Abstract
Context:
Long noncoding RNAs (lncRNAs) regulate pathological processes, yet their potential roles in papillary thyroid carcinoma (PTC) are poorly understood.
Objective:
To profile transcriptionally dysregulated lncRNAs in PTC and identify lncRNAs associated with clinicopathological characteristics.
Design:
We performed RNA sequencing of 12 paired PTC tumors and matched noncancerous tissues and correlated the expression of lncRNAs with clinical parameters. The 2 most significantly dysregulated lncRNAs were studied in an Ohio PTC cohort (n = 109) and in PTC data (n = 497) from The Cancer Genome Atlas.
Setting:
A combination of laboratory-based studies and computational analysis using clinical data and samples and a publically available database.
Main Outcome Measures:
Correlation between expression values and clinical parameters.
Results:
We identified 218 lncRNAs showing differential expression in PTC (fold change ≥ 2.0, P < .01). Significant correlation was observed between the expression of 2 lncRNAs (XLOC_051122 and XLOC_006074) and 1) lymph node metastasis (N stage) and 2) BRAF(V600E) mutation. Among patients with wild-type BRAF, the expression of these 2 lncRNAs showed significantly higher levels in the patients with lymph node metastasis. In silico analysis of these lncRNAs pinpointed cell movement and cellular growth and proliferation as targeted functions.
Conclusions:
Comprehensive expression screening identified 2 novel lncRNAs associated with risk factors of adverse prognosis in PTC patients. These lncRNAs may be novel players in PTC carcinogenesis.
RNA-Sequencing analysis identified differentially expressed lncRNAs in PTC tumor. Two lncRNAs were significantly associated with lymph node metastasis and BRAF(V600E) mutation.
Thyroid cancer represents approximately 1% of newly diagnosed cancers and is the most common endocrine malignancy. Thyroid cancer is one of the few cancers that has increased in incidence over recent years (1). It is estimated that 64 300 individuals in the United States will be diagnosed with thyroid cancer in 2016 (http://seer.cancer.gov/statfacts/html/thyro.html). Most all thyroid cancers are classified as nonmedullary thyroid carcinoma. Papillary thyroid carcinoma (PTC) is the main form of nonmedullary thyroid carcinoma, accounting for approximately 80% of all thyroid cancers. Generally, PTC is relatively indolent, and its long-term outcome is favorable with a survival rate more than 90%. However, PTCs with certain clinical and pathological features, such as diagnosis at older age, larger tumor size, larger lymph node (LN) metastasis, gross extrathyroidal extension, or BRAF mutations, are associated with a more aggressive clinical course (2, 3). Cervical LN metastases are quite common in PTC and have been found in 20%–50% of patients. LN metastases are known to be important prognostic factors for regional and distant metastasis (2, 4–6). Unlike other cancers where the involvement of LNs usually means a worse prognosis, positive LNs in the neck of patients with PTC do not decrease survival but do signal a greater risk of recurrence or residual disease. Some studies have reported that the number of metastatic nodes, extranodal extensions, and the size of the metastatic foci are independent prognostic factors (7, 8). Recently, the revised American Thyroid Association management guidelines for differentiated thyroid cancer described several clinicopathologic features of metastatic LNs in determining the risk of recurrence (9).
Although molecular and genetic pathobiological concepts are emerging, including the role of radiation, microRNAs and a few long noncoding RNAs (lncRNAs), the molecular mechanisms underlying PTC development are not yet completely understood. LncRNAs, typically more than 200 nucleotides, are increasingly reported to play key roles in numerous biological processes, such as imprinting, epigenetic regulation, nuclear import, cell cycle control, cell differentiation, alternative splicing, and RNA decay and transcription. Thus, lncRNAs are critical for normal development and, in many cases, are dysregulated in diseases such as cancer. Altered lncRNA expression levels have been observed in several cancer types, including PTC, indicating that aberrant expression of some lncRNAs contributes to carcinogenesis (10–12). Recent evidence suggests that lncRNAs are frequently cell type specific and may interact with chromatin remodeling enzymes such as enhancer of zeste homolog 2 (13). Indeed, further examples indicate that lncRNAs may be essential players in thyroid cancer biology. Previously, our group described 4 lncRNA genes (NAMA, PTCSC1, PTCSC2, and PTCSC3) having roles in PTC predisposition and tumorigenesis (14–17). The recent efforts to genetically characterize PTCs by The Cancer Genome Atlas (TCGA) Research Network Thyroid Working Group and others have demonstrated that noncoding RNAs may play an important role in PTC initiation and progression.
In this study, we analyzed paired PTC tumor and noncancerous samples to profile differentially expressed lncRNAs using RNA sequencing (RNA-Seq) technology. We then analyzed whether differentially expressed lncRNAs correlated with clinical parameters of the PTC patients. We focused on 2 lncRNAs that were significantly associated with risk factors of recurrence in PTC patients.
Materials and Methods
This study was approved by the Cancer Institutional Review Boards at The Ohio State University Medical Center. All subjects gave written informed consent for participation.
Patients and sample collection
Thyroid tumor tissue samples (n = 109) and paired noncancerous tissue samples (n = 20) were obtained from PTC patients undergoing surgical resection. All patients had total thyroidectomy and lymphadenectomy. The samples were snap frozen in liquid nitrogen and stored at −80°C. Clinical data and information corresponding to the specimens are shown in Supplemental Table 1.
