Abstract
Context:
The dipeptidyl peptidase-4 inhibitor, linagliptin, possesses pleiotropic vasodilatory, antioxidant, and anti-inflammatory properties in animals, independent of its glucose-lowering properties. Although large, randomized clinical trials are being conducted to better evaluate the efficacy and safety of linagliptin on cardiovascular outcomes, little is known about its effects on vascular function in humans.
Objective:
This study sought to evaluate the effect of linagliptin on surrogates of vascular and mitochondrial function.
Design and Setting:
This was a randomized, double-blind, placebo-controlled trial at a tertiary care center with a large type 2 diabetes referral base.
Patients and Intervention:
Forty participants with type 2 diabetes were included in a 12-wk treatment of either linagliptin 5mg/d or placebo.
Main Outcome Measures:
Micro- and macrovascular functions were assessed using laser Doppler coupled with iontophoresis and with brachial flow-mediated dilation, respectively. Mitochondrial function was assessed by phosphorus-31 metabolites changes in the calf muscle measured by magnetic resonance spectroscopy. Circulating endothelial progenitor cells, as well as inflammatory cytokines, growth factors, and biomarkers of endothelial function were also quantified.
Results:
Linagliptin was associated with an increase in axon reflex-dependent vasodilation, a marker of neurovascular function (P = .05). A trend indicating increased endothelium-dependent microvascular reactivity was observed (P = .07). These were associated with decreases in concentrations of IFNγ (P < .05), IL-6 (P = .03), IL-12 (P < .03), and MIP-1 (P < .04) following linagliptin treatment when compared with placebo.
Conclusions:
This study demonstrates that linagliptin tends to improve endothelial and neurovascular microvascular function and is associated with decreased markers of inflammation in patients with type 2 diabetes. There was no significant effect of linagliptin on mitochondrial function, macrovascular function, or endothelial progenitor cells.
This randomized, placebo-controlled trial shows that linagliptin decreases inflammation and improves microvascular function in patients with type 2 diabetes.
The fundamental gluco-regulatory role of the intestinal-derived incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide, led to the development of two classes of new antidiabetic agents. Inhibitors of the dipeptidyl peptidase-4 (DPP-4), an enzyme that rapidly inactivates both GLP-1 and gastric inhibitory peptide, are recommended treatments for patients with type 2 diabetes mellitus (1). Beyond improving glycemic control, DPP-4 inhibitors may also have beneficial cardiovascular effects through both incretin-dependent and -independent mechanisms (2, 3). An extensive amount of preclinical data demonstrates these positive effects of DPP-4 inhibition on atherosclerosis, vascular injury, and cardio-protection in experimental models of cardiovascular disease (3, 4). In addition, improved endothelial function and decreased proinflammatory cytokines have been ascribed to DPP-4 inhibition (5). In contrast, several recent large, randomized controlled clinical trials evaluating the effect of DPP-4 inhibitors on cardiovascular outcomes did not show any reduction in the rate of ischemic events for saxagliptin, sitagliptin, and alogliptin (6–8). Furthermore, a statistically significant increase in the risk of hospitalization for heart failure has also been reported with saxagliptin (6, 9) but not with alogliptin or sitagliptin (7, 10). Similarly, studies exploring the effect of DPP-4 inhibition on endothelial function in humans report conflicting results (11–15). This may be attributed to intra-class differences between the different DPP-4 inhibitors.
Interestingly, the DPP-4 inhibitor, linagliptin, possesses pleiotropic vasodilatory, antioxidant, and anti-inflammatory properties in animals, independent of its glucose-lowering properties (16), which may in turn lead to improved cardiovascular outcomes in patients with type 2 diabetes. Although large, randomized trials are currently being conducted to better assess the efficacy and safety profile of linagliptin treatment for improving cardiovascular outcomes, little is known about its effects on vascular function in humans. Therefore, the aim of this prospective, randomized, placebo-controlled, parallel study is to explore the effects of linagliptin on peripheral micro and macrovascular function, circulating endothelial progenitor cells (EPCs) and markers of inflammation in patients with type 2 diabetes. As insulin resistance and the diabetic state are also characterized by mitochondrial dysfunction (17–19), which is associated with inflammation and lower-extremity complications (20), we further hypothesized that if linagliptin improves muscle microcirculation, it may improve muscle oxygenation and mitochondrial function.
Research Design and Methods
Study participants
Males and females age 30–70 years with type 2 diabetes, diagnosed according to the American Diabetes Association Guidelines (21), were recruited to participate in this present study. Participants were included on the basis that they had stable glucose control and no recent changes in diabetes treatment; and if glycated hemoglobin A1c (HbA1c) was ≤10%. Patients who were smokers or had previous history of myocardial ischemia, angina, macroalbuminuria, or any other serious illness were excluded. The full list of exclusion criteria is available as online Supplemental Figure 1. The protocol was approved by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Review Board and all participants provided written informed consent before commencing the protocol. The study was listed on ClinicalTrials.gov as trial number NCT01969084.
