Abstract
Astrocytes are closely related to the amyloid-β (Aβ) deposition in the brain and play crucial roles in Alzheimer’s disease (AD) pathology. Meanwhile, inflammation in the CNS has been increasingly demonstrated as a prominent hallmark in AD. Our data from animal models and subjects with Alzheimer’s disease (AD) showed GFAP immunoreactivity altered in different stage of AD and had a positive correlation with neprilysin (NEP), suggesting astrocytes might take a protective role in pathogenetic course of AD. Here, we investigate the role of astrocyte in the mechanism of Aβ removal. ELISA and western blotting were performed to determine the ability of astrocyte to clear Aβ1-42. In this study, we demonstrated that cultured astrocytes removed extracellular oligomeric Aβ. However, cultured astrocytes from an AD mouse model showed less capacity to clear extracellular Aβ42 but with hyper-expression of NEP protein than normal astrocytes. In addition, LPS-induced inflammation rather than continuous Aβ stimuli inhibited the capacity of Aβ clearance by astrocytes indicating that inflammation possibly contributed to astrocytic dysfunction. Lastly, HOEC which exhibited anti-inflammatory effects restored the capacity of injured or aged astrocytes to clear Aβ. In conclusion, astrocytes have been shown to exert a direct role in Aβ clearance and undergo functional impair associated with inflammation in the pathogenesis of AD. Therefore, anti-inflammatory treatments aimed at restoring astrocyte functions may represent an appropriate approach to treat AD.
Keywords: Alzheimer’s disease, astrocyte, Aβ clearance, neprilysin, inflammation, HOEC
Introduction
Alzheimer’s disease (AD) is a progressive, age-related neurodegenerative disorder characterized by extracellular and intracellular deposition of the amyloid β-protein (Aβ) [1]. The Aβ plaques are surrounded by a sphere of reactive astrocytes and activated microglia, and cultured astrocytes isolated from human [2,3] and mice [4] can bind and uptake Aβ, suggesting that glial activation is an endogenous defensive mechanism against plaque deposition. On the other hand, however, the associated inflammation resulted by persistent activation of glia cells may also contribute to the progression of AD [5]. It’s been well established that astrocytes are involved in Aβ clearance as mentioned above. Transplanted exogenous astrocytes co-localize with and ingest deposited human Aβ in vivo, while endogenous astrocytes may not be able to respond to and clear the excessive amount of Aβ in the AD mimicking mice [6]. So it is reasonable to assume that the capacity of astrocytes to internalize Aβ may be impaired or saturated in AD. Moreover, it is found that fetal astrocytes are able to engulf Aβ1-42 more efficient than adult astrocytes [3]. The evidence suggests that the ability of Aβ clearance of astrocytes in AD brain might be quite different from the astrocytes in normal brains, and constant Aβ accumulation may gradually result in dysfunction of astrocytes in Aβ clearance mechanism in AD.
Aβ-degrading proteases such as neprilysin (NEP), insulin-degrading enzyme (IDE), endothelin-converting enzyme (ECE), angiotensin-converting enzyme (ACE), plasminogen activators and the matrix metalloproteinases-9 and 2 (MMP-9, MMP-2) [7] play important roles in Aβ clearance. With abundant expression in human astrocytes [8], NEP appears to be the predominant Aβ protease in Aβ clearance in astrocytes both in vivo [9-11] and ex vivo [6]. Particular regional and subcellular localization profiles of different proteases define distinct pools of Aβ, which may contribute differentially to the pathogenesis of AD [4]. Among the subcellular organelles, a portion of endosomes, a place involving the generation [12], accumulation [13] and secretion [14] of Aβ, is a pivotal compartment in Aβ metabolism. However, little is known about co-localization of NEP with endosomes in astrocytes.
