Abstract
Context and Objective:
End-stage renal disease patients have a higher risk of thyroid disease compared with those without kidney disease. Although thyroid dysfunction is associated with higher death risk in the general population and those undergoing hemodialysis, little is known about the effect of thyroid disease upon mortality in patients treated with peritoneal dialysis (PD).
Design, Setting, Participants, and Main Outcome:
We examined the association of thyroid status, assessed by serum TSH, with all-cause mortality among PD patients from a large national dialysis organization who underwent one or more TSH measurements over 5 years (January 2007 to December 2011). Thyroid status was categorized as overt-hyperthyroid, subclinical-hyperthyroid, low-normal, high-normal, subclinical-hypothyroid, and overt-hypothyroid range (TSH < 0.1, 0.1–<0.5, 0.5–<3.0, 3.0–<5.0, 5.0–<10.0, and ≥10.0 mIU/L, respectively). We examined the association between TSH and mortality using case mix–adjusted time-dependent Cox models to assess short-term thyroid function–mortality associations and to account for changes in thyroid function over time.
Results:
Among 1484 patients, 7 and 18% had hyperthyroidism and hypothyroidism, respectively, at baseline. We found that both lower and higher time-dependent TSH levels were associated with higher mortality (reference: TSH, 0.5–<3.0 mIU/L): adjusted hazard ratios (95% confidence intervals) 2.09 (1.08–4.06), 1.53 (0.87–2.70), 1.05 (0.75–1.46), 1.63 (1.11–2.40), and 3.11 (2.08–4.63) for TSH levels, <0.1, 0.1–<0.5, 0.5–<3.0, 3.0–<5.0, 5.0–<10.0, and ≥10.0 mIU/L, respectively.
Conclusion:
Time-dependent TSH levels < 0.1 mIU/L and ≥ 5.0 mIU/L were associated with higher mortality, suggesting hyper- and hypothyroidism carry short-term risk in PD patients. Additional studies are needed to determine mechanisms underlying the thyroid dysfunction–mortality association, and whether normalization of TSH with treatment ameliorates mortality in this population.
Among 1484 peritoneal dialysis patients from a large national dialysis organization, both lower (<0.1mIU/L) and higher serum thyrotropin levels (≥5.0mIU/L) were associated with higher mortality risk.
Patients with chronic kidney disease (CKD), including those receiving hemodialysis, have a higher prevalence of thyroid disease compared with the general population (1–5). Epidemiologic data have shown that hypothyroidism is progressively more common as kidney function worsens in several large population-based studies, affecting as many as 23% of patients with moderate-to-advanced CKD (ie, stage 3 CKD or higher) (2, 4). Recent data from a large dialysis provider has also shown a similarly high prevalence of hypothyroidism (∼22%) among a national cohort of hemodialysis patients (5).
Although there has been comparatively less study of the peritoneal dialysis (PD) population, patients treated with PD may also be at heightened risk of thyroid functional disease via unique pathways (6). For example, given that the vast majority (>99%) of thyroid hormone in the form of T3 and T4 is bound to carrier proteins such as T4-binding globulin, transthyretin, and serum albumin [molecular weights 54 kDa, 54 kDa, and 65 kDa, respectively (7)], PD patients may be susceptible to total body thyroid hormone depletion with substantial peritoneal effluent protein-hormone losses (8, 9). Indeed, data from several small-sized PD cohorts suggest that ∼15–25% and 4% of patients may have underlying hypo- and hyperthyroidism, respectively (10, 11).
In the general population, hypothyroidism has been associated with adverse cardiovascular sequelae, including dyslipidemia, endothelial dysfunction, and accelerated atherosclerosis (12–15). Conversely, hyperthyroidism is a known risk factor for atrial fibrillation, congestive heart failure (CHF), and coronary ischemia, which are also common cardiovascular complications in CKD patients who have an exceedingly high death risk (13, 16–18). Although there has been considerable debate as to whether thyroid functional test abnormalities in CKD are a physiologic adaptation, marker of illness, or pathologic entity, an increasing body of data suggest that hypothyroidism, defined by serum TSH elevation, is associated with higher mortality risk in hemodialysis patients (3, 5, 19). There has not, however, been prior examination of the association between thyroid dysfunction, defined by TSH, with mortality in the PD population. Thus, to better inform the field, we conducted a study of the association between thyroid disease and all-cause mortality among a national cohort of PD patients who underwent repeated measures of TSH over time. We hypothesized that both hypothyroidism and hyperthyroidism were independently associated with higher death risk in this nationally representative PD cohort from a large U.S. dialysis organization.
Materials and Methods
Source cohort
We conducted an observational study using data from a large national dialysis organization in the United States with detailed patient-level information on sociodemographics, comorbidities, laboratory tests, and vital status (20, 21). Our source population was a mixed incident and prevalent cohort of 7323 adult dialysis patients who solely received PD from one of the facilities operated by the dialysis provider over the period of January 1, 2007 to December 31, 2011. Patients were included provided that they underwent at least one TSH measure(s) any time during the study period. Patients were excluded from the study if they underwent treatment with a dialysis modality other than PD at any time during followup or they did not have a TSH measurement (n = 5839). The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor–UCLA, University of California–Irvine Medical Center, and University of Washington.
