Abstract
Context:
Immune checkpoint blockade is associated with endocrine-related adverse events. Thyroid dysfunction during pembrolizumab therapy, an anti-programmed cell death 1 (PD-1) receptor monoclonal antibody, remains to be fully characterized.
Objective:
To assess the incidence and characteristics of pembrolizumab-associated thyroid dysfunction.
Design and Setting:
Thyroid function was monitored prospectively in melanoma patients who initiated pembrolizumab within an expanded access program at a referral oncology center. 18Fluorodeoxyglucose uptake on positron emission tomography/computed tomography (18FDG-PET/CT) was reviewed in cases compatible with inflammatory thyroiditis.
Patients:
Ninety-nine patients with advanced melanoma (age, 26.3–93.6 years; 63.6% females) who received at least one administration of pembrolizumab.
Main Outcome Measures:
Patient characteristics, thyroid function (TSH, free T4), thyroid autoantibodies, and 18FDG-PET/CT.
Results:
Eighteen adverse events of thyroid dysfunction were observed in 17 patients. Thyrotoxicosis occurred in 12 patients, of which nine evolved to hypothyroidism. Isolated hypothyroidism was present in six patients. Levothyroxine therapy was required in 10 of 15 hypothyroid patients. Thyroid autoantibodies were elevated during thyroid dysfunction in four of 10 cases. Diffuse increased 18FDG uptake by the thyroid gland was observed in all seven thyrotoxic patients who progressed to hypothyroidism.
Conclusions:
Thyroid dysfunction is common in melanoma patients treated with pembrolizumab. Hypothyroidism and thyrotoxicosis related to inflammatory thyroiditis are the most frequent presentations. Serial measurements of thyroid function tests are indicated during anti-PD-1 monoclonal antibody therapy. Thyrotoxicosis compatible with inflammatory thyroiditis was associated with diffuse increased 18FDG uptake by the thyroid gland. The prospective role of thyroid autoantibodies should be further investigated, together with the histopathological correlates.
We studied 99 advanced melanoma patients treated with pembrolizumab (anti-PD-1) and found frequent hypo- and hyperthyroidism, the latter associated with diffuse increased 18FDG uptake by the thyroid.
The cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) receptor are immune checkpoint receptors that inhibit the function of T cells. These receptors are important in maintaining self-tolerance and are therapeutically targeted by immune checkpoint-inhibiting monoclonal antibodies (mAbs) to enhance antitumor immune responses (1). Immune checkpoint blockade is associated with a risk for immune-related adverse events (irAEs) potentially affecting the endocrine organs (2, 3). Pembrolizumab, an IgG4 PD-1-directed mAb, improves the overall survival of patients with advanced melanoma and has been registered for this indication by the European Medicines Agency and the U.S. Food and Drug Administration (4, 5). Pembrolizumab therapy has been associated with a 57–79.5% incidence of any irAE, among which are hypothyroidism (5–10.1%) and hyperthyroidism (<2–6.5%) (6–9). In KEYNOTE-002 and -006, two phase III pembrolizumab trials in melanoma, hypo- and hyperthyroidism occurred in 5–10.1% and 3.2–6.5% of patients, respectively (8, 9). In a retrospective analysis of 92 pembrolizumab-treated cancer patients at the Mayo Clinic, abnormal thyroid function tests were detected in 14% of patients, mainly involving cases of hypothyroidism and thyroiditis (10). Anti-thyroid peroxidase antibodies (TPOAbs) were elevated in 50% of evaluable patient cases. In a case series of 10 patients with painless thyroiditis syndrome on anti-PD-1 mAb therapy, all were diagnosed with hypothyroidism, which was preceded by transient thyrotoxicosis in six of the patients. TPOAbs were detected in 66% of available patient cases (11). A systematic prospective analysis of thyroid function and thyroid autoantibodies in pembrolizumab-treated melanoma patients has yet to be reported. It remains unknown which patients are at risk for developing thyroid-related adverse events (AEs). It is unclear how the thyroid function evolves over time during pembrolizumab treatment and what the role is for thyroid autoantibodies. Finally, additional insight into the toxicity mechanisms and the specific role of PD-1 and its ligand in thyroid irAE could enable a better understanding of the pathophysiology of autoimmune thyroid disease. The aim of the present study is to investigate the incidence and characteristics of thyroid-related AEs in a “real-life” cohort of melanoma patients treated with pembrolizumab at the Oncology Center of the University Hospital of Brussels.
Patients and Methods
Patients
Patients with advanced/unresectable (American Joint Committee on Cancer [AJCC] stage III/IV) melanoma who initiated pembrolizumab treatment between September 3, 2014, and January 4, 2016, in an expanded access program (EAP) were recruited in a therapeutically noninterventional academia-sponsored clinical trial (12, 13). This study was approved by the Ethical Committee of the University Hospital of Brussels; written informed consent was obtained from all patients. The ClinicalTrials.gov identifier is NCT02673970. Patient characteristics (age, gender, history of thyroid disorder, prior immunotherapy, melanoma staging), thyroid function tests, and thyroid autoantibodies were retrieved from the medical records along with the washout period between the last dosing of prior immunotherapy and the first pembrolizumab dosing.