Exposure of astrocytes to Aβ could cause neuroinflammation showing upregulation of inflammatory cytokines and increase of the nitric oxide release [15]. Growing number of evidences demonstrate that neuroinflammation and oxidative stress induced by the interaction of amyloid plaques and disturbed astrocytic homeostasis [16] are implicated in the pathological process of AD [17-19]. What’s more, emerging significant epidemiological evidence shows that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) exhibited beneficial effects on Alzheimer’s disease [20]. HOEC, (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate, isolated from Incarvillea maireivargranditlora (Wehrhahn) Griersonis (a Chinese herb), exhibits anti-inflammatory effects by inhibiting 5-lipoxygenase in rodent animals [21]. Several reports including ours support that there was significant protective effects of HOEC against hydrogen peroxide or lipopolysaccharide induced injuries and modulation of inflammation-related signaling [22,23]. An impressing results showing cognitive improvement of HOEC on AD-mimic animal model have been obtained in our lab (data not shown). So we hypothesized that HOEC might affect the function of glial cells.
In this study, we first investigated the functional alteration of astrocyte undergone multiple treatment of Aβ which mimics the microenvironment of astrocytes of AD in vitro. Next, we verified the differences in function of cultured astrocytes from wide-type mice and AD model mice including the capacity of clearing Aβ and the expression of Aβ-degrading proteases in the brains of patients and transgenic mice, and whether continuous exposure to Aβ or a trigger of inflammation can alter astrocytic ability of clearing Aβ in vitro. Last, we explored whether HOEC, a natural anti-inflammatory agent, could restore the capacity of injured or aged astrocytes to remove Aβ in vitro.
Materials and methods
Animals
APPswe/PS1dE9 double-transgenic mice overexpressing a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and human mutant presenilin 1 (PS1-dE9), as well as wild-type C57Bl/6J mice were obtained from Jackson Laboratory. Animals were housed at a temperature of 20-25°C, relative humidity of 50-60%, and a 12 h light-dark cycle environment with free access to food and water. All procedures were carried out in accordance with the guidelines of Care and Use of Laboratory Animals of China for animal experimentation. Astrocyte cultures were prepared either from C57Bl/6J mice or APPswe/PS1dE9 double-transgenic mice.
Case and clinical features
Frozen frontal cortex from 6 NCI, 10 MCI and 12 AD were obtained from participants in the Religious Orders Study of the Rush Alzheimer Disease Center (P30AG10161) [24]. All individuals had undergone a uniform structured clinical evaluation that included a medical history, neurologic examination, neuropsychological performance testing, and diagnostic classification for dementia and AD, and MCI; NCI referred to those individuals without dementia or MCI. The study was approved by the Human Investigations Committee of Rush University and signed an informed consent and Anatomic Gift Act or organ donation.
Multiplexed immunohistochemistry
Paraffin embedded frontal cortex tissue sections mounted on glass slides from NCI, MCI and AD or from control C56 WT mice and various ages of transgenic APP/PS1 mice were taken through deparaffinization and rehydration. Slides were blocked with endogenous peroxidase blocking buffer (Peroxidazed, Biocare Medical LLC), protein blocking buffer, and alter- native antigen retrieval. For multiple labeling, the primary antibodies were applied as a cocktail (rat anti-NEP (15 μg/ml, R&D) in antibody diluent (Renoir Red, Biocare Medical, LLC) overnight at 4°C. Sections were washed with TBS and then incubated with goat anti-mouse HRP polymer for 30 minutes at room temp and goat anti-rabbit AP polymer (Mach 2 Rabbit AP-Polymer) for 60 minutes at room temp. Immunoreactivity was visualized with brown chromogen (Betazoid DAB Chromogen Kit) and red chromogen (Warp Red Chromogen Kit Biocare Medical, LLC) for 7 minutes. After rinsing thoroughly with distilled water, antibodies were denatured (Denaturing Solution Kit Biocare Medical, LLC) for 3 minutes. The third primary antibody was applied (rabbit anti GFAP, 1:200, EnCor Biotechnology) in Renoir Red diluent for 60 minutes at room temp. After washing, goat anti-rabbit HRP polymer was applied for 30 minutes at room temp. The third chromogen (Vina Green Chromogen Kit, Biocare Medical, LLC) was incubated for 10 minutes. Slides were counterstained with hematoxylin, rinsed with distilled water, dehydrated through graded alcohols and cleared in xylene and coverslipped. The slides were quantitatively analyzed using state-of-the-art imaging systems VectraTM or NuanceTM (Perkin Elmer Waltham, MA). By using a spectral library, co-localized signals from different antibodies were unmixed and quantified inrandomly selected regions of interest (ROI) for analysis.