Exposure ascertainment
The exposure of interest was thyroid status defined by TSH level. We sought to examine the association between time-dependent thyroid function and all-cause mortality in which thyroid status was time updated with repeated TSH measures in order 1) to ascertain the short-term association of thyroid function with death risk, and 2) to account for changes in thyroid function over time (22). Using this approach, patients who were found to have a change in TSH testing immediately crossed over to the new exposure category, with the reasoning that change in thyroid function had occurred during the prior exposure period.
In primary analyses, we examined thyroid function using granular categories of TSH, defined according to the usual TSH ranges for these designations as overt-hyperthyroid range (<0.1 mIU/L), subclinical-hyperthyroid range (0.1–<0.5 mIU/L), low-normal (0.5–<3.0 mIU/L), high-normal (3.0–<5.0 mIU/L), subclinical-hypothyroid range (5.0–<10.0 mIU/L), and overt-hypothyroid range TSH levels (≥10.0 mIU/L) (3, 5). We also conducted secondary analyses in which we defined thyroid function according to thresholds used in the general population: hyperthyroidism, euthyroidism, and hypothyroidism (defined as TSH <0.5, 0.5–<5.0, and ≥5.0 mIU/L, respectively) (19). In sensitivity analyses, to flexibly examine TSH as a continuous predictor of mortality, we conducted restricted cubic spline analyses with knots defined at the 33rd and 66th percentiles of observed TSH values (corresponding to TSH levels of 1.8 and 3.5 mIU/L, respectively). The median (interquartile range [IQR]) frequency of TSH measurements per patient was 3 (1–8) for the time-dependent analyses.
Among patients who underwent two or more TSH measurements, we conducted sensitivity analyses examining the association between change in TSH level (from the baseline [first] to second TSH measurement) with mortality based on the following thyroid function patterns: persistently high, increase in TSH, persistently normal, decline in TSH, and persistently low (Supplemental Figures 1 and 2).
Outcome ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after the baseline quarter of TSH measurement. Patients were censored for kidney transplantation, transfer to a dialysis facility operated by another provider, or at the end of the study (December 31, 2011).
Laboratory data collection and peritoneal dialysis characteristics
Serum samples for laboratory testing were drawn within the outpatient dialysis clinics of the large dialysis organization using uniform techniques, and were transported to a single central laboratory in Deland, Florida typically within 24 hours of collection. All laboratory testing was conducted using automated and standardized methods. We also considered peritoneal equilibration test (PET) data, which included 1) the ratio of the dialysate to plasma creatinine (D/PCr ratio) at the end of a 4-hour dwell (dichotomized as <0.65 vs ≥0.65, respectively), as well as 2) the ultrafiltration volume at the end of the 4-hour dwell, defined as “4-hour PET ultrafiltration volume = 4-hour drain volume − 4-hour infusion volume” (23).
Statistical analyses
We estimated the association between thyroid function and mortality using Cox proportional hazards models with three hierarchical levels of covariate adjustment. In time-dependent analyses, all laboratory data were examined as time-dependent covariates summarized over 91-day periods (ie, mean or median values over the quarter for each patient): 1) Minimally adjusted model: adjusted for patient's calendar quarter of entry into the cohort; 2) case mix–adjusted model: adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, and baseline diabetes status; and 3) expanded case mix + laboratory test–adjusted model: adjusted for covariates in the case-mix model, as well as vintage, cause of end-stage renal disease (ESRD), baseline comorbidities including CHF and atherosclerotic disease, serum albumin, serum bicarbonate, and residual kidney function (defined by 24-h urine urea clearance).
We a priori defined the case mix–adjusted model as our preferred model, which included core sociodemographic measures and other confounders of the association between thyroid function and mortality. To explore potential causal pathways that might mediate the association between hypothyroidism and mortality, we conducted sensitivity analyses in which we added covariate terms for potential pathway intermediates to the expanded case mix + laboratory-adjusted model and observed for attenuation of effect estimates. These covariates included CHF, atherosclerotic disease, and residual kidney function (3, 14). To determine whether the PD prescription and transport function characteristics may influence thyroid function–mortality associations, we additionally examined an expanded case mix + laboratory + PD characteristics model, which included covariates in the expanded case mix + laboratory test model, as well as the use of automated PD during the baseline quarter and PET characteristics (D/PCr ratio and 4-h PET ultrafiltration volume). Proportional hazards assumptions were checked by graphical and formal testing.
We conducted subgroup analyses of thyroid function (categorized as hyperthyroidism, euthyroidism, and hypothyroidism) across clinically relevant categories of sociodemographics, comorbidity status, PET characteristics, and laboratory measures. Missing data were handled using methods that included multiple imputation. There were no missing values for age, sex, diabetes, vintage, cause of ESRD, CHF, atherosclerotic heart disease, or automated PD status. The remaining covariates ascertained at baseline had less than or equal to 1% missing values, except for residual kidney function (14%), D/PCr ratio (25%), and 4-hour PET ultrafiltration volume (30%). Analyses and Figures were generated using SAS version 9.4 (SAS Institute, Inc.), Stata version 13.1 (StataCorp), and SigmaPlot Version 12.5 (Systat Software).