Pembrolizumab immunotherapy
Pembrolizumab (Keytruda; Merck Sharp & Dohme Corp.) was administered in accordance with manufacturer-approved guidelines for the use of pembrolizumab in the EAP (2 mg/kg every 3 weeks). All patients received at least one administration of pembrolizumab. No patient had to be excluded for active autoimmune disease, which was our main eligibility criterion for initiating pembrolizumab treatment in the EAP. Baseline tumor staging was according to the AJCC Staging Manual, 7th edition (14). Performance status was classified using the Eastern Cooperative Oncology Group (ECOG) score (15). Response to immunotherapy was assessed by immune-related response criteria (16). Patients received three cycles of pembrolizumab followed by radiological assessment. On progressive disease (PD), consecutive radiological assessments at least 4 weeks apart (according to immune-related response criteria) were performed. If PD persisted, pembrolizumab was discontinued. Pembrolizumab was further discontinued based on manufacturer-approved guidelines (17) or the investigator's clinical judgment.
Thyroid function tests and thyroid-related AEs
TSH and free T4 (fT4) were prospectively assessed at baseline and before each pembrolizumab administration. Thyrotoxicosis was defined as a suppressed TSH level with an elevated fT4 and/or free T3 (fT3) level. Hypothyroidism was defined as an elevated TSH level with a decreased fT4 level. Subclinical hypothyroidism or thyrotoxicosis was defined as an elevated or suppressed TSH level, respectively, with normal fT4 and fT3 levels. Thyroid irAEs were graded according to Common Terminology Criteria for Adverse Events, version 4.03 (18). This classification uses the term hyperthyroidism; in this manuscript, we preferred the term thyrotoxicosis in order to be unambiguous for the endocrinologist. Our institutional laboratory's Department for Hormonology & Tumor Markers measured the serum TSH, fT4, fT3, TPOAb, and TSH receptor antibodies (TRAbs) using the Elecsys electrochemiluminescence immunoassays on a Cobas 6000 immunoanalyzer (Roche Diagnostics). The within-run and between-run coefficients of variation were <2% and <6.5% for TSH, ≤2% and <5% for fT4, ≤1.6% and 2.15% for fT3, <5% and ≤7% for TPOAb, and ≤6.6 and 10.1% for TRAb. Reference laboratory values were: TSH, 0.27–4.20 mIU/L; fT4, 11.6–22.0 pmol/L; fT3, 4.0–6.8 pmol/L; TPOAb, <34 kIU/L; and TRAb, <1.75 U/L.
Thyroid autoantibody subanalysis
The measurement of thyroid autoantibodies was performed at the time of onset of thyroid dysfunction. In addition, evaluable plasma samples obtained before the introduction of pembrolizumab were analyzed for baseline TPOAb status in patients whose TPOAb status during thyroid-related AE was identified. These plasma samples were prospectively collected after obtaining written informed consent. Blood samples were collected in 10-mL EDTA tubes and immediately centrifuged at a relative centrifugal force of 1410.63 × g during 15 minutes at room temperature. Plasma was separated and stored in 1-mL aliquots at −80°C (19).
18FDG-PET/CT subanalysis
In patients with thyrotoxicosis compatible with inflammatory thyroiditis due to rapid onset and spontaneous progression to hypothyroidism, 18fluorodeoxyglucose-positron emission tomography/computed tomography (18FDG-PET/CT) studies were retrieved from the medical records and investigated post hoc for increased 18FDG uptake in the thyroid gland. When evaluable, 18FDG-PET/CT studies obtained before the first pembrolizumab dosing were compared to imaging results obtained closest after the onset of thyrotoxicosis. 18FDG uptake in the thyroid was assessed both visually and semiquantitatively by measuring the peak standardized uptake value corrected for body weight in a three-dimensional region of interest placed over the thyroid. Before 18FDG administration, patients were instructed to fast for at least 6 hours to minimize glucose-related competition of 18FDG uptake and to reduce serum insulin to near basal levels. Blood glucose levels were checked before the imaging procedures. None of the patients presented with glucose levels exceeding 200 mg/dL. Whole-body 18FDG-PET/CT images were acquired 60 minutes after iv tracer administration (18FDG activity range, 255–355 MBq; average, 311 MBq) using a high-end PET/CT scanner (Gemini TF64 PET/CT; Philips). PET images were reconstructed (using the vendor's standard BLOB-OS-TF algorithm) corrected for attenuation, scatter, and random coincidences.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 23 (IBM Corp.). Patient, tumor, and treatment variables were compared using the Fisher's exact test (gender, history of thyroid disorder, prior immunotherapy), the Mann-Whitney-Wilcoxon test (age, melanoma staging, baseline lactate dehydrogenase [LDH], time between last ipilimumab and first pembrolizumab dosing), the Kruskal-Wallis test (ECOG performance status, metastasis stage), and the unpaired two-sample t test (baseline mean TSH/fT4). Significance was defined as a P value of <.05.
