Abstract
Context:
It is not known whether decreases in trophoblast invasion promoting the peptide, adrenomedullin2 (ADM2) system is associated with preeclampsia (PreE).
Objective:
The objective of the study was to assess the changes in ADM2 levels in plasma, placenta, and amniotic fluid (AF) and its receptor components in placenta from PreE pregnancy compared with the age-matched normal and study the effect of ADM2 on the synthesis of nitric oxide (NO), endothelial nitric oxide synthase (eNOS), and matrix-metalloproteinase (MMP)-2 and MMP-9 in trophoblast cells.
Results:
PreE is associated with a decreased expression of ADM2 in plasma and placenta (P < .05); ADM2 interacts with a seven-transmembrane G protein-coupled receptor, calcitonin receptor-like receptor (CRLR) in HTR-8/SVneo cells; placental expression of ADM2/CRLR complex is lower in PreE; mRNA for CRLR and receptor activity-modifying protein-3 are lower, whereas receptor activity-modifying protein-2 is higher in the PreE placenta (P < .05); ADM2 levels in the second trimester are lower in the AF from pregnant women who develop PreE later in gestation (P < .05); ADM2 is localized to the epithelium of the amnion and the ectoderm and mesoderm of the chorion in term fetal membranes; ADM2 increases NO production, eNOS, and MMP2/9-immunoreactivity, whereas ADM2 knockdown inhibits the expression of eNOS and MMP2/9 mRNA and S-nitrosylation in HTR-8/SVneo cells; and ADM2-induced increases in MMP2/9 activity is inhibited by L-nitro-arginine methyl ester in HTR-8SV/neo cells.
Conclusion:
Decreases in the ADM2 system in PreE at term, in AF from pregnant women during the second trimester who develop PreE later in gestation, and ADM2-induced increases in the NO and MMP-2/9 levels in trophoblast cells suggest a potential role for ADM2 via the NO-MMP system in the pathophysiology of PreE.
Current study shows that expression of ADM2 system is altered in preeclampsia and ADM2 increases the expression of nitric oxide (NO) and matrix-metalloproteinase (MMP)2 and MMP9 in trophoblast cells.‘
Preeclampsia (PreE) is characterized by deficient trophoblast invasion, endothelial dysfunction, and new onset of hypertension and endothelial dysfunction. It affects 5%–10% of pregnancies worldwide and confers an increased risk of fetal and maternal mortality and morbidity (1). The etiology of PreE is not known, but defects in trophoblast/placental function (2, 3) is suggested to play a role. PreE is associated with abnormal cytotrophoblast differentiation, which, in turn, causes a reduction in the invasion of trophoblasts and therefore an incomplete transformation of the maternal spiral arteries. This results in impaired spiral artery remodeling, inadequate uteroplacental circulation, maternal hypertension, and fetal intrauterine growth restriction (4).
Vascular remodeling is thought to result from the loss of musculoelastic structure induced by cytotrophoblasts (CTBs) through the actions of matrix metalloproteases (MMP)-2 and MMP9 secreted by CTBs. The functional role for nitric oxide (NO) is implicated in human CTB differentiation (5–7) and survival (8). Differentiation of CTBs into the invasive phenotype in early pregnancy correlates with an increase in the activity of nitric oxide synthase and MMPs, and NO regulates the expression and activity of MMP2/9 in trophoblast cells during embryo implantation and placental development (9–11). S-nitrosylation of MMP2/9 at the tip of invading trophoblasts cells is a critical requirement for the invasive capacity of the trophoblast cells (12). Inefficient trophoblast invasion, impaired NO, MMP2 and MMP9 production, and endothelial dysfunction result in inefficient spiral artery remodeling and adverse pregnancy outcome such as PreE (9, 12, 13), and inhibition of NO is shown to reduces the cGMP concentration and generate characteristics of PreE (9, 14–16).
Recently we reported an expression- and invasion-promoting role for a recently discovered peptide adrenomedullin2 (ADM2) /intermedin (IMD) in human pregnancy (17, 18). ADM2 was discovered in 2004 as a hypotensive peptide belonging to calcitonin/calcitonin gene-related peptide (CGRP) family with approximately 28% structural homology with adrenomedullin (ADM) and approximately 20% with CGRP (17, 19–23). CGRP, ADM, and ADM2 share a single seven-transmembrane G protein-coupled receptor (GPCR), calcitonin receptor-like receptor (CRLR), whose ligand binding is dictated by one of the three receptor activity-modifying proteins (RAMP1, RAMP2, or RAMP3) (24, 25). Coexpression of CRLR with RAMP1 forms a CGRP receptor; with RAMP2 or RAMP3 produces an ADM receptor, and coexpression of CRLR with any of the three RAMPs mediates ADM2 signaling. However, the majority of ADM2 effects are mediated through CRLR in with RAMP2 or RAMP3. However, it is yet to be demonstrated that CRLR is the GPCR for ADM2 actions in the placenta.
We have shown that ADM2 is expressed in trophoblast cells throughout the human pregnancy (17), it increases the invasive capacity of first-trimester trophoblast cells (17, 18, 26), and that sensitivity of omental arteries to ADM2 is increased pregnant women compared with the nonpregnant woman (27). In addition, lower serum and placental levels of ADM2 are associated with spontaneous abortion (18). The importance of ADM2 in the establishment of a successful pregnancy was further demonstrated in our ADM2 antagonist infusion studies showing reduced number of implantation sites accompanied by the down-regulation of the NO and MMP2/9 system along with impaired placental vasculature (21, 28). Therefore, based on the human and rat studies, we hypothesized that PreE is associated with a decreased expression of ADM2 and its receptors in placenta and that ADM2 effects are mediated through the NO-MMP system in human trophoblast cells. Therefore, the objective of this study was to compare the expression of ADM2 and its receptor levels in PreE and normotensive control pregnancies and identify the involvement of the NO-MMP2/9 system in ADM2-induced effects in trophoblast cells
Subjects and Methods
The protocol for this study was approved by the Baylor College of Medicine Institutional Review Board and was conducted according to Declaration of Helsinki principles. Pregnant patients undergoing cesarean delivery were enrolled (Table 1). All patients gave informed written consent. Blood samples and placental tissues were collected from subjects who consented and had PreE and gestational age-matched normal pregnancies. Patients were excluded from the study if they had any of the following: diabetes, fetal anomalies, multifetal pregnancy, or clinical evidence of maternal or fetal infection.
