Abstract
Context:
Recently, two patients with primary ovarian insufficiency (POI) delivered healthy babies after in vitro activation (IVA) treatment followed by auto-transplantation of frozen-thawed ovarian tissues.
Objective:
This study sought to report the first case of live birth after IVA treatment following fresh ovarian tissue grafting in patients with POI, together with monitoring of follicle development and serum hormonal changes.
Design:
This was a prospective observational cohort study.
Setting:
We performed IVA treatment in 14 patients with POI with mean age of 29 years, mean duration since last menses of 3.8 years, and average basal FSH level of 94.5 mIU/mL.
Interventions:
Prior to IVA treatment, all patients received routine hormonal treatments with no follicle development. We removed one ovary from patients with POI and treated them with Akt stimulators. We improved upon early procedures by grafting back fresh tissues using a simplified protocol.
Main Outcome Measures:
In six of the 14 patients (43%), a total of 15 follicle development waves were detected, and four patients had successful oocyte retrieval to yield six oocytes. For two patients showing no spontaneous follicle growth, human menopausal gonadotropin treatment induced follicle growth at 6–8 months after grafting. After vitro fertilization of oocyte retrieved, four early embryos were derived. Following embryo transfer, one patient became pregnant and delivered a healthy baby boy, with three other embryos under cryopreservation.
Conclusion:
IVA technology can effectively activate residual follicles in some patients with POI and allow them to conceive their own genetic offspring. IVA may also be useful for treating patients with ovarian dysfunction including aging women and cancer survivors.
We performed in vitro activation treatment following fresh ovarian tissue grafting in primary ovarian insufficiency patients, and achieved the first case of live birth with this method.
Primary ovarian insufficiency (POI) is a cause of infertility in women, affecting 1% of the population. It is characterized by amenorrhea, hypoestrogenism, and elevated gonadotropin levels in women younger than 40 years of age (1, 2).
Ovarian tissue transplantation can restore fertility and endocrine functions in patients with cancer with ovarian dysfunction induced by chemo- or radiation therapies (3–6). Depending on the type of tissues grafted, ovarian tissue transplantation can be categorized as ovarian cortex transplantation or whole-ovary transplantation. Depending on the transplantation sites, ovary transplantation can be categorized as orthotopic or heterotopic. Based on sources of transplanted ovarian tissues, the procedure can be categorized as autologous, allogeneic, or xeno-transplantation (7, 8). Currently, autologous ovarian tissue transplantation is primarily used in patients with malignant cancers. Before chemo- and radiotherapy are performed, normally functioning ovarian tissue is cryopreserved. At the end of therapy, the ovarian tissue is returned to the patient's body to restore ovarian endocrine and reproductive functions (9). According to data from five centers providing auto-ovarian tissue transplantation, the proportion of patients who conceived was 29%. It has been recommended that this technology be implemented in cancer centers for fertility preservation, especially in prepubertal girls or when chemotherapy cannot be delayed (10).
From the traditional perspective, autologous transplantation of ovarian tissue in patients with POI is not useful because ovarian endocrine and reproductive functions have ceased based menstrual bleeding history. Thus, some patients with POI underwent restoration of their ovarian endocrine and reproductive functions through allogeneic transplantation of ovarian tissues from siblings (4). Furthermore, most POI patients underwent oocyte donation but they would not conceive genetically related children.
In 2010, phosphatase and tensin homolog (PTEN) enzyme inhibitors and phosphatidylinositol-3 kinase activators were used to activate the AKT pathway in dormant follicles in murine and human ovaries, suggesting the possibility that patients with POI with residual follicles could be activated to develop into preovulatory follicles for egg retrieval (11). Subsequent studies suggested that ovarian fragmentation could interfere with the ovarian Hippo signaling pathway, also leading to ovarian follicle growth (12). Kawamura et al (12) combined these two methods in an in vitro activation (IVA) approach to treat infertility in patients with POI. After ovariectomy, residual follicles were activated in vitro using AKT stimulators, followed by ovarian tissue auto-transplantation, leading to the delivery of two healthy babies (12, 13). After improvement of the original IVA method, we also successfully obtained mature oocytes from patients with POI before performing in vitro fertilization (IVF) and embryo transfer. We now report details of follicle growth, serum hormonal profiles for patients showing both spontaneous and hormonal-induced follicle growth after IVA treatment, together with one successful pregnancy and delivery.