RNA isolation and quality assessment
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The purity of extracted RNA was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc), and the concentration was assessed with Qubit 2.0 Fluorometer (Agilent Technologies) using an RNA HS Assay kit. Samples with RNA Integrity Number greater than 4 as assessed by a BioAnalyzer (Agilent) with no visible sign of genomic DNA contamination from the HS Nanochip tracings were used for total RNA library generation.
Preparation of strand-specific RNA-Seq libraries and RNA-Seq
RNA-Seq libraries were prepared using 12 paired PTC tumor and noncancerous tissue samples. The clinical information from these cases is provided in Supplemental Table 2. To accurately determine RNA levels in the PTC samples, we used the Illumina TruSeq Stranded Total RNA Sample Prep kit with Ribo-Zero Gold (catalog number RS-122-2201), which transformed RNA into cDNA. This kit employed Ribo-Zero to remove rRNA and mitochondrial RNA before library preparation. The protocol consisted of random primer and stranded RNA extraction, RNA fragmentation, reverse transcription, and 100-bp paired-end sequencing using the Illumina HiSeq 2500 system.
Prealignment data quality control (QC) were assessed with FastQC. Postalignment data quality was assessed with an in-house QC pipeline/database for RNA-Seq database (18). First, RNA-Seq data were trimmed for any adapter sequences using Adapter Removal (19). Any residual rRNA and mitoRNA in RNA-Seq reads was removed by aligning to a custom human contaminome containing these high abundance sequences using Bowtie2 (20). The RNA-Seq data have been deposited in the Gene Expression Omnibus repository and is accessible through Gene Expression Omnibus series accession number GSE83520.
Gene expression estimate and differential gene expression analysis
To obtain expression estimates, we mapped the RNA-Seq reads to the human genome using TopHat and quantified the reads using Cufflinks software. The relative abundance of transcripts was measured in fragments per kilobase of exons per million fragments mapped (FPKM). We obtained RNA-Seq expression estimates by using GENCODE v.19 Gene Transfer Format file as a transcript reference (GENCODE annotation). In addition, we performed analysis using the Cufflinks RABT assembly method and using the UCSC Gene Transfer Format file to identify potential novel lncRNA genes (de novo transcript assembly) (21, 22).
To identify transcripts expressed differentially between tumor and noncancerous samples in PTC, the estimated expression values obtained with GENCODE and de novo assembly annotations were analyzed separately. To eliminate bias due to very low expression levels, genes with FPKM values of 0 in more than 80% of the samples and genes with FPKM values below 2 across all the samples were filtered out.
Semiquantitative and quantitative RT-PCR
Total RNA was first treated with deoxyribonuclease I and then reverse transcribed to cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Target genes and an endogenous control gene glyceraldeyde-3-phosphate dehydrogenase (GAPDH) were included in the same PCR reaction for semiquantitative RT-PCR. Quantitative real-time PCR was performed by using an ABI PRISM 7700 DNA Sequence Detection System (Applied Biosystems) and a SYBR Green PCR kit (Applied Biosystems). GAPDH was included as a control. The comparative threshold cycle method was used to calculate the relative gene expression. Primers for amplification of the tested lncRNA genes and GAPDH are listed in Supplemental Table 3.
TCGA thyroid cancer RNA-Seq data
In TCGA consortium database, there are thyroid cancer RNA-Seq data from 505 PTC patients (December 2015). We downloaded the clinical information and the level 3 RNA-Seq expression values of coding mRNA genes from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). In addition, we downloaded RNA-Seq expression values for the selected lncRNAs of 497 TCGA PTC patients and 59 matched normal samples from the atlas of ncRNA in cancer (TANRIC) data base (http://ibl.mdanderson.org/tanric/_design/basic/index.html) (23). For the differential gene expression analysis, only tumors with a matched normal sample were used. For the correlation analysis, all the tumor samples (n = 497) were used. Clinical data and information corresponding to the TCGA samples are shown in Supplemental Table 4.
Computational analysis of the potential functions of selected lncRNAs
We first identified the coexpressed coding genes with each of the selected lncRNAs by correlation analysis. These coding genes were then analyzed with Ingenuity Pathway Analysis (IPA) program.
Statistical and bioinformatics analysis
In order to identify differentially expressed genes, paired t test was applied after transforming expression estimates (X) with the transformation log2(X+0.1) (24). The Benjamini and Hochberg method was used to adjust P values for multiple comparisons. The median fold change of tumor (T) vs noncancerous (N) of pairs for each gene was estimated. LncRNA genes with adjusted P < .01 and fold change more than or equal to 2 were selected as differentially expressed.
The Spearman rank correlation of expression levels between lncRNAs and coding genes were obtained. Coding genes with absolute correlation coefficients more than or equal to 0.6 were used in the IPA functional analysis program. Association between the expression levels and the patient's clinical parameters were evaluated by applying the nonparametric Mann-Whitney, Kruskal, and Spearman rank correlation tests.