Table 1.
Clinical Parameters of Female Participants for Studies With Blood and Placenta Collected at Term Pregnancy
| Clinical Parameters | Control | PreE | Severe PreE |
|---|---|---|---|
| Term placenta GA, wk | 36.7 ± 0.38 (n = 8) | 37.8 ± 0.25 (n = 8) | None |
| Blood pressure | <140/90 mm Hg | ≥ 140/90 mm Hg | |
| Protein urea | Absent | 2+ | |
| Mode of delivery | C/S | C/S | |
| Parity | 1.87 ± 0.09 | 1.7 ± 0.1 |
Abbreviations: C/S, cesarean section; GA, gestational age. Data are represented as mean ± SD, and maternal age of participants ranged from 18 to 41 years.
Collection of amniotic fluid and fetal membranes
A cohort of 731 women with singleton nonanomalous pregnancies undergoing second-trimester genetic amniocentesis at gestational age 17 ± 1 weeks was followed up for their status of pregnancy until delivery, as previously reported in a different study (29). From this cohort, pregnant women with early-onset PreE (n = 17; parity 1.61) diagnosed before 34 week's gestation were compared with the pregnant women from the same cohort that had uncomplicated pregnancy (n = 12; parity 2). In this group of PreE patients, 18% were diagnosed with severe PreE. ADM2 concentrations in amniotic fluid (AF) were measured by an enzyme-linked immunoassay (EIA). ADM2 immunoreactivity in unrelated amnion and chorionic tissues collected from healthy pregnant women at term was assessed by immunohistochemistry.
HTR-8/SVneo cells culture
The HTR-8SV/neo cells gifted by Dr Charles H. Graham (Queen's University, Kingston, Ontario, Canada) were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS). Cells were starved for overnight (RMPI 1640 + 2% FBS) prior to treating with ADM2 (10−9 M to 10−7 M) in presence or absence of L-nitro-arginine methyl ester (L-NAME; 10 μM) for 24 hours followed by assessment of NO levels using 4,5-diaminofluorescein diacetate (Molecular Probes).
For immunofluorescent staining of the invaded HTR-8/SVneo cells, cells were then seeded onto the Beta Mercaptoethanol-coated invasion chambers (0.5 × 105 cells/well) in presence or absence ADM2 (10−8 M) for 24 hours at 37°C. At the end of the incubation period, invaded cells on the Matrigel-coated inserts were fixed with methanol and acetone mixture (1:1) and assessed for their immunoreactivity for endothelial nitric oxide synthase (eNOS), MMP2, and MMP9 by immunofluorescent staining.
For the proximity ligation assay, cells (7000/well) were seeded in 16-well plates and treated with or without ADM2 10−8 M for 2 minutes followed by fixing in 4% paraformaldehyde.
Short hairpin RNA (shRNA) transfection
HTR-8SV/neo cells were cultured in RPMI 1640 with 10% FBS and 1% penicillin/streptomycin. The knockdown transfection was performed with four ADM2 shRNAs and one scramble control shRNA plasmid (OriGene) using Lipofectamine 2000 following the manufacturer's protocol (Invitrogen). All transfections were done in three to four different experiments following the manufacturer's instructions. Transfected cells were harvested after 72 hours for mRNA and protein extraction followed by real-time quantitative PCR and Western blot analysis, respectively. Knockdown efficiency was greater than 70% compared with the scrambled shRNA (data not shown).
Quantitative real-time PCR
Real-time quantitative RT-PCR was performed by using TaqMan probes for CRLR, RAMP1, RAMP2, and RAMP3 produced by Life Technologies and ADM2, eNOS, MMP2, and MMP9 primer assay from SA Biosciences. Amplification of housekeeping GAPDH (forward primer: 5′-GGTCTCCTCTGACTTCAACA-3′, and reverse primer: 5′-AGCCAAATTCGTTGTCATAC-3′) served as an endogenous control to standardize the amount of sample RNA added to a reaction. All experiments were performed in triplicate. Negative controls were performed by replacing RNA templates with nuclease-free water. For no-reverse transcription control, nuclease-free water was used in place of the reverse transcriptase. Results were calculated using the 2-δδcycle threshold method and expressed in folds increase/decrease of the gene of interest.
Proximity ligation assay (PLA)
An in situ PLA is a modification of traditional immunoassays, which is capable of detecting protein-protein interactions with high specificity and sensitivity (29). Two antibodies are used in these assays, which are raised in different species against the target antigen. PLA probes facilitate the binding of the primary antibodies to the secondary antibody only when PLA probes are in close proximity. Each interaction between the two proteins results in bright fluorescent dots. We used a PLA to demonstrate protein-protein interaction suggestive of a complex formation between ADM2 and CRLR using a Duolink II fluorescence kit (Olink Biosciences) according to the manufacturer's instructions. Briefly, first-trimester HTR-8/SVneo cells sparsely grown on 16-well Lab-Tek chamber slides were treated with or without ADM2 (10−8 M) for 2 minutes, fixed using 4% paraformaldehyde, and incubated with primary antibodies (one monoclonal antibody and one polyclonal antibody) followed by incubation with PLA probes. A PLA on the villous tissue was performed in a similar way on frozen sections after fixation in paraformaldehyde and antigen retrieval with 1% sodium dodecyl sulfate (SDS). Images are observed under a fluorescence microscope (U-TV1 X; Olympus), and red spots were counted using Image-Pro Plus software (Media Cybernetics) in 10 randomly selected images per replicate. Appropriate negative controls included no primary antibody or a negative IgG isotype of primary antibody.