Materials and Methods
Patients
From August 2014 to July 2015, we performed IVA therapy in a total of 14 patients with POI. Basic patient information is shown in Table 1. The diagnostic criteria of POI are as follows: 1) younger than 40 years of age; 2) at least 1 year of amenorrhea, 3) two or more instances in which the serum FSH level is greater than 35 U/L (ie, two analyses at intervals of 1 mo or more), and 4) serum estradiol levels less than 20 pg/mL. All patients included have received prior ovarian stimulation with different gonadotropin treatment protocols without showing follicle development based on serum estradiol levels or ultrasound monitoring. All patients have signed informed consent.
Table 1.
Patient Information
| No. | Age, y | FSH, mIU/mL | AMH, ng/mL | Menopause, y | Residual Follicles by Biopsy | Follicle Development |
|---|---|---|---|---|---|---|
| 1 | 35 | 138.4 | N.D. | 2 | Yes | Yes |
| 2 | 27 | 118.4 | N.D. | 2 | No | Yes |
| 3 | 38 | 83.5 | 0.09 | 5 | Yes | Yes |
| 4 | 30 | 37.36 | N.D. | 5 | No | Yes |
| 5 | 32 | 35.8 | N.D. | 5 | Yes | Yes |
| 6 | 27 | 66.5 | N.D. | 1 | Yes | Yes |
| 7 | 30 | 85.9 | N.D. | 6 | No | No |
| 8 | 29 | 94.6 | 0.21 | 6 | Yes | No |
| 9 | 25 | 110.2 | N.D. | 4 | No | No |
| 10 | 27 | 83.8 | N.D. | 4 | No | No |
| 11 | 32 | 109.5 | N.D. | 5 | Yes | No |
| 12 | 30 | 99.3 | N.D. | 2 | No | No |
| 13 | 23 | 132.0 | N.D. | 4 | No | No |
| 14 | 24 | 128.4 | 1.63 | 2 | Yes | No |
N.D., not detectable.
In vitro activation of ovarian follicles and auto-transplantation
We performed IVA following the published protocol (13) except using fresh ovarian tissue instead of frozen-thawed ones. We also improved grafting procedure by using an applicator (Endometrial Sampling Device) to transplant multiple ovarian tissue cubes beneath the serosa of both fallopian tubes. Briefly, after preoperative patient examinations, we removed one ovary from each patient (under general anesthesia) through laparoscopy. Depending on the actual surgical circumstances, the larger ovary was removed. The removed ovary was immediately transferred to the laboratory in an incubator with a constant temperature of 37°C.
After removing the ovary, we immediately dissected the ovarian cortex from the medulla and cut it into strips. We further cut a portion of the ovarian tissue strips into small cubes (1 × 1 mm2), and then incubated them in medium containing a PTEN inhibitor [bpV(Hopic), Merck Millipore] and a phosphatidylinositol-3 kinase activator (740YP, Tocris). The ovarian cubes were cultured for 2 days (12) and the remaining ovarian tissues were cryopreserved for future use using the vitrification method as described (13). At the same time, 10–20% volumes of each ovarian stripe were used for histological analysis to determine the presence of residual follicles. After 2 days of in vitro activation, fresh ovarian tissues were thoroughly rinsed and then transplanted laparoscopically beneath the serosa of both fallopian tubes. We improved upon earlier grafting procedures by making three to four graft sites with each site containing 20–30 small tissue cubes delivered in bulk using a tissue applicator.
