively correlated with circulating levels of androgens, resembling the increased intra-abdominal fat accumulation observed in female-to-male gender reassignment patients receiving testosterone (46). This intra-abdominal fat deposition also positively correlated with fasting insulin and lipid levels (ie, TG, total cholesterol and non-HDL-cholesterol) as risk factors for metabolic syndrome, linking intra-abdominal fat mass, hyperinsulinemia, and hyperandrogenism.
As alternate fat storage depots, SC abdominal and gluteo-femoral fat masses are protective against insulin resistance (19) and in our study were similar between PCOS and NL women. Moreover, SC abdominal fat mass in all women combined was positively correlated with BMI, and remained positively correlated with other measures of adiposity (ie, total body fat, percent total body fat, gynoid and android fat masses) after adjusting for BMI.
SC abdominal adipose tissue consists of adipocytes and other cell types (including adipose stem cells, endothelial cells, macrophages, and immune cells) surrounded by extracellular matrix proteins that through paracrine interactions regulate energy balance and whole body homeostasis (47). Although enlarged (hypertrophic) adipocytes in this fat depot are viewed as a biological marker of metabolic disease, recent studies implicate both large and small SC abdominal adipocytes with insulin resistance (28). Our results demonstrate that SC abdominal fat of normal weight PCOS contained an increased proportion of small adipocytes, without changes in the large adipocyte population. Furthermore, the increased proportion of small SC abdominal adipocytes was positively correlated with serum T and A4 levels in all women combined, whereas no correlation was detected between androgen levels and average cell size measures (28, 48, 49, 50). This finding agrees with an increased proportion of small SC abdominal adipocytes present in prenatally T-treated adult rhesus monkeys and sheep with insulin resistance, suggesting that a constrained capacity of SC adipose to store fat may be determined in utero (48, 49). If so, absence of enlarged SC abdominal adipocytes in our young normal weight PCOS women mostly from the general community may represent their overall good health, given that their insulin sensitivity remained within the lower-normal range.
Based upon these findings, energy intake in some normal weight PCOS women could theoretically exceed a constrained capacity of SC adipose to safely store fat, given an increased population of small adipocytes. Under this circumstance, excess FFAs could then deposit in abnormal locations, such as muscle and liver, where increased oxidative and endoplasmic reticulum stress could be tightly linked with insulin resistance and inflammation through lipotoxicity as disease progresses (19, 21, 22, 23, 51–53). Such a hypothesis could explain why insulin resistance occurs in some (4, 10, 24), but not all (11, 25, 26), normal weight PCOS women. In support of this theory, sonographic studies of normal weight PCOS women with insulin resistance show decreased SC abdominal and increased intra-abdominal fat thicknesses (29), whereas SC abdominal fat of overweight PCOS women develops enlarged adipocytes, along with decreased insulin-mediated glucose utilization, reduced GLUT-4 expression and lipolytic catecholamine resistance from diminished protein levels of β2-adrenergic receptor, hormone-sensitive lipase, and protein kinase A regulatory-IIβ component (3, 14, 17, 20).
Strengths of this study are the use of young, normal weight PCOS women by NIH criteria who were matched by age and BMI to NL women who were recruited from a large cohort of subjects to eliminate multiple confounders affecting insulin resistance. Recruitment of non-Hispanic Caucasian subjects from the general population also eliminated ethnic variations of body size, composition, and fat distribution (1), while simultaneously studying a less severe PCOS phenotype within the general community rather than from a referral setting (54). Use of sophisticated techniques for radiographic and hormonal analysis also permitted stringent assessment of body fat distribution and androgen excess as additional mediators of insulin resistance, whereas simultaneous FSIGT testing, rather than use of surrogate indices of insulin action, allowed precise quantification of insulin sensitivity and pancreatic β-cell responsiveness to glucose infusion (1, 32). On the other hand, limitations of our study include a small number of normal weight PCOS women, in whom a subtle reduction of Si to within the lower-normal range likely represents diminished statistical power. Another limitation is our use of SC abdominal adipose biopsy as the only site for fat analysis, since adipose cells differ in size within and between depots, which could influence fat storage capacity. Furthermore, our findings in study participants who were non-Hispanic Caucasian in ethnicity may not apply to other ethnic groups.