Results
Patient characteristics
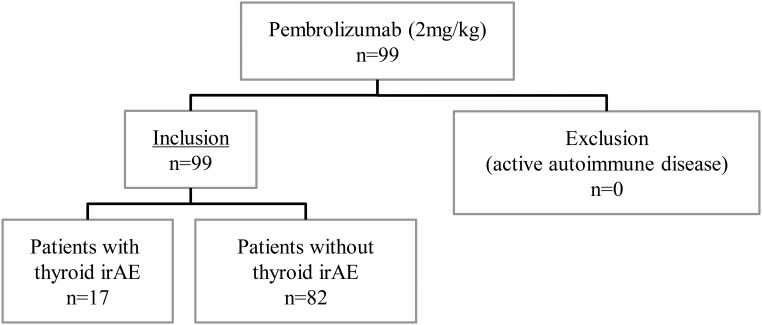
A total of 99 patients with advanced/unresectable (AJCC stage III/IV) melanoma initiated pembrolizumab treatment between September 3, 2014, and January 4, 2016 (Figure 1). Table 1 illustrates the baseline characteristics of the study population. The median age was 60.3 years (range, 26.3–93.6); 63 patients (63.6%) were female. Of the 99 included patients, 92 (92.9%) presented with metastatic disease, of which 42 (42.4%) had an elevated LDH level at baseline. Median follow-up was 20.7 weeks (range, 1.1–72.6) after pembrolizumab introduction. Total duration of follow-up was limited: 21, 25, and 33 patients were followed for <6, 9, and 12 weeks, respectively. Prior history of a thyroid disorder not requiring active therapy was present in 11 patients (11.1%) and consisted of three patients with a multinodular goiter, one patient with an unspecified goiter, one patient with a thyroid nodule, one patient with amiodarone-induced thyrotoxicosis, and five patients with a thyroid-related AE on prior ipilimumab (transient thyrotoxicosis, n = 4; Graves' disease, n = 1). The mean baseline values ± SD for TSH and fT4 were 1.32 ± 0.94 mIU/L and 15.5 ± 3.67 pmol/L, respectively. There was no significant difference between groups with/without thyroid-related AE for age, gender, tumor stage, metastasis stage, baseline LDH, history of thyroid disorder, prior ipilimumab, time between last ipilimumab and first pembrolizumab dosing, or baseline mean TSH/fT4. Baseline ECOG performance status was significantly better in the 17 patients who later developed a thyroid-related AE.
Figure 1.
Overview of the investigated cohort of pembrolizumab-treated melanoma patients, displaying inclusion criteria and overall thyroid dysfunction.
Table 1.
Overview of Patient Characteristics
| Characteristic | Pembrolizumab | Thyroid irAE | No Thyroid irAE | P Value |
|---|---|---|---|---|
| n | 99 | 17 | 82 | |
| Age, y | ||||
| Median ± SD | 60.3 ± 14 | 51.7 ± 12.3 | 60.7 ± 14.2 | |
| Range | 26.3–93.6 | 32.0–71.3 | 26.3–93.6 | |
| <40 | 10 (10.1) | 3 (17.6) | 7 (8.5) | .369 |
| ≥40 | 89 (89.9) | 14 (82.4) | 75 (91.5) | |
| Gender | ||||
| Female | 63 (63.6) | 12 (70.6) | 51 (62.2) | .589 |
| Male | 36 (36.4) | 5 (29.4) | 31 (37.8) | |
| Follow-up, wka | ||||
| Median (range) | 20.7 (1.1–72.6) | 30.0 (11.0–61.0) | 19.1 (1.1–72.6) | |
| ≤6 | 21 | 0 | 21 | |
| ≤9 | 25 | 0 | 25 | |
| ≤12 | 33 | 1 | 32 | |
| Baseline ECOG status | ||||
| 0 | 58 (58.6) | 15 (88.2) | 43 (52.4) | .012 |
| 1 | 29 (29.3) | 1 (5.9) | 28 (34.1) | |
| 2 | 11 (11.1) | 1 (5.9) | 10 (12.2) | |
| Missing | 1 (1.0) | 0 | 1 (1.2) | |
| Tumor staging | ||||
| III | 7 (7.1) | 1 (5.9) | 6 (7.3) | .999 |
| IV | 92 (92.9) | 16 (94.1) | 76 (92.7) | |
| Metastasis stage | ||||
| M1a | 7 (7.1) | 1 (5.9) | 6 (7.3) | .699 |
| M1b | 8 (8.1) | 1 (5.9) | 7 (8.5) | |
| M1c | 77 (77.8) | 14 (82.4) | 63 (76.8) | |
| LDH level | ||||
| Elevatedb | 42 (42.4) | 6 (35.3) | 36 (43.9) | .594 |
| Normal | 56 (56.6) | 11 (64.7) | 45 (54.9) | |
| Unknown | 1 (1.0) | 0 | 1 (1.2) | |
| History of thyroid disorder | ||||
| Yes | 11 (11.1) | 2 (11.8) | 9 (11.0) | .999 |
| No | 88 (88.9) | 15 (88.2) | 73 (89.0) | |
| Baseline TSH, mIU/L | 1.32 ± 0.94 | 1.70 ± 1.18 | 1.24 ± 0.87 | .144 |
| Baseline fT4, pmol/L | 15.5 ± 3.67 | 14.2 ± 2.79 | 15.8 ± 3.78 | .098 |
Data are expressed as number (percentage) or mean ± SD, unless specified otherwise.
Follow-up after start of pembrolizumab.
Elevated LDH denotes above the upper limit of the normal range.