Postoperative followup, IVF, and embryo transfer
Protocols
Two weeks after autologous transplantation of the ovarian tissue, we performed biweekly postoperative followups, primarily using vaginal ultrasound to examine uterine size and endometrial thickness, particularly focusing on whether there was follicle development in the bilateral annex area. To rule out follicle growth in grafts beneath fallopian tubes, growing follicles in the graft were detected based on their unique morphology characterized by the absence of a neighboring medulla (13).
We also measured serum gonadotropin and estradiol levels. If increases in estrogen levels accompanied by follicle development were found, patients were asked to visit daily until growth of preovulatory follicles was observed. After administrating human chorionic gonadotropin, (10 000∼15 000 IU) to promote oocyte maturation, oocyte retrieval was performed approximately 34∼36 hours later. If there was no follicle development at 3–4 months after surgery, patients were treated with estrogen (progynova, 3 mg/d for 21 d) and progesterone (dydrogesterone, 20 mg/d for the last 5 d) to induce menstruation. If serum FSH level was less than 20 mIU/mL on the second day of menstruation, patients were administered human menopausal gonadotropin (HMG) or recombinant FSH 300–450 IU/d to promote follicle development. If serum FSH level was greater than 20 mIU/mL on the second day of menstruation, patients were treated with a single injection of the GnRH agonist (Triptorelin, 0.1 mg) to decrease serum levels of FSH before HMG or recombinant FSH injections for 15–30 days, depending on the follicle growth pattern. If follicle growth was evident, oocyte retrieval was performed when follicles reached 16–18 mm in diameter and serum estradiol levels did not decline below 50 pg/mL. After oocyte retrieval, fertilization was achieved through intra-cytoplasmic sperm injection. Depending on individual patient's uterine condition, day 2 embryo transfer or embryo freezing by vitrification was performed.
After gaining more experience on follicle activation, we now routinely suppress serum gonadotropin levels before grafting back ovarian fragments using estrogen replacement for at least 1 month, followed by 10–14 days of estrogen plus progesterone treatments to insure that serum LH and FSH levels are less than 10 mIU/mL. This treatment stops the day before ovary autografting to induce withdrawal bleeding soon after the surgery. Within several days after initiation of withdrawal bleeding, injection of a GnRH agonist (Triptorelin, 0.1 mg/d) will be initiated to maintain serum LH levels to less than 10 mIU/mL, followed by daily purified urinary HMG or recombinant FSH injections (300–450 IU maintain circulating levels of FSH to >25 mIU/mL). Estrogen replacement and GnRH agonist treatment continue throughout ovarian stimulation for up to 4 weeks. If no follicle growth is detected, withdrawal bleeding will be induced by progesterone.
Results
A total of 14 patients with POI were included in the study. The average patient age was 29.2 years, the mean time from menstrual cessation was 3.8 years, and the mean serum FSH level was 94.5 mIU/mL. As revealed by histological analyses, 7 of the 14 patients (50%) had remaining follicles in their removed ovaries (Table 1).