Cell culture and immunocytochemistry
C6 rat glioma cells were obtained from the American Type Culture Collection (ATCC). Human U87 astrocytoma cell was from Dr. Jianmiao Liu’s lab. Cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS in a humidified atmosphere of 5% CO2 at 37°C, fed every 2 days and subcultured once they reached 80-90% confluence into 7 cm cell culture dishes. Experiments were carried out 12-24 h after cells were seeded. For immunocytochemical staining, cells were plated onto coverslips in cell culture plates (Jet Bio-Filtration Products Co., Ltd., Guangzhou, China) at a density of 1×105 cells/mL. After 24 h, coverslips were washed three times in D-Hanks, fixed in 4% paraformaldehyde/PBS, then blocked and permeabilized in the buffer containing 5% heat inactivated normal bovine serum/PBS/0.3% Triton-X100. To examine the localization of Aβ-degrading proteases in late endosomes, cells were double stained with late endosomes proteins (Rab7, 1:500, Santa Cruz) and anti-neprilysin (CD10) antibody (1:50, Abcam), washed, and incubated with corresponding AlexaFluor488/594-conjugated secondary antibody (1:4000, Invitrogen). Nuclei of each cell were stained with Hoechst 33342 (1 µg/mL, Sigma). After a final wash and mount with glycerol, cells were imaged with a fluorescence Nikon Eclipse E800 microscope and Spot advanced digital camera (Diagnostic Instruments, Sterling Heights, MI).
β-galactosidase staining
Astrocytes underwent different treatments were rinsed with PBS one time and fixed in fixative solution for 30 min at room temperature. After two-time rinse with PBS, β-galactosidase staining solution is added and the plate is incubated at 37°C overnight. Check the cells under a microscope (200× magnification) for the development of blue color which represents the degree of senescence in astrocyte cultures. The senescence β-galactosidase staining kit (Cell Signaling Technology) identifies senescent cells in culture [25]. Cells were observed using a microscope (Nikon Eclipse TS100) and digitally photographed with a camera (Nikon digital sightDS-U3).
Neonatal and adult astrocyte cultures
Cultured astrocytes were prepared from the cerebral cortex of newborn wildtype mice or 12-month-old APP/PS1 mice as described previously [26], with slight modifications. In brief, newborn mice were sacrificed by decapitation. The brains were aseptically removed and the midbrain, meninges and blood vessels were dissected. The remaining cerebral cortices were mechanically dissociated by pipetting for 2 min in 40 mL of DMEM/F12 (Dulbecco’s modified Eagle medium/Ham’s F-12, Invitrogen) containing a mixture of penicillin/streptomycin 100 U/0.1 mg/mL (SunBio, CA) or enzymatic dissociated by medium containing papain (1 mg/mL, Sigma-Aldrich), dispase II (1.2 U/mL, Roche) and DNase I (20 U/mL, Invitrogen) for 20 min. The suspension was filtered through a 70-μm pore size nylon mesh cell strainer (Biologix, CA). Then, the cells were plated in 75 cm2 culture flasks (Corning) at 15×106 cells/flask and maintained in DMEM/F12 supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 2 mM L-glutamine (Invitrogen) at 37°C in a 5% CO2/95% air incubator. The culture medium was changed every other day. When an astrocytic monolayer formed, the flasks were shaken at 200 rpm on a rotary shaker at 37°C for 24 h to dislodge microglia and oligodendrocytes. The medium was immediately discarded and replaced with a fresh medium. 98% attached cells are astrocytes, as assessed by immunocytochemical staining with anti-GFAP antibody (glial fibrillary acidic protein, 1:1000 dilution; EnCor Biotechnology Inc.).