In conclusion, hyperandrogenism in normal weight PCOS women is associated with preferential intra-abdominal fat deposition and an increased population of small SC abdominal adipocytes that could constrain SC adipose storage and promote metabolic dysfunction through lipotoxicity.
Acknowledgments
The authors thank Erica Keller and Karla Largaespada for the support and design of subject recruitment strategies that were crucial for the success of these studies.
This study was funded by the Department of Obstetrics and Gynecology, University of California, Los Angeles; a grant from The Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (NIH) through cooperative agreement P50 HD071836 and NIH P51 ODO11092 through the Oregon National Primate Research Center; the UCLA Clinical and Translational Science Institute, UL1TR000124; and the Santa Monica Bay Woman's Club.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A4
- androstenedione
- AIRg
- pancreatic β-cell response to glucose infusion
- BMI
- body mass index
- CRP
- C-reactive protein
- CV
- coefficient of variation
- DHEAS
- dehydroepiandrosterone sulfate
- DHT
- dihydrotestosterone
- DI
- β-cell compensation index
- DXA
- dual-energy x-ray absorptiometry
- E1
- estrone
- E2
- estradiol
- FFA
- free fatty acid
- GLUT-4
- glucose transporter type 4
- FSIGT
- frequently sampled IV glucose tolerance
- HDL
- high-density lipoprotein
- HMW
- high molecular weight
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LDL
- low-density lipoprotein
- MRI
- magnetic resonance imaging
- NIH
- National Institutes of Health
- NL
- normoandrogenic ovulatory
- P4
- progesterone
- PCOS
- polycystic ovary syndrome
- Si
- insulin sensitivity index
- Sg
- glucose effectiveness index
- TG
- triglycerides.
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460. [DOI] [PubMed] [Google Scholar]
- 3. Dunaif AA, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. [DOI] [PubMed] [Google Scholar]
- 4. Diamanti-Kandarakis E, Mitrakou A, Hennes MM, et al. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism. 1995;44:525–531. [DOI] [PubMed] [Google Scholar]
- 5. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–784. [DOI] [PubMed] [Google Scholar]
- 6. Ciampelli M, Fulghesu AM, Cucinelli F, et al. Heterogeneity in β cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod. 1997;12:1897–1901. [DOI] [PubMed] [Google Scholar]
- 7. Moghetti P, Tosi F, Bonin C, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E628–E637. [DOI] [PubMed] [Google Scholar]
- 8. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–637. [DOI] [PubMed] [Google Scholar]
- 9. Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95–109. [DOI] [PubMed] [Google Scholar]
- 10. Rebuffé-Scrive M, Cullberg G, Lundberg PA, Lindstedt G, Björntorp P. Anthropometric variables and metabolism in polycystic ovarian disease. Horm Metab Res. 1989;21:391–397. [DOI] [PubMed] [Google Scholar]
- 11. Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–1058. [DOI] [PubMed] [Google Scholar]
- 12. Teede HJ, Joham AE, Paul E, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21:1526–1532. [DOI] [PubMed] [Google Scholar]
- 13. Carmina E, Bucchieri S, Esposito A, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500–2505. [DOI] [PubMed] [Google Scholar]
- 14. Mannerås-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–E311. [DOI] [PubMed] [Google Scholar]
- 15. Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:68–76. [DOI] [PubMed] [Google Scholar]
- 16. Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. [DOI] [PubMed] [Google Scholar]
- 17. Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003;88:2269–2273. [DOI] [PubMed] [Google Scholar]
- 18. Marsden PJ, Murdoch A, Taylor R. Severe impairment of insulin action in adipocytes from amenorrheic subjects with polycystic ovary syndrome. Metab Clin Exp. 1994;43:1536–1542. [DOI] [PubMed] [Google Scholar]
- 19. McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993;264:E197–E202. [DOI] [PubMed] [Google Scholar]
- 21. de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20:418–423. [DOI] [PubMed] [Google Scholar]
- 22. Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008;6:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. [DOI] [PubMed] [Google Scholar]
- 24. Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81:2854–2864. [DOI] [PubMed] [Google Scholar]
