Abstract
Atherosclerosis is a chronic inflammatory disease of the arterial intima, occurring usually in the aged populations who are suffering from hypertension, dyslipidemia and diabetes for a long time. Research on atherosclerosis has shown that macrophage foam cell formation, inflammation, dyslipidemia and immune cells infiltration are all involved in regulating the onset and progression of atherosclerosis. Mesenchymal stem cells (MSCs) originated from different kinds of tissue are a group of cells possessing well-established self-renewal and multipotent differentiation properties as well as immunomodulatory and anti-inflammatory roles. Recent studies have displayed their dyslipidemia regulation functions. Transplantation of MSCs to atherosclerotic patients might be a new multifactorial therapeutic strategy to improve atherosclerosis. This review updates the advancement on MSCs and atherosclerosis.
Keywords: Mesenchymal stem cells, foam cells, macrophages, differentiation, t cells, atherosclerosis
Introduction
Despite the remarkable achievements have been made in the past decades, cardiovascular diseases still remain to be one of the leading causes of death worldwide, leading to immense health and economic burdens globally [1]. Atherosclerosis is the major cause of cardiovascular diseases.
Although the pathogenesis of atherosclerosis is not completely clear, current evidence indicates that macrophage at least involves in the pathogenesis. Under some conditions, the monocytes are recruited into the vascular intima, where they take up of modified low-density lipoprotein (LDL), particularly low-density lipoprotein cholesterol (LDL-C), to form macrophage foam cells. These lipid overloading foam cells mark the initiation of atherosclerosis and their accumulation and necrosis or apoptosis further promote the development of atheromatous plaques and eventually lead to serious cardiovascular diseases [2].
Lipid lowering, especially by statins, has been the most effective way to reduce risk of atherosclerotic cardiovascular diseases currently [3]. Despite the great progress achieved in the pharmacologic treatments with statins, several large controlled clinical trials, from the long-term intervention with pravastatin in ischemic disease study to the improve-it trial, from the cholesterol and recurrent events trial to the Scandinavian simvastatin survival study, all support that cardiovascular risk reduction including treatments with statins remains far from satisfactory [4-6]. Moreover, approximately two thirds of patients under the treatment of statins continue to suffer from the expected cardiovascular disease events and many patients cannot tolerate statin or follow a long term stains treatment to reach optimal LDL-C levels [3]. Thus, additional therapies for cardiovascular diseases, particularly for effective lipid lowering to inhibit foam cells formation so as to improve atherosclerosis are needed.
Inflammation and immunity are also known to be intimately involved in all stages of atherosclerosis, which links multiple risk factors covering aging, hypertension, dyslipidemia and diabetes for atherosclerosis. Moreover, inflammatory and immunological signaling can alter the behavior of the intrinsic cells of the artery wall like endothelium and smooth muscle cells, leading to lipid peroxidation, endothelium dysfunction and further recruitment of inflammatory and immune cells to the vascular intima [2,7-10]. The roles of anti-inflammation and anti-immunity therapies in mitigation of atherosclerosis have been support by increasing studies [11-13].
Given the underlying pathological and physiological development of atherosclerosis, current and future treatment strategies should more focus on lowering plasma cholesterol and attenuating inflammation and balancing immunity. Mesenchymal stem cells (MSCs), also known as multipotent mesenchymal stromal cells, are a cluster of well-established cells characterized with non-hematopoietic, self-renewal and multipotent differentiation properties. Bone marrow is considered to be original source of MSCs. More studies have showed that MSCs can be isolated from the different tissues including umbilical cord, placenta, adipose tissue and human gingiva [14-16]. Recently, the anti-inflammatory and immunomodulatory effects of MSCs on autoimmune and inflammatory diseases have been increasingly appreciated [17-22]. Additionally, MSCs also took part in the lipid metabolism, reducing serum cholesterol strikingly [23]. These properties of MSCs may open a new avenue for the treatment of atherosclerosis. This review will update the study progress and discuss the possibility to apply MSCs for improving atherosclerosis. The review also proposes some questions that are needed to be overcome.