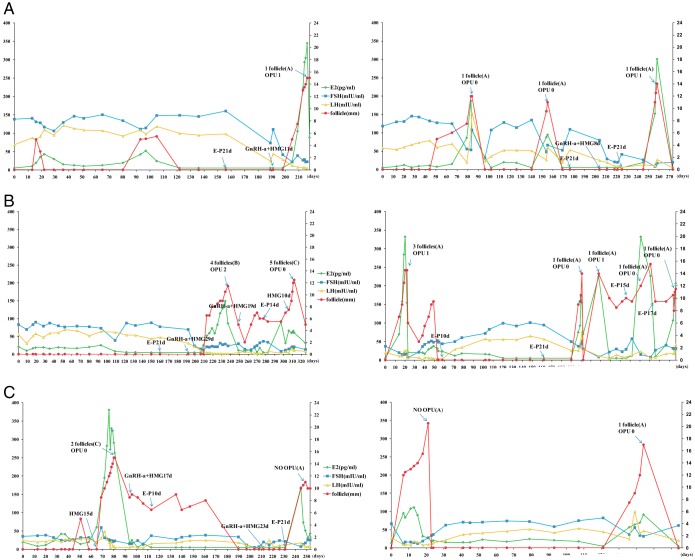
During the 1-year follow up period, six of the 14 patients showed 15 follicular development waves reaching the preovulatory stage (Figure 1). When follicles reached the preovulatory stage, human chorionic gonadotropin treatment was initiated. Oocyte retrieval was cancelled or unsuccessful in 10 of the 15 follicle growth waves due to premature luteinization of follicles or missing oocytes. In five other waves, six oocytes were obtained. Delay of spontaneous preovulatory follicle growth after grafting ranged from 20 days to 10 months (Figure 1, spontaneous growth waves designated as panel A). Some patients were treated with a GnRH agonist followed by HMG (Figure 1, Group B) whereas other patients were treated with HMG with or without pretreatment with estradiol/progesterone (Figure 1, Group C). In 11 spontaneous follicle growth waves found in four patients, three successful oocyte pickups were achieved. In contrast, a total of four HMG-induced follicle growth waves (Groups B and C) were found in three patients. For two patients (1 and 3) showing no spontaneous follicle growth, HMG treatment induced follicle growth at 6–8 months after grafting. Among the four HMG-induced waves, two successful oocyte pickups were all found in growth waves after GnRH agonist pretreatment (Group B).
Figure 1.
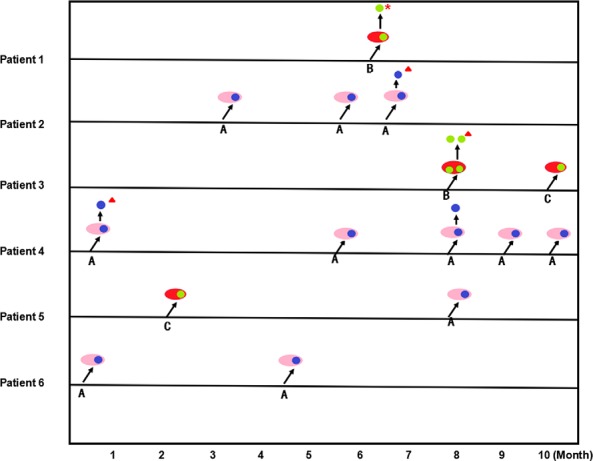
Follicle growth patterns and oocyte retrieval for six POI patients after IVA treatment. Pink ovals with an inner dot indicate spontaneous follicle growth whereas red ovals with an inner dot indicate hormonal induced follicle growth. Right upward arrows indicate follicle growth. Upward arrows pointing to one or two dots indicate successful retrieval of oocyte. A, spontaneous follicle growth with or without E/P. B, GnRH agonist followed by HMG treatment. C, HMG treatment with or without E/P pretreatment.  spontaneous;
spontaneous;  induced;
induced;  live birth;
live birth;  frozen embryo.
frozen embryo.
After IVF of retrieved oocytes, four day-2 cleavage-stage embryos developed. Following fresh embryo transfer, one healthy baby boy was delivered (Patient 1) with embryos from three other patients still vitrified, pending transfer. A male baby (birth weight, 2.9 kg) was delivered at 37 weeks of pregnancy by Caesarean section. Physical features of the baby are normal, together with normal placenta and umbilical cord.
