Abstract
Context:
The aldosterone to renin ratio (ARR) is recommended to screen for primary aldosteronism (PA).
Objective:
To evaluate whether dietary sodium restriction results in misinterpretation of PA screening.
Participants:
Untreated hypertensives with ARR more than 20 on a high dietary sodium intake (HS) were also evaluated on a low dietary sodium intake (LS) (n = 241). Positive screening for PA was defined as: plasma renin activity (PRA) less than or equal to 1.0 ng/mL · h with serum aldosterone more than or equal to 6 ng/dL. PA was confirmed by a 24-hour urinary aldosterone excretion more than or equal to 12 mcg with urinary sodium more than 200 mmol.
Results:
Only 33% (79/241) of participants with an ARR more than 20 had a positive PA screen on HS. On LS, 56% (44/79) of these participants no longer met criteria for positive PA screening. When compared with participants with positive PA screening on both diets, participants with a positive screen on HS but negative on LS exhibited a significantly higher PRA on both diets. Remarkably, of the 48/79 participants who had PA confirmed, 52% had negative PA screening on LS. The distinguishing feature of these participants with “discordant” screening results was a larger rise in PRA on LS resulting in normalization of the ARR and higher Caucasian race prevalence.
Conclusions:
Sodium restriction is recommended in hypertension; however, it can significantly raise PRA, normalize the ARR, and result in false interpretation of PA screening. Milder phenotypes of PA, where PRA is not as suppressed, are most susceptible to dietary sodium influences on renin and ARR. Optimal screening for PA should occur under conditions of HS.
Dietary sodium restriction can significantly raise renin, normalize the ARR, and result in false-negative interpretation of mild cases of primary aldosteronism.
Primary aldosteronism (PA) is the most common cause of secondary hypertension. The prevalence of PA is reported to be 3%–4% in the primary care setting, approximately 10% in tertiary care centers, and up to 20% in populations with resistant hypertension (1–3). Excessive activation of the renal and extrarenal mineralocorticoid receptor is associated with a higher incidence of vascular dysfunction, myocardial dysfunction, metabolic syndrome, and cardiovascular mortality (4, 5). The Endocrine Society recommends broad screening for PA in populations with resistant hypertension, hypertension, and hypokalemia, incidentally discovered adrenal masses, and with suggestive family histories (6).
The most recommended tool for PA screening is the aldosterone to renin ratio (ARR), which is used to identify angiotensin II-independent aldosteronism expected in PA (a high ARR in the context of a suppressed renin). The ARR is influenced by specific antihypertensive medications, serum potassium levels, the phase of the menstrual cycle, and posture (6–8). However, dietary sodium intake and total body sodium balance are also crucial and underrated modulators of the ARR. Although guidelines from The Endocrine Society suggest that testing of the ARR should occur under conditions of “unrestricted dietary salt intake” (6), specific recommendations for assessing dietary sodium intake and sodium balance, and the consequences of variable dietary sodium intake on the ARR, are not available. This may be particularly relevant for milder forms of PA where renin and ARR values may be more susceptible to modulations by dietary sodium restriction. Diagnosing PA early, when the disease is mild or “subclinical,” is of public health importance to prevent comorbidities associated with aldosterone excess. Therefore, improving our understanding of how dietary sodium intake influences the ARR in PA may improve our ability to accurately detect even mild-to-moderate cases of PA (9–11).
We hypothesized that dietary sodium restriction could result in sufficient modification of the ARR, particularly by influencing renin, such that false-negative ARR screening could occur even in bone fide cases of PA. Here, we present investigations where a dietary sodium restriction intervention was used to assess the variability of PA screening and how this may influence the interpretation of confirmed cases of PA.
Materials and Methods
Overall study population and protocol
We retrospectively identified hypertensive participants who might have PA from the HyperPATH cohort (11, 12). Each participant in the HyperPATH cohort underwent detailed physiologic evaluation of the renin-angiotensin-aldosterone system under conditions of controlled posture (participants were studied after overnight supine rest in a supervised Clinical Research Center), medications (participants were studied after stopping all antihypertensive medications), and diurnal variation (participants were all studied in the morning at 8 am). Participants who used antihypertensive medications underwent a washout before assessment: angiotensin converting enzyme inhibitors and angiotensin receptor blockers were stopped for 3 months, adrenergic receptor antagonists, calcium-channel blockers, and diuretics were stopped for 1 month, as previously described (11). All study assessments were undertaken after completion of 2 dietary interventions. Participants were first administered a high dietary sodium intake (HS) for 1 week, and adherence to this diet was confirmed with a 24-hour urinary sodium excretion of more than 150 mmol. Subsequently, all participants were administered a low dietary sodium intake (LS) for 1 week, and adherence to this diet was confirmed with a 24-hour urinary sodium excretion of less than 50 mmol. Study procedures included measurement of morning plasma renin activity (PRA) and serum aldosterone, and serum and urinary sodium and potassium, as we have described before (13). All participants provided informed consent and our protocol was approved and monitored by our institutional human research committee. Although previous analyses and results from the HyperPATH cohort have been reported, the current study is independent and unique from previous studies.