MSCs inhibit foam cell formation
Multiple lines of evidence, from genetic, experimental, epidemiological to clinical studies, have converged on plasma cholesterol, particularly low density lipoprotein cholesterol (LDL-C), as the primary driver of the initiation and progression of the atherosclerotic plaque. After being recruited to the intima by activated or damaged endothelial cells, the monocytes differentiate into macrophages. These macrophages are then able to take up modified low-density lipoproteins (LDL) particles such as oxidized LDL (ox-LDL) and thereby transform into foam cells. Foam cells, mostly arising from monocytes, are recognized as the early pathological changes of atherosclerosis [24]. During the foam cell formation, two steps are critical in maintaining lipid homeostasis in macrophages: 1) cholesterol uptake mediated by scavenger receptors such as CD36 and scavenger receptor A (SR-A), and 2) cholesterol efflux mediated by ABCA1/ABCG1 [25]. When the balance was disturbed, the foam cells are formated.
Interestingly, a recent study shows that bone marrow derived mesenchymal stem cells (BM-MSCs) can inhibit the formation of macrophage foam cells in vitro and in ApoE-KO mice, and the mechanism underlying this therapeutic effect might partly be related to the downregulation of scavenger receptors CD36 and SRA in response to infusion of BM-MSCs (Figure 1). Also, anti-inflammatory cytokines IL-10, which is thought to be able to modulate the lipid metabolism and protect from atherogenesis, was significantly upregulated in the BM-MSC treatment [25,26]. Moreover, BM-MSCs-treated mice displayed a significant reduction in circulating monocytes and serum cholesterol level [23].
Figure 1.
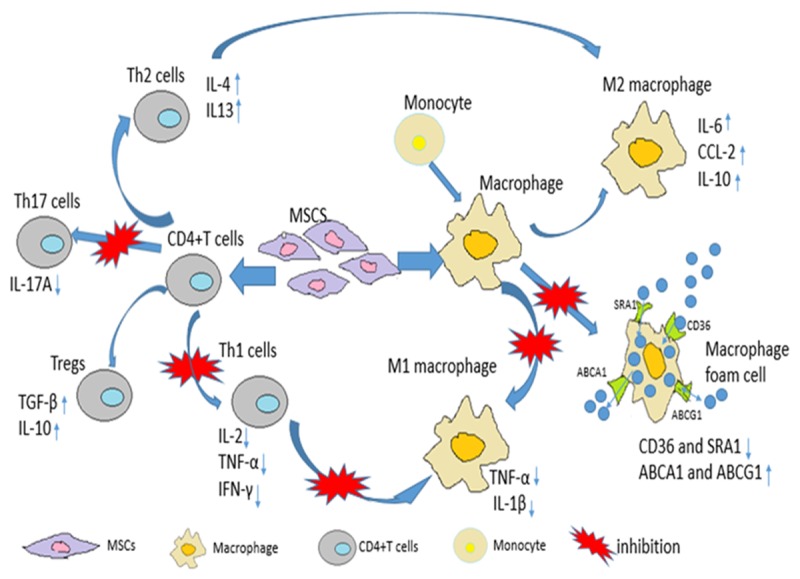
The immunomodulatory and anti-inflammatory effects of MSCs on immune cells involved in atherosclerosis. MSCs suppress Th1, Th17 whereas promote Th2 and Treg cells. MSCs also regulate the balance between M1 and M2 macrophage. MSCs inhibit M1 but facilitate M2 differentiation. MSCs also curb the formation of foam cells from macrophage in atherosclerosis.
MSCs induce the polarization of M2 macrophage
Inflammation plays an important role in all stages of atherosclerosis, which involves different kinds of immune cells. Inside the atherosclerotic plaque, macrophages also account for the vast majority of immune cells [27,28]. Normally, arterial endothelial cells resist the attachment of leukocytes cells streaming past them. When the endothelium is subjected to harmful stimulus such as dyslipidemia and hypertension, monocytes are recruited to the intima where they differentiate into M1 macrophages, produce and release pro-inflammatory cytokines. Further, some of them engulf lipoprotein to become macrophage foam cells.
Interestingly, some studies showed that mesenchymal stem cells can reprogram macrophages into anti-inflammatory phenotypes, M2 macrophages [29,30]. When co-cultured with macrophages, human gingiva-derived mesenchymal stem cells (GMSCs) can convert macrophages into M2 phenotype, inducing the secreting of IL-6, CCL-2, IL-10, and decreasing the production of TNF-α. After systematically injection, GMSCs can home to the wound site to accelerate the wound healing by secreting anti-inflammatory cytokines and enhance macrophage phagocytic capacity [29]. Additionally, cardiac adipose tissue-derived mesenchymal stromal cells (AT-MSCs) also can polarize macrophages toward an M2 anti-inflammatory phenotype, and this function was mediated partly by IL-6 (Figure 1). Interestingly, these AT-MSCs are shown to weaken macrophage phagocytic capacity [30]. Whether macrophage phagocytic capacity is weaker or stronger when co-cultured with MSCs and the signaling pathways by which MSCs play to reprogram macrophage may need further research.