Results
Study population
Among 1484 patients meeting eligibility criteria, we observed that 7% (n = 104) had hyperthyroidism, 18% (n = 270) had hypothyroidism, and 75% (n = 1110) were euthyroid defined by baseline TSH levels. In the overall cohort, the mean ± SD and median (IQR) of observed TSH levels were 5.0 ± 14.2 and 2.5 (1.5–4.1) mIU/L, respectively. We compared baseline characteristics among patients who were included vs excluded on the basis of TSH measurement vs nonmeasurement, and we found that those who underwent TSH measurement were more likely to be older, female, and non-Hispanic white; were less likely to be non-Hispanic Black; were more likely to have underlying diabetes or atherosclerotic disease; were less likely to be receiving automated PD at study entry; and had a lower proportion of deaths (Supplemental Table 1). The causes of ESRD, prevalence of CHF, and laboratory characteristics were largely similar between the two groups.
Comparison of baseline characteristics among patients categorized by baseline TSH level is shown in Table 1. Compared with patients in the lowest TSH category (<0.1 mIU/L), those in the highest TSH category (≥10.0 mIU/L) were older and less likely to be non-Hispanic Black; were more likely to have diabetes and less likely to have hypertension as the cause of their ESRD; were more likely to have underlying diabetes; and were less likely to have CHF and atherosclerotic heart disease.
Table 1.
Baseline Characteristics of Peritoneal Dialysis Patients According to Baseline Thyroid Functional Status Defined by Serum TSH Level
| Characteristic | Overall | TSH Category, mIU/L |
|||||
|---|---|---|---|---|---|---|---|
| TSH <0.1 | TSH 0.1–<0.5 | TSH 0.5–<3.0 | TSH 3.0–<5.0 | TSH 5.0–<10.0 | TSH ≥10.0 | ||
| N | 1484 | 31 | 73 | 777 | 333 | 173 | 97 |
| TSH level, mIU/L, mean ± sd | 5.0 ± 14.2 | 0.04 ± 0.03 | 0.3 ± 0.1 | 1.8 ± 0.7 | 3.9 ± 0.6 | 6.8 ± 1.4 | 37.0 ± 44.1 |
| Age, mean ± sd | 60 ± 15 | 60 ± 13 | 60 ± 12 | 59 ± 15 | 61 ± 16 | 61 ± 16 | 64 ± 15 |
| Female, % | 52 | 61 | 59 | 48 | 54 | 57 | 66 |
| Race/ethnicity, % | |||||||
| Non-Hispanic white | 67 | 68 | 61 | 63 | 72 | 74 | 69 |
| Non-Hispanic Black | 13 | 13 | 18 | 16 | 8 | 10 | 9 |
| Hispanics | 5 | 13 | 10 | 11 | 13 | 12 | 18 |
| Asians | 12 | 0 | 4 | 6 | 5 | 3 | 2 |
| Other | 3 | 6 | 7 | 4 | 2 | 1 | 2 |
| Vintage in mo, mean ± sd | 7.2 ± 9.5 | 6.3 ± 10.9 | 7.1 ± 10.2 | 7.4 ± 9.6 | 7.3 ± 9.8 | 6.6 ± 8.6 | 6.7 ± 9.2 |
| Cause of ESRD, % | |||||||
| Diabetes | 40 | 23 | 36 | 39 | 40 | 45 | 47 |
| Glomerulonephritis | 17 | 42 | 23 | 25 | 26 | 21 | 24 |
| Hypertension | 25 | 16 | 14 | 19 | 16 | 15 | 9 |
| Cystic disease | 5 | 3 | 7 | 5 | 7 | 4 | 3 |
| Other | 13 | 16 | 21 | 12 | 11 | 17 | 17 |
| Automated PD, % | 51 | 48 | 52 | 46 | 57 | 58 | 50 |
| Comorbidities, % | |||||||
| Diabetes | 64 | 48 | 67 | 63 | 68 | 65 | 62 |
| CHF | 7 | 19 | 8 | 6 | 9 | 7 | 6 |
| Atherosclerotic disease | 16 | 26 | 19 | 15 | 17 | 16 | 14 |
| Laboratory variables, median, IQR | |||||||
| Peritoneal urea clearance, peritoneal Kt/V | 1.42 (1.16–1.67) | 1.54 (1.22–1.69) | 1.41 (1.14–1.63) | 1.42 (1.16–1.68) | 1.39 (1.14–1.67) | 1.44 (1.20–1.65) | 1.39 (1.14–1.81) |
| 24-h urine urea clearance, mL/min | 1.1 (0.5–1.7) | 1.0 (0.6–1.6) | 1.1 (0.6–1.8) | 1.1 (0.5–1.7) | 1.1 (0.5–1.6) | 1.0 (0.4–1.7) | 0.8 (0.3–1.5) |
| 4-h PET D/P creatinine | 0.64 (0.55–0.72) | 0.66 (0.61–0.7) | 0.59 (0.52–0.69) | 0.63 (0.56–0.72) | 0.65 (0.55–0.72) | 0.65 (0.56–0.72) | 0.61 (0.53–0.72) |
| 4-h PET ultrafiltration volume, mL | 300 (150–450) | 300 (150–500) | 375 (200–451) | 300 (150–500) | 300 (100–400) | 300 (200–500) | 300 (150–400) |