Results
Differentially expressed lncRNAs in PTC tumor
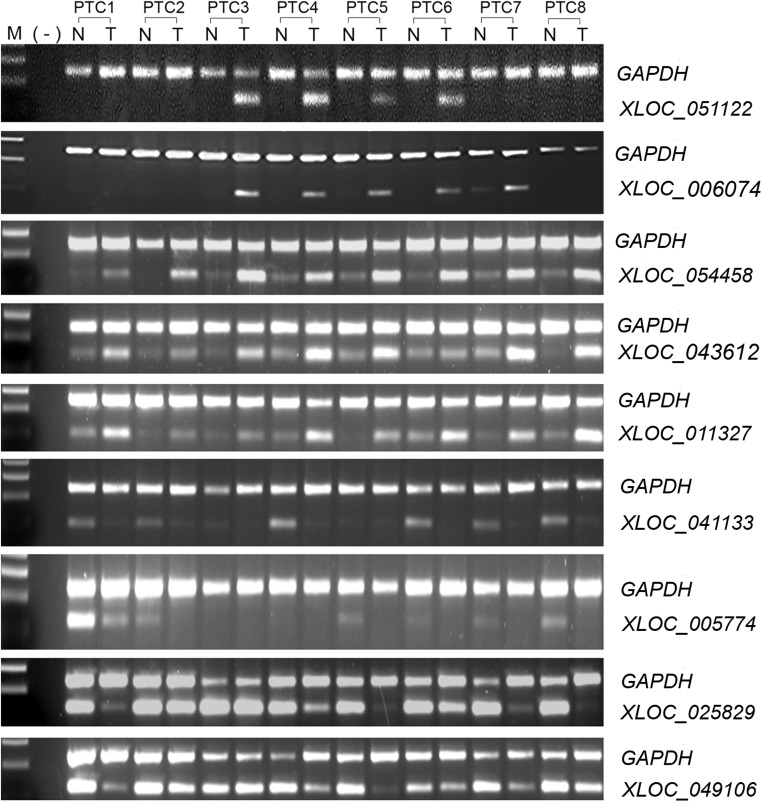
Initially we annotated the RNA-Seq data using GENCODE (v19) as a reference and obtained a total of 183 lncRNA genes showing differential expression in PTC tumors (P < .01, fold ≥ 2). Among them, 69 lncRNAs were up-regulated, whereas 114 were down-regulated in PTC tumor tissue in comparison with unaffected tissue. The known lncRNA PTCSC3 gene occurred as down-regulated in PTC tumors. This group of lncRNAs could be categorized as 5 classes: intergenic lncRNAs (lincRNAs) (n = 79), antisense (n = 65), sense overlapping and sense intronic (n = 20), processed transcripts (n = 17), and 3 prime overlapping (n = 2). To further identify uncharacterized lncRNA transcripts, we annotated RNA-Seq reads by de novo transcript assembly and obtained an additional 55 differentially expressed multiexonic lncRNAs (P < .01, fold ≥ 2). Among them, 22 lncRNAs were up-regulated, whereas 33 were down-regulated. These lncRNAs were annotated with a class code of “u” by the Cufflink program, indicating unknown intergenic transcripts. The top 10 up-regulated and top 10 down-regulated lncRNAs in each of the 2 annotations are listed in Table 1. To validate the expression, we performed semiquantitative RT-PCR of 9 lncRNAs with a new set of 8 paired PTC samples. The RT-PCR results confirmed the RNA-Seq results with respect to the up or down direction of the gene expression in PTC (Figure 1). All the 9 lncRNAs showed significant differential expression in tumors (P < .01, fold ≥ 2). We noticed that lncRNAs XLOC_051122 and XLOC_006074 were highly expressed in 4 and 5 out of 8 tumors, respectively, whereas they were undetectable or very weak in the other tumors and in all the noncancerous samples (Figure 1).
Table 1.