Thyroid-related AEs
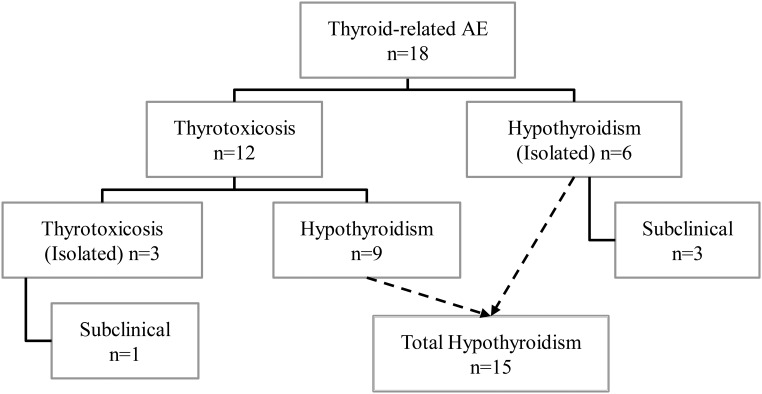
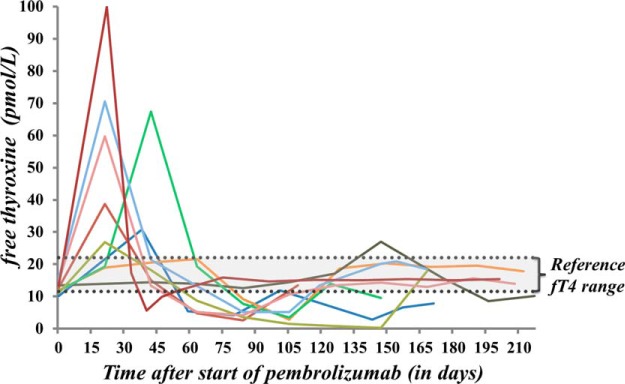
A total of 18 AEs related to an abnormal thyroid function test were observed in 17 patients. Figure 2 summarizes all thyroid-related AEs during pembrolizumab therapy. One patient developed both transient thyrotoxicosis and isolated hypothyroidism, which were counted as two separate events due to a euthyroid interval of 7 months in between. Thyrotoxicosis and hypothyroidism were observed in 12 and 15% of patients, respectively. Thyrotoxicosis was observed in 12 patients (three of grade 1, eight of grade 2, one of grade 3). In nine of these, initial thyrotoxicosis spontaneously progressed to hypothyroidism. There was no correlation between the severity of thyrotoxicosis and the development of subsequent hypothyroidism. Isolated thyrotoxicosis occurred in three patients (including two cases of transient and one of subclinical thyrotoxicosis). Maximum fT4 values ranged between 21.6 and 100 pmol/L. One notable patient presented with severe thyrotoxicosis (grade 3) and had an unmeasurable high fT4 value. Complementary technetium-99m (Tc99m) thyroid scintigraphy showed a diffuse low uptake, suggesting a destructive or subacute thyroiditis. Treatment with symptomatic β-blocker (propranolol, 60 mg/d) was initiated; thionamide was started initially but was discontinued after scintigraphy results were obtained. Hypothyroidism developed in 15 patients (three of grade 1, 12 of grade 2). Isolated hypothyroidism was observed in six patients, of whom three had subclinical hypothyroidism. Thyroid hormone replacement therapy was required in 10 of 15 hypothyroid patients. The median time of onset after the initiation of pembrolizumab therapy was: for all cases of thyroid dysfunction, 6 weeks (range, 3–40); for all cases of hypothyroidism, 5.7 weeks (range, 3–40); for cases of thyrotoxicosis progressing to hypothyroidism, 3.1 weeks (range, 3–21); and for isolated thyrotoxicosis, 8.6 weeks (range, 6–11.1). The time course of fT4 levels in thyrotoxic patients progressing to hypothyroidism is shown in Figure 3. Isolated hypothyroidism was observed in one patient with multinodular goiter, whereas thyrotoxicosis developed in one patient with a history of isolated thyrotoxicosis on prior ipilimumab. Baseline TSH/fT4 levels were normal in both of these patients. Pembrolizumab immunotherapy was temporarily interrupted for one case of grade 3 thyrotoxicosis. Pembrolizumab was not interrupted or discontinued in all other thyroid-related AEs (n = 13), but it was discontinued nonetheless for PD in three patients and interrupted for a nonendocrine irAE in one patient.
Figure 2.
Overview of thyroid-related AEs in pembrolizumab-treated melanoma patients. A total of 18 events of abnormal thyroid function tests were observed in 17 patients. Hypothyroidism and thyrotoxicosis due to suspected inflammatory thyroiditis (spontaneous evolution of thyrotoxicosis to hypothyroidism) were the most frequent types of thyroid dysfunction.
Figure 3.
Graphic representation of fT4 values in patients with suspected inflammatory thyroiditis on pembrolizumab. The evolution of fT4 levels is plotted against the time (in days) after the introduction of pembrolizumab. Early transient thyrotoxicosis develops, followed by spontaneous progression to hypothyroidism.