| Serum albumin, g/dL | 3.7 (3.3–4.0) | 3.6 (3.3–4.1) | 3.7 (3.4–4.0) | 3.7 (3.4–4.0) | 3.6 (3.3–4.0) | 3.6 (3.2–3.9) | 3.6 (3.1–3.9) |
| Serum bicarbonate, mEq/L | 25 (23–27) | 25 (25–27) | 25 (23–27) | 25 (23–27) | 25 (23–27) | 25 (22–26) | 25 (23–27) |
| Serum creatinine, mg/dL | 5.5 (4.1–7.6) | 4.8 (4.0–6.5) | 5.4 (4.1–7.4) | 5.6 (4.2–7.7) | 5.4 (4.0–7.6) | 5.4 (4.1–7.2) | 5.4 (3.8–7.4) |
Time-dependent thyroid function and mortality
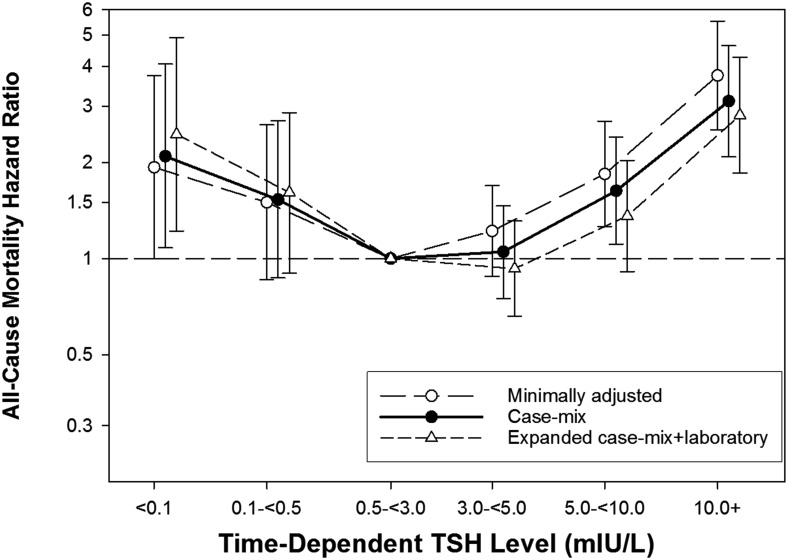
Patients contributed a total of 1953 years of follow up during which time 258 deaths occurred (crude death rate [95% confidence interval (CI)] 132 [117–149] deaths per 1000 person-years; Supplemental Table 2). Median (IQR) at-risk time was 1.0 (0.5–1.9) years. In our primary analyses, we observed a U-shaped association between time-dependent TSH category and mortality, such that TSH levels <0.1 mIU/L and ≥5.0 mIU/L were associated with higher death risk in case-mix adjusted Cox models (reference: TSH 0.5–<3.0 mIU/L): adjusted hazard ratios (95% confidence intervals) 2.09 (1.08–4.06), 1.53 (0.87–2.70), 1.05 (0.75–1.46), 1.63 (1.11–2.40), and 3.11 (2.08–4.63) for TSH levels <0.1, 0.1–<0.5, 3.0–<5.0, 5.0–<10.0, and ≥10.0 mIU/L, respectively (Figure 1 and Table 2). With incremental adjustment for covariates in the expanded case mix + laboratory and expanded case mix + laboratory + PD characteristics models, associations became stronger for TSH levels <0.1 mIU/L, whereas associations were attenuated but remained significant for TSH levels ≥10.0 mIU/L and were attenuated to the null for TSH 5.0–<10.0 mIU/L.
Figure 1.
Association between time-dependent TSH category with all-cause mortality in peritoneal dialysis patients. Minimally adjusted model adjusted for patient's calendar quarter of entry into the cohort. Case mix–adjusted model adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, and baseline diabetes status. Expanded case mix + laboratory test–adjusted model adjusted for covariates in the case-mix model, as well as vintage, cause of ESRD, baseline comorbidities including CHF and atherosclerotic disease, serum albumin, serum bicarbonate, and residual kidney function.
Table 2.
Association Between Time-dependent and Baseline TSH Gradations With All-cause Mortality
| TSH, mIU/L | Time-dependent TSH Level |
|||||||
|---|---|---|---|---|---|---|---|---|
| Minimally Adjusted |
Case Mix Adjusted |
Expanded Case Mix + Laboratory Adjusted |
Expanded Case Mix + Laboratory + PD Characteristics Adjusted |
|||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| <0.1 | 1.93 (1.00–3.73) | .05 | 2.09 (1.08–4.06) | .03 | 2.45 (1.22–4.92) | .01 | 2.29 (1.13–4.67) | .02 |
| 0.1–<0.5 | 1.50 (0.86–2.62) | .2 | 1.53 (0.87–2.70) | .1 | 1.61 (0.90–2.86) | .1 | 1.51 (0.85–2.70) | .2 |
| 0.5–<3.0 | 1 (ref) | N/A | 1 (ref) | N/A | 1 (ref) | N/A | 1 (ref) | N/A |
| 3.0–<5.0 | 1.22 (0.88–1.69) | .2 | 1.05 (0.75–1.46) | .8 | 0.93 (0.66–1.31) | .7 | 0.93 (0.66–1.31) | .7 |
| 5.0–<10.0 | 1.84 (1.26–2.69) | .002 | 1.63 (1.11–2.40) | .01 | 1.36 (0.91–2.03) | .1 | 1.35 (0.90–2.01) | .2 |
| ≥10.0 | 3.74 (2.53–5.53) | <.001 | 3.11 (2.08–4.63) | <.001 | 2.81 (1.85–4.27) | <.001 | 2.85 (1.87–4.34) | <.001 |
| Hyperthyroid | 1.55 (1.01–2.39) | .05 | 1.69 (1.09–2.62) | .02 | 1.93 (1.23–3.02) | .004 | 1.83 (1.16–2.87) | .009 |
| Euthyroid | 1 (ref) | N/A | 1 (ref) | N/A | 1 (ref) | N/A | 1 (ref) | N/A |
| Hypothyroid | 2.29 (1.73–3.04) | <.001 | 2.08 (1.56–2.78) | <.001 | 1.84 (1.36–2.50) | <.001 | 1.84 (1.36–2.50) | <.001 |
Abbreviation: HR, hazard ratio.