We performed detailed analyses of serum gonadotropin and estradiol levels together with follicle size monitoring using ultrasound (Figure 2). For Patient 1, three waves of follicle growth were found but the first two waves (d 15 and 100) did not reach the preovulatory stage with unsustained serum estradiol increases. Of interest, elevated serum LH levels accompanied follicle growth. The third wave was preceded by estrogen/progesterone and GnRH agonist injections before daily HMG treatment. This approach suppressed serum LH levels and likely led to successful egg retrieval. Following IVF and fresh day-2 embryo transfer, this patient became pregnant, and delivered successfully.
Figure 2.
Hormonal profiles for six POI patients showing follicle growth after the IVA procedure. Serum levels of estradiol, FSH, and LH (left y-axis) were measured and plotted together with follicle diameters (right y-axis) estimated based on ultrasound monitoring. X-axis represents the number of days after ovarian grafting. Hormonal treatment protocols are also shown. A, spontaneous follicle growth. B, GnRH agonist followed by HMG treatment. C, HMG treatment with or without E/P pretreatment. Abbreviations: E-P, pretreatment with estrogen and progesterone; GnRH-a, GnRH agonist; opu, ovum pickup.
For Patient 2, three waves of spontaneous follicle growth accompanied by serum estradiol increases were found (d 80, 155, and 260). The first two waves were associated with elevated serum LH levels. Although attempts were made for ovum pickup, no oocytes were retrieved. Egg retrieval for the third wave of follicle growth was successful and was associated with lower serum LH levels.
For Patient 3, no follicle growth was found after almost 8 months of grafting. After estrogen/progesterone and GnRH agonist pretreatments, HMG injections led to the growth of four preovulatory follicles, leading to the retrieval of two mature eggs. Subsequent hormonal treatments, however, led to increases in follicle sizes without sustained estradiol increases, accompanied by failed egg retrieval.
For Patient 4, five spontaneous follicle growth waves were found, together with an unsustained growth wave at approximately day 45 after grafting. At 220 days after grafting, oocyte retrieval was successful.
For Patient 5, multiple follicle growth waves were found with or without HMG treatment. However, elevated estradiol levels did not accompany several growth curves. One oocyte retrieval on day 80 was not successful.
For Patient 6, two spontaneous waves were found based on increased serum estradiol levels and follicle growth. No ovum pickup was performed in the first wave whereas oocyte retrieval was unsuccessful in the second wave that is characterized by elevated serum LH levels.
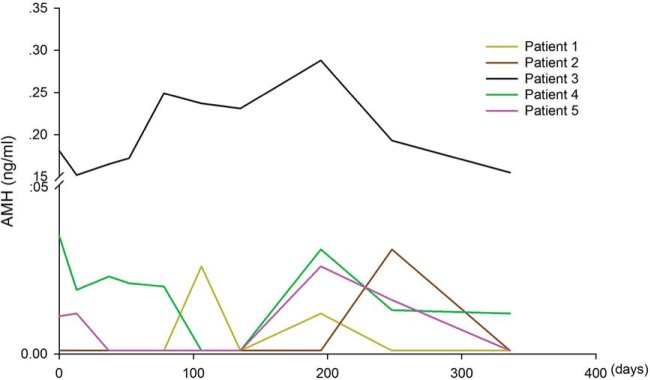
In Table 2, patients were divided into two groups based on detection or absence of follicle growth after ovarian tissue transplantation. No differences in age were found between the two groups. Compared with patients without follicle development, those with follicle development had lower basal FSH levels at the time of IVA therapy and had a shorter duration since cessation of menses. In contrast, serum AMH, anti-Mullerian hormone (AMH) levels at the time of IVA therapy do not correlate with the success of follicle growth. Only one of six patients showing follicle growth had detectable AMH levels whereas three of eight patients without follicle growth had detectable serum AMH. In addition, 67% of patients showing follicle development had residual follicles in the ovarian cortex based on histological analyses. However, only 38% patients showing no follicle growth had residual follicles (Table 2). To investigate whether IVA treatment and ovarian tissue grafting could alter serum AMH levels, we monitored AMH levels in five patients showing follicle growth. As shown in Figure 3, only Patient 3 showed continuous elevation of AMH levels before and after grafting. Additional comparison between serum AMH and FSH levels in these patients (Supplemental Figure 1) suggested a lack of correlation between serum AMH and FSH levels (r2 = 0.032).