For the current analysis, we created a retrospective cohort of HyperPATH participants who had hypertension but no known diagnosis of PA or hypokalemia. Hypertension was defined as a seated systolic blood pressure (SBP) more than 140 mm Hg in the absence of any antihypertensive medications, or a SBP of more than or equal to 130 mm Hg while taking only 1 or more antihypertensive medications on the initial recruitment visit before medication washout. We included only participants with an estimated glomerular filtration rate of more than 60 mL/min 1.73 m2. Further, we selected only hypertensive participants who had the potential to have a positive screen for PA, defined as an ARR (serum aldosterone in ng/dL divided by PRA in ng/mL · h) of more than 20 while on HS. We used this ARR cutoff because it is highly sensitive and therefore ensured that we would not miss potential cases of PA among our population of mild hypertensives on a high dietary sodium balance (6). The number of participants who met the aforementioned criteria and were included in our study population was 241.
Assessment of positive screening for PA
We first analyzed our study population of 241 hypertensives with an ARR more than 20 on HS for those who had a “positive screen for PA” and those who had a likely “negative screen for PA.” Participants with a positive screen for PA were defined as those who had a HS ARR more than 20(ng/dL of serum aldosterone divided ng/mL/h of PRA) and a low or suppressed HS PRA less than or equal to 1.0 ng/mL · h and a HS serum aldosterone more than or equal to 6.0 ng/dL; these thresholds have been previously suggested to be the most sensitive in detecting cases of PA and excluding cases of secondary hyperaldosteronism (6–8, 13, 14). Participants with a negative screen for PA were defined as those who had a HS ARR more than 20 but without evidence for angiotensin II-independent aldosteronism: a nonsuppressed HS PRA more than 1.0 ng/mL · h and/or a HS serum aldosterone less than 6.0 ng/dL. Participants with a likely negative screen for PA were excluded from subsequent analyses.
Assessment of concordance of PA screening with dietary sodium interventions
We next evaluated whether the results of PA screening differed between the dietary interventions. We evaluated how many participants had a “concordant screen” for PA, as defined by a positive screen for PA on both HS and LS, and also how many participants had a “discordant screen” for PA, as defined by a positive screen for PA on HS but a negative PA screen on LS.
Confirmation of PA and the ARR
We evaluated how many participants with a positive screen for PA on HS were confirmed to have PA. All participants performed a 24-hour urinary collection on HS, which was used to measure the aldosterone excretion rate (AER) as well as sodium and creatinine. PA was “confirmed” in participants who had a 24-hour AER more than or equal to 12 mcg with a urinary sodium of more than or equal to 200 mmol (6, 15). In a small number of participants (n = 8) who had a urinary sodium more than or equal to 200 mmol, the urinary AER was not available; however, all 8 of these participants demonstrated a very high ARR on HS (>50) in combination with a very low PRA (<0.2 ng/ml/h) and a high serum aldosterone on HS (>15 ng/dL), and were therefore also confirmed to have PA (16). In contrast, PA was “not confirmed” when the 24-hour AER was less than 12 mcg in the setting of a urinary sodium of more than or equal to 200 mmol.
Correlation between confirmed PA status and screening concordance with dietary sodium modulation
Lastly, we assessed the correlation between the likelihood that a participant would have concordance in their screening parameters between dietary sodium interventions with the likelihood that a participant had confirmation of PA.
Measurements
PRA was measured via the Diasorin, Inc RIA, which has a dynamic range of 0.1–50 ng/mL · h. The intraassay variation is 4.6%–10% and the interassay variation is 5.6%–7.6%. Serum aldosterone was measured using the Siemens radioimmunometric assay, which has a dynamic range of 2.5–120 ng/dL. The intraassay variation is 2.5%–5.4% and the interassay variation is 3.8%–15.7%. Both aldosterone and PRA where measured in duplicate on HS and LS and averaged for our analyses. BP was measured by registering 3 consecutive readings at 2-minute intervals on both diets (Dinamap; Critikon).