Thyroid function category definitions: hyperthyroid (TSH <0.5 mIU/L), euthyroid (TSH 0.5–<5.0 mIU/L), hypothyroid (TSH ≥ 5.0 mIU/L).
Minimally adjusted model adjusted for patient's calendar quarter of entry into the cohort. Case mix–adjusted model adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, and baseline diabetes status. Expanded case mix + laboratory test–adjusted model adjusted for covariates in the case-mix model, as well as vintage, cause of ESRD, baseline comorbidities including CHF and atherosclerotic disease, serum albumin, serum bicarbonate, and residual kidney function. Expanded case mix + laboratory + PD characteristics model adjusted for covariates in the expanded case mix + laboratory test model, as well as the use of automated PD during the baseline quarter and 4-hour peritoneal equilibrium test characteristics including 4-hour D/PCr ratio and ultrafiltration volume.
In our secondary analyses, we observed that both hyperthyroidism and hypothyroidism were associated with higher mortality risk in case mix–adjusted Cox models (reference: euthyroidism): adjusted hazard ratios (95% confidence intervals) 1.69 (1.09–2.62) and 2.08 (1.56–2.78), respectively (Supplemental Figure 3 and Table 2). The hyperthyroidism-mortality associations were magnified in the expanded case-mix + laboratory and expanded case-mix + laboratory + PD characteristics models, whereas there was a slight attenuation in the estimates for hypothyroidism and mortality.
In restricted cubic spline analyses of time-dependent TSH as a continuous predictor and mortality, we observed that TSH levels >6.0 mIU/L were monotonically associated with higher death risk, whereas levels ∼1.5–3.0 mIU/L were associated with lower death risk, below which the survival benefit plateaued in case-mix analyses (Figure 2B).
Figure 2.
Association between continuous time-dependent TSH gradations and all-cause mortality in peritoneal dialysis patients. Figures present hazard ratios (short-dashed lines indicate 95% CIs) for TSH analyzed as a spline with knots at the 33rd and 66th percentiles of observed values (TSH levels 1.8 mIU/L and 3.5 mIU/L, respectively). A histogram of observed time-dependent TSH values and a hazard reference ratio of 1 (horizontal solid line) is overlaid. Minimally adjusted model adjusted for patient's calendar quarter of entry into the cohort (A). Case mix–adjusted model adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, and baseline diabetes status (B).
Subgroup analyses
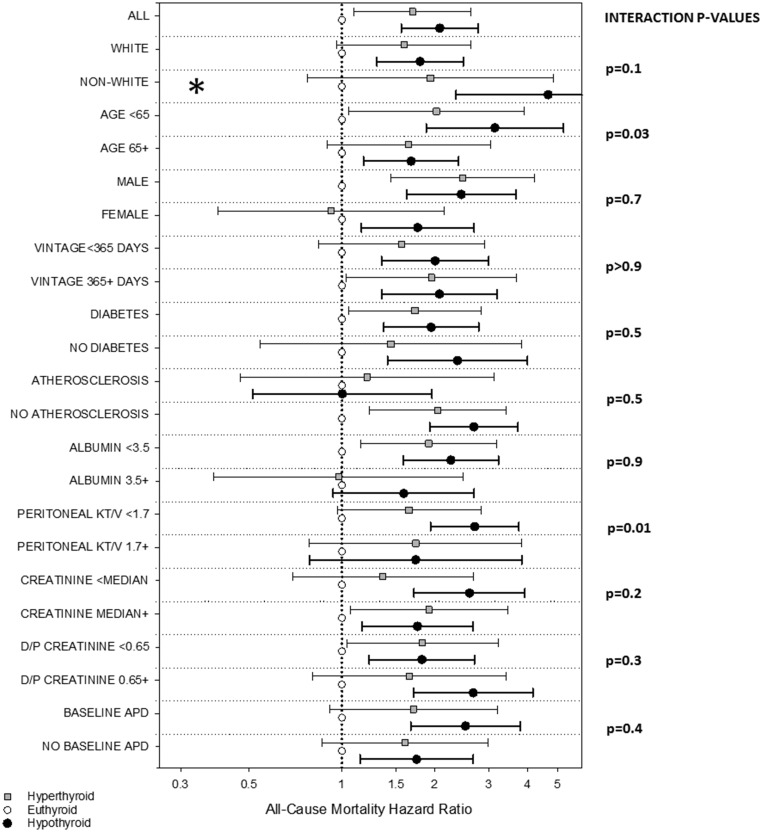
We also examined the association between thyroid function (defined as hyperthyroidism, euthyroidism, and hypothyroidism; reference: euthyroidism) across clinically relevant subgroups. In time-dependent analyses adjusted for case-mix covariates, interaction tests demonstrated that differences in estimates of the thyroid function–mortality association across subcategories were statistically significant for subgroups of age and peritoneal urea clearance (P-interaction = 0.03 and 0.01, respectively; Figure 3 and Supplemental Table 3).