Gelatinase zymography
Gelatinase zymography was performed in 10% NOVEX precast SDS polyacrylamide gel (Invitrogen Corp) in the presence of 0.1% gelatin under nonreducing conditions. Culture media (20 μL) were mixed with sample buffer and loaded for SDS-PAGE with Tris glycine SDS buffer as suggested by the manufacturer (Novex). Recombinant MMP2 and MMP9 (Sigma) were run concurrently and approximate molecular weights of the clear bands were determined.
Immunohistochemical and immunofluorescent staining
Fetal membranes were fixed in formalin (24–72 h) and paraffin embedded. Sections were dewaxed and rehydrated before antigen retrieval immunostaining with ADM2 antibody (Alpha Diagnostics). Staining was developed with diaminobenzidine (Dako). Counterstaining was performed with hematoxylin.
Optimum cutting temperature-embedded frozen tissue sections or HTR-8/SVneo cell were fixed with methanol and acetone mixture (1:1) (30). The antibodies used were eNOS, MMP2, MMP9 (Abcam), or IMD (Alpha Diagnostics) at a dilution of 1:150 as reported earlier or as per the manufacturer's instructions (17). Absence of antibody, preimmune serum, or mouse IgG (Ready-to-Use; Dako) was used as a negative control. Mouse IgG1 (Dako), IMD antibody neutralized by IMD peptide, or secondary antibody alone was also used as a negative control (data not shown).
Enzyme-linked immunoassay
Stored AF from amniocenteses during the second trimester and serum samples from normotensive and PreE pregnancies, which were collected at term, were analyzed for ADM2 levels by an EIA as per published protocol (Phoenix Pharmaceuticals). The intraassay variation was 6% and the interassay variation was 10% (n = 3). Spike recovery studies using human plasma and recombinant ADM2 confirmed the specificity of the EIA (data not shown).
Measurement of intracellular NO production
NO production in HTR-8/SVneo cells was measured using 4,5-diaminofluorescein diacetate, a cell membrane-permeable NO sensitive fluorescent dye, which converts to 4,5-diaminofluorescein diacetate and reacts with NO to form the fluorescent triazole 4,5-diaminofluorescein-2T (31). Prior to treatment, HTR-8/SVneo cells were incubated with 5 μM 4,5-diaminofluorescein diacetate for 15 minutes at room temperature. The increase in fluorescence intensity was monitored at 485 nm excitation and 515 nm emission using a fluorescence microplate reader (BMG spectrophotometer) at 37°C. Fluorescence intensities were normalized based on the number of cells per sample.
Statistical analysis
All results are expressed as the mean ± SEM. The experimental data were analyzed by a Student's t test or a one-way ANOVA. Each RT-PCR experiment was done in triplicate. Values were considered significant at P ≤ .05.
Results
Expression of ADM2, CRLR, and RAMPs in PreE compared with age-matched normal pregnancy
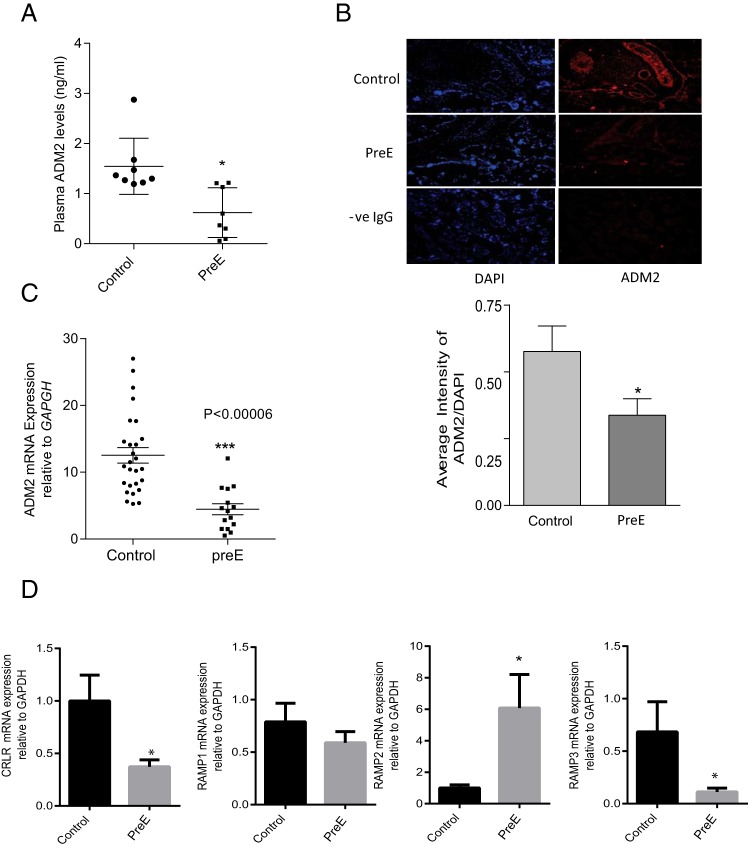
Figure 1A shows that ADM2 concentrations in plasma collected from PreE women are 38% of that found in the plasma of the normotensive pregnant women (1.56 ± 0.22, for control vs 0.58 ± 0.25 for PreE; n = 7–8; P = .019). Expression of ADM2 mRNA in villous tissue from PreE was assessed by quantitative PCR. As shown in Figure 1B, villi from PreE have lower levels of ADM2 mRNA compared with those obtained from normal pregnancy (12.30 ± 1.14 for normal pregnancy vs 3.91 ± 0.67 for PreE; n = 8; P = .0001). Similar to the mRNA expression, ADM2-specific immunoreactivity was also lower throughout the villous tissue from PreE placenta compared with those from gestational age-matched control (Figure 1C; magnification, ×200; n = 3; P < .05).
Figure 1.