Statistical analysis
Data are presented as means with SDs for continuous variables and percentages of the total sample for categorical variables. Comparisons of means and frequencies were performed using the Student's t test and χ2 testing, respectively. Normality was assessed using the Kolmogorov-Smirnov test. In the situation where a variable was not normally distributed (eg, PRA), a bootstrapping procedure with 1000 iterations was performed. Logistic regression was used to evaluate independent predictors of concordance in PA screening between diet interventions, where the odds ratio (OR) and 95% confidence intervals were used to represent the association. Goodness of fit was determined by the Hosmer-Lemeshow test and a priori selected interactions were examined. A 2-sided P < .05 was considered statistically significant. All analyses were performed using SPSS 21 and STATA 13 statistical packages.
To supplement our primary comparisons, we conducted sensitivity analyses using stricter criteria to increase confidence in the likelihood that a positive screen for PA was “truly positive” by restricting positive screens to only those with an ARR more than 30 and PRA less than 1.0 ng/mL · h and serum aldosterone more than 9 ng/dL (6). We also conducted sensitivity analyses by restricting confirmed PA cases to only those with an available urinary AER demonstrating 24-hour AER more than or equal to 12 mcg in the setting of urinary sodium more than or equal to 200 mmol.
Results
Assessment of positive screening for PA
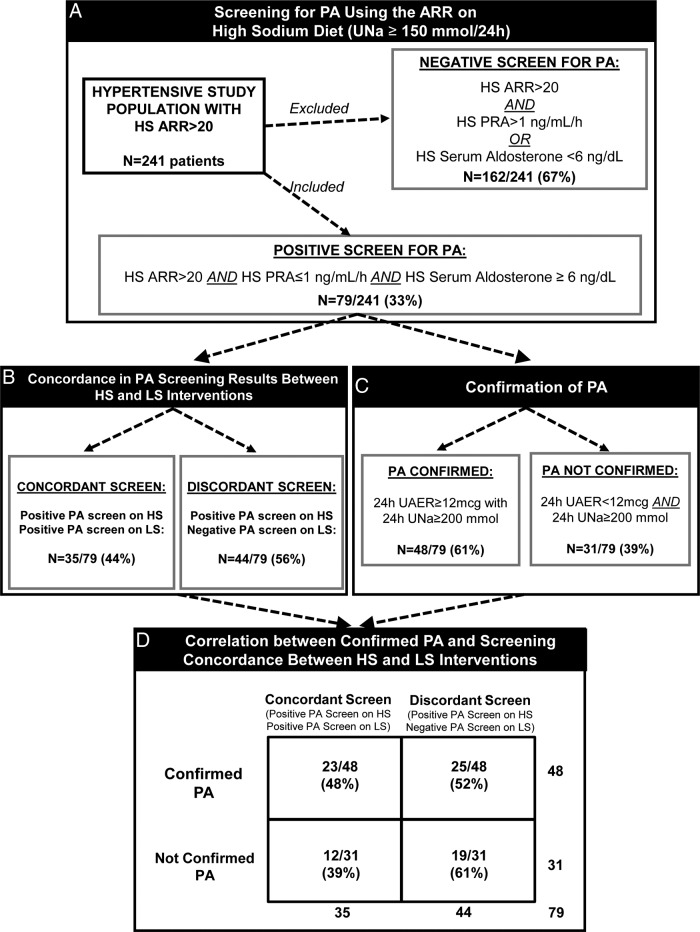
Of the initial 241 hypertensive study participants, only 33% (79/241) had a positive screen for PA on HS despite the fact that they all had ARR more than 20 (Figure 1A). The remaining 67% (162/241) were deemed to have a likely negative screen for PA and were excluded from further analyses. The most common pattern among participants with a negative screen for PA was a very low serum aldosterone (<5 ng/dL) in the context of a highly suppressed PRA that resulted in an ARR more than 20.
Figure 1.
An illustrated depiction of participants with likely PA that were selected for the current study (A), the concordance in PA screening results on HS and LS (B), the number of participants with confirmed PA (C), and the correlation between confirmed PA cases and PA screening results with dietary sodium modulation (D). UNa, urinary sodium excretion (mmol/d); UAER, urinary aldosterone excretion.