Figure 3.
Associations between time-dependent thyroid function with all-cause mortality across clinically relevant subgroups of peritoneal dialysis patients. Multivariable Cox models adjusted for case-mix covariates: patient's calendar quarter of entry into the cohort, age, sex, race/ethnicity, and baseline diabetes status. *, Upper bound of 95% CI exceeds figure limits.
Change in thyroid function and mortality
Among a subcohort of patients (n = 733) who underwent two or more TSH measurements, we also examined the association between change in TSH level from the baseline to second measurement with mortality (Supplemental Table 4). Patients with persistently high TSH levels (n = 28) and persistently low TSH levels (n = 97) each had a higher mortality risk compared with those with persistently normal TSH levels (n = 430) in case mix–adjusted analyses. However, these associations were attenuated to the null in expanded case mix + laboratory and expanded case mix + laboratory + PD characteristics models.
Discussion
In the largest study of thyroid function and mortality in PD patients to date, we observed a U-shaped association between time-dependent serum TSH levels and mortality risk independent of case-mix covariates. We found that lower TSH levels in the overt-hyperthyroid range were associated with a 2-fold higher mortality risk, and that higher TSH levels in the subclinical-hypothyroid and overt-hypothyroid range were associated with a 1.6- and 3-fold higher mortality, respectively.
Several studies have shown that hypothyroidism, defined by serum TSH, is associated with higher mortality risk in hemodialysis patients (1, 3, 5). In a study of 2715 hemodialysis and PD patients from two tertiary care centers in Boston, hypothyroidism ascertained at baseline was associated with a 35% higher mortality risk (3). In exploratory analyses that adjusted for potential cardiovascular intermediates, dramatic attenuation of the associations to the null suggested cardiovascular pathways may mediate the association between hypothyroidism and mortality. Although a subsequent study of 1000 diabetic hemodialysis patients from the Die Deutsche Diabetes Dialyze Studie (4D Trial) showed that neither subclinical hypothyroidism assessed separately nor in conjunction with overt hypothyroidism was associated with cardiovascular events or mortality, the low prevalence (1.8%) of hypothyroidism in the cohort may have resulted in limited power (1). More recently, among 8840 national hemodialysis patients from a large U.S. dialysis provider, time-dependent hypothyroidism was associated with higher mortality, and incrementally higher TSH levels were associated with an increasingly stronger death risk, including those in the high-normal, moderately high, and severely high range (>3.0–5.0, >5.0–10.0, and >10.0 mIU/L, respectively): 1.42-, 1.67-, and 2.21-fold higher death risks, respectively, compared with low-normal levels (5). It has been suggested that hemodialysis and PD patients are akin to non-CKD populations with high cardiovascular risk, who may have heightened susceptibility to thyroid-related perturbations (14). Indeed, Third National Health and Nutrition Examination Survey data have shown that hypothyroidism (TSH >4.5 mIU/L), as well as TSH levels in the subclinical-hypothyroid range (>4.5–10.0 mIU/L) were associated with higher mortality in participants with CHF, but not in those without (24).
To our knowledge, ours is the first study to examine hypothyroidism defined by longitudinal TSH measurements and mortality in a national cohort of dialysis patients exclusively receiving PD. Although three small studies have shown that low T3 and/or T4 levels are associated with higher mortality in PD patients, inference from these studies is limited by their 1) exclusion of patients with known thyroid functional disease (25) or biochemical evidence of overt hypothyroidism or hyperthyroidism (25–27), or 2) reliance upon thyroid functional metrics confounded by mortality determinants (ie, T3 levels are influenced by malnutrition, inflammation, and uremia) (28–30) or that have impaired performance in advanced CKD (ie, in low protein states or conditions where circulating substances such as uremic toxins impair hormone-protein binding, routinely-used free T4 assays may result in spurious levels) (8). Although some TSH alterations may be observed in uremia (31, 32), it is the most sensitive and specific metric of thyroid function given its negative logarithmic association with T3/T4 (14).