ADM2, CRLR, and RAMPs levels in preeclamptic and normotensive pregnant women. A, An EIA showing a lower level of ADM2 at term in preeclamptic pregnant women (PreE) compared with the gestational age-matched normal (control) (P < .05; n = 7–8). B, Expression of ADM2 mRNA in the villous tissue from PreE compared with the gestational age-matched normal placenta (control). C, ADM2 immunoreactivity in placental villi from normal (control) and PreE pregnancy. As shown, immunoreactivity for ADM2 is lower throughout the villous tissue in PreE compared with the control villi. IgG was used as negative control (-ve IgG) and 4′, 6-diamidino-2-phenylindole (DAPI) was used for blue nuclear staining. Bar graph shows ADM2 immunostaining relative to DAPI. Data are presented as mean ± SEM. *, P < .05 (magnification, ×200, n = 3). D, Expression of CRLR and RAMP mRNA in the placental villous tissue. The bar graphs represent mRNA levels of CRLR and RAMP1, RAMP2, and RAMP3 in villous tissue from normal pregnant women (control, n = 8) and women with PreE (n = 8) normalized to GAPDH mRNA expression. Data are presented as mean ± SEM. *, P < .05.
Furthermore, Figure 1D demonstrates that PreE is associated with changes in the mRNA levels of CRLR and RAMPs in villous tissue. The mRNA levels of receptor components CRLR, RAMP1, RAMP2, and RAMP3 in the villous tissues from PreE placenta were analyzed and compared with those from normal gestational age-matched control placenta. As shown, mRNA levels of CRLR and RAMP3 were lower, whereas RAMP2 was higher, in PreE compared with the controls (n = 8; P < .05). There was no change in the mRNA levels of RAMP1 in PreE placenta compared with the age-matched normal.
Expression of ADM2 with CRLR (ligand/receptor) complex in trophoblast cells and its association with PreE
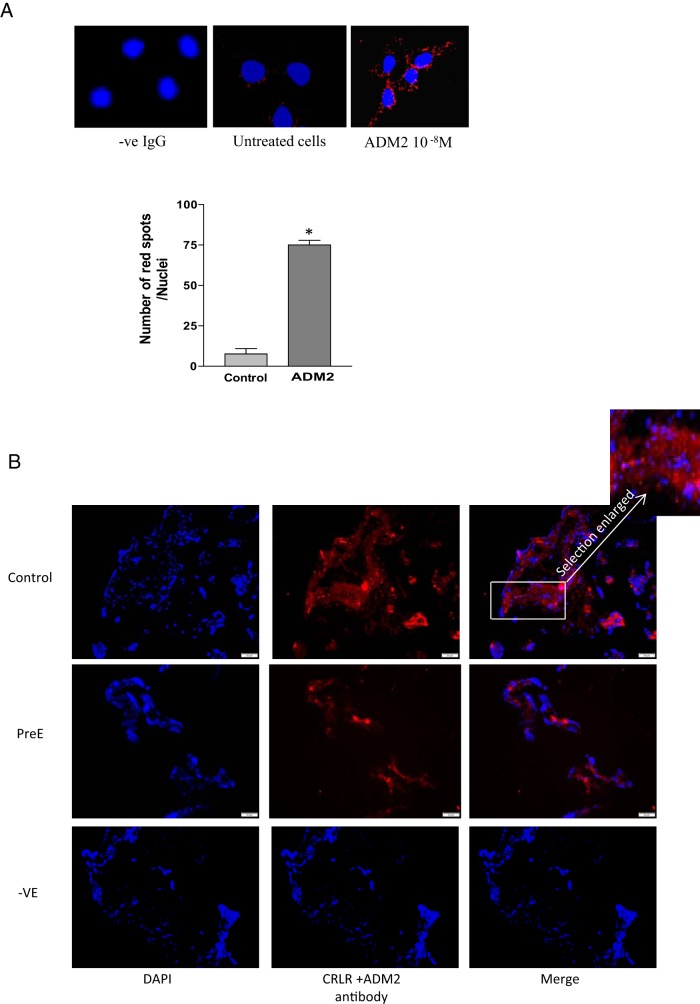
An in situ PLA demonstrates that the addition of ADM2 10−8 M to HTR-8/SVneo cells resulted in a greater number of red fluorescent spots, demonstrating binding of ADM2 to CRLR (Figure 2A; P < .05; n = 3). Furthermore, Figure 2B demonstrates that expression of this ligand-receptor complex is reduced in villous tissue from PreE placenta compared with the controls (n = 3, magnification, ×200).
Figure 2.
PLA showing protein-protein interaction between ADM2 and CRLR. A, Increases in the number of red fluorescent dots in ADM2 (10−8 M)-treated cells indicate that ADM2 binds to CRLR in HTR-8/SVneo cells. Bar graph presents the number of spots per nuclei (4′, 6-diamidino-2-phenylindole [DAPI]) that correspond to the number of interactions between CRLR and ADM2. Nuclei staining was done with DAPI. Data are presented as mean ± SEM for three replicate experiments. *, P < .05. B, PLA in the villous explants. Figure shows decreases in the red fluorescence pertaining to ADM2/CRLR association in the villous tissue section from PreE compared with that in the normal placenta (control), thus suggesting PreE-associated decreases in the expression of ligand receptor complex in placenta. Selection in the box is enlarged to show the red fluorescent spots. Absence of antibodies served as negative control (-ve). DAPI was used for nuclear staining (magnification, ×200; n = 3).