Prior medical therapy associated with thyroid dysfunction
In total, 76 patients (76.8%) were pretreated with ipilimumab mAb therapy; 23 patients (23.2%) were ipilimumab-naive. Ipilimumab and pembrolizumab were always administered sequentially. Table 2 provides an overview of prior ipilimumab immunotherapy. The duration of washout after the last ipilimumab dosing was ≤4 weeks in 17 patients; ≤6, 9, and 12 weeks, respectively, in 25, 37 and 44 patients. Thyroid-related AEs developed in five of 17 patients (29%) with an ipilimumab washout ≤4 weeks vs 10 of 59 patients (17%) with a washout period >4 weeks. In patients with a washout ≤6, 9, and 12 weeks, the incidence of thyroid-related AEs was 24, 22, and 23%, respectively. In cases of thyrotoxicosis progressing to hypothyroidism, five of nine patients (56%) had a washout >4 weeks vs ≤4 weeks in three of nine patients (33%), whereas the one remaining patient (11%) was ipilimumab-naive. The half-life of ipilimumab is 14.7 days (20). Its plasma concentration would be negligible after 5 times the half-life, which equals 10.5 weeks. Of 15 ipilimumab-pretreated patients with a thyroid-related AE, seven had an ipilimumab washout of >10.5 weeks. Of note, two patients were on amiodarone before and during pembrolizumab therapy, one of which had developed amiodarone-induced thyrotoxicosis 8 years before melanoma diagnosis. None developed thyroid dysfunction on pembrolizumab.
Table 2.
Overview of Prior Ipilimumab Immunotherapy
| Characteristics | Pembrolizumab | Thyroid irAE | No Thyroid irAE | P Value |
|---|---|---|---|---|
| n | 99 | 17 | 82 | |
| Prior ipi | ||||
| Yes | 76 (76.8) | 15 (88.2) | 61 (74.4) | .345 |
| No | 23 (23.2) | 2 (11.8) | 21 (25.6) | |
| Last ipi − first pem, wka | ||||
| ≤4 | 17 | 5 (29) | 12 (71) | .304 |
| >4 | 59 | 10 (17) | 49 (83) | |
| ≤6 | 25 | 6 (24) | 19 (76) | .549 |
| >6 | 51 | 9 (18) | 42 (82) | |
| ≤9 | 37 | 8 (22) | 29 (78) | .777 |
| >9 | 39 | 7 (18) | 32 (82) | |
| ≤10.5 | 41 | 8 (20) | 33 (80) | .958 |
| >10.5 | 35 | 7 (20) | 28 (80) | |
| ≤12 | 44 | 10 (23) | 34 (77) | .564 |
| >12 | 32 | 5 (16) | 27 (84) |
Abbreviations: ipi, ipilimumab; pem, pembrolizumab. Data are expressed as number or number (percentage).
Time between last ipilimumab and first pembrolizumab dosing.
Thyroid autoantibody subanalysis
Thyroid autoantibodies (TPOAb and/or TRAb) could be assessed in 10 of 17 patients with a thyroid-related AE and were elevated in four of 10 patients (40%) during thyroid dysfunction. TPOAb serology was available in all 10 patients and was elevated in three patients. TRAb was tested in five patients and was elevated in one patient at the time of thyrotoxicosis. Positive antithyroid antibodies were exclusively observed in cases of thyrotoxicosis progressing to hypothyroidism. In the one case of positive TRAb, thyrotoxicosis swiftly evolved into hypothyroidism without antithyroid therapy. This patient might have had Graves' disease rapidly shifting to hypothyroidism due to a switch in her antibody subpopulation. Additional serological analyses (on plasma samples obtained before the introduction of pembrolizumab) identified the baseline TPOAb status in four of 10 patients whose TPOAb status during thyroid dysfunction had been analyzed. Baseline TPOAb was elevated in two patients with an elevated TPOAb level during thyroid dysfunction, whereas baseline TPOAb remained unchanged in two patients in whom a normal TPOAb level was identified during thyroid dysfunction.
18FDG uptake in subgroup with suspected inflammatory thyroiditis
The uptake of 18FDG in the thyroid gland could be analyzed in seven of nine thyrotoxic patients who progressed to hypothyroidism and in whom comparable 18FDG-PET/CT results were available. A diffuse increase of 18FDG uptake (visually and/or semiquantitatively) after the onset of thyrotoxicosis was observed in all seven evaluated patients. Interestingly, there was no increased 18FDG uptake in six of these patients before the introduction of pembrolizumab, whereas the one remaining patient also had an increased tracer uptake at baseline. Table 3 summarizes the visual and semiquantitative 18FDG uptake in the thyroid gland.
Table 3.