Assessment of concordance of PA screening with dietary sodium intake interventions
When the 79 participants with a positive screen for PA on HS underwent the LS intervention, 44% continued to demonstrate a positive screen for PA (concordant screen) (Figure 1B). In contrast, the other 56% of participants who had a positive screen on HS no longer met criteria for a positive screen after 1 week of LS (discordant screen) (Figure 1B). The characteristics of participants with concordant and discordant screening for PA are outlined in Table 1 and suggest that those with concordant PA screening had a more severe phenotype of PA, as evidenced by higher SBP, lower serum potassium, and higher ARR and lower PRA values on HS diet. The main determinant of a discordant screen was the modulation of PRA, independent of serum aldosterone, by the LS intervention (Table 1). Participants with discordant PA screening exhibited multifold elevations of PRA when on LS that normalized the ARR, whereas participants with concordant PA screening maintained a suppressed PRA and elevated ARR on both dietary conditions. Serum and urinary aldosterone measures were not different between participants with concordant vs discordant screening results.
Table 1.
Characteristics of Participants With Concordant vs Discordant Screening for PA
| Discordant Screen (Positive PA Screen on HS; Negative PA Screen on LS); 44/79 (56%) | Concordant Screen (Positive PA Screen on HS; Positive PA Screen on LS); 35/79 (44%) | P Value | |
|---|---|---|---|
| Age (y) | 49.4 ± 8.1 | 49.6 ± 6.7 | .920 |
| Caucasian race (%) | 90 | 62 | .002 |
| BMI (kg/m2) | 28.4 ± 3.9 | 28.7 ± 3.5 | .762 |
| Gender (% male) | 64 | 66 | .850 |
| SBP on HS (mm Hg) | 145.8 ± 21.6 | 157.2 ± 23.2 | .030 |
| SBP on LS (mm Hg) | 127.2 ± 16.3 | 140.2 ± 21.0 | .005 |
| ARR on HS | 54.5 ± 49.2 | 77.2 ± 36.8 | .008a |
| ARR on LS | 12.6 ± 5.7 | 54.3 ± 38.2 | <.001a |
| HS plasma potassium (mmol/L) | 4.11 ± 0.3 | 3.96 ± 0.3 | .039 |
| LS plasma potassium (mmol/L) | 4.18 ± 0.3 | 4.20 ± 0.2 | .955 |
| HS aldosterone (ng/dL) | 11.1 ± 5.2 | 10.3 ± 4.1 | .755 |
| LS aldosterone (ng/dL) | 19.6 ± 12.3 | 17.0 ± 8.5 | .567 |
| HS PRA (ng/mL · h) | 0.25 ± 0.1 | 0.16 ± 0.1 | .001a |
| LS PRA (ng/mL · h) | 2.03 ± 1.9 | 0.44 ± 0.3 | <.001a |
| HS urinary sodium (24 h mmol) | 237.2 ± 61.3 | 242.3 ± 58.5 | .708 |
| LS urinary sodium (24 h mmol) | 14.2 ± 9.9 | 16.7 ± 7.4 | .246 |
| HS urinary aldosterone (μg/24 h) | 14.7 ± 7.0 | 14.9 ± 6.6 | .906 |
| LS urinary aldosterone (μg/24 h) | 38.6 ± 22.4 | 24.4 ± 10.9 | .001 |
| Aldosterone fold changes (HS to LS) (magnitude and %) | 1.92 ± 1.0 (92%) | 1.77 ± 0.9 (77%) | .508 |
| PRA fold changes (HS to LS) (magnitude and %) | 10.9 ± 11.8 (+909%) | 3.5 ± 4.9 (+250%) | .006a |
Data as mean ± SD, except as noted. Positive screening: ARR > 20, aldosterone > 6 ng/dL, PRA < 1 ng/mL · h.
Boostrapped.
There was an inverse relationship between PRA and 24-hour urinary sodium balance on LS (r = −0.263, P < .001), suggesting that despite the narrow distribution of restricted sodium balance, greater sodium restriction predicted a higher PRA. Further, there was no difference in the degree of the inverse relation between PRA and urinary sodium balance on LS between the discordant and concordant groups (P-interaction = 0.30); however, PRA values were higher across the entire spectrum sodium balance in the discordant group, suggesting that this group may have a milder form of PA or less mineralocorticoid receptor activation when compared with the concordant group.
Another notable determinant of PA screening concordance was race. Discordant PA screening was strongly associated with a higher prevalence of Caucasian race (90% vs 62%; P = .002), whereas other notable demographic factors such as age, gender, and body mass index (BMI) were comparable between groups.