PreE-associated changes in the levels of ADM2 in second-trimester AF and expression of ADM2 in fetal membranes
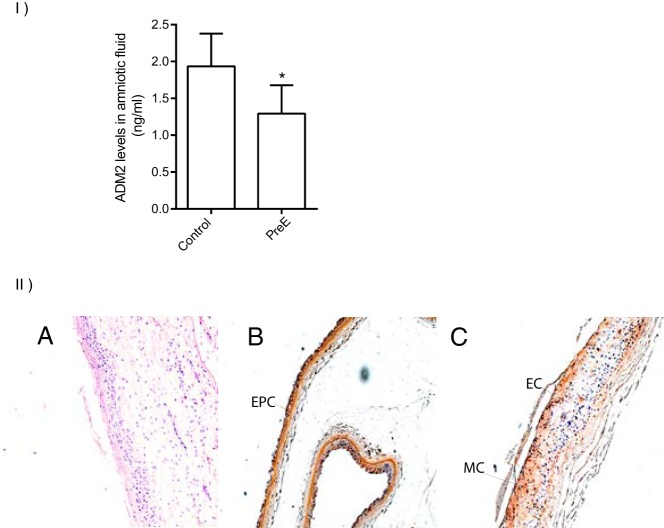
ADM2 concentrations in the AF of pregnant women undergoing second-trimester genetic amniocentesis are presented in Figure 3I with respect to their pregnancy outcome. ADM2 levels in the second-trimester AF were lower in women who later developed PreE (Figure 3I; SEM ± 1.29 ± 0.11; n = 17) compared with the normotensive pregnant women who delivered normally at term (1.93 ± 0.10; n = 12; P = .004). Immunohistochemical staining in Figure 3II demonstrated that ADM2 protein is localized in the epithelium of amnion and ectodermal and mesodermal cells of the chorion in the fetal membranes collected at term from women with normal pregnancy (magnification, ×100, n = 3).
Figure 3.
ADM 2 peptide levels in second-trimester AF of pregnant women who either had normal pregnancy or developed early PreE and ADM2 immunoreactivity in fetal membranes. I, Enzyme-linked immunoassay of ADM2 levels in the AF during the second trimester in women who developed PreE (n = 17) and those who had a normal pregnancy (n = 12). Levels of ADM2 in the PreE group were lower compared with the control (P < .05). Data are presented as mean ± SEM. *, P < .05. II, ADM2 immunoreactivity in fetal membranes from normal pregnant women at term using 3,3′-diaminobenzidine (DAB) staining. B, Immunohistochemical staining of fetal membrane in term placenta. EPC epithelial cells of amnion. C, Ectodermal cells (EC) and mesodermal cells (MC) of chorion. In panel A, IgG was used as a negative control (-ve IgG) (magnification, ×100, n = 3).
Effect of ADM2 on the synthesis of NO in HTR-8/SV/neo cells
Figure 4A demonstrates that ADM2 dose dependently increases NO levels in HTR-8/SVneo cells and that this increase is inhibited in presence of L-NAME (n = 3; P < .05).
Figure 4.
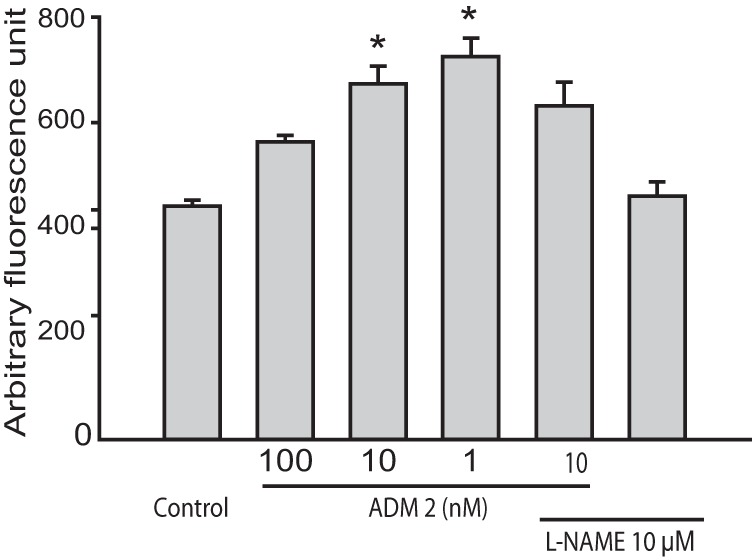
Effect of ADM2 on NO production in HTR-8/SVneo cells. 4,5-Diaminofluorescein diacetate fluorometric assay showing NO content in the HTR-8/SVneo cells treated for 2 hours with ADM2 (10−9 M to10−7 M) in presence or absence of the NO synthase inhibitor L-NAME (10−3 M). As shown, addition of L-NAME prevented ADM2-induced increase in NO formation in HTR-8/SVneo cells. *, Significant difference compared with the control cells (n = 3; P < .05).
Effect of ADM2 on the expression of eNOS, MMP2, MMP9, and S-nitrosylation of proteins in HTR-8/SVneo cells
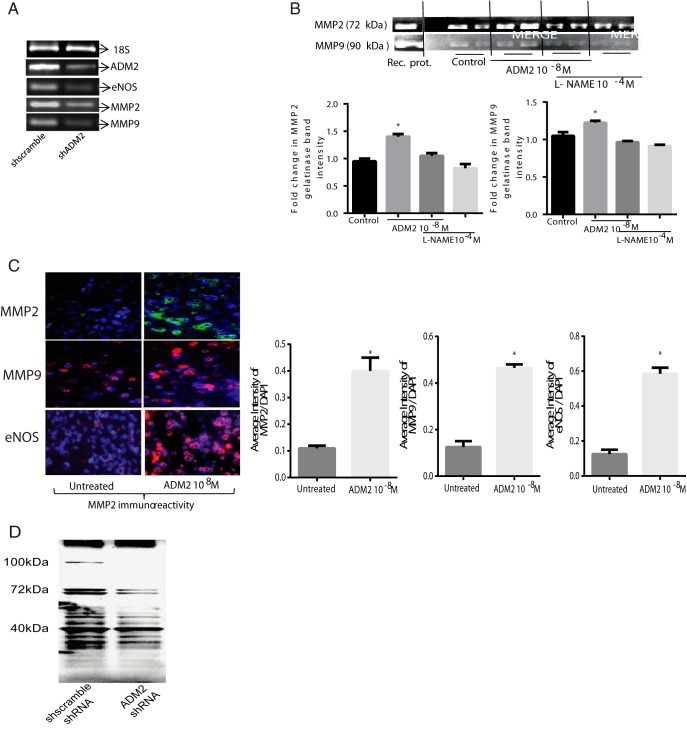
As shown in Figure 5A, silencing of the ADM2 gene in HTR-8/SVneo cells by ADM2-specific shRNA inhibits the expression of eNOS, MMP2, and MMP9 compared with the cells transfected with the scramble shRNA (n = 3). The knockdown efficiency tested was greater than 70% (data not shown). Furthermore, a zymogram gel picture in Figure 5B demonstrates that ADM2 treatment increases the gelatinase activity of both MMP2 and MMP9 in HTR-8/SVneo cells, which is inhibited in presence of L-NAME. In addition, cells that invaded the Matrigel-coated inserts in the presence ADM2 (10−8 M) showed increased eNOS, MMP2, and MMP9 immunoreactivity compared with the untreated controls (Figure 5C; magnification, ×200, n = 3). Furthermore, knockdown of ADM2 results in decreases in the S-nitrosylation of protein in HTR-8/SVneo cells (n = 3).