- 25. Vrbíková J, Cibula D, Dvoráková K, et al. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:2942–2925. [DOI] [PubMed] [Google Scholar]
- 26. Ovesen P, Moller J, Ingerslev HJ, et al. Normal basal and insulin-stimulated fuel metabolism in lean women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1993;77:1636–1640. [DOI] [PubMed] [Google Scholar]
- 27. McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–1715. [DOI] [PubMed] [Google Scholar]
- 28. McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: Relationship to insulin resistance and body fat. Obesity (Silver Spring). 2014;22:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1358–1364. [DOI] [PubMed] [Google Scholar]
- 30. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. [DOI] [PubMed] [Google Scholar]
- 31. Rosenzweig JL, Ferrannini E, Grundy SM, et al. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3671–3689. [DOI] [PubMed] [Google Scholar]
- 32. Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–256. [DOI] [PubMed] [Google Scholar]
- 33. Sokal RR, Rohlf FJ. Biometry, the principles and practice of statistics in biological research. 3rd ed New York: WH Freeman and Co., 1995. [Google Scholar]
- 34. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome: a systemic review and meta-analysis. Hum Reprod Updates. 2010;16:347–363. [DOI] [PubMed] [Google Scholar]
- 35. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. [DOI] [PubMed] [Google Scholar]
- 36. Moran LJ, Norman RJ, Teede HJ. Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol Metab. 2015;26:136–143. [DOI] [PubMed] [Google Scholar]
- 37. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280–285. [DOI] [PubMed] [Google Scholar]
- 38. Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16:1255–1260. [DOI] [PubMed] [Google Scholar]
- 39. Good C, Tulchinsky M, Mauger D, Demers LM, Legro RS. Bone mineral density and body composition in lean women with polycystic ovary syndrome. Fertil Steril. 1999;72:21–25. [DOI] [PubMed] [Google Scholar]
- 40. Faloia E, Canibus P, Gatti C, et al. Body composition, fat distribution and metabolic characteristics in lean and obese women with polycystic ovary syndrome. J Endocrinol Invest. 2004;27:424–429. [DOI] [PubMed] [Google Scholar]
- 41. Dumesic DA, Abbott DH, Eisner JR, et al. Pituitary desensitization to gonadotropin releasing-hormone increases abdominal adiposity in hyperandrogenic women. Fertil Steril. 1998;70:94–101. [DOI] [PubMed] [Google Scholar]
- 42. Tosi F, Di Sarra D, Kaufman JM, et al. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:661–669. [DOI] [PubMed] [Google Scholar]
- 43. Baillargeon JP, Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril. 2007;88:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. [DOI] [PubMed] [Google Scholar]
- 45. Dolfing JG, Stassen CM, van Haard PM, Wolffenbuttel BH, Schweitzer DH. Comparison of MRI-assessed body fat content between lean women with polycystic ovary syndrome (PCOS) and matched controls: less visceral fat with PCOS. Hum Reprod. 2011;26:1495–1500. [DOI] [PubMed] [Google Scholar]
- 46. Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82:2044–2047. [DOI] [PubMed] [Google Scholar]
- 47. Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keller E, Chazenbalk GD, Aguilera P, et al. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;7:2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veiga-Lopez A, Moeller J, Patel D, et al. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jernås M, Palming J, Sjöholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. [DOI] [PubMed] [Google Scholar]
- 51. Sørensen TI, Virtue S, Vidal-Puig A. Obesity as a clinical and public health problem: is there a need for a new definition based on lipotoxicity effects? Biochim Biophys Acta. 2010;1801:400–404. [DOI] [PubMed] [Google Scholar]
- 52. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome – an allostatic perspective. Biochim Biophys. 2010;1801:338–349. [DOI] [PubMed] [Google Scholar]
- 53. González F. Nutrient-induced inflammation in polycystic ovary syndrome: role in the development of metabolic aberration and ovarian dysfunction. Semin Reprod Med. 2015;33:276–286. [DOI] [PubMed] [Google Scholar]
- 54. Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E1088–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]