Top 40 Differentially Expressed lncRNAs in PTC Tumor
| LncRNA ID | Genomic Coordinates (hg19) | Gene Category | P Valuea | Fold Change T/Nb |
|---|---|---|---|---|
| De novo transcript assembly | ||||
| XLOC_051122 | chr9:109984408-110018228 | LincRNA | .0019 | 51.70 |
| XLOC_006074 | chr10:54643921-54681370 | LincRNA | .0082 | 27.80 |
| XLOC_054471 | chrX:124213404-124217383 | LincRNA | .0000 | 23.83 |
| XLOC_054487 | chrX:124265067-124294007 | LincRNA | .0000 | 20.88 |
| XLOC_002597 | chr1:116048086-116095605 | LincRNA | .0002 | 15.61 |
| XLOC_026790 | chr2:123144937-123162652 | LincRNA | .0002 | 10.49 |
| XLOC_022931 | chr18:45219886-45221402 | LincRNA | .0048 | 7.50 |
| XLOC_011352 | chr12:43604156-43605916 | LincRNA | .0046 | 6.49 |
| XLOC_001585 | chr1:14584411-14643085 | LincRNA | .0062 | 5.42 |
| XLOC_007231 | chr10:59134031-59171466 | LincRNA | .0101 | 4.88 |
| XLOC_021094 | chr17:41393281-41394741 | LincRNA | .0033 | −7.90 |
| XLOC_040384 | chr5:49819666-49850906 | LincRNA | .0047 | −7.95 |
| XLOC_008252 | chr11:36720677-36776338 | LincRNA | .0005 | −8.12 |
| XLOC_049604 | chr8:51838871-51847332 | LincRNA | .0009 | −8.73 |
| XLOC_034124 | chr3:161634454-161641317 | LincRNA | .0084 | −8.93 |
| XLOC_006356 | chr10:89891479-90023521 | LincRNA | .0003 | −9.50 |
| XLOC_029023 | chr2:134772444-134788920 | LincRNA | .0039 | −10.87 |
| XLOC_007949 | chr11:9634738-9635849 | LincRNA | .0001 | −11.17 |
| XLOC_034079 | chr3:161318134-161350884 | LincRNA | .0005 | −14.45 |
| XLOC_028936 | chr2:120485609-120488531 | LincRNA | .0011 | −14.77 |
| GENCODE annotation | ||||
| ENSG00000223914.1 | chr12:40550040-40561509 | LincRNA | .0001 | 84.09 |
| ENSG00000237463.1 | chr1:165446077-165551713 | Antisense | .0005 | 31.29 |
| ENSG00000260943.1 | chr12:40534727-40536678 | LincRNA | .0006 | 25.56 |
| ENSG00000250748.2 | chr12:65672422-66036152 | LincRNA | .0001 | 24.37 |
| ENSG00000223813.2 | chr7:29019582-29603286 | Antisense | .0001 | 20.94 |
| ENSG00000272482.1 | chr1:12678905-12679250 | LincRNA | .0003 | 18.59 |
| ENSG00000268307.1 | chr19:58911587-58912046 | LincRNA | .0027 | 18.11 |
| ENSG00000237512.2 | chr10:72972326-73062621 | Antisense | .0062 | 15.34 |
| ENSG00000251002.3 | chr14:22849082-22951948 | Processed transcript | .0041 | 15.13 |
| ENSG00000250343.1 | chr5:146559766-146614422 | Antisense | .0003 | 14.44 |
| ENSG00000228031.2 | chr7:137029675-137039229 | LincRNA | .0004 | −10.99 |
| ENSG00000230587.1 | chr2:43324883-43329829 | LincRNA | .0008 | −11.52 |
| ENSG00000248479.1 | chr4:66567919-66571271 | LincRNA | .0004 | −13.59 |
| ENSG00000223414.2 | chr6:165740775-166401536 | LincRNA | .0045 | −13.83 |
| ENSG00000206129.3 | chr18:53548918-53858493 | LincRNA | .0004 | −15.72 |
| ENSG00000267712.1 | chr18:53548918-53858493 | LincRNA | .0005 | −16.99 |
| ENSG00000254489.1 | chr11:30406039-30652055 | Antisense | .0010 | −17.44 |
| ENSG00000233705.2 | chr7:107294420-107358254 | Antisense | .0060 | −23.73 |
| ENSG00000258117.1 | chr12:63686408-63753817 | LincRNA | .0001 | −33.27 |
| ENSG00000261185.1 | chr1:182610950-182612442 | LincRNA | .0002 | −42.11 |
T, tumor; N; noncancerous tissue.
Paired t test, Benjamini and Hochberg adjusted P values with log2 transformed expression values.
Median fold change.
Figure 1.
Differential gene expression of lncRNAs. Semiquantitative RT-PCR was performed in paired thyroid tumor and noncancerous tissues. GAPDH was used as loading control. N, noncancerous tissue; T, tumor tissue.
LncRNAs associated with clinicopathologic characteristics
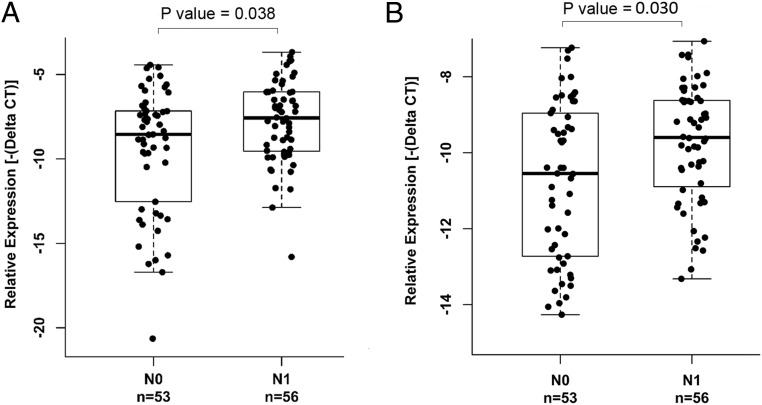
The expression patterns of XLOC_051122 and XLOC_006074 prompted us to assess whether the levels of these 2 lncRNAs and other differentially expressed lncRNAs correlated with clinicopathologic characteristics (age, gender, N stage, T stage, tumor size, multifocality, and extrathyroidal extension). We performed the initial analysis using the RNA-Seq data of 12 cases and found 6 lncRNAs to be correlated with LN metastasis, and one of them was also correlated with multifocality. The most remarkable correlation was that 2 lncRNAs (XLOC_051122 and XLOC_006074) were significantly associated with LN metastasis (N stage) with P = .013 and P = .009, respectively. Both XLOC_051122 and XLOC_006074 were predicted to be novel lincRNAs; they were listed among the top 10 up-regulated lncRNAs annotated by de novo transcript assembly (Table 1). The annotated genomic loci of these 2 genes are shown in Supplemental Figure 1. We decided to focus on these 2 lncRNAs to further assess the correlation between the expression and the clinical parameters. We performed quantitative real-time RT-PCR in an Ohio PTC tumor sample cohort (n = 109) and found that indeed these 2 lncRNAs were significantly associated with LN metastasis (N stage) (P < .05) (Figure 2). Both XLOC_051122 and XLOC_006074 also showed significant correlation with BRAF(V600E) mutation status (P < .001), but no association with age, gender, tumor size, T stage, extrathyroidal extension, or multifocality (Table 2 and Supplemental Figures 2 and 3). To further validate the differential lncRNA expression and association between the lncRNA expression and clinicopathologic characteristics, we downloaded the expression values of these 2 lncRNAs and the relevant clinical information from the TCGA and TANRIC websites and performed differential analysis between 59 tumor-normal pairs and correlation analysis on 497 PTC tumors. The results confirmed the observation that the 2 lncRNAs were overexpressed in tumor and most significantly associated with N stage as well as BRAF(V600E) mutation (all the P < .001) (Table 2). Both XLOC_051122 and XLOC_006074 were also significantly correlated with extrathyroidal extension (P < .001) and T stage (P < .05) (Table 2).