Research design
Following a screening visit, eligible participants completed a randomized, double-blind, placebo-controlled, parallel trial that consisted of two visits over a 12-week period. The screening visit was conducted following an overnight fast (≥8 h) at the BIDMC General Clinical Research Center and included an examination of the participant's current physical health and past medical history, as well as a review of the inclusion/exclusion criteria. Participants were then randomly assigned to the linagliptin or placebo treatment groups. Each participant presented to visit one (baseline) in an overnight fasted state (8–12 h) where assessment of mitochondrial function, muscle oxygenation, cutaneous microvascular function, macrovascular function, inflammatory cytokines, growth factors, and biomarkers of endothelial function were performed. The same sequence of testing was repeated following 12 weeks of linagliptin (5 mg once daily) or matched placebo treatment.
Measurements
Mitochondrial function and muscle oxygenation
Mitochondrial function was assessed by magnetic resonance spectroscopy that specifically measured changes in calf muscle energy reserves (phosphorus-31 metabolites) in response to a graded exercise test, as previously described (20). Briefly, prior to the beginning of the exercise test each participant rested in a supine position while their dominant leg was positioned on a plantar flexion ergometer and restrained at the knee. Participants then performed a one repetition maximum strength measurement of the plantar flexion movement to set the ergometer resistance for the exercise test at 50% of the one repetition maximum value. The exercise test began following baseline magnetic resonance spectroscopy measurements and consisted of 30 repetitions of the plantar flexion movement per minute until voluntary muscle exhaustion or calf pain, or for a maximum period of 7 minutes. Phosphorus-31 metabolites including inorganic phosphorous and phosphocreatine (PCr) concentrations were measured at 10-second intervals for 1 minute before exercise with measurements continuing during the exercise test, as well as for 6 minutes throughout the recovery period that immediately followed the conclusion of the exercise test. The main variable of interest was the time from the conclusion of the exercise test to the recovery of PCr to 63% of baseline concentrations (the exponential recovery time constant).
Participants then remained in a supine position while changes in lower leg muscle oxygenation in response to an occlusion test were assessed using the blood oxygenation level–dependent (BOLD) magnetic resonance imaging (MRI) technique. In brief, the BOLD MRI technique is based on the principle that hemoglobin changes its magnetic qualities depending on whether it is oxygenated or deoxygenated with a stronger MRI signal being interpreted as increased oxygenation of blood throughout the muscle (22). Following 1 minute of baseline recordings, a sphygmomanometer cuff positioned at the midthigh was inflated to 50 mm Hg above the participant's resting brachial systolic blood pressure (BP) to occlude the lower leg circulation. The ischemia-inducing occlusion was maintained for 4 minutes before the cuff was rapidly released inducing reactive hyperemia. Muscle oxygenation was measured throughout baseline and ischemia, as well as for 6 minutes during reactive hyperemia with the time elapsed from the end of ischemia until muscle oxygenation returned to 63% of baseline concentrations being a key variable of interest.
Cutaneous microvascular function
Cutaneous microvascular function was assessed using laser Doppler perfusion imaging (LDPI) and laser Doppler flowmetry (LDF) in conjunction with transdermal iontophoresis. In brief, iontophoresis involves the delivery of a pharmacologically charged drug to the cutaneous microcirculation using a low-charge electrical current to induce changes in skin blood flow (23). Prior to iontophoresis, baseline measurements of resting cutaneous microvascular blood flux were performed on the ventral surface of the left forearm at two drug delivery sites separated by 5 cm using a PeriScan PIM II LDPI system (Perimed, Järfälla) with a wavelength of 633 nm.
Endothelium-dependent function was then assessed with iontophoresis of acetylcholine using an MIC1 iontophoresis system (Moor Instruments Ltd., Millwey) that delivered an anodal electrical current of 0.2 mA for 60 seconds. Cutaneous microvascular blood flux was monitored for 40 seconds (baseline) before, for 60 seconds during, and for 90 seconds following the conclusion of transdermal iontophoresis using a DRT4 LDF system (Moor Instruments Ltd., Millwey). Immediately following the conclusion of LDF measurements, changes in cutaneous microvascular blood flux in response to transdermal iontophoresis of acetylcholine were then also assessed with LDPI. This sequence of testing was repeated for transdermal iontophoresis of sodium nitroprusside, which assessed endothelium-independent function, using a cathodal current of 0.2 mA for 60 seconds.
Neurovascular function was measured with a second LDF probe placed outside but near (5 mm) the iontophoresis solution chamber. During iontophoresis, the application of a mild, non-noxious electrical current increases skin blood due to the stimulation of the C nociceptor fibers, and may be used as a marker of the axon reflexpen]mediated vasodilation (24).
Data were expressed as the mean blood flux in arbitrary units at baseline and following transdermal iontophoresis, and the percentage change from baseline measurements in response to transdermal iontophoresis of acetylcholine and sodium nitroprusside.