Figure 5.
Effect of ADM2 on the expression of eNOS, MMP2, MMP9, and protein S-nitrosylation in HTR-8/SVneo cells. A, Agarose gel picture showing that knockdown of ADM2 mRNA expression in HTR-8/SVneo cells results in decreased expression of eNOS, MMP2, and MMP9 mRNA compared with the control (sh scramble). B, A zymogram gel picture showing an effect of ADM2 (10−8 M) supplementation on the gelatinase activity of MMP2 and MMP9 in HTR-8/SVneo cells in presence or absence of L-NAME (10−4 M). Bar graph representing mean ± SEM of the fold change shows that addition of ADM2 increases the gelatinase activity of MMP2 and MMP9 in HTR-8/SVneo cells, which is inhibited in the presence of L-NAME. *, Significant difference compared with the control (n = 3; P < .05). C, Immunofluorescent staining showing effect of ADM2 on the eNOS, MMP2, and MMP9 immunoreactivity in HTR-8/SVneo cells cultured on inserts coated with Matrigel. As shown, addition of ADM2 (10−8 M) increases the immunoreactivity of eNOS, MMP2, and MMP9 in the HTR-8/SVneo cells that invaded the Matrigel compared with the untreated controls. 4′, 6-Diamidino-2-phenylindole (DAPI) was used for blue nuclear staining and IgG was used as a negative control. Bar graph shows ADM2 immunostaining relative to DAPI. Data are presented as mean ± SEM. *, P < .05 (magnification, ×200, n = 3). D, Western blot showing that silencing of ADM2 gene using ADM2-specific shRNA transfection decreases the expression of S-nitrosylated proteins in HTR-8/SVneo cells compared with the cells transfected with scramble shRNA. As shown, the decrease is more prominent in proteins with weight of 70 kDa or greater. The blot is representative of three experiments.
Discussion
The current study shows the following: 1) PreE is associated with decreases in the expression of circulating and placental ADM2 levels compared with the normal pregnancy, 2) CRLR is the GPCR for ADM2 actions in trophoblast cells and decreases in the expression of ADM2/CRLR complex in villous tissue is associated with PreE, 3) PreE is associated with decreases in the expression of CRLR and RAMP3 mRNA and increases in RAMP2 mRNA in the villous tissue, 4) fetal membranes express ADM2 and its levels are significantly lower in second-trimester AF of pregnancies that developed PreE in a later phase of gestation, 6) ADM2 promotes the synthesis of NO in HTR-8/SVneo cells, and 7) blocking ADM2 synthesis decreases eNOS, MMP2, and MMP9 mRNA levels along with decreases in the protein S-nitrosylation in HTR-8/SVneo cells, and the addition of ADM2 increases eNOS, MMP2, and MMP9 immunoreactivity, and ADM2-induced increased MMP2/9 gelatinase activity in the invasive trophoblast cells is inhibited by L-NAME. Taken together, these results suggest that changes in the expression of the ADM2/receptor system are associated with the pathophysiology of PreE and implicate a potential physiological role for ADM2 via its effect on NO-MMP-mediated regulation of trophoblast function to support placental development in human pregnancy.
We reported earlier that ADM2 increases the invasive capacity of first-trimester cytotrophoblast cells; and elevated levels of ADM2 in the first-trimester human placenta coinciding with the invasive phase of placental formation suggest its involvement in regulating early placental development in human pregnancy (17, 26, 28, 30, 32). Thus, any impairment in ADM2 function is likely to have an adverse effect on pregnancy outcome. This is supported by our human and rat studies showing an association of lower ADM2 levels in first-trimester spontaneous abortion in humans and fetoplacental growth restriction in pregnant rats infused with ADM2 antagonist (ADM217–47). The current study is the first to demonstrate an association of lower levels of the ADM2 system in circulation and placenta (Figure 1, A–D) with PreE pregnancy. Furthermore, ADM2 interacts with GPCR CRLR to function in trophoblast cells and this ligand/receptor complex is lower in PreE placenta (Figure 2).
Senna et al (33) showed decreases in plasma levels of ADM, an ADM2 related peptide in PreE, and Caron et al speculated that complete deficiency of ADM might contribute to a PreE phenotype in the mouse placenta with decreased remodeling of placental vasculature and recruitment of the maternal uterine natural killer cells that are important for vascular remodeling. The authors proposed ADM as a potential marker or therapeutic target (34, 35). Similar effects in vascular remodeling and maternal uterine natural killer cell recruitment were observed by Dackor et al (36) in CRLR knockout mice, suggesting an involvement of CRLR in ADM functions. The current study shows that ADM2 also interacts with CRLR in human trophoblast cells (Figure 2) and that mRNA expression of CRLR and RAMP3 are lower, whereas RAMP2 are higher, in PreE placenta compared with the normal (Figure 1D). Although CRLR is capable of mediating ADM2 function through RAMP1, RAMP2, or RAMP3, the majority of ADM2 effects are mediated through CRLR and RAMP3, whereas ADM effects are through CRLR and RAMP2 (19, 24). It is likely that the increase in RAMP2 in PreE may be a compensatory effect of the pathology. However, decreases in the expression of CRLR and RAMP3 receptor components (Figure 1D) in addition to PreE-associated decreases in the expression of ADM2 /CRLR receptor complex in PreE placenta (Figure 2B) suggest an impaired ADM2 signaling in PreE.