Overview of 18FDG-PET/CT Imaging in Inflammatory Thyroiditis
| Patient No. | Baseline 18FDG-PET Time Before First Pem, d |
Thyroiditis 18FDG-PET (after thyroiditis) |
||||||
|---|---|---|---|---|---|---|---|---|
| Visual | PeakSUV | Time, d | Time, d | Visual | PeakSUV | Δ From Baseline | ||
| 1 | 0 | No increase | 1.16 | 38 | 59 | No increase | 1.75 | 0.59 (51%) |
| 2 | −4 | No increase | 1.25 | 42 | 128 | Increased | 2.80 | 1.55 (124%) |
| 3 | −4 | No increase | 1.43 | 147 | 172 | Increased | 3.53 | 2.10 (147%) |
| 4 | −144 | Increased | 2.95 | 42 | 118 | Increased | Unavailable | |
| 5 | −56 | No increase | 2.04 | 21 | No imaging | |||
| 6 | −7 | No increase | 1.96 | 21 | 60 | Increased | 3.66 | 1.70 (87%) |
| 7 | −92 | No increase | 1.85 | 21 | 63 | Increased | 3.29 | 1.44 (78%) |
| 8 | −27 | No increase | 1.67 | 22 | 44 | Increased | 3.7 | 2.03 (122%) |
| 9 | No imaging | 21 | No imaging | |||||
| Mean ± SD | 1.79 ± 0.57 | 3.12 ± 0.75 | 1.57 ± 0.55 | |||||
Abbreviations: pem, pembrolizumab; peakSUV, peak standardized uptake value (corrected for body weight). Overview of 18FDG-PET/CT imaging in thyrotoxic patients with compatible inflammatory thyroiditis. Time is denoted in days before/after first pembrolizumab dosing. Δ signifies the difference in peakSUV (corrected for body weight) after onset of thyroiditis/before first pembrolizumab dosing, followed by the difference compared to the tracer uptake at baseline. A diffuse increased 18FDG uptake (visually and/or semiquantitatively) was observed in all seven evaluable patients after the onset of thyrotoxicosis. Interestingly, there was no increased 18FDG uptake in six of these patients before the introduction of pembrolizumab.
Discussion
Thyroid dysfunction is a common AE in the present cohort of pembrolizumab-treated melanoma patients. Hypothyroidism and thyrotoxicosis due to suspected inflammatory thyroiditis were the most frequent clinical presentations. Thyroid dysfunction was mostly observed within the first weeks after the initiation of pembrolizumab. The time interval for the development of thyrotoxicosis progressing to hypothyroidism was particularly short, often following the first pembrolizumab dosing. Thyrotoxicosis was rarely severe, and hypothyroidism was manageable with thyroid hormone replacement therapy. Therefore, pembrolizumab was not interrupted despite the thyroid-related AE in all but one patient. The underlying mechanisms of thyroid dysfunction were further unraveled by the measurement of thyroid autoantibodies and by reviewing 18FDG-PET/CT imaging studies. Thyroid autoantibodies were detected in nearly half of the patients with thyrotoxicosis progressing to hypothyroidism. Interestingly, we discovered that these positive TPOAb during thyroid dysfunction were already present at baseline in two evaluable patient cases. The usefulness of 18FDG-PET/CT imaging was demonstrated in a subanalysis of patients with thyrotoxicosis progressing to hypothyroidism. A diffuse increase of 18FDG uptake in the thyroid gland was found in all patients who were clinically suspected to have a more severe form of pembrolizumab-associated inflammatory thyroiditis.
One of the strengths of this study is the large number of evaluated melanoma patients. Thyroid dysfunction was clearly defined, and all patients were systematically surveyed. This likely contributed to the higher than previously reported incidence of thyroid disorder in pembrolizumab-treated patients. In the KEYNOTE-002 trial, in which melanoma patients received a similar pembrolizumab regime (2 mg/kg every 3 weeks), hypothyroidism was only observed in 5% of patients (8). Thyroid dysfunction has also been reported at a lower rate in non-small cell lung cancer patients. Hypothyroidism developed in 6.9% of non-small cell lung cancer patients (2 or 10 mg/kg, every 2 or 3 weeks) in a phase I pembrolizumab trial (6). In a subsequent phase II/III study, hypothyroidism was observed in 8% (2 or 10 mg/kg) and hyperthyroidism in 4–6% (2 or 10 mg/kg) of patients (7). Our findings highlight the importance of a systematic follow-up of thyroid function tests in this population. Thyrotoxicosis due to suspected inflammatory thyroiditis developed rapidly after the first pembrolizumab dosing. The vigorous onset and the short lag time for evolution toward hypothyroidism are compatible with inflammatory destruction rather than stimulation of the thyroid related to stimulatory autoantibodies. Results of 18FDG-PET/CT imaging analysis were also in favor of an inflammatory thyroiditis because diffuse increased 18FDG uptake in the thyroid gland was observed. This finding has also been reported in other forms of inflammatory thyroiditis including Graves' disease, incidental chronic lymphocytic thyroiditis (21–23), and in a case report on two transiently thyrotoxic patients receiving nivolumab (anti-PD-1) immunotherapy (24). When reporting 18FDG-PET/CT in patients treated with pembrolizumab, clinicians should be aware that this finding might indicate inflammatory thyroiditis. Further systematic assessment needs to confirm our findings and explore the usefulness of 18FDG uptake in all patients with thyroid dysfunction on pembrolizumab. In thyrotoxic patients on pembrolizumab, we suggest the use of radioiodine/Tc99m scintigraphy for the evaluation of pembrolizumab-associated inflammatory thyroiditis in future studies. Thyroid scintigraphy would demonstrate a decreased tracer uptake during the thyrotoxic period, such as the one patient case evaluated by Tc99m thyroid scan in this cohort. On the subject of pembrolizumab-associated hypothyroidism, it is unclear whether discontinuation of levothyroxine is achievable on the long-term. In Hashimoto's thyroiditis, cessation of thyroid replacement therapy is often not possible (25). Further evaluation is needed to determine whether this also holds true for pembrolizumab-associated hypothyroidism.