Macrovascular function
Endothelium-dependent and endothelium-independent macrovascular function was assessed at the brachial artery using flow-mediated dilation (FMD) and sublingual administration of nitroglycerine, respectively. Vascular responses were quantified using a high-resolution ultrasound with a 10.0-MHz linear array transducer (Aloka Prosound α7, Hitachi Aloka Medical, Ltd.) according to standard guidelines (25). Briefly, the subject rested in a supine position while a sphygmomanometer cuff was wrapped around the left forearm and the ultrasound probe was positioned between the antecubital fossa and axillary region. Baseline measurements of brachial artery diameter were then performed before the sphygmomanometer cuff was inflated to 220 mm Hg. This occlusion was maintained for a period of 5 minutes before the cuff was rapidly deflated inducing reactive hyperemia and subsequent FMD. Measurements of brachial artery diameter were repeated 60 seconds following the end of the occlusion. Participants then rested for 10 minutes to allow the brachial artery to return to its basal diameter before baseline measurements were performed again. The participants then received a sublingual administration of nitroglycerine and measurements of brachial artery diameter were performed again 4 minutes after the nitroglycerine pill had dissolved. Data were reported as the brachial artery diameter (mm) at rest and the percentage change in brachial artery diameter from baseline in response to FMD and nitroglycerine administration.
Circulating EPCs
Concentrations of peripheral plasma circulating EPCs were assessed using fluorescence-activated cell sorting analysis at the BIDMC Flow Cytometry Core Facility. Immunofluorescent cell staining was performed on peripheral blood with the use of the fluorescent conjugated antibody CD34–fluorescein isothiocyanate (Becton Dickinson), type 2 vascular endothelial growth factor receptor 2/kinase insert domain receptor) –phycoerythrin (Miltenyi Biotec), CD133-allophycocyanin (Miltenyi Biotec), and CD45-Peridinin Chlorophyll Protein (MiltenyiBiotec) according to standard techniques. The methods have been further described previously (26).
Inflammatory cytokines, growth factors, and biomarkers of endothelial function
Plasma biomarkers of endothelial dysfunction and inflammation, including circulating cytokines, metallopeptidases, adhesion molecules and growth factors were measured at visit one and again at visit two using Luminex technology. Substance P (R&D Systems Inc., Minneapolis, MN) and Stromal Cell-Derived factor 1 (SDF-1) (ThermoFisher Scientific Inc.) were quantified using ELISA.
Statistical analysis
The primary endpoint of this study was the effect of 12 weeks of linagliptin treatment on mitochondrial function as assessed by the time to recovery of PCr to 63% of baseline concentrations immediately following a graded exercise test. However, given that this was an exploratory study, it was also an aim to explore new possible effects of linagliptin, develop new hypotheses, and collect preliminary data that could help in the design of more definitive trials in the future. Additional endpoints included the effect of linagliptin treatment on muscle oxygenation, and endothelium-dependent and -independent function in the micro and macrocirculation, as well as the effect of linagliptin treatment on concentrations of inflammatory cytokines, growth factors, and biomarkers of endothelial dysfunction, which are elevated in those with type 2 diabetes and are associated with diabetic complications.
Data were expressed as the mean ± SD for normally distributed data or the median (25th:75th percentiles) for nonnormally distributed data. The primary statistical analysis evaluated the difference in the mean change from visit one (baseline) to visit two (post-treatment) measures between the linagliptin and placebo treatment groups. Between-group comparisons were performed using the Student t test or nonparametric tests (Mann-Whitney U) when data were not normally distributed. Relationships between quantitative variables were assessed with Pearson's correlation test or Spearman's rank correlation test if the data did not follow a normal distribution. A post-hoc analysis was performed using the general linear model to assess the influence of treatment with ACE inhibitors on Substance P concentration (log transformation was used). P < .05 was considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (IBM Corp.) and the Minitab Statistical Package Version 14.0 (Minitab, Inc.).