To assess whether decreases in ADM2 levels precede the development of PreE, we show (Figure 3I) that the levels of ADM2 in second-trimester AF are lower in the patients who develop PreE later in gestation compared with those who do not develop PreE. In addition, Figure 3II demonstrates that ADM2 is localized to the epithelial cells of the amnion and ectodermal and mesodermal cells of the chorion in normal pregnancy, providing an additional evidence for a potential role for ADM2 in placental function. This allows us to speculate that decreases in the ADM2 system in the early phase of human pregnancy may affect trophoblast invasion in early placental formation (17, 18, 26) and thus contribute to the development of PreE pathology.
Shallow trophoblast invasion and failure of uterine spiral artery transformation in PreE are thought to result from the loss of musculoelastic structure by the invading CTBs. This involves the actions of MMPs, MMP2 and MMP9, secreted by CTBs. Several reports suggest an interaction between NO and MMPs (12, 37), and S-nitrosylation of MMP9 at the tip of invading trophoblast cells is a critical requirement for the invasive capacity of the trophoblast cells (12). The current study demonstrates that ADM2 treatment increases NO levels (Figure 4) along with increases in eNOS immunoreactivity (Figure 5C) in HTR-8/SVneo cells, and blocking ADM2 synthesis results in the inhibition of eNOS mRNA expression (Figure 5A) along with decreases in the S-nitrosylation of proteins (Figure 5D) with a prominent effect on S-nitrosylation of proteins at molecular mass of 70 kDa or greater. Furthermore, similar to the effect observed in our rat model infused with ADM2 antagonist (28), blocking ADM2 synthesis in HTR-8/SVneo cells resulted in decreases in the MMP2 and MMP9 mRNA (Figure 5A), and the addition of ADM2 resulted in increased MMP2 and MMP9 immunoreactivity in the invading trophoblast cells (Figure 5C). In addition, ADM2 treatment increased the gelatinase activity of MMP2 and MMP9 in HTR-8/SVneo cells, which was inhibited in presence of L-NAME (Figure 5B). Thus, based on our data showing ADM2-induced increases in NO production, ADM2 knockdown induced inhibition of S-nitrosylation of proteins and inhibition of ADM2-induced increase in the gelatinase activity by L-NAME, allows us to speculate that the invasive effects of ADM2 may involve regulation of NO-MMP2/MMP9 in early placental development. Thus, inhibition or lack of ADM2 function in human pregnancy may contribute to impaired trophoblast invasion resulting in abnormal functioning of the placenta. This inference is supported by our earlier reports in which infusion of ADM2 antagonist during early pregnancy in rats resulted in impaired fetoplacental growth with impaired placental vasculature (28, 30).
Although ADM2 and ADM both are suggested to support fetoplacental growth (25), unlike ADM expression, which is relatively lower in the first trimester and increases with advancing gestation, ADM2 levels are higher during the first trimester of gestation compared with the second and third trimesters. Thus, ADM and ADM2 may act differently to support different functions at different stages of fetoplacental growth (17, 18, 34, 35, 38). However, it is beyond the scope of this study to determine whether the changes in ADM2 and its receptors are a cause or consequence of the PreE pathology. Nevertheless, the current study together with our earlier reports showing an association of first-trimester spontaneous abortion with lower levels of ADM2 (18) allows us to speculate that the aberrant expression of ADM2 in the early phase of placental development may contribute to the PreE pathology.
In summary, this study demonstrates that decreases in the circulatory and placental ADM2 levels along with the decreases in the expression of ADM2 receptor components, CRLR and RAMP3, in the placenta are associated with PreE in the human pregnancy. In addition, the precedence of lower levels of ADM2 in second-trimester AF with the early onset of PreE pathology and ADM2-induced regulation of NO, MMP2, and MMP9 in trophoblast cells suggests a potential role for ADM2 in the pathophysiology of PreE via its effects on the NO/MMP system in human placenta. This study warrants future prospective studies in a cohort of pregnant women starting from the first trimester following through delivery and postpartum to assess whether PreE-associated changes in the ADM2 system are the cause or effect of the PreE pathology.
Acknowledgments
We thank Ms Sandra Garcia Dale for her incredible administrative assistance.
This work was supported by the National Institutes of Health through Grant HD 054867 (to M.C.) and Grant HL58144 (to C.Y.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADM
- adrenomedullin
- ADM2
- ADM 2
- AF
- amniotic fluid
- CGRP
- calcitonin gene-related peptide
- CRLR
- calcitonin receptor-like receptor
- CTB
- cytotrophoblast
- EIA
- enzyme-linked immunoassay
- eNOS
- endothelial NO synthase
- FBS
- fetal bovine serum
- GPCR
- G protein-coupled receptor
- IMD
- intermedin
- L-NAME
- L-nitro-arginine methyl ester
- NO
- nitric oxide
- PLA
- proximity ligation assay
- PreE
- preeclampsia
- RAMP
- receptor activity-modifying protein
- SDS
- sodium dodecyl sulfate
- shRNA
- small interfering RNA.