This study also adds new knowledge by demonstrating that hyperthyroidism is associated with higher death risk in PD patients for the first time. A secondary analysis of the 4D Trial has shown that, compared with euthyroidism, subclinical hyperthyroidism ascertained at a single point in time was associated with higher risk of sudden cardiac death in diabetic hemodialysis patients (1). When follow-up time was parsed into short- vs longer-term intervals, higher mortality risk was only observed over short-term followup (although marginally nonsignificant); the abrogation of associations over longer-term follow up may have been due to change in thyroid function over time (ie, baseline thyroid function may not reflect thyroid functional status 2–4 y later). When we broadly defined hyperthyroidism as TSH <0.5 mIU/L using repeated measures of thyroid function, hyperthyroidism was associated with higher mortality. However, when we examined finer gradations of TSH, we observed a potent association between TSH levels in the overt-hyperthyroid range and mortality, whereas the association between TSH levels in the subclinical-hyperthyroid range were attenuated to the null, which may have been due to lack of biological effect or limited power of the subclinical-hyperthyroid range category. Although a growing body of evidence suggests that thyroid hormone deficiency is linked with higher mortality in dialysis patients due to alterations in cardiovascular pathways, little is known about the mechanisms underlying thyroid hormone excess and death in kidney disease (33–37). In the general population, hyperthyroidism even in the subclinical range has been associated with conditions that are common and fatal in dialysis patients, including atrial fibrillation, coronary ischemia, CHF, and fracture risk (13, 17, 18, 38). Given that T3 replacement in dialysis patients with low T3 levels may result in protein degradation, thyroid hormone excess could lead to protein-energy wasting, a potent mortality predictor in this population (39, 40).
Strengths of our study include its examination of a large cohort of PD patients whose mortality profile is representative of the greater U.S. population (crude death rates 132 vs 134 deaths per 1000 person-years, respectively) (41), as well as comprehensive availability of patient-level data on sociodemographics, comorbidities, and longitudinal laboratory data collected in the outpatient setting and uniformly measured in a single laboratory. Several limitations of our study bear acknowledgment. First, inclusion in the study required that patients have one or more TSH measurements. Comparison of baseline characteristics among patients included vs excluded on the basis of TSH measurement suggests that our study population had less favorable sociodemographics and comorbidity characteristics, which may affect the study's generalizability. However, although the indications for TSH testing in this study cohort are unknown, this requirement applied equally to patients irrespective of thyroid function and should not impair the study's internal validity. Second, given the sparsity of free T4 measurements and their unclear accuracy in kidney disease (8), we stratified patients according to TSH ranges that typically reflect the spectrum of overt to subclinical thyroid disease (19). Although it remains uncertain as to whether TSH changes alone in kidney disease reflect true alterations in thyroid function (42), evidence has increasingly shown adverse outcomes in CKD patients with moderately abnormal TSH levels (3, 5, 14). Third, our dataset lacked information on most outpatient pharmacotherapies, preventing examination of thyroid hormone replacement or suppressive therapy, nor other medications that may influence thyroid function. Lastly, given our study's observational design, we cannot exclude the possibility of residual confounding.
In conclusion, our study found that both hypothyroidism and hyperthyroidism were independently associated with higher mortality in a national PD cohort, consistent with data in the hemodialysis population. Given the high prevalence of thyroid functional disease and exceedingly high mortality of the dialysis population, additional studies are needed to determine the underlying mechanisms by which thyroid functional disease affects mortality, whether thyroid hormone modulating therapies ameliorate mortality risk, and the precise TSH targets associated with improved outcomes in the dialysis population.
Acknowledgments
Portions of these data have been presented as an abstract at the 15th International Thyroid Congress, October 18–23, 2015, Lake Buena Vista, FL; and at the American Society of Nephrology Kidney Week Meeting, November 3–8, 2015, San Diego, CA.
This work was supported by the research grants from the NIH/NIDDK including K23-DK102903 (C.M.R.), K24-DK091419 (K.K.-Z.), R01-DK09568 (R.M. and K.K.-Z.), R01-DK078106 (K.K.-Z.), R01-DK096920 (C.P.K. and K.K.-Z.), U01-DK102163 (K.K.-Z. and C.P.K.), UL1TR000153 (D.V.N.), and philanthropist grants from Mr Harold Simmons, Mr Louis Chang, and Dr Joseph Lee.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 4D Trial
- Die Deutsche Diabetes Dialyze Studie
- CHF
- congestive heart failure
- CI
- confidence interval
- CKD
- chronic kidney disease
- D
- dialysate
- ESRD
- end-stage renal disease
- IQR
- interquartile range
- PCr
- plasma creatinine
- PD
- peritoneal dialysis
- PET
- peritoneal equilibration test.