Results
Description of the study population
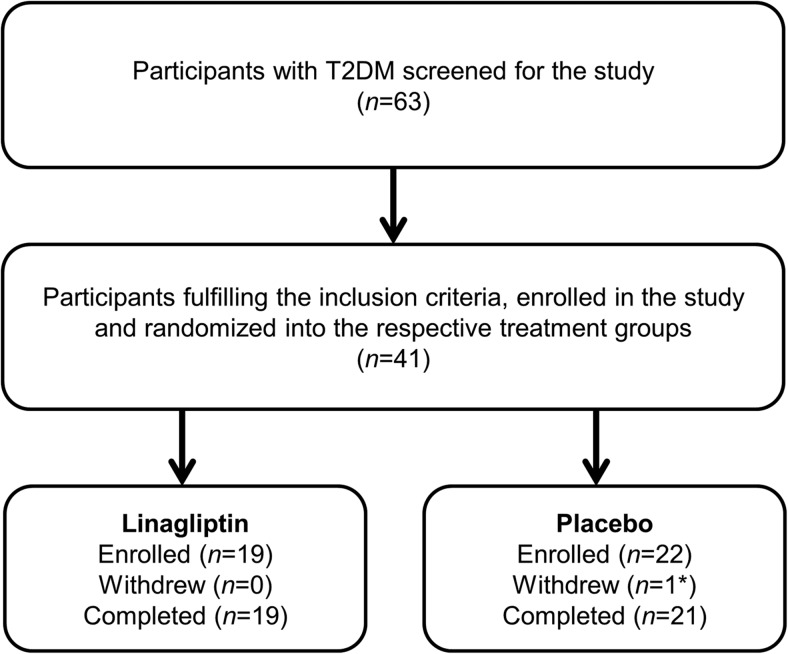
In total, 63 patients were screened to participate in this study, of whom 41 fulfilled the inclusion and exclusion criteria and were randomly assigned to either the linagliptin or placebo treatment groups (Figure 1). Of the 22 participants randomized onto the placebo-treatment group, one participant withdrew from the study due to an unrelated serious adverse event. The baseline clinical characteristics are presented in Table 1. No differences were observed between treatment groups except for a trend toward higher use of ACE inhibitors in the linagliptin group. The most common antidiabetic medication used by the participants included in the study was metformin (90%), followed by insulin (30%) and sulfonylureas (27.5%).
Figure 1.
Scheme of the clinical trial. T2DM, type 2 diabetes mellitus. *, Withdrew due to unrelated serious adverse event.
Table 1.
Baseline Clinical Characteristics of Participants Included in the Study
| Characteristic | Linagliptin | Placebo | P |
|---|---|---|---|
| n | 19 | 21 | |
| Age, y | 60.79 ± 5.75 | 56.76 ± 6.79 | .1 |
| Men | 12 (63.16%) | 11 (52.38%) | .49 |
| Diabetes duration, y | 10.15 ± 7.56 | 12.14 ± 7.30 | .4 |
| Weight, kg | 95.22 ± 20.75 | 100.57 ± 21.06 | .42 |
| BMI, kg/m2 | 32.40 ± 4.87 | 36.12 ± 9.19 | .14 |
| Waist circumference, cm | 111.50 ± 11.34 | 116.31 ± 16.71 | .31 |
| Systolic BP, mm Hg | 136.53 ± 14.92 | 134.67 ± 19.65 | .74 |
| Diastolic BP, mm Hg | 75.89 ± 12.26 | 75.95 ± 13.96 | .99 |
| Fasting blood glucose, mg/dL | 131.21 ± 30.19 | 126.62 ± 39.28 | .68 |
| HbA1c, % | 7.08 ± 0.82 | 7.16 ± 0.78 | .76 |
| Total cholesterol, mg/dL | 146.37 ± 30.65 | 159.29 ± 38.84 | .25 |
| LDL cholesterol, mg/dL | 80.21 ± 26.29 | 81.90 ± 22.36 | .83 |
| HDL cholesterol, mg/dL | 46.89 ± 10.35 | 50.71 ± 14.37 | .34 |
| Triglycerides, mg/dL | 96.42 ± 38.69 | 125.90 ± 85.30 | .18 |
| Creatinine, mg/dL | 0.87 ± 0.20 | 0.91 ± 0.22 | .55 |
| Microalbumin/creatinine ratio, mg/g creatinine | 26.45 ± 30.80 | 19.64 ± 15.21 | .37 |
| Insulinemia, μIU/mL | 38.29 ± 102.63 | 13.05 ± 6.49 | .27 |
| Diabetes treatment | |||
| Insulin | 4 (21.05%) | 8 (38.1%) | .24 |
| Metformin | 16 (84.21%) | 20 (95.24%) | .25 |
| Sulfonylureas | 5 (26.32%) | 6 (28.57%) | .87 |
| ACEi | 11 (57.9%) | 6 (28.57%) | .06 |
| ARBs | 2 (10.5%) | 1 (4.8%) | .60 |
| Statins | 11 (57.9%) | 13 (61.9%) | .80 |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers.
Data are expressed as mean ± SD or n (%).
Following the 12-week treatment period, median fasting blood glucose decreased by −19 (−29:7) mg/dL in the linagliptin group and increased by 11 (−3:31) in the placebo group (P < .01). Median HbA1c decreased by −0.3% (−0.9:1.0%) in the linagliptin group and −0.2% (−0.35:0.15%) in the placebo group (P = .12). There was also a trend toward an increase in body weight (0.70 [−1.40:1.50]) vs −1.70 [−2.70:0.20] kg in the linagliptin and placebo groups, respectively; P = .06) and waist circumference (1.43 [−0.10:3.43] vs −0.35 [−2.23:1.67] cm in the linagliptin and placebo groups, respectively; P = .09).