Table 2.
Information for POI Patients With or Without Follicle Development after IVA Therapy
| With Follicle Development | Without Follicle Development | P | |
|---|---|---|---|
| No. | 6 | 8 | |
| Age, y | 31.5 ± 4.42 | 27.5 ± 3.25 | .45 |
| Basal FSH, mIU/mL | 80.0 ± 42.07 | 105.5 ± 18.04 | .04 |
| Menopause (time from last cycle), y | 3.33 ± 1.86 | 4.13 ± 1.55 | .24 |
| AMH detectable | 16.7% (1/6) | 37.5% (3/8) | .58 |
| Residual follicles by biopsy | 66.7% (4/6) | 37.5% (3/8) | .59 |
Values are Mean ± sd unless otherwise indicated.
Figure 3.
Serum AMH levels in five patients with POI showing follicle growth. Serum AMH was measured before and at different times after grafting.
Discussion
After menopause, there are still approximately 1000 primordial follicles in the ovary (14). For some patients with POI, ovarian follicles are not exhausted, and there have been reports of patients with POI naturally ovulating and conceiving (15). However, in most cases, normal development of residual follicles is impaired and none or few ovulations were detected (16, 17). The present study showed that residual follicles in some patients with POI could be activated using the IVA approach as a potential therapy. Although successful follicle growth was found here, larger-scale randomized control studies are needed to demonstrate the efficacy of the present intervention.
In mice with oocyte-specific deletion of the PTEN gene, primordial follicles in the follicular pool were activated, leading to follicle depletion in early adulthood (18). After incubating murine ovaries with a PTEN inhibitor and a PI3K enhancer to stimulate AKT signaling and activate dormant follicles, grafting of ovaries allowed the retrieval of mature oocytes. After IVF and embryo transfer, normal pups were obtained (11). Subsequently, Kawamura and colleagues (12) performed the IVA treatment for patients with POI. First, one or both ovaries were removed through laparoscopic surgery, and then only the cortex was cut into small blocks and treated with AKT stimulators. Subsequently, the activated ovarian tissues were cryopreserved before thawing and grafting back into the patient. In this process, ovarian tissues were cut into small pieces, and studies have confirmed that this process disrupts ovarian Hippo signaling pathway, thereby stimulating rapid follicle growth (12).
Among14 patients with POI included in our study, seven patients (50%) were found to have residual follicles, close to the proportion reported earlier (20/37 = 54%) (13). Six of our 14 patients (43%) showed follicle development, also similar to the earlier report (9/20, 45%). Compared with patients without follicle development, the average time since menopause in patients with follicle development was shorter, and the proportion of patients with residual follicles in their ovarian tissue slices was higher, consistent with a recent report (13). In contrast with the original IVA method that included cryopreservation of ovarian tissues (13), we performed ovarian tissue transplantation immediately after 2 days of in vitro activation by skipping the vitrification step. In addition to avoiding potential loss of follicles associated with cryopreservation, the present fresh tissue grafting without vitrification also minimizes costs and total length of hospital stay.
We found that the average time needed to detect follicle growth after grafting was 6 months, ranging from 20 to 240 days. In some patients (2, 4, and 6), spontaneous follicle growth occurred as early as 20–80 days after transplantation, presumably due to growth of existing secondary follicles in grafts. Among a total of 15 waves of follicle growth, 10 waves occurred between 5.5 and 10 months after grafting. After primordial follicle activation following IVA drug treatment, some follicles took 4–6 months to reach the preovulatory stage similar to growth duration estimated in earlier reports (6, 19) whereas the growth of other follicles could be further delayed due to local factors affecting Hippo signaling (20).