The treatment of astrocyte and assay of Aβ clearance
For preparation of Aβ42 oligomers, synthetic human Aβ (ChinaPepetides) were dissolved in ddH2O at 1×10-4 mol/L and incubated at 37°C for 3-4 days. Prior to cell experiments, astrocytes were seeded at 1×105 cells/mL in 24-well plates in DMEM/F12 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Cells were allowed to adhere and recover (normally 48 h) and then variously treated in serum-free DMEM/F12 medium containing 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Lipopolysaccharide is commonly accepted as a potent pro-inflammatory agent. Astrocytes were treated with 1 µg/mL LPS (L-2654, Sigma) for 24 h alone or pretreated with 10 μM HOEC for 2 h before assay of Aβ clearance. HOEC was provided by department of Natural product chemistry, Second Military Medical University. The purity of the synthetic HOEC was more than 97%. For all experiments, HOEC was dissolved freshly in DMSO and diluted with the media for cell culture before using.
For analysis of Aβ clearance, cells were exposed to 0.2 μM human Aβ1-42. At various time points, Aβ levels in cell-culture supernatants and adherent cells were determined by ELISA (Human Aβ42 enzyme-linked immune sorbent assay kit, Invitrogen) or immunoblotting with antibody B-4 (against amino acids 672-714 of amyloid A4 representing full length β-Amyloid of human origin, 1:200 dilution; Santa Cruz Biotechnology).
Western blotting
Both supernatants and cell pellets were collected to determine Ab levels. Cells were washed two times in ice-cold D-Hanks, and then lysed in ice-cold lysis cell protein extraction buffer (50 mM Tris HCl pH = 7.5, 2 mM EDTA, 1% NP-40, 150 mM NaCl, 0.1% SDS, 0.25% sodium deoxycholate) containing protease inhibitors (1 mM PMSF and Cocktail, Sigma). Both supernatant and cell lysate samples were stored at -80°C until analysis.
For Western blot analysis, aliquots of the protein extracts were mixed with sample buffer and boiled for 5 min. Proteins were separated via 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis, and transferred to polyvinylidine difluoride (PVDF) membrane (0.22 μm, Millipore). The membranes were blocked with 5% BSA, 0.05% Tween-20 in Tris-buffered saline (TBS) for 1 h at RT followed by overnight incubation with the following primary antibodies: mouse monoclonal B-4 (amino acids 672-714 of Aβ peptide, 1:200, Santa Cruz) or mouse monoclonal anti-CD10 antibody (1:1000, Abcam). After three washes in TBST buffer, membranes were incubated with HRP-conjugated anti-mouse antibody (1:5000, Santa Cruz) for 2 h at room temperature. The immunolabeled protein bands were visualized by using ECL Western blotting detection system (Tanon5200S, CA) and autoradiography film.
ELISA
The levels of Aβ42 in supernatant and cell pellet were quantified using ELISA (a sandwich enzyme immunosorbant assay) kit following manufacturer’s instructions and detected by EnSpire® Multimode Plate Reader.
Image analysis and statistics
All the Multiplexed images were analyzed by Vectra/Nuance systems. Image J program were used to analyze the images of Western blotting, immunofluorescent staining. Data were expressed as mean ± standard error of the mean (SEM) unless otherwise specified. Statistical analyses were performed with one-way ANOVA followed by least significant difference post hoc analysis(multiple comparisons) and liner regress analysis with threshold of P < 0.05, *P < 0.05 and **P < 0.01.
Results
Astrocytes altered and correlated with NEP protein levels in the progression of AD
To investigate the roles of astrocyte and neprilysin (NEP) in Aβ clearance during the process of AD, immunohistochemical staining was used to detect cortical NEP and GFAP (a marker of astrocyte) in the persons of NCI, MCI and AD, as well as APP/PS1 transgenic mice. Immunopositive signals of NEP and GFAP were visualized and analyzed with a digital imaging system (VectraTM or NuanceTM). Our data showed that immunoreactivity of cortical NEP in MCI was higher than NCI, while GFAP immunoreactivity in AD dementia was lower than NCI suggesting astrocyte injury or lose in AD (Figure 1A, 1B). Correlation analysis found a positive correlation between NEP and GFAP immunoreactivity levels in cortex of