Effect of linagliptin on mitochondrial function and muscle oxygenation
We did not observe an improvement in mitochondrial function with linagliptin treatment, as indicated by no change in the time to phosphocreatine recovery between the baseline visit and post-treatment visit following the graded exercise test. Similarly, linagliptin treatment was not associated with an improvement in muscle oxygenation recovery time (Table 2). Adjustments on body weight did not significantly change the results (Supplemental Table 1).
Table 2.
Effect of Linagliptin Compared with Placebo on Mitochondrial Function, Muscle Oxygenation, and Vascular Function
| Baseline |
P | Difference Between Week 12 and Baseline |
P | Mean Difference (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Linagliptin | Placebo | Linagliptin | Placebo | ||||
| PCr recovery time (s) | 46.4 (30.3:75.8) | 50.6 (26.9:102.2) | .63 | 0.0 (−23.3:15.3) | 1.01 (−36.8:14.0) | .66 | 20.0 (−10.1, 50.2) |
| Muscle oxygenation recovery time (s) | |||||||
| Medial gastrocnemius | 13.0 (6.6:19.0) | 11.6 (5.2:19.7) | 1 | 2.6 (−6.2:13.9) | 7.1 (−7.1:10.1) | .96 | 37.4 (−45.6, 120.6) |
| Lateral gastrocnemius | 13.7 (8.5:25.1) | 13.7 (9.2:22.8) | .98 | 2.8 (−6.6:11.3) | 2.2 (−9.1:8.1) | .51 | −0.74 (−20.3, 18.9) |
| Soleus | 8.8 (5.7:13.5) | 8.6 (7.6:11.4) | .92 | 0.3 (−3.6:3.9) | −1.6 (−3.9:2.2) | .78 | 0.13 (−4.3, 4.6) |
| Tibialis anterior | 9.1 (4.4:14.8) | 11.3 (7.3:16.5) | .62 | 2.9 (1.2:27.1) | 2.40 (−6.4:8.6) | .61 | 218.4 (−161.2, 597.5) |
| Peroneus Longus | 8.2 (3.8:14.5) | 8.2 (5.5:10.6) | .95 | 0.4 (−4.0:7.1) | −0.12 (−2.0:2.6) | 1 | −49.2 (−159.2, 60.7) |
| Microvascular function | |||||||
| Pre-Ach iontophoresis, LDPI, AU | 0.78 (0.65:0.97) | 0.73 (0.61:0.83) | .36 | 0.01 (−0.10:0.07) | −0.02 (−0.07:0.18) | .94 | 0.03 (−0.11, 0.18) |
| Post-Ach iontophoresis LDPI, AU | 0.88 (0.78:1.15) | 0.92 (0.85:1.00) | .98 | 0.06 (−0.05:0.22) | −0.03 (−0.11:0.11) | .07 | 0.09 (−0.01,0.20) |
| ACh LDPI, % change | 15.0 (6.0:38.5) | 24.0 (8.9:36.5) | .54 | 14.2 (−9.5:25.4) | −6.1 (−15.3:22.6) | .21 | 7.2 (−22.4, 36.8) |
| Pre-Ach iontophoresis, axon reflex, AU | 11.4 (9.4:24.5) | 12.7 (10.4: 25.3) | .54 | 0.1 (−5.9:13.0) | 0.9 (−17.3:6.2) | .1 | 7.0 (−15.0, 29.1) |
| Post-Ach iontophoresis, axon reflex, AU | 34.9 (13.0:55.5) | 50.6 (19.6:76.4) | .07 | 5.1 (−18.0:55.5) | −11.9 (−46.0:24.0) | .05 | 32.3 (−0.3, 65.0) |
| ACh axon reflex, % change | 98.1 (25.2:226.7) | 211.5 (56.8:289.8) | .31 | 48.5 (−47.8:125.7) | −7.4 (−259.2:153.2) | .52 | 21.8 (−156.4, 200.0) |
| Macrovascular function | |||||||
| Resting brachial artery diameter, mm | 4.49 ± 0.77 | 4.44 ± 0.77 | .81 | −0.10 (−0.37:0.17) | −0.08 (−0.19:0.11) | .65 | −0.04 (−0.23, 0.15) |
| FMD, % change | 6.51 ± 2.05 | 7.07 ± 1.20 | .29 | 0.67 (−0.58:1.58) | .36 (−0.38:1.21) | .24 | 0.52 (−0.37, 1.41) |
| NMD, % change | 14.59 ± 3.64 | 14.75 ± 3.80 | .89 | 0.27 (−0.78:1.62) | .11 (−0.91:1.21) | .53 | 0.31 (−0.68, 1.31) |
Abbreviations: % change, percentage change from pre-iontophoresis or from resting brachial artery diameter; CI, confidence interval; NMD, nitrate-mediated dilation.
Data expressed as median (25th:75th percentiles) or mean ± SD.