References
- 1. Drechsler C, Schneider A, Gutjahr-Lengsfeld L, et al. Thyroid function, cardiovascular events, and mortality in diabetic hemodialysis patients. Am J Kidney Dis. 2014;63(6):988–996. [DOI] [PubMed] [Google Scholar]
- 2. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67(3):1047–1052. [DOI] [PubMed] [Google Scholar]
- 3. Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2013;8(4):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhee CM, Kalantar-Zadeh K, Streja E, et al. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30(2):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee CM, Kim S, Gillen DL, et al. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J Clin Endocrinol Metab. 2015;100(4):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee CM. Low-T3 syndrome in peritoneal dialysis: Metabolic adaptation, marker of illness, or mortality mediator? Clin J Am Soc Nephrol. 2015;10(6):917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benvenga S. Thyroid hormone transport proteins and the physiology of hormone binding. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's the thyroid. 10th ed Philadelphia: Lippincott Williams and Wilkins; 2013;93–103. [Google Scholar]
- 8. Soldin OP. Measuring serum thyroid-stimulating hormone, thyroid hormones, thyroid-directed antibodies, and transport proteins. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's the thyroid. 10th ed Philadelphia: Lippincott Williams and Wilkins; 2013;279–297. [Google Scholar]
- 9. Robey C, Shreedhar K, Batuman V. Effects of chronic peritoneal dialysis on thyroid function tests. Am J Kidney Dis. 1989;13(2):99–103. [DOI] [PubMed] [Google Scholar]
- 10. Lin YS, Tarng DC. Abnormal thyroid function in peritoneal dialysis patients: Lots of smoke but no fire. J Chin Med Assoc. 2012;75(2):47–48. [DOI] [PubMed] [Google Scholar]
- 11. Ng YY, Wu SC, Lin HD, et al. Prevalence of clinical and subclinical thyroid disease in a peritoneal dialysis population. Perit Dial Int. 2012;32(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. [DOI] [PubMed] [Google Scholar]
- 13. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;15 344(7):501–509. [DOI] [PubMed] [Google Scholar]
- 14. Rhee CM, Brent GA, Kovesdy CP, et al. Thyroid functional disease: An under-recognized cardiovascular risk factor in kidney disease patients. Nephrol Dial Transplant. 2015;30(5):724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wheeler DC, Haynes R, Landray MJ, Baigent C. Cardiovascular aspects of kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, eds. Taal: Brenner and Rector's the kidney. 9th ed Philadelphia: Elsevier Saunders; 2012;2060–2075. [Google Scholar]
- 17. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: An individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ladenson PW. Diagnosis of hypothyroidism. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's the thyroid. 10th ed Philadelphia: Lippincott Williams and Wilkins; 2013;606–611. [Google Scholar]
- 20. Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30(7):1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shapiro BB, Streja E, Chen JL, Kovesdy CP, Kalantar-Zadeh K, Rhee CM. The relationship between ultraviolet light exposure and mortality in dialysis patients. Am J Nephrol. 2014;40(3):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: Time-dependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–997. [DOI] [PubMed] [Google Scholar]
- 23. Mehrotra R, Ravel V, Streja E, et al. Peritoneal equilibration test and patient outcomes. Clin J Am Soc Nephrol. 2015;10(11):1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. Subclinical hypothyroidism and survival: The effects of heart failure and race. J Clin Endocrinol Metab. 2013;98(6):2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang TI, Nam JY, Shin SK, Kang EW. Low triiodothyronine syndrome and long-term cardiovascular outcome in incident peritoneal dialysis patients. Clin J Am Soc Nephrol. 2015;10(6):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enia G, Panuccio V, Cutrupi S, et al. Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2007;22(2):538–544. [DOI] [PubMed] [Google Scholar]
- 27. Jung HY, Cho JH, Jang HM, et al. Free thyroxine level as an independent predictor of infection-related mortality in patients on peritoneal dialysis: A prospective multicenter cohort study. PloS One. 2014;9(12):e112760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langton JE, Brent GA. Nonthyroidal illness syndrome: Evaluation of thyroid function in sick patients. Endocrinol Metab Clin North Am. 2002;31(1):159–172. [DOI] [PubMed] [Google Scholar]
- 29. Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23(1):22–26. [DOI] [PubMed] [Google Scholar]
- 30. Meuwese CL, Dekkers OM, Stenvinkel P, Dekker FW, Carrero JJ. Nonthyroidal illness and the cardiorenal syndrome. Nat Rev Nephrol. 2013;9(10):599–609. [DOI] [PubMed] [Google Scholar]
- 31. Carrero JJ, Stenvinkel P, Lindholm B. Endocrine aspects of chronic kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, eds. Taal: Brenner and Rector's the kidney. 9th ed Philadelphia: Elsevier Saunders; 2012;2122–2137. [Google Scholar]
- 32. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996;17(1):45–63. [DOI] [PubMed] [Google Scholar]
- 33. Jaroszyński AJ, Głowniak A, Chrapko B, et al. Low-T3 syndrome and signal-averaged ECG in haemodialysed patients. Physiol Res. 2005;54(5):521–526. [PubMed] [Google Scholar]
- 34. Kang EW, Nam JY, Yoo TH, et al. Clinical implications of subclinical hypothyroidism in continuous ambulatory peritoneal dialysis patients. Am J Nephrol. 2008;28(6):908–913. [DOI] [PubMed] [Google Scholar]
- 35. Meuwese CL, Carrero JJ, Cabezas-Rodriguez I, et al. Nonthyroidal illness: A risk factor for coronary calcification and arterial stiffness in patients undergoing peritoneal dialysis? J Intl Med. 2013;274(6):584–593. [DOI] [PubMed] [Google Scholar]
- 36. Tatar E, Kircelli F, Asci G, et al. Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(9):2240–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Chang Y, Ryu S, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: The Kangbuk Samsung Health Study. Intl J Epidemiol. 2014;43(5):1624–1632. [DOI] [PubMed] [Google Scholar]
- 38. Ross DS. Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid. 1994;4(3):319–326. [DOI] [PubMed] [Google Scholar]
- 39. Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001;38(4 Suppl 1):S80–S84. [DOI] [PubMed] [Google Scholar]
- 40. Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. United States Renal Data System. USRDS 2014 annual data report: Atlas of end-stage renal disease in the United States. Bethesda, MD; 2014. [Google Scholar]
- 42. Kaptein EM, LoPresti JS, Kaptein MJ. Is an isolated TSH elevation in chronic nonthyroidal illness “subclinical hypothyroidism”? J Clin Endocrinol Metab. 2014;99(11):4015–4026. [DOI] [PubMed] [Google Scholar]