Skin-derived MSCs (S-MSCs) are also able to migrate to the atherosclerotic plaque to modulate the function of macrophages after tail-vein injection and reduce the formation of atherosclerotic plaque in Apo E-/- mice. This modulatory function of S-MSCs in macrophage is thought to partly depend on the impairment of the NF-κβ signaling pathway in S-MSCs and the increased release of COX-2 or PGE2 from S-MSCs. In turn, these changes stimulated the release of anti-inflammatory cytokine IL-10 and decreased the release of inflammatory cytokines TNF-α and IL-1β, leading to the reduction of atherosclerotic lesions in Apo E-/- mice eventually [31].
However, it is noteworthy that aortic smooth muscle cells can be transformed into a dysfunctional macrophage-like phenotype by cholesterol loading [32]. When one tries to evaluate the macrophage inside atherosclerosis, it should be taken into consideration to avoid underestimating the function of macrophage involved in the atherosclerotic plaque [32].
Effects of MSCs on T cells
It was widely thought that immune cells played little role in atherogenesis until Hansson et al reported the presence of lymphocytes within atherosclerotic lesions before 1986 [2]. Although in much lower number than macrophages, accumulating evidences now show that adaptive immune cells, mainly T and B lymphocytes, also exist and involve in atherosclerotic plaque and atherosclerosis. To date, the CD4+ effector T cells that play a role in atherosclerosis include Th1, Th2, Th17 cells and Tregs [2,33-35].
Among these cells, Th1 cells are predominately involved in aggravating atherosclerosis, no matter whether in a plaque of human or mouse [36]. A variety of inflammatory cytokines are produced by Th1 cells, such as IFN-γ, TNF-α and IL-2. Among these inflammatory cytokines, IFN-γ is closely related to the instability of atherosclerotic plaque and reduces the collagen production of smooth muscle cells. IFN-γ also increases the expression of adherence factors and the lipid absorption of macrophage and further increases the rupture of unstable plaques. Cleaning up of CD4+ T cells showed a 70% reduction in plaque size [37], further highlighting the importance of T cells in the pathogenesis of atherosclerosis.
In contrast, few Th2 cells are found in atherosclerotic plaques, their roles in atherosclerosis remains unclear [38-40]. However, polarizing leukocytes to a Th2-like profile can inhibit of experimental atherosclerosis [41,42]. Clinical evidence also supports a protective role of Th2 cells in cardiovascular disease such as myocardial infarction [34].
Th17 cells also exist in atherosclerotic plaques, studies to date are inclined to support that Th17 cells play an atherogenic role in atherosclerosis [33,39,43-45]. However, the role of IL-17A, one cytokine mainly produced by Th17 cells, remains somewhat controversial in atherosclerosis. Some studies suggested that IL-17A is one of the pathogenic factors in atherosclerosis, and inhibiting of IL-17A can reduce atherosclerotic lesion development in ApoE-deficient mice via weakening its widespread of pro-inflammatory and pro-apoptotic effects in atherosclerosis [46]. In contrast, Gistera and her colleagues observed that IL-17A can induce a stable plaque phenotype by stimulating the collagen production of human vascular smooth muscle cells, and blocking IL-17A receptor could increase the cardiovascular events in patients [47].
T regulatory cells (Tregs) are another subset of T cells. They play an important role in maintaining the immune tolerance and immune homeostasis [48-51]. The protective function of Tregs in atherosclerosis has been confirmed in multiple studies [43,52-55]. In unstable atherosclerotic lesions, the number of Tregs is much lower compared to stable ones [54]. Accordingly, an increase in Tregs can alleviate atherosclerosis in animal models [43,53].