One of the shortcomings of our study is the limited total duration of follow-up after the first pembrolizumab dosing, mainly in patients without a thyroid-related AE. The overall incidence of thyroid dysfunction may be underestimated. Nevertheless, we describe a higher than previously reported incidence of thyroid-related AE, which could be biased by prior ipilimumab immunotherapy. After all, most patients were not immunotherapy-naive when pembrolizumab was initiated, although checkpoint blockade therapy was always administered sequentially and never combined. This is very important because the combination of anti-PD-1 and anti-CTLA-4 mAb therapy is associated with an increased risk of thyroid irAE. For instance, in a phase III melanoma study, hypothyroidism was observed in 8.6% of patients treated with nivolumab (anti-PD-1) alone, which almost doubled to 15% when combined with ipilimumab (anti-CTLA-4) immunotherapy (26). A washout period of at least 4 weeks between the last dosing of the most recent therapy (ipilimumab, B-Raf, or MEK inhibitor) and the first pembrolizumab dosing was implemented in the KEYNOTE-002 trial to account for the possible increased risk of irAE if pembrolizumab was initiated shortly after the last ipilimumab dosing (8). In our study, a relative increase in the incidence of thyroid-related AEs was observed in patients with an ipilimumab washout period ≤4 weeks. Most of the observed thyroid-related AEs are likely induced by pembrolizumab. Many patients either were ipilimumab-naive or had a washout period of prior ipilimumab therapy >5 times its half-life. The latter is in agreement with a phase II trial in which ipilimumab did not induce objective responses or endocrine-related AEs of any grade when administered at a low dose of 0.3 mg/kg (27). However, it is impossible to exclude the possibility that the effect of prior ipilimumab mAb therapy carries over, even without therapeutic plasma levels. Another consideration is the presence of confounding factors not related to checkpoint blockade therapy. For example, the use of radiographic contrast media is associated with thyroid dysfunction (28), and iodine-induced hyperthyroidism has been described in patients without a history of thyroid disorder (29–31). Severe melanoma progression associated with a predominantly low fT3, suggestive of euthyroid sick syndrome, cannot be definitively excluded because fT3 was not routinely analyzed. Curiously, neither a history of preexisting thyroid disease nor thyroid dysfunction related to prior ipilimumab immunotherapy was identified as a major risk factor for thyroid dysfunction on pembrolizumab therapy in this cohort.
Future studies should focus on the underlying mechanisms of thyroid dysfunction. Pembrolizumab mAb therapy activates peripheral antitumor effector T cells by blocking the PD-1 receptor which, together with its ligand, is an important negative immune regulatory signal (1, 32, 33). We hypothesize a destructive thyroiditis mediated by autoreactive T cells against the thyroid gland, given the nature of the immune-stimulating mechanism of immune checkpoint blockade. However, histopathological correlates are not yet available. We suggest a bystander role for thyroid autoantibodies because these were not detectable in all patients, both in our cohort and in other reports. In a retrospective analysis of pembrolizumab-treated cancer patients at the Mayo Clinic, TPOAbs were observed in 50% of analyzed patients. However, TPOAbs were not elevated in their reported cases of thyroiditis (10). In the case series on painless thyroiditis syndrome after anti-PD-1 mAb therapy, Orlov et al detected TPOAbs in 67% of patients (11). The predictive role of thyroid autoantibodies remains unsettled. It would be of interest to prospectively evaluate thyroid autoantibodies at baseline and during follow-up because their presence could be a risk factor for inflammatory thyroiditis on pembrolizumab. In autoimmune thyroid disease, the presence of antithyroid antibodies correlates well with a T-cell infiltration in the thyroid gland (34), and patients with subclinical hypothyroidism and positive TPOAb are at increased risk of developing overt hypothyroidism (35). Pembrolizumab is an IgG4 mAb, an Ig subclass not associated with antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity (36). Therefore, it is unlikely that pembrolizumab directly triggers an immune reaction via the interaction with the PD-1 receptor expressed by the thyroid gland.
Patients receiving novel immune checkpoint inhibitors should be surveyed closely for the induction of autoimmune AEs as illustrated in the present cohort study. Pathophysiological mechanisms underlying the clinical observation of thyroid dysfunction need further investigation to underpin the processes involved.
Conclusion
Thyroid dysfunction is common in pembrolizumab-treated melanoma patients; hypothyroidism and thyrotoxicosis due to inflammatory thyroiditis are the most frequent presentations. Thyroid hormone replacement therapy is often required for pembrolizumab-associated hypothyroidism. Serial measurements of thyroid function tests are indicated, especially during the first weeks of pembrolizumab therapy. A diffuse increase of 18FDG uptake in the thyroid gland of pembrolizumab-treated patients can further point to the diagnosis of an inflammatory thyroiditis. The predictive role of thyroid autoantibodies should be investigated further, as well as the histopathological correlates.
Acknowledgments
The authors thank Prof. Dr. Ronald Buyl and Dr. Jose Parra for their advice concerning the statistical analyses, Prof. Dr. Ellen Anckaert for her advice about the description of the thyroid laboratory analyses, software engineer Yves Thorrez for his help in retrieving the thyroid laboratory results from the University Hospital of Brussels' data information system, Dr. Isabelle Nubourgh for her encouraging advice concerning an early draft of the introduction and methods, and data nurses Katrien Van den Bossche and Kathleen Mooren for their help with the data collection and regulatory administration. They also thank all involved patients for their consent and cooperation.
Clinical Trial Registration: ClinicalTrials.gov NCT02673970.