In sensitivity analyses where we implemented even stricter criteria for a positive PA screen (ARR > 30 with a PRA < 1.0 ng/mL · h and a serum aldosterone ≥ 9 ng/dL) (6), we observed a recapitulation of trends suggesting that discordant PA screening was associated with a potentially milder form of PA where PRA was more likely to rise on LS and result in normalization of the ARR (Supplemental Table 1).
Adjusted logistic regression analyses
We performed logistic regression analyses adjusted for race, HS PRA, HS BP, and HS potassium to evaluate the independent determinants of PA screening concordance. The adjusted odds for discordant PA screening on LS remained significant for Caucasian race (OR = 8.3 [95% confidence intervals 2.1–32.2]) and PRA more than 0.3 ng/mL · h on HS (OR = 3.7 [1.1–12.5]).
Correlation between PA confirmation status and PA screening concordance
Among the 79 participants with a positive screen for PA on HS, 61% (48/79) were confirmed to have PA (Figure 1C). We therefore evaluated the likelihood of having concordant vs discordant PA screening with dietary interventions among those with confirmed PA. As depicted in Figure 1D, although 48% of participants with confirmed PA had a positive screen on both dietary conditions, a remarkable 52% of participants with confirmed PA had a negative screen for PA with the LS intervention. Once again, the main determinants for having a negative PA screen despite having a confirmed diagnosis of PA appeared to be Caucasian race and a “milder” phenotype of PA whereby PRA was not as suppressed and more likely to rise with LS intervention thereby resulting in normalization of the ARR (Table 2).
Table 2.
Characteristics of Participants With Confirmed PA Based on Whether They Had Concordant or Discordant Screening for PA
| Confirmed PA and Discordant Screen 25/48 (52%) | Confirmed PA and Concordant Screen 23/48 (48%) | P Value | |
|---|---|---|---|
| Age (y) | 47.2 ± 6.9 | 49.7 ± 7.7 | .257 |
| Caucasians (%) | 96 | 65 | .006 |
| BMI (kg/m2) | 28.7 ± 3.4 | 28.5 ± 3.8 | .820 |
| SBP on HS (mm Hg) | 146.4 ± 23.5 | 162.9 ± 23.2 | .022 |
| SBP on LS (mm Hg) | 126.1 ± 17.6 | 146.1 ± 20.8 | .001 |
| ARR on HS | 63.2 ± 52.8 | 75.5 ± 38.8 | .447a |
| ARR on LS | 12.5 ± 6.2 | 54.3 ± 39.2 | .01a |
| HS potassium (mmol/L) | 4.05 ± 0.2 | 3.97 ± 0.3 | .367 |
| LS potassium (mmol/L) | 4.18 ± 0.3 | 4.26 ± 0.1 | .385 |
| HS aldosterone (ng/dL) | 13.1 ± 5.9 | 10.9 ± 4.3 | .136a |
| LS aldosterone (ng/dL) | 23.1 ± 13.7 | 19.0 ± 8.8 | .232a |
| HS PRA (ng/mL · h) | 0.27 ± 0.1 | 0.19 ± 0.1 | .058a |
| LS PRA (ng/mL · h) | 2.50 ± 2.4 | 0.50 ± 0.3 | .021a |
| HS urinary Na (24 h mmol) | 227.9 ± 37.8 | 248.9 ± 57.0 | .146 |
| LS urinary Na (24 h mmol) | 13.2 ± 9.7 | 15.0 ± 5.2 | .632 |
| HS urinary aldosterone (μg/24 h) | 26.5 ± 5.1 | 18.7 ± 4.7 | .265 |
| LS urinary aldosterone (μg/24 h) | 44.05 ± 23.9 | 27.0 ± 12.1 | .005 |
| Aldosterone fold changes (HS to LS) (magnitude and %) | 1.99 ± 1.1 (99%) | 1.85 ± 0.9 (85%) | .636a |
| PRA fold changes (HS to LS) (magnitude and %) | 13.2 ± 14.1 (+1220%) | 3.07 ± 1.9 (+207%) | .011a |
Data reported as mean ± SD, except as noted. Confirmed PA, urinary aldosterone > 12 μg/24 on HS.
Boostrapped.
Sensitivity analyses using stricter screening and confirmation criteria demonstrated a recapitulation of trends suggesting that misinterpretation of PA screening on LS was associated with Caucasian race and a milder phenotype of PA (Supplemental Table 2).