Mean differences between groups and their 95% CIs are provided as estimates of treatment effects.
Effect of linagliptin on macro- and microvascular function
Linagliptin treatment was associated with an increase in axon reflex–dependent vasodilation, a marker of neurovascular function (P = .05). A similar trend was observed toward increased endothelium-dependent microvascular reactivity after linagliptin treatment (Table 2). However, there were no significant differences in the change in markers of endothelium-independent microvascular function from the baseline visit to the post-treatment visit between the two groups. Linagliptin did not have a significant effect on FMD (Supplemental Figure 1; Table 2).
Post-hoc analyses were conducted to test whether these changes were related to glycemic control (expressed as change in HbA1c between week 12 and baseline). We observed no significant correlation between changes in HbA1c and changes in microvascular reactivity indices over the study period.
Effect on concentrations of inflammatory cytokines, growth factors and biomarkers of endothelial dysfunction
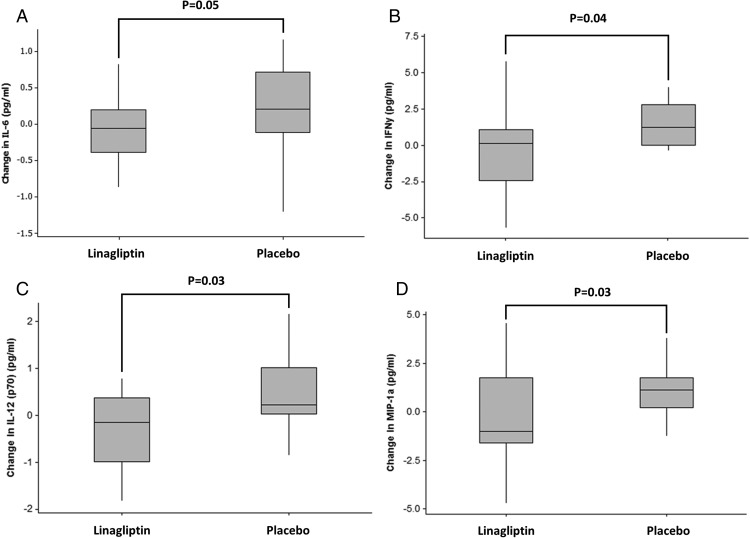
Assessment of plasma concentrations of inflammatory cytokines, growth factors, and biomarkers of endothelial function revealed a decrease in concentrations of interferon gamma (IFNγ) (P < .05), IL-12 (p70) (P = .03), and macrophage inflammatory protein-1a (MIP-1) (P < .04) following the 12-week linagliptin treatment period when compared with the placebo group (Figure 2). In addition, there were trends that suggested a decrease in fractalkine (P = .07) and ILIL-6 (P = .052) following linagliptin treatment. There were no other differences in inflammatory cytokines, growth factors, and biomarkers of endothelial function between the linagliptin and placebo groups (Supplemental Table 2).
Figure 2.
Changes in (A) IL-6, (B) IFNγ, (C) IL-12 (p70), and (D) MIP-1a following a 12-week treatment period with linagliptin compared with treatment with the placebo.
Change in substance P over the study period was not significantly different between the two groups in the total population (Supplemental Table 2). Given that ACE inhibition is known to inhibit degradation of substance P, subgroup post-hoc analyses was conducted. Significantly higher Substance P concentration was found after 12 weeks in patients receiving ACE inhibitors (Supplemental Table 3). Considering the imbalance in treatment with ACE inhibitors between the linagliptin and the placebo groups, we further tested the effects of ACE inhibitor therapy within each group. In the subgroup of patients without ACE inhibitors, we observed a significantly higher serum concentration of Substance P in patients treated with linagliptin compared with placebo (Supplemental Table 4).
There was a strong correlation between change in HbA1c and change in IFNγ (ρ = 0.55; P = .002), IL-12 (ρ = 0.66; P < .001), and MIP-1a (ρ = 0.67; P < .001). In multivariate analyses, changes in HbA1c and the group were not independent predictors of change in the concentration of each of those cytokines.
Effect of linagliptin on circulating endothelial progenitor cell phenotypes
Linagliptin treatment was not associated with any significant change in the concentrations of circulating EPC phenotypes. Details about the concentrations of the 20 phenotypes that were assessed at baseline and after 12 weeks are provided as Supplemental Table 5.
Adverse events
There were no major differences in the serious adverse events and the nonserious adverse events between the two groups. None of these adverse events was judged to be likely related with linagliptin.