Figure 2.
Correlation between the expression of XLOC_051122 and XLOC_006074 and LN metastasis in an Ohio PTC tumor cohort. Gene expression was assessed by real-time RT-PCR. The relative expression level was normalized using GAPDH as an internal control. The sample size (n) and the N stage (N0 or N1) are shown. Nonparametric Wilcoxon tests were performed. A, Expression of XLOC_051122. B, Expression of XLOC_006074.
Table 2.
Correlation Between Expression Levels of 2 lncRNAs and Clinicopathologic Characteristics of PTC
| LncRNA ID | Age at Diagnosis (Age <45, Age ≥45) | Gender | Multifocality | Tumor Size | T Stage | N Stage | Extrathyroidal Extension | BRAF (V600E) |
|---|---|---|---|---|---|---|---|---|
| Ohio cohort (n = 109) | ||||||||
| XLOC_051122 | 0.546 | 0.749 | 0.230 | 0.478 | 0.155 | 3.79E-02 | 0.084 | 1.55E-09 |
| XLOC_006074 | 0.429 | 0.195 | 0.152 | 0.289 | 0.26 | 1.47E-02 | 0.908 | 6.40E-09 |
| TCGA cohort (n = 497) | ||||||||
| XLOC_051122 | 0.481 | 0.694 | 0.563 | 0.679 | 0.004 | 4.84E-14 | 1.15E-07 | 1.49E-28 |
| XLOC_006074 | 0.088 | 0.506 | 0.433 | 0.648 | 0.019 | 1.54E-10 | 3.50E-07 | 4.98E-42 |
| TCGA classic PTC (n = 350) | ||||||||
| XLOC_051122 | 0.569 | 0.662 | 0.527 | 0.829 | 0.167 | 3.73E-05 | 0.064 | 4.83E-12 |
| XLOC_006074 | 0.024 | 0.527 | 0.498 | 0.868 | 0.567 | 2.52E-03 | 0.279 | 1.01E-20 |
| TCGA fvPTC (n = 101) | ||||||||
| XLOC_051122 | 0.59 | 0.494 | 1 | 0.983 | 0.072 | 1.95E-03 | 0.029 | 5.14E-06 |
| XLOC_006074 | 0.789 | 0.042 | 0.316 | 0.657 | 0.271 | 0.019 | 0.016 | 4.24E-09 |
| TCGA Tall Cell PTC (n = 36) | ||||||||
| XLOC_051122 | 0.019 | 0.016 | 0.914 | 0.125 | 0.272 | 0.371 | 0.876 | 0.168 |
| XLOC_006074 | 0.985 | 0.349 | 0.971 | 0.612 | 0.425 | 0.389 | 0.218 | 0.562 |
The P values of nonparametric analysis are listed. The gene expression levels were determined by real-time RT-PCR for the Ohio samples or downloaded from the TANRIC website for the TCGA samples. n, sample size.
We further analyzed the expression of these 2 lncRNAs in the histological subgroups of the TCGA PTC cohort, including classic PTC, follicular variant PTC (fvPTC), and tall cell PTC. The expression of both XLOC_051122 and XLOC_006074 were significantly lower in fvPTC samples when comparing with classic PTC or tall cell PTC (P < .001). In contrast, tall cell PTC samples showed the highest expression (P < .05) (Supplemental Figure 4). This observation prompted us to analyze the correlation between the expression of the 2 lncRNAs and the clinical characteristics in each of the subgroups. In the classic PTC group (n = 350), the expression of the 2 lncRNAs showed significant association with LN metastasis (N stage) and BRAF(V600E) mutation (P < .05) (Table 2 and Supplemental Figure 5). In the fvPTC group (n = 101), the expression of both of the 2 lncRNAs showed significant association with LN metastasis (N stage), BRAF(V600E) mutation, and extrathyroidal extension (P < .05) (Table 2 and Supplemental Figure 5). Neither classic PTC nor the fvPTC groups showed any association with age, gender, tumor size, T stage, or multifocality, except that the expression of XLOC_006074 was significantly associated with age in classic PTC and gender in fvPTC (P < .05) (Table 2). In the tall cell group (n = 36), no significant correlation was found except that the expression of XLOC_051122 was significantly associated with age and gender (P < .05) (Table 2).