Discussion
PA is relatively common within the hypertensive population and is a treatable form of mineralocorticoid receptor overactivation that can result in cardiovascular and metabolic diseases and death. Therefore, the current consensus guidelines recommend broad screening for PA to identify those patients who might have a form of angiotensin II-independent aldosteronism, as evidenced by a suppressed renin and relatively high serum aldosterone. Although seemingly simple in premise, measuring and interpreting the ARR can be challenging given the multiple factors that can modulate renin and aldosterone levels, including dietary sodium balance. In the present study, we hypothesized that measurement of the ARR under conditions of dietary sodium restriction could alter the ARR sufficiently as to result in misinterpretation of confirmed cases of PA. We observed that approximately half of all cases of confirmed PA could have been misinterpreted as having a negative screen for PA when the ARR was performed on a restricted dietary sodium intake. Importantly, our data suggest that individuals with a milder phenotype of PA were most susceptible to false misinterpretation of PA screening due to restricted dietary sodium intake, as evidenced by their lower BP, higher potassium and PRA, and higher likelihood of increasing PRA with sodium restriction. This observation has important implications for widespread clinical practice because hypertensive patients are routinely advised to restrict their intake of dietary sodium (17); failure to recognize milder forms of PA earlier in the course of the disease could expose a large proportion of these patients to preventable and unnecessary cardiovascular and metabolic risk.
It is known that dietary sodium intake can modify the renin-angiotensin-aldosterone system (18), and therefore the current The Endocrine Society guidelines (6) do recommend that screening for PA should be conducted under liberalized dietary salt intake; however, specific guidelines on how much dietary sodium intake to recommend and whether it is important to confirm a high dietary sodium balance are lacking. Further, there is scarce information regarding the effects of intraindividual variability in dietary sodium intake in PA and how it might influence renin, aldosterone, and the ARR. For example, in a carefully designed protocol, Schwartz and Turner (19) showed that oral sodium loading to induce a high sodium balance did not adversely affect the interpretation of the ARR in detecting PA cases when compared with measuring the ARR on ad libitum dietary sodium intake; however, this study did not assess the effect of dietary sodium restriction on the ARR in the same subjects. In this regard, our data suggest that encouraging and verifying a sufficiently high sodium balance when measuring the ARR for PA screening is important to minimize false-negative case detection of PA. From a public health standpoint, the global prevalence of individuals with hypertension and potential PA attempting to adhere to a low sodium diet because of its cardioprotective effects is high (20). For example, the American Heart Association recommends a restricted dietary sodium intake of less than 1500 mg (<65 mmol/d) in hypertension (21). Although the low dietary sodium intervention used in our study (<50 mmol/d) was lower than this recommendation, it is easy to imagine how adherence to the American Heart Association's recommended sodium restriction could result in sufficient increases in renin and lowering of the ARR into the “normal” range. Verification of a high sodium balance at the time of PA screening with an ARR using either a spot or 24-hour urine sodium assessment is worth considering and should be assessed in future studies. Although a spot sodium-to-creatinine assessment is not as accurate as measuring the 24-hour sodium excretion, it is far more convenient and cost-effective and has been shown to correlate strongly with the 24-hour assessment (22).
Recent evidence suggests that recognizing mild forms of PA, that may be subclinical with nonsevere hypertension and without hypokalemia, could be of critical importance considering the long-term risks associated with this condition. Among patients who do not have “overt PA,” greater degrees of nonsuppressible aldosterone associate with cardiovascular and metabolic risk factors, impaired renal hemodynamics, and increased risk for future cardiovascular events (11, 23). In 2004, Vasan et al (24) reported that even among normotensive participants in the Framingham Heart Study who did not have known PA, higher plasma aldosterone levels associated with a higher risk of incident hypertension over 4 years of follow-up. Subsequently in 2007 (25), the same group of investigators showed that normotensives with low renin levels demonstrated a higher risk of developing hypertension when compared with those without low renin, even when serum aldosterone levels were far lower than what we currently consider to be consistent with overt PA, thereby adding further support for a potential state of subclinical or mild PA that may warrant early recognition to implement preventative care. In this regard, PA may exist in a continuum between a preclinical state, normotensive or mildly hypertensive individuals with subtle angiotensin II-independent aldosteronism, which may or may not advance to a more overt and classical presentation of PA as we traditionally recognize it, severe or resistant hypertension with or without hypokalemia (26). We now understand a potential basic and cellular corollary to these epidemiologic and clinical observations. Nishimoto et al have elegantly shown that a substantial proportion of morphologically normal adrenal glands from patients without known PA contain abnormal clusters of CYP11B2 (cytochrome P450 family 11 subfamily B member 2, or aldosterone synthase) expression (termed aldosterone-producing cell clusters) that harbor somatic mutations in channels known to cause aldosterone hypersecretion (27, 28). Further, the heterogeneous acquisition of somatic mutations resulting in increased aldosterone synthesis appears to be much more common than we previously realized (29). Although much more research is needed to understand the true prevalence and clinical significance of aldosterone-producing cell clusters, they provide a tantalizing cellular explanation for nonsuppressible aldosterone levels in participants with cardiometabolic risk factors and the concept of subclinical or mild PA (9).