Although multiple spontaneous follicle growth waves were found, follicle growth (four of 15) also occurred after hormonal treatments. For spontaneous growth waves, ovum pickup was successful in three of the 11 follicle growth waves (27%). For the four hormone-treated waves, oocytes were retrieved in two waves (50%). We used two hormonal treatment protocols: HMG treatment following pretreatment with estrogen/progesterone plus a GnRH agonist (Group B) and HMG treatment with or without estrogen/progesterone pretreatment (Group C). Three oocytes were retrieved in two waves found in Group B, with Patient 3 showing four preovulatory follicles in a single wave. In contrast, ovum pickup was not successful in Group C. It is likely that elevated serum LH levels found in spontaneous follicle growth waves and HMG-treated waves without GnRH pretreatments are associated with premature luteinization of follicles, leading to unsustained elevation of estradiol secretion and suboptimal oocytes. In pituitary “down-regulation” cycles, both FSH and LH levels were reduced by the use of the GnRH agonist, which might minimize premature luteinization of follicles and/or resensitize FSH receptors in granulosa cells of activated follicles to exogenous FSH. It is well known that patients with polycystic ovary syndrome have elevated serum LH to FSH ratios associated with follicle arrest (21). In patients with polycystic ovary syndrome undergoing HMG therapy, a high basal LH/FSH ratio also has an adverse effect on the number of growing follicles and oocyte quality (22). Our findings are consistent with the notion that suppression of elevated LH in patients with POI could be useful in obtaining functional oocytes after IVA therapy. However, more studies are needed to verify this hypothesis.
AMH is mainly secreted by granulosa cells of secondary, and early antral follicles of less than 4 mm in diameter (23, 24) and serum AMH level is considered as a biomarker for ovarian reserve (25). Given that there are few residual follicles in patients with POI, even after IVA treatment, AMH level did not change dramatically after grafting. Although Patient 3 showed higher serum AMH levels than other patients, no correlation between AMH levels and follicle growth or serum FSH levels was evident. Our findings suggest that serum AMH is not suitable for predicting follicle growth in grafts and are consistent with a recent study for POI patients receiving ovarian tissue grafting from their monozygotic twin's sister (6). After grafting, menstrual cycle and fertility function could be restored; however, serum AMH elevation was only observed a short time after grafting, and decreased rapidly to undetectable levels.
Currently, oocyte donation is commonly used to treat infertility in patients with POI. In oocyte donation cycles, the donor must be injected with ovarian stimulation drugs and may be at risk for ovarian hyperstimulation and other complications. For recipients, artificial menstrual cycles are required to induce endometrial growth, with the potential of developing complications (26). More importantly, patients with POI are unable to conceive genetically related offspring through oocyte donation. Another therapy for patients with POI to achieve pregnancy is allogeneic transplantation with ovarian tissues from siblings. However, this therapy is extremely restrictive due to the need of sibling ovaries (7).
It has been difficult for us to enroll patients with POI onto a control group not undergoing IVA treatment due to patients' strong desire to have their own children. Future randomized controlled studies are needed to evaluate the efficacy of the present IVA treatment protocol. Future improvements are also needed to improve the efficiency of IVA, including the selection of optimal graft sites and preventing grafting associated follicle loss (27–29). In addition, new methods are needed to predict the presence of residual follicles in grafts and to estimate the outcome of IVA procedure. Currently, oocyte vitrification has been used in patients with cancer for postoperative fertility treatment (30). However, this procedure cannot be used in all patients with cancer due to the need for immediate radiation or chemotherapy or complications associated with high estrogen levels caused by ovarian stimulation. Our data showed that, even in patients with POI, multiple spontaneous follicle growth waves could be found without ovarian stimulation, indicating that IVA is also a potential fertility therapy for cancer survivors. For patients with cancer with nonovarian malignancy, their ovaries should contain large numbers of healthy follicles ready to grow after IVA and grafting, even without incubation with IVA drugs (31).