Correlation between the expression levels of the 2 lncRNAs (XLOC_051122 and XLOC_006074) and LN metastasis in patients with or without BRAF(V600E) mutation
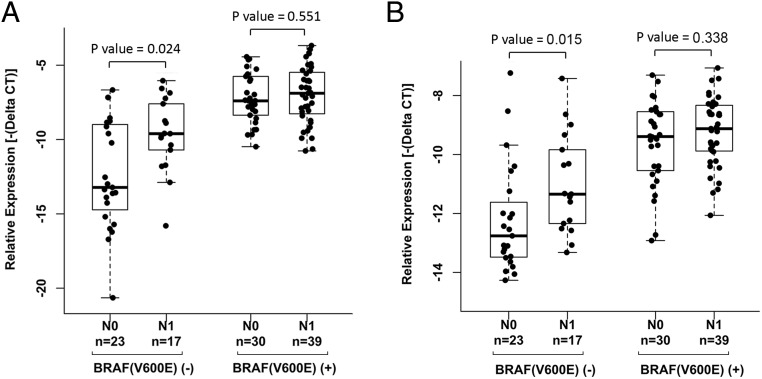
To further stratify the correlation between the 2 lncRNAs and BRAF(V600E) mutation status, we sorted the PTC patients into groups of BRAF(V600E) mutation and BRAF wild type and performed correlation analysis for N stage. Noticeably, among the Ohio PTC patients without the BRAF(V600E) mutation, both XLOC_051122 and XLOC_006074 showed significantly increased expression in patients of N1 stage (P < .05), whereas no significant differences between N0 and N1 stage were found among the Ohio PTC patients with BRAF(V600E) mutation (Figure 3). We performed correlation analysis for tumor size and extrathyroid extension; no significant association was observed in the presence or absence of BRAF mutation between the expression of these 2 lncRNAs and tumor size (Supplemental Figure 6) and extrathyroidal extension (Supplemental Figure 7).
Figure 3.
Correlation between the expression of XLOC_051122 and XLOC_006074 and LN metastasis in the presence or absence of BRAF(V600E) mutation. The gene expression and clinical parameters were the same as described in Figure 2. BRAF(V600E) (−), without mutation; BRAF(V600E) (+), with mutation. Nonparametric Wilcoxon tests were performed. A, Expression of XLOC_051122. B, Expression of XLOC_006074.
We further performed correlation analysis for LN metastasis in the TCGA cohort and made the same observation in the TCGA patients without BRAF(V600E) mutation, in that both XLOC_051122 and XLOC_006074 showed significantly increased expression in patients of N1 stage with P < .001 (Supplemental Figure 8, A and B). In the TCGA patients with BRAF(V600E) mutation, there was also an increased expression of XLOC_051122 in patients of N1 stage (P < .05), whereas XLOC_006074 showed no difference between N1 and N0 patients (Supplemental Figure 8, A and B). We also performed correlation analysis among the classic PTC and fvPTC but not the tall cell groups, because of the small sample size of the tall cell group. In the classic PTC patients without BRAF(V600E) mutation, both XLOC_051122 and XLOC_006074 showed significantly increased expression in patients of N1 stage (P < .001) but no differences among the patients with BRAF mutation (P > .05) (Supplemental Figure 8, C and D). In the fvPTC patients, only the expression of XLOC_051122 showed significantly increased expression in patients without BRAF(V600E) mutation (P < .05) (Supplemental Figure 8, E and F).
Annotation of the functions of the 2 lncRNAs (XLOC_051122 and XLOC_006074)
It is widely accepted that lncRNAs can regulate the expression of adjacent or overlapping protein-coding genes. To this end, the function of lncRNAs may be reflected by their associated protein-coding genes. Therefore, computational functional analysis of the coexpressed coding genes to the lncRNAs may provide insight into the function of the lncRNAs. We performed expression correlation analysis and selected the significantly coexpressed coding genes to each of the 2 lncRNAs with absolute correlation coefficient of more than or equal to 0.6 and P < .001. The top 40 coexpressed genes to each of the lncRNAs are listed as Supplemental Tables 5 and 6. Biological functional analysis and pathway analysis was carried out using IPA software. Both XLOC_051122 and XLOC_006074 are predicted to have biological functions related to cancer and cellular movement (Supplemental Figure 9). Among the top 5 categories of “Molecular and Cellular Functions,” 4 categories, namely cellular movement, cellular growth and proliferation, cellular assembly and organization, and cellular function and maintenance, are the same for the 2 lncRNAs. In addition, XLOC_051122 is involved in cell-to-cell signaling and Interaction, whereas XLOC_006074 is involved in cell morphology (Table 3).
Table 3.