Our results also suggested that Caucasians were more susceptible to increases in renin with dietary sodium restriction and therefore had a higher risk of “false negative” results than non-Caucasians (which were predominantly of African descent in our study). Although the distribution of race in our study was too narrow to make broad conclusions, one potential interpretation is that non-Caucasians tended to exhibit a more severe PA phenotype, whereas Caucasians tended to have a greater proportion of milder PA phenotypes. The differences we observed in race-specific renin modulation by sodium restriction could be related to dietary/environmental and/or genetic disparities that warrant further study.
Our study must be interpreted in the context of its design and associated strengths and limitations. An important strength was that all study participants were evaluated in a Clinical Research Center after strict control of potential confounders of the renin-angiotensin-aldosterone system, including medications, posture, diurnal variation, and dietary sodium and potassium. A notable limitation that warrants commentary is that all of our PRA and serum aldosterone measurements were done while participants were supine. Because clinical guidelines and recommendations are calibrated for ARR and PRA interpretations in the seated position, our results cannot be directly applied to clinical practice but should instead serve as a physiologic demonstration of the important influence of dietary sodium restriction on renin and ARR. It should be noted, however, that supine posture suppresses the renin-angiotensin-aldosterone system and should therefore have biased our findings towards the null. In this regard, it is remarkable that we observed nearly 50% of confirmed PA cases with a negative PA screen on dietary sodium restriction; despite the suppressive effect of supine posture on renin, a majority still exhibited substantial elevations in PRA that lowered the ARR. We strongly suspect that had our study measurements been performed in the seated position, the frequency of significant renin elevations on sodium restriction might have been even higher and the rate of PA screening misinterpretation may have been higher as well. Another limitation is that participants in our study were all diagnosed with PA incidentally and did not undergo abdominal imaging or adrenal venous sampling as a part of our study procedures; therefore, we are unable to comment on the distribution of unilateral vs bilateral PA and whether the laterality of disease may have predicted the eventual results. Finally, our restricted dietary sodium intervention was extreme. We recognize that even adherent patients can seldom achieve a sodium balance that is less than 50 mmol/d and approaching less than 20 mmol/d. In this regard, our findings may reflect an overestimation of how potent the “typical” sodium-restricted diet influence might be. On the other hand, the American Heart Association recommends a dietary sodium intake of less than 65 mmol/d, which is also fairly extreme and we suspect that many patients who adhere to this recommendation may exhibit physiologic changes such as those we report herein. Regardless, future studies to confirm and extend our work are needed to directly impact the clinical workplace: the assessment of a more “moderate” dietary sodium restriction, and in the seated position, may provide results that can be directly applied to the daily practice of medicine.
In summary, dietary sodium restriction in individuals with established PA can significantly raise PRA and normalize the ARR, thereby resulting in the potential to misclassify a substantial proportion of true PA cases. Our results suggest that the PA cases that are most susceptible to this dietary sodium restriction induced effect are those with a milder phenotype of PA where the PRA is not maximally suppressed. Further, because renin is arguably the most important determinant of the ARR, our findings again highlight the limitations of using the ARR without ensuring a suppressed renin. Because PA is usually diagnosed from the hypertensive population that is routinely advised to restrict dietary sodium intake, these results indicate that a substantial proportion of individuals with milder forms of PA may be at risk of being misdiagnosed or under diagnosed. We emphasize and extend The Endocrine Society recommendation (6) that using the ARR to screen for PA should optimally occur after prescribing a HS, and ideally with some confirmation that this has occurred.
Acknowledgments
We thank our funding sources and research volunteers and staff.