Discussion
We conducted a 12-week, randomized, placebo-controlled, parallel trial to explore the peripheral vascular effects of linagliptin in patients with type 2 diabetes. Our results show that linagliptin decreased markers of inflammation, and that such effect is related to improved glycemic control. In contrast, treatment with linaglitpin tended to improve microvascular neurovascular, and endothelium-dependent function independently of glycemic control. However, this did not translate into increased mitochondrial function or muscle oxygenation. Yet, indices of endothelial function in the microcirculation (vascular responses to iontophoresis of acetylcholine [ACh]) and in the large vessels (FMD of the brachial artery) significantly correlated with the time to phosphocreatine recovery, an index of mitochondrial function (ρ = −0.41, P = .01; and ρ = −0.40, P = .01, respectively). However, we hypothesize that the effect was too moderate to observe any change in mitochondrial function. Indeed, the small sample size only allowed to detect a large effect size for the primary outcome (>0.9), which is a limitation of our study. Similarly, we did not see any effect of linagliptin on macrovascular reactivity or on circulating EPC phenotypes.
The effects of DPP-4 inhibitors on the microcirculation have been explored in vitro and in animal models, but data in humans remain relatively scarce. Consistent with our findings, a recent randomized controlled study showed improved microvascular retinal blood flow assessed with laser Doppler flowmetry after 12 weeks of treatment with linagliptin in nondiabetic patients with arterial hypertension (27). A similar effect on retinal blood flow was observed after a 6-week treatment with another DDP-4 inhibitor, saxagliptin (28). However, to our knowledge, our study is the first to investigate skin microvascular reactivity, an index of systemic microvascular function, following DPP-4 inhibitor treatment. The mechanism through which DPP-4 inhibitors improve peripheral microvascular perfusion has not been fully elucidated yet. In vitro, activation of the GLP-1 receptor in endothelial cells increases endothelial Nitric Oxide Synthase phosphorylation and nitric oxide production (29), which is consistent with the trend toward improved vascular reactivity to iontophoresis of acetylcholine that was observed in the linagliptin group, as compared with the placebo group. Endothelium-dependent reactivity was also negatively correlated with markers of inflammation, which were found to be decreased in the linagliptin group. The reduction in inflammatory biomarkers that was observed with linagliptin treatment in the present study is consistent with preclinical data and with previous findings in humans (30).
Other vascular effects of DPP-4 inhibitors may involve GLP-1-independent pathways. Indeed, DPP-4 has been localized to endothelial cells in different tissues and its substrates include a variety of peptides, some of which are vasodilators in the microcirculation. For example, Substance P is involved in cutaneous axon reflex vasodilation (23), which was enhanced by linagliptin in the present study. This is consistent with the trend toward increased circulating Substance P after treatment with linagliptin.
Another peptide cleaved by DPP-4 is SDF-1, which plays a role in angiogenesis by promoting migration and homing of EPCs in response to hypoxia (29). In patients treated with linagliptin, we did not see any significant change neither in circulating SDF-1 concentration, nor in any EPC phenotype. A recent randomized, crossover study that involved a much shorter (4-d) treatment period, demonstrated that linagliptin significantly increases CD34+CD133+ and CD34+KDR+ EPCs, concomitantly with SDF-1 (30). This discrepancy might be explained by a transient effect of linagliptin, which raises the question of the clinical significance of such a transient increase on cardiovascular outcomes. Indeed, higher levels of circulating EPCs are associated with better cardiovascular outcomes (31, 32), but the long-term effect of a transient increase in EPCs on cardiovascular events is uncertain. The discrepancy between these findings could also be due to chance, considering the relatively small sample size of both studies.
In the present study, 12 weeks of treatment with linagliptin did not significantly improve large vessel endothelial function, assessed with FMD of the brachial artery, when compared with placebo treatment. Previous studies have reported conflicting results. In a series of randomized, 6-week crossover studies with sitagliptin and alogliptin, DPP-4 inhibition was associated with decreased FMD in patients with type 2 diabetes (11). In contrast, two other 12-week trials have shown a beneficial effect of sitagliptin on FMD (12, 14), further confirmed by a single-arm, observational study conducted over 52 weeks (13). These discrepancies could be explained by suboptimal study designs (none of these studies were randomly assigned and double-blind), and by the fact that despite efforts to optimize and standardize the test (25, 33, 34), FMD still suffers from a lack of homogeneity and high interoperator variability.
Besides the limitation of the small sample size discussed above, the multiplicity of comparisons is another issue that may inflate the risk of type 1 error. Yet, the significant differences we found between the two groups are consistent with preclinical data, and strongly correlate with each other. Therefore, it seems unlikely that these differences are only due to chance.
Another limitation of our study was that despite randomization there was an imbalance between the two groups regarding the use of ACE inhibitors that influences the results, especially those regarding Substance P concentration. Indeed, Substance P is a substrate of both DPP-4 and ACE, and concomitant use of DDP-4 inhibitors and ACE inhibitors has been shown to have an influence on circulating Substance P concentration (35). Therefore, post-hoc analyses were conducted to test the influence of ACE inhibitor therapy. Interestingly, in patients who were not taking ACE inhibitors, linagliptin was associated with a significant increase in serum Substance P concentration. Although the interaction was not significant, possibly because of a lack of power, these findings suggest that the effect of linagliptin treatment was masked in patients receiving ACE inhibitors. The fact that almost half of the patients included in the study were receiving ACE inhibitors may explain why no significant differences were initially observed. Excluding patients with ACE inhibitors was not planned prospectively, given that this interaction was not known to be clinically meaningful at the time the study started. In addition, excluding such participants would have considerably affected recruitment.