Molecular and Cellular Functions of lnRNA Coexpressed Coding Genes
| LncRNA ID | Function | P Value | Number of Molecules |
|---|---|---|---|
| XLOC_051122 | Cellular movement | 1.60E-03-8.50E-12 | 82 |
| Cellular growth and proliferation | 1.83E-03-2.62E-09 | 112 | |
| Cell-to-cell signaling and interaction | 1.83E-03-8.80E-09 | 51 | |
| Cellular assembly and organization | 1.83E-03-2.20E-07 | 55 | |
| Cellular function and maintenance | 1.23E-03-2.20E-07 | 65 | |
| XLOC_006074 | Cellular movement | 7.07E-03-3.56E-09 | 108 |
| Cellular growth and proliferation | 7.77E-03-4.32E-08 | 171 | |
| Cell morphology | 7.07E-03-1.72E-07 | 118 | |
| Cellular assembly and organization | 7.07E-03-4.31E-07 | 91 | |
| Cellular function and maintenance | 7.07E-03-4.31E-07 | 85 |
Discussion
LncRNAs do not encode proteins yet recent work has proved their involvement in cancer predisposition and tumorigenesis. It has been reported that several cancer risk loci are transcribed into lncRNAs and these transcripts play key roles in the respective cancers (25). We previously reported several lncRNA genes termed PTC susceptibility candidate 1–3 (PTCSC1, PTCSC2, and PTCSC3) (15–17). We showed that these lncRNAs play roles in PTC predisposition and are significantly down-regulated in thyroid tumors, implying a role as tumor suppressor. We also reported a noncoding RNA named NAMA associated with the MAPK pathway and growth arrest; down-regulation of NAMA is highly associated with the activating BRAF mutation V600E in PTC (14). In the present study, our data further characterize differential expression of lncRNAs in PTC tumors, providing a base for further functional research of the role of lncRNAs in thyroid cancer (26).
Cancer is a disease of aberrant gene expression (25). The aberrant expression of specific lncRNAs in cancer could provide markers of disease progression and prognosis. To explore the potential clinical implication of the differentially expressed lncRNAs in PTC, we performed correlation analysis between lncRNA expression and clinicopathological characteristics of PTC patients and identified 2 novel lncRNAs (XLOC_051122 and XLOC_006074) significantly associated with LN metastasis. These 2 lncRNAs were significantly overexpressed in PTC tumors, suggesting a possible oncogenic role in cancer. Patients with PTC have a high incidence (30%–100%) of LN metastases at presentation, which is an independent risk factor for residual or recurrent disease (27). Our findings provide a new clue to the underlying molecular mechanism of LN metastasis, which might provide a rationale for therapeutic targeting lncRNAs in PTC. Further study of the 2 lncRNAs described in this study and other differentially expressed lncRNAs may reveal potential biomarkers, which in turn may facilitate further stratification of the patients.
The BRAF(V600E) mutation has long been considered to be a risk factor for PTC progression and prognosis (28, 29). Early work suggested that the BRAF mutation acted together with dysregulated lncRNAs, triggering cascade signaling in the ERK pathway (14, 30). Expression of a BRAF-activated lncRNA (BANCR) was significantly up-regulated in PTC tumors. BANCR-knockdown led to inhibition of cell growth and cell cycle arrest (30). Another lncRNA (NAMA) was down-regulated in PTC with BRAF(V600E) mutation. NAMA was inducible by knockdown of BRAF (14). In the present study, we observed that the expression of XLOC_051122 and XLOC_006074 was significantly associated with BRAF(V600E) mutation. However, among the patients with wild-type BRAF, there was a subgroup in stage N1 having significant overexpression of these 2 lncRNAs (Figure 3 and Supplemental Figure 8). This could mean that the 2 lncRNAs, but not the BRAF(V600E) mutation, are involved in LN metastasis in a subset of patients.
LncRNAs are a heterogeneous group of RNAs that regulate gene expression at the epigenetic, transcriptional, or posttranscriptional level (31–33). Growing evidence has shown that lncRNAs are important factors controlling gene expression in cis (ie, of flanking neighboring genes) or in trans (distant genes) (34). The specific interacting genes, however, are not easily predicted based on sequence. Because lncRNAs do not have protein encoding abilities, their function is closely related with their transcript abundance and could be realized by their coexpressed coding genes (35, 36). We analyzed the biological functions of the coexpressed coding genes of XLOC_051122 and XLOC_006074, separately. The top biological functions listed by IPA analysis of both XLOC_051122 and XLOC_006074 were very similar; involving cellular movement, cellular growth and proliferation, cellular assembly and organization, and cellular function and maintenance (Table 3). Additional studies will be needed to determine whether XLOC_051122 and XLOC_006074 might be potential targets in therapies for PTC.
In conclusion, we successfully screened for lncRNAs differentially expressed in PTC and observed 2 lncRNAs (XLOC_051122 and XLOC_006074) significantly overexpressed in tumors and also associated with LN metastasis and BRAF(V600E) mutation. A subset of patients with LN metastasis was associated with higher expression of these 2 lncRNAs but independent of BRAF(V600E) mutation. Gene coexpression analysis suggested that these 2 lncRNAs are oncogenic by regulating cellular movement and cellular growth and proliferation. Our results emphasize the potential value of lncRNAs both as prognostic markers and as potential therapeutic targets.