Accumulating studies showed that MSCs can wake up the immune activity of different kinds of immune cells including T cells, B cells, NK cells and so on. This immunosuppressive effect provides MSCs an advantage in cell-based therapy. In vitro, studies indicated that GMSCs are able to suppress the activation and proliferation of Th1 and Th17, and enhance the differentiation of regulatory T cells [56]. Also, BM-MSCs are found to promote the expansion of Tregs as well as improve the function of Tregs in atherosclerotic mice [23]. Moreover, BM-MSCs also inhibit the inflammatory cytokines secreted by effector T cells [57]. All together, these studies indicate that MSCs are able to regulate the balance between inflammatory effector T cells and anti-inflammatory Tregs to maintain a stable plaque or reduced atherosclerosis, implicating that MSCs maybe a potent candidate for atherosclerosis therapies (Figure 1).
Impairment of MSCs by age and age-related diseases
Atherosclerosis is a multifactorial-induced chronic disease and usually accompanied by age and age-related diseases. However, age and age-associated conditions also impair the properties and functions of MSCs [58-62]. Although transplantation of human MSCs from all patients can improve the heart function in rats with myocardial infarction (MI), the ability to ameliorate MI is significantly reduced in MSCs from aged subjects than that from younger ones [58]. Also, the angiogenic potential of adipose-derived mesenchymal stromal cells from aged patients declines, even though they can maintain stable mesenchymal stromal cell properties [60]. Moreover, human MSC-mediated T-cell suppression also markedly reduced in aged, T2DM and atherosclerotic subjects [61]. Further, MSCs isolated from experimental type II diabetes displayed the impaired regenerative capacity in post-ischemic neovascularization [62]. The hyperinsulinemia-induced oxidant stress brought by experimental type II diabetes in MSCs may help to explain the impairment of MSCs. Nonetheless, hypoxic stimulation conversely promotes the immunomodulatory properties of GMSCs by inhibiting the proliferation of PBMCs as well as increasing the apoptosis of PBMCs, which was thought to associate with the Fas ligand (FasL) expression of GMSCs [63].
Given to the anti-inflammatory and immune regulatory function, as well as their effect on restoring endothelial function [64], one can conclude that MSCs may be a promising candidate for the treatment of atherosclerosis. Since the vast majority of patients that may benefit from MSCs therapies in atherosclerosis are elderly individuals, clarifying the underlying molecular mechanism by which MSCs function and by which aged-MSCs are weaken, as well as proper MSCs donors selection are important to maximize the therapeutic effect of MSCs in atherosclerosis.
Acknowledgements
This work was supported by the Major Research Plan of the National Natural Science Foundation of Guangdong, China (2014A030308005) and Science and Technology Program of Guangzhou, China (201508020060), Project from the Department of Education in Guangdong Province and Special Project on the Integration of Industry, Education and Research as well as the National Natural Science Foundation of China (81370447, 81671611) and Science and Technology Planning Project of Guangdong Province (2016A050502014).
Disclosure of conflict of interest
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta. 2012;413:1562–1568. doi: 10.1016/j.cca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro MD, Fazio S. From Lipids to Inflammation. Circ Res. 2016;118:732–749. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 4.Hague W, Forder P, Simes J, Hunt D, Tonkin A. Effect of pravastatin on cardiovascular events and mortality in 1516 women with coronary heart disease: results from the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) study. Am Heart J. 2003;145:643–651. doi: 10.1067/mhj.2003.1. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. The Forgotten Majority: unfinished Business in Cadiovascular Risk Reduction. J Am Coll Cardiol. 2005;46:1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Serban MC, Banach M, Mikhailidis DP. Clinical implications of the IMPROVE-IT trial in the light of current and future lipid-lowering treatment options. Expert Opin Pharmacother. 2016;17:369–380. doi: 10.1517/14656566.2016.1118055. [DOI] [PubMed] [Google Scholar]
- 7.Major AS, Harrison DG. What fans the fire: insights into mechanisms of inflammation in atherosclerosis and diabetes mellitus. Circulation. 2011;124:2809–2811. doi: 10.1161/CIRCULATIONAHA.111.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jager SC, Pasterkamp G. Crosstalk of lipids and inflammation in atherosclerosis: the PRO of PGRN? Cardiovasc Res. 2013;100:4–6. doi: 10.1093/cvr/cvt199. [DOI] [PubMed] [Google Scholar]