Disclosure Summary: B.N. has received financial compensation for public speaking and participation in advisory board meetings from Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, Novartis, and Amgen. J.d.F., Y.J., M.S., H.E., B.V., and B.B. have nothing to declare. No external funding was obtained for this study.
Footnotes
- AE
- adverse event
- CT
- computed tomography
- CTLA-4
- cytotoxic T-lymphocyte antigen 4
- EAP
- expanded access program
- 18FDG
- 18fluorodeoxyglucose
- fT3
- free T3
- fT4
- free T4
- irAE
- immune-related AE
- LDH
- lactate dehydrogenase
- mAb
- monoclonal antibody
- PD
- progressive disease
- PD-1
- programmed cell death 1
- PET
- positron emission tomography
- Tc99m
- technetium-99m
- TPOAb
- thyroid peroxidase antibody
- TRAb
- TSH receptor antibody.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–1375. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Food and Drug Administration. Pembrolizumab. http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm412861.htm. Updated January 9, 2015 Accessed December 12, 2015.
- 5. European Medicines Agency. Keytruda—pembrolizumab. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003820/human_med_001886.jsp&mid=WC0b01ac058001d124 Accessed April 30, 2016.
- 6. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 8. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 10. Delivanis D, Merten MM, Kottschade L, Ryder M. Immune therapies targeting the thyroid: new insights from a comprehensive review of pembrolizumab-induced thyroiditis cases at Mayo Clinic. Presented at 85th Annual Meeting of the American Thyroid Association: Lake Buena Vista, FL; October 20, 2015 Abstract 88. [Google Scholar]
- 11. Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100:1738–1741. [DOI] [PubMed] [Google Scholar]
- 12. Jansen Y, Schreuer M, Seremet T, Awada G, Wilgenhof S, Neyns B. Single-center experience with pembrolizumab in patients with ipilimumab pretreated advanced melanoma. In: Proceedings of European Association of Dermato Oncology; October 29, 2015; Marseille, France Abstract. [Google Scholar]
- 13. Jansen Y, Corthals J, Wilgenhof S, et al. A randomized controlled phase II clinical trial on autologous monocyte-derived mRNA electroporated dendritic cells for stage III/IV melanoma patients who are disease-free following the local treatment of macrometastases. In: Proceedings of Society for Melanoma Research International Congress; November 18–21, 2015; San Francisco, CA Abstract. [Google Scholar]
- 14. American Joint Committee on Cancer. AJCC Cancer Staging Manual. In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. 7th ed New York, Dordrecht, Heidelberg, London: Springer; 2010. [Google Scholar]
- 15. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16. Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration. Keytruda (pembrolizumab). Risk Evaluation and Mitigation Strategy (REMS) Review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125514Orig1s000RiskR.pdf. Published July 25, 2014 Accessed February 11, 2016.
- 18. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Published June 14, 2010 Accessed December 16, 2015.
- 19. Schreuer M, Meersseman G, Van Den Herrewegen S, et al. Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med. 2016;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T. 2012;37:503–530. [PMC free article] [PubMed] [Google Scholar]
- 21. Chen W, Parsons M, Torigian DA, Zhuang H, Alavi A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun. 2009;30:240–244. [DOI] [PubMed] [Google Scholar]
- 22. Karantanis D, Bogsrud TV, Wiseman GA, et al. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896–901. [DOI] [PubMed] [Google Scholar]
- 23. Chen YK, Chen YL, Liao AC, Shen YY, Kao CH. Elevated 18F-FDG uptake in skeletal muscles and thymus: a clue for the diagnosis of Graves' disease. Nucl Med Commun. 2004;25:115–121. [DOI] [PubMed] [Google Scholar]
- 24. van Kooten MJ, van den Berg G, Glaudemans AW, Hiltermann TJ, Groen HJ, Links TP. Transient thyrotoxicosis during nivolumab treatment. Poster presented at ENDO2016, April 3, 2016; Boston, MA https://endo.confex.com/endo/2016endo/webprogram/Paper25877.html Accessed April 6, 2016. [PubMed] [Google Scholar]
- 25. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391–397. [DOI] [PubMed] [Google Scholar]
- 26. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. [DOI] [PubMed] [Google Scholar]
- 28. Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172:153–159. [DOI] [PubMed] [Google Scholar]
- 29. Leger AF, Massin JP, Laurent MF, et al. Iodine-induced thyrotoxicosis: analysis of eighty-five consecutive cases. Eur J Clin Invest. 1984;14:449–455. [DOI] [PubMed] [Google Scholar]
- 30. Skare S, Frey HM. Iodine induced thyrotoxicosis in apparently normal thyroid glands. Acta Endocrinol (Copenh). 1980;94:332–336. [DOI] [PubMed] [Google Scholar]
- 31. Fradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore). 1983;62:1–20. [DOI] [PubMed] [Google Scholar]
- 32. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. [DOI] [PubMed] [Google Scholar]
- 33. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 8th ed Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 34. Yoshida H, Amino N, Yagawa K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–862. [DOI] [PubMed] [Google Scholar]
- 35. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. [DOI] [PubMed] [Google Scholar]
- 36. Davies AM, Sutton BJ. Human IgG4: a structural perspective. Immunol Rev. 2015;268:139–159. [DOI] [PMC free article] [PubMed] [Google Scholar]