In conclusion, our study suggests that treatment with linagliptin decreases markers of inflammation, and tends to improve endothelial and neurovascular microvascular function in patients with type 2 diabetes. However, there was no effect of linagliptin treatment on mitochondrial function, macrovascular function and EPCs. Larger trials are needed to examine with greater precision these findings in patients at high risk for cardiovascular disease.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This study was registered in ClinicalTrials.gov as trial number NCT01969084.
This was an Investigator Initiated Research study that was supported by a research grant from Boehringer Ingelheim Pharmaceuticals Inc (IIS2012–10164) to A.V. The project was also supported by the Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources (National Institutes of Health).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ach
- acetylcholine
- BIDMC
- Beth Israel Deaconess Medical Center
- BOLD
- blood oxygenation level-dependent
- BP
- blood pressure
- DPP-4
- dipeptidyl peptidase-4
- EPC
- endothelial progenitor cell
- FMD
- flow-mediated dilation
- IFNγ
- interferon gamma
- GLP-1
- glucagon-like peptide 1
- HbA1c
- glycated hemoglobin A1c
- KDR
- kinase insert domain receptor
- LDF
- laser Doppler flowmetry
- LDPI
- laser Doppler perfusion imaging
- MIP
- macrophage inflammatory protein-1a
- MRI
- magnetic resonance imaging
- PCr
- phosphorous and phosphocreatine
- SDF
- Stromal Cell-Derived factor 1.
References
- 1. American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2016;39.26223240 [Google Scholar]
- 2. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. [DOI] [PubMed] [Google Scholar]
- 3. Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. [DOI] [PubMed] [Google Scholar]
- 4. Zhong J, Maiseyeu A, Davis SN, Rajagopalan S. DPP4 in cardiometabolic disease recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015;116:1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–276. [DOI] [PubMed] [Google Scholar]
- 6. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 7. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 8. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 9. Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. The Lancet. 2015;385:2067–2076. [DOI] [PubMed] [Google Scholar]
- 11. Ayaori M, Iwakami N, Uto-Kondo H, et al. Dipeptidyl Peptidase-4 Inhibitors Attenuate Endothelial Function as Evaluated by Flow-Mediated Vasodilatation in Type 2 Diabetic Patients. J Am Heart Assoc. 2013;2:e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kubota Y, Miyamoto M, Takagi G, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J Korean Med Sci. 2012;27:1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung M, Leung DY, Wong VW. Effects of dipeptidyl peptidase-4 inhibitors on cardiac and endothelial function in type 2 diabetes mellitus: A pilot study. Diab Vasc Dis Res. 2016;13:236–243. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noda Y, Miyoshi T, Oe H, et al. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non- diabetic subjects: A preliminary report. Cardiovasc Diabetol. 2013;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kroller-Schon S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl peptidase-4 inhibition. Cardiovasc Res. 2012;96:140–149. [DOI] [PubMed] [Google Scholar]
- 17. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. [DOI] [PubMed] [Google Scholar]
- 19. Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tecilazich F, Dinh T, Lyons TE, et al. Postexercise phosphocreatine recovery, an index of mitochondrial oxidative phosphorylation, is reduced in diabetic patients with lower extremity complications. J Vasc Surg. 2013;57:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–S20. [DOI] [PubMed] [Google Scholar]
- 22. Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34:373–384. [DOI] [PubMed] [Google Scholar]
- 24. Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 26. Tecilazich F, Dinh T, Pradhan-Nabzdyk L, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8:e83314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forst T, Michelson G, Diessel S, Jahnke J, Kapitza C. Microvascular effects of the inhibition of dipeptidylpeptidase IV by linagliptin in nondiabetic hypertensive patients. J Hypertens. 2016;34:345–350. [DOI] [PubMed] [Google Scholar]
- 28. Ott C, Raff U, Schmidt S, et al. Effects of saxagliptin on early microvascular changes in patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fadini GP, Bonora BM, Cappellari R, et al. Acute effects of linagliptin on progenitor cells, monocyte phenotypes, and soluble mediators in type 2 diabetes. J Clin Endocrinol Metab. 2016;101:748–756. [DOI] [PubMed] [Google Scholar]
- 31. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- 32. Rigato M, Avogaro A, Fadini GP. Levels of circulating progenitor cells, cardiovascular outcomes and death: A meta-analysis of prospective observational studies. Circ Res. 2016;118:1930–1939. [DOI] [PubMed] [Google Scholar]
- 33. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Devin JK, Pretorius M, Nian H, Yu C, Billings FT, 4th, Brown NJ. Substance P increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. Hypertension. 2014;63:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]