- 9.Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson ME, Zhang XY, Edfeldt K, Lundberg AM, Levin MC, Boren J, Li W, Yuan XM, Folkersen L, Eriksson P, Hedin U, Low H, Sviridov D, Rios FJ, Hansson GK, Yan ZQ. Innate immune receptor NOD2 promotes vascular inflammation and formation of lipid-rich necrotic cores in hypercholesterolemic mice. Eur J Immunol. 2014;44:3081–3092. doi: 10.1002/eji.201444755. [DOI] [PubMed] [Google Scholar]
- 11.Khan R, Spagnoli V, Tardif JC, L’Allier PL. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis. 2015;240:497–509. doi: 10.1016/j.atherosclerosis.2015.04.783. [DOI] [PubMed] [Google Scholar]
- 12.Mendel I, Yacov N, Harats D, Breitbart E. Therapies targeting innate immunity for fighting inflammation in atherosclerosis. Curr Pharm Des. 2015;21:1185–1195. doi: 10.2174/1381612820666141013133322. [DOI] [PubMed] [Google Scholar]
- 13.Asciutto G, Dias NV, Edsfeldt A, Alm R, Fredrikson GN, Goncalves I, Nilsson J. Low levels of IgG autoantibodies against the apolipoprotein B antigen p210 increases the risk of cardiovascular death after carotid endarterectomy. Atherosclerosis. 2015;239:289–294. doi: 10.1016/j.atherosclerosis.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 15.Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, Mao N. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15:539–547. doi: 10.1038/sj.cr.7290323. [DOI] [PubMed] [Google Scholar]
- 16.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 17.Maria AT, Toupet K, Maumus M, Fonteneau G, Le Quellec A, Jorgensen C, Guilpain P, Noel D. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun. 2016;70:31–39. doi: 10.1016/j.jaut.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol. 2016;22:6036–6048. doi: 10.3748/wjg.v22.i26.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 20.Tan L, Dai T, Liu D, Chen Z, Wu L, Gao L, Wang Y, Shi C. Contribution of dermal-derived mesenchymal cells during liver repair in two different experimental models. Sci Rep. 2016;6:25314. doi: 10.1038/srep25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesne J, Cheminant-Muller MA, Sagan C, Magnan A, Lemarchand P. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells. 2016;34:1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- 22.Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014;10:4730–4741. doi: 10.1016/j.actbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Frodermann V, van Duijn J, van Pel M, van Santbrink PJ, Bot I, Kuiper J, de Jager SC. Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development. Sci Rep. 2015;5:15559. doi: 10.1038/srep15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 25.Han X, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost. 2015;113:505–512. doi: 10.1160/TH14-06-0509. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZX, Wang CQ, Li XY, Feng GK, Zhu HL, Ding Y, Jiang XJ. Mesenchymal stem cells alleviate atherosclerosis by elevating number and function of CD4(+)CD25(+)FOXP3 (+) regulatory T-cells and inhibiting macrophage foam cell formation. Mol Cell Biochem. 2015;400:163–172. doi: 10.1007/s11010-014-2272-3. [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adutler-Lieber S, Ben-Mordechai T, Naftali-Shani N, Asher E, Loberman D, Raanani E, Leor J. Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. J Cardiovasc Pharmacol Ther. 2013;18:78–86. doi: 10.1177/1074248412453875. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Sun W, Wang X, Zhang K, Xi W, Gao P. Skin-Derived Mesenchymal Stem Cells Alleviate Atherosclerosis via Modulating Macrophage Function. Stem Cells Transl Med. 2015;4:1294–1301. doi: 10.5966/sctm.2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Wang Y, Li H, Shen G, Hu S. Ox-LDL influences peripheral Th17/Treg balance by modulating Treg apoptosis and Th17 proliferation in atherosclerotic cerebral infarction. Cell Physiol Biochem. 2014;33:1849–1862. doi: 10.1159/000362963. [DOI] [PubMed] [Google Scholar]
- 34.Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, Bjorkbacka H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33:637–644. doi: 10.1161/ATVBAHA.112.300871. [DOI] [PubMed] [Google Scholar]