Acknowledgments
We thank all the patients included in this study.
This work was supported by National Natural and Science Foundation of China (No. 31471404) and Key Projects of Medical Science and Technology Project of Henan Province (No. 201402013).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Mullerian hormone
- HMG
- human menopausal gonadotropin
- IVA
- in vitro activation
- IVF
- in vitro fertilization
- POI
- primary ovarian insufficiency
- PTEN
- phosphatase and tensin homolog.
References
- 1. Kovanci E, Schutt AK. Premature ovarian failure: Clinical presentation and treatment. Obstet Gynecol Clin North Am. 2015;42:153–161. [DOI] [PubMed] [Google Scholar]
- 2. Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. [DOI] [PubMed] [Google Scholar]
- 3. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. [DOI] [PubMed] [Google Scholar]
- 4. Silber SJ, Lenahan KM, Levine DJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. [DOI] [PubMed] [Google Scholar]
- 5. Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. [DOI] [PubMed] [Google Scholar]
- 6. Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod Biomed Online. 2015;30:643–650. [DOI] [PubMed] [Google Scholar]
- 7. Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. [DOI] [PubMed] [Google Scholar]
- 8. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnez J, Jadoul P, Squifflet J, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. [DOI] [PubMed] [Google Scholar]
- 10. Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: Time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097–1098. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Kawamura K, Cheng Y, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki N, Yoshioka N, Takae S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. [DOI] [PubMed] [Google Scholar]
- 14. Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28:1188–1197. [DOI] [PubMed] [Google Scholar]
- 15. Dragojević-Dikić S, Rakić S, Nikolić B, Popovac S. Hormone replacement therapy and successful pregnancy in a patient with premature ovarian failure. Gynecol Endocrinol. 2009;25:769–772. [DOI] [PubMed] [Google Scholar]
- 16. Bidet M, Bachelot A, Bissauge E, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96:3864–3872. [DOI] [PubMed] [Google Scholar]
- 17. van Kasteren YM, Schoemaker J. Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. [DOI] [PubMed] [Google Scholar]
- 18. Reddy P, Liu L, Adhikari D, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. [DOI] [PubMed] [Google Scholar]
- 19. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- 20. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fauser BC, Pache TD, Lamberts SW, Hop WC, de Jong FH, Dahl KD. Serum bioactive and immunoreactive luteinizing hormone and follicle-stimulating hormone levels in women with cycle abnormalities, with or without polycystic ovarian disease. J Clin Endocrinol Metab. 1991;73:811–817. [DOI] [PubMed] [Google Scholar]
- 22. Tarlatzis BC, Grimbizis G, Pournaropoulos F, et al. The prognostic value of basal luteinizing hormone:follicle-stimulating hormone ratio in the treatment of patients with polycystic ovarian syndrome by assisted reproduction techniques. Hum Reprod. 1995;10:2545–2549. [DOI] [PubMed] [Google Scholar]
- 23. Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. [DOI] [PubMed] [Google Scholar]
- 24. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- 25. La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: Is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf). 2006;64:603–610. [DOI] [PubMed] [Google Scholar]
- 26. Tarlatzis BC, Pados G. Oocyte donation: Clinical and practical aspects. Mol Cell Endocrinol. 2000;161:99–102. [DOI] [PubMed] [Google Scholar]
- 27. Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–749. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17:605–611. [DOI] [PubMed] [Google Scholar]
- 29. Cordeiro CN, Christianson MS, Selter JH, Segars JJ. In vitro activation: A possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci. 2016. [DOI] [PubMed] [Google Scholar]
- 30. Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. [DOI] [PubMed] [Google Scholar]
- 31. Kawamura K, Cheng Y, Sun YP, et al. Ovary transplantation: To activate or not to activate. Hum Reprod. 2015;30:2457–2460. [DOI] [PubMed] [Google Scholar]