Acknowledgments
We thank Ms Jan Lockman and Ms Barbara Fersch for administrative help and The Ohio State University Comprehensive Cancer Center Nucleic Acid Shared Resource for genotyping and real-time PCR. Tissue samples were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute.
This work was supported by National Cancer Institute Grants P30CA16058 and P50CA168505. This work was also supported in part by an allocation of computing time from The Ohio Supercomputer Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FPKM
- fragments per kilobase of exons per million fragments mapped
- fvPTC
- follicular variant PTC
- GAPDH
- glyceraldeyde-3-phosphate dehydrogenase
- IPA
- Ingenuity Pathway Analysis
- lincRNA
- intergenic lncRNA
- LN
- lymph node
- lncRNA
- long noncoding RNA
- PTC
- papillary thyroid carcinoma
- QC
- quality control
- RNA-Seq
- RNA sequencing
- TCGA
- The Cancer Genome Atlas.
References
- 1. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. [DOI] [PubMed] [Google Scholar]
- 2. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–531. [DOI] [PubMed] [Google Scholar]
- 3. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. [DOI] [PubMed] [Google Scholar]
- 4. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98:31–40. [DOI] [PubMed] [Google Scholar]
- 5. Suman P, Wang CH, Abadin SS, Moo-Young TA, Prinz RA, Winchester DJ. Risk factors for central lymph node metastasis in papillary thyroid carcinoma: a National Cancer Data Base (NCDB) study. Surgery. 2016;159:31–39. [DOI] [PubMed] [Google Scholar]
- 6. Sun Y, Shi C, Shi T, Yu J, Li Z. Correlation between the BRAF(v600E) gene mutation and factors influencing the prognosis of papillary thyroid microcarcinoma. Int J Clin Exp Med. 2015;8:22525–22528. [PMC free article] [PubMed] [Google Scholar]
- 7. Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol. 2016;12:981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun W, Lan X, Zhang H, et al. Risk factors for central lymph node metastasis in CN0 papillary thyroid carcinoma: a systematic review and meta-analysis. PLoS One. 2015;10:e0139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urken ML, Haser GC, Likhterov I, Wenig BM. The impact of metastatic lymph nodes on risk stratification in differentiated thyroid cancer: have we reached a higher level of understanding? Thyroid. 2016;26:481–488. [DOI] [PubMed] [Google Scholar]
- 10. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. [DOI] [PubMed] [Google Scholar]
- 12. Matouk IJ, Raveh E, Abu-lail R, et al. Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta. 2014;1843:1414–1426. [DOI] [PubMed] [Google Scholar]
- 13. Zhou Q, Chen J, Feng J, Wang J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumour Biol. 2015;1–9. [DOI] [PubMed] [Google Scholar]
- 14. Yoon H, He H, Nagy R, et al. Identification of a novel noncoding RNA gene, NAMA, that is downregulated in papillary thyroid carcinoma with BRAF mutation and associated with growth arrest. Int J Cancer. 2007;121:767–775. [DOI] [PubMed] [Google Scholar]
- 15. He H, Nagy R, Liyanarachchi S, et al. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Res. 2009;69:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jendrzejewski J, He H, Radomska HS, et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci USA. 2012;109:8646–8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He H, Li W, Liyanarachchi S, et al. Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J Clin Endocrinol Metab. 2015;100:E164–E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroll KW, Mokaram NE, Pelletier AR, et al. Quality control for RNA-Seq (QuaCRS): an integrated quality control pipeline. Cancer Inform. 2014;13(suppl 3):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stocks MB, Moxon S, Mapleson D, et al. The UEA sRNA workbench: a suite of tools for analysing and visualizing next generation sequencing microRNA and small RNA datasets. Bioinformatics. 2012;28:2059–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Meth. 2012;9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–2329. [DOI] [PubMed] [Google Scholar]
- 22. Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, Han L, Roebuck P, et al. TANRIC: an interactive open platform to explore the function of lncRNAs in cancer. Cancer Res. 2015;75:3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warden DC, Yuan Y-C, Wu X. Optimal calculation of RNA-Seq fold-change values. Int J Comput Bioinfo In Silico Model. 2013;2:285–292. [Google Scholar]
- 25. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang LY, Palmer FL, Nixon IJ, et al. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid. 2014;24:1594–1599. [DOI] [PubMed] [Google Scholar]
- 28. Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–471; discussion 470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xing M, Alzahrani AS, Carson KA, et al. Association between braf v600e mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng H, Wang M, Jiang L, et al. BRAF-activated long noncoding RNA modulates papillary thyroid carcinoma cell proliferation through regulating thyroid stimulating hormone receptor. Cancer Res Treat. 2016;48:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khachane AN, Harrison PM. Mining mammalian transcript data for functional long non-coding RNAs. PLoS One. 2010;5:e10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao Q, Liu C, Yuan X, et al. Large-scale prediction of long non-coding RNA functions in a coding-non-coding gene co-expression network. Nucleic Acids Res. 2011;39:3864–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hao Y, Wu W, Shi F, et al. Prediction of long noncoding RNA functions with co-expression network in esophageal squamous cell carcinoma. BMC Cancer. 2015;15:168. [DOI] [PMC free article] [PubMed] [Google Scholar]