References
- 1. Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance—United States, 1991–1999. MMWR Surveill Summ. 2003;52(2):1–8. [PubMed] [Google Scholar]
- 2. van BE, Ekhart TH, Schiffers PM, van EJ, Peeters LL, de Leeuw PW. Persistent abnormalities in plasma volume and renal hemodynamics in patients with a history of preeclampsia. Am J Obstet Gynecol. 1998;179(3 Pt 1):690–696. [DOI] [PubMed] [Google Scholar]
- 3. Rossi AC, Mullin PM. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):9–16. [DOI] [PubMed] [Google Scholar]
- 4. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(suppl A):S25–S30. [DOI] [PubMed] [Google Scholar]
- 5. Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279(2):219–226. [DOI] [PubMed] [Google Scholar]
- 6. Gagioti S, Scavone C, Bevilacqua E. Participation of the mouse implanting trophoblast in nitric oxide production during pregnancy. Biol Reprod. 2000;62(2):260–268. [DOI] [PubMed] [Google Scholar]
- 7. Lyall F, Jablonka-Shariff A, Johnson RD, Olson LM, Nelson DM. Gene expression of nitric oxide synthase in cultured human term placental trophoblast during in vitro differentiation. Placenta. 1998;19(4):253–260. [DOI] [PubMed] [Google Scholar]
- 8. Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res. 2003;287(2):314–324. [DOI] [PubMed] [Google Scholar]
- 9. Zhu JY, Pang ZJ, Yu YH. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol. 2012;5(3–4):e137–e143. [PMC free article] [PubMed] [Google Scholar]
- 10. Novaro V, Colman-Lerner A, Ortega FV, et al. Regulation of metalloproteinases by nitric oxide in human trophoblast cells in culture. Reprod Fertil Dev. 2001;13(5–6):411–420. [DOI] [PubMed] [Google Scholar]
- 11. Novaro V, Pustovrh C, Colman-Lerner A, et al. Nitric oxide induces gelatinase A (matrix metalloproteinase 2) during rat embryo implantation. Fertil Steril. 2002;78(6):1278–1287. [DOI] [PubMed] [Google Scholar]
- 12. Harris LK, McCormick J, Cartwright JE, Whitley GS, Dash PR. S-nitrosylation of proteins at the leading edge of migrating trophoblasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res. 2008;314(8):1765–1776. [DOI] [PubMed] [Google Scholar]
- 13. Bolnick JM, Kilburn BA, Bolnick AD, et al. Sildenafil stimulates human trophoblast invasion through nitric oxide and guanosine 3′,5′-cyclic monophosphate signaling. Fertil Steril. 2015;103(6):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90(1):278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buhimschi I, Yallampalli C, Dong Y-L, Garfield RE. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol. 1995;172(5):1577–1584. [DOI] [PubMed] [Google Scholar]
- 16. Bahtiyar MO, Buhimschi C, Ravishankar V, et al. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol. 2007;196(1):72–76. [DOI] [PubMed] [Google Scholar]
- 17. Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod. 2009;81(4):777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Havemann D, Balakrishnan M, Borahay M, et al. Intermedin/adrenomedullin 2 is associated with implantation and placentation via trophoblast invasion in human pregnancy. J Clin Endocrinol Metab. 2012;98(2):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279(8):7264–7274. [DOI] [PubMed] [Google Scholar]
- 20. Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004;556(1–3):53–58. [DOI] [PubMed] [Google Scholar]
- 21. Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148(4):1727–1735. [DOI] [PubMed] [Google Scholar]
- 22. Smith RS, Jr, Gao L, Bledsoe G, Chao L, Chao J. Intermedin is a new angiogenic growth factor. Am J Physiol Heart Circ Physiol. 2009;297(3):H1040–H1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi K, Morimoto R, Hirose T, Satoh F, Totsune K. Adrenomedullin 2/intermedin in the hypothalamo-pituitary-adrenal axis. J Mol Neurosci. 2011;43(2):182–192. [DOI] [PubMed] [Google Scholar]
- 24. McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. [DOI] [PubMed] [Google Scholar]
- 25. Yallampalli C, Chauhan M, Sathishkumar K. Calcitonin gene-related family peptides in vascular adaptations, uteroplacental circulation, and fetal growth. Curr Vasc Pharmacol. 2013;11(5):641–654. [DOI] [PubMed] [Google Scholar]
- 26. Chauhan M, Balakrishnan M, Yallampalli U, et al. Adrenomedullin 2/intermedin regulates HLA-G in human trophoblasts. Biol Reprod. 2011;85(6):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong Y, Betancourt A, Chauhan M, et al. Pregnancy increases relaxation in human omental arteries to the CGRP family of peptides. Biol Reprod. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats. Regul Pept. 2011;170(1–3):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banadakoppa M, Vidaeff A, Yallampalli U., Ramin SA, Belfort MA, Yallampalli C. Complement split products in amniotic fluid in pregnancies subsequently developing early-onset preeclampsia. Dis Markers. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75(6):940–947. [DOI] [PubMed] [Google Scholar]
- 31. Kojima H, Nakatsubo N, Kikuchi K, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70(13):2446–2453. [DOI] [PubMed] [Google Scholar]
- 32. Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senna AA, Zedan M, el-Salam GE, el-Mashad AI. Study of plasma adrenomedullin level in normal pregnancy and preeclampsia. Medscape J Med. 2008;10(2):29. [PMC free article] [PubMed] [Google Scholar]
- 34. Caron K, Hagaman J, Nishikimi T, Kim HS, Smithies O. Adrenomedullin gene expression differences in mice do not affect blood pressure but modulate hypertension-induced pathology in males. Proc Natl Acad Sci USA. 2007;104(9):3420–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98(2):615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol. 2006;26(7):2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Sullivan S, Medina C, Ledwidge M, Radomski MW, Gilmer JF. Nitric oxide-matrix metaloproteinase-9 interactions: biological and pharmacological significance—NO and MMP-9 interactions. Biochim Biophys Acta. 2014;1843(3):603–617. [DOI] [PubMed] [Google Scholar]
- 38. Matson BC, Corty RW, Karpinich NO, et al. Midregional pro-adrenomedullin plasma concentrations are blunted in severe preeclampsia. Placenta. 2014;35(9):780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]