This work was supported by Fondo Nacional de Desarrollo Cientifico y Tecnologico (FONDECYT) Grants 1150437, 1150327, 1160836, and 1160695 (to R.B.) and the Corporacion de Fomento de la Produccion de Chile (CORFO) Grant 13CTI-21526-P1 (to R.B.); by a Chilean Society of Endocrinology and Diabetes scholarship (F.J.G.); and by the National Institutes of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health Award R01 DK107407, the Doris Duke Charitable Foundation Grant 2015085, and the National Heart, Lung, and Blood Institute of the National Institutes of Health Award K23HL111771 (to A.V.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AER
- aldosterone excretion rate
- ARR
- aldosterone to renin ratio
- BMI
- body mass index
- HS
- high dietary sodium intake
- LS
- low dietary sodium intake
- OR
- odds ratio
- PA
- primary aldosteronism
- PRA
- plasma renin activity
- SBP
- systolic blood pressure.
References
- 1. Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20:129–136. [DOI] [PubMed] [Google Scholar]
- 2. Funder JW. Primary aldosteronism and low-renin hypertension: a continuum? Nephrol Dial Transplant. 2013;28:1625–1627. [DOI] [PubMed] [Google Scholar]
- 3. Vongpatanasin W. Resistant hypertension: a review of diagnosis and management. JAMA. 2014;311:2216–2224. [DOI] [PubMed] [Google Scholar]
- 4. Piaditis G, Markou A, Papanastasiou L, Androulakis II, Kaltsas G. Progress in aldosteronism: a review of the prevalence of primary aldosteronism in pre-hypertension and hypertension. Eur J Endocrinol. 2015;172:R191–R203. [DOI] [PubMed] [Google Scholar]
- 5. Hanslik G, Wallaschofski H, Dietz A, et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn's Registry. Eur J Endocrinol. 2015;173:665–675. [DOI] [PubMed] [Google Scholar]
- 6. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016:101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 7. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012;44:170–176. [DOI] [PubMed] [Google Scholar]
- 8. Raizman JE, Diamandis EP, Holmes D, Stowasser M, Auchus R, Cavalier E. A renin-ssance in primary aldosteronism testing: obstacles and opportunities for screening, diagnosis, and management. Clin Chem. 2015;61:1022–1027. [DOI] [PubMed] [Google Scholar]
- 9. Lalli E, Barhanin J, Zennaro MC, Warth R. Local control of aldosterone production and primary aldosteronism. Trends Endocrinol Metab. 2016;27:123–131. [DOI] [PubMed] [Google Scholar]
- 10. Ito Y, Takeda R, Karashima S, Yamamoto Y, Yoneda T, Takeda Y. Prevalence of primary aldosteronism among prehypertensive and stage 1 hypertensive subjects. Hypertens Res. 2011;34:98–102. [DOI] [PubMed] [Google Scholar]
- 11. Vaidya A, Underwood PC, Hopkins PN, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baudrand R, Pojoga LH, Vaidya A, et al. Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation. 2015;132:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pimenta E, Stowasser M, Gordon RD, et al. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest. 2013;143:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rye P, Chin A, Pasieka J, So B, Harvey A, Kline G. Unadjusted plasma renin activity as a “first-look” test to decide upon further investigations for primary aldosteronism. J Clin Hypertens (Greenwich). 2015;17:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young WF, Jr, Hogan MJ, Klee GG, Grant CS, van Heerden JA. Primary aldosteronism: diagnosis and treatment. Mayo Clin Proc. 1990;65:96–110. [DOI] [PubMed] [Google Scholar]
- 16. Pimenta E, Calhoun DA. Primary aldosteronism: diagnosis and treatment. J Clin Hypertens (Greenwich). 2006;8:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 18. Koch M, Aker S, Haastert B, Rump LC. Clinical relevance of dietary salt intake on aldosterone and the aldosterone-to-renin ratio as screening parameters for primary aldosteronism. Clin Nephrol. 2010;74:182–189. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. [DOI] [PubMed] [Google Scholar]
- 20. Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk - measurement matters [published online ahead of print June 1, 2016]. N Engl J Med. 10.1056/NEJMsb1607161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antman EM, Appel LJ, Balentine D, et al. Stakeholder discussion to reduce population-wide sodium intake and decrease sodium in the food supply: a conference report from the American Heart Association Sodium Conference 2013 Planning Group. Circulation. 2014;129:e660–e679. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. [DOI] [PubMed] [Google Scholar]
- 23. Brown JM, Underwood PC, Ferri C, et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. [DOI] [PubMed] [Google Scholar]
- 25. Newton-Cheh C, Guo CY, Gona P, et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49:846–856. [DOI] [PubMed] [Google Scholar]
- 26. Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2012;26:485–495. [DOI] [PubMed] [Google Scholar]
- 27. Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. [DOI] [PubMed] [Google Scholar]
- 28. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112:E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nanba K, Chen AX, Omata K, et al. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]