- 35.Li N. CD4+ T cells in atherosclerosis: regulation by platelets. Thromb Haemost. 2013;109:980–990. doi: 10.1160/TH12-11-0819. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96:427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- 37.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-gamma: new insights and therapeutic targets. Trends Cardiovasc Med. 2014;24:45–51. doi: 10.1016/j.tcm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelbertsen D, Rattik S, Knutsson A, Bjorkbacka H, Bengtsson E, Nilsson J. Induction of T helper 2 responses against human apolipoprotein B100 does not affect atherosclerosis in ApoE-/- mice. Cardiovasc Res. 2014;103:304–312. doi: 10.1093/cvr/cvu131. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Gao S, Xu W, Zhao S, Zhou J, Wang N, Yuan Z. Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice. J Mol Cell Cardiol. 2014;72:20–27. doi: 10.1016/j.yjmcc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Cheng X, Chen Y, Xie JJ, Yao R, Yu X, Liao MY, Ding YJ, Tang TT, Liao YH, Cheng Y. Suppressive oligodeoxynucleotides inhibit atherosclerosis in ApoE(-/-) mice through modulation of Th1/Th2 balance. J Mol Cell Cardiol. 2008;45:168–175. doi: 10.1016/j.yjmcc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Cao M, Figueroa JA, Cobos E, Uretsky BF, Chiriva-Internati M, Hermonat PL. AAV2/8-hSMAD3 gene delivery attenuates aortic atherogenesis, enhances Th2 response without fibrosis, in LDLR-KO mice on high cholesterol diet. J Transl Med. 2014;12:252. doi: 10.1186/s12967-014-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellison S, Gabunia K, Kelemen SE, England RN, Scalia R, Richards JM, Orr AW, Traylor JG Jr, Rogers T, Cornwell W, Berglund LM, Goncalves I, Gomez MF, Autieri MV. Attenuation of experimental atherosclerosis by interleukin-19. Arterioscler Thromb Vasc Biol. 2013;33:2316–2324. doi: 10.1161/ATVBAHA.113.301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding JW, Zheng XX, Zhou T, Tong XH, Luo CY, Wang XA. HMGB1Modulates the Treg/Th17 Ratio in Atherosclerotic Patients. J Atheroscler Thromb. 2016;23:737–745. doi: 10.5551/jat.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014;40:153–165. doi: 10.1016/j.immuni.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C, He S, Peng Y, Kushwaha KK, Lin J, Dong J, Wang B, Lin J, Shan S, Liu J, Huang K, Li D. TSLPR deficiency attenuates atherosclerotic lesion development associated with the inhibition of TH17 cells and the promotion of regulator T cells in ApoE-deficient mice. J Mol Cell Cardiol. 2014;76:33–45. doi: 10.1016/j.yjmcc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 47.Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 48.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 49.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 50.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2014;111:E3432–3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, Wu Q, Chen Z, Gao Z, Fan H, Tsun A, Shen J, Chen G, Liu Z, Lou Z, Olsen NJ, Zheng SG, Li B. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A. 2015;112:E3246–3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 53.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015;35:280–287. doi: 10.1161/ATVBAHA.114.303568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohm I, Atiskova Y, Drobnik S, Fritzenwanger M, Kretzschmar D, Pistulli R, Zanow J, Krönert T, Mall G, Figulla HR, Yilmaz A. Decreased regulatory T cells in vulnerable atherosclerotic lesions: imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediators Inflamm. 2015;2015:364710. doi: 10.1155/2015/364710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasib L, Lundberg AK, Zachrisson H, Ernerudh J, Jonasson L. Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease. J Intern Med. 2016;279:63–77. doi: 10.1111/joim.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laranjeira P, Pedrosa M, Pedreiro S, Gomes J, Martinho A, Antunes B, Ribeiro T, Santos F, Trindade H, Paiva A. Effect of human bone marrow mesenchymal stromal cells on cytokine production by peripheral blood naive, memory, and effector T cells. Stem Cell Res Ther. 2015;6:3. doi: 10.1186/scrt537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Liu T, Han J, Yang Z, Xue X, Jiang H, Wang H. Advanced age impairs cardioprotective function of mesenchymal stem cell transplantation from patients to myocardially infarcted rats. Cardiology. 2014;128:209–219. doi: 10.1159/000360393. [DOI] [PubMed] [Google Scholar]
- 59.Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc. 2012;1:e002238. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med. 2014;3:32–41. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kizilay Mancini O, Shum-Tim D, Stochaj U, Correa JA, Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res Ther. 2015;6:140. doi: 10.1186/s13287-015-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan J, Tie G, Xu TY, Cecchini K, Messina LM. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev. 2013;9:360–372. doi: 10.1007/s12015-013-9433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang CM, Liu J, Zhao JY, Xiao L, An S, Gou YC, Quan HX, Cheng Q, Zhang YL, He W, Wang YT, Yu WJ, Huang YF, Yi YT, Chen Y, Wang J. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. 2015;94:69–77. doi: 10.1177/0022034514557671. [DOI] [PubMed] [Google Scholar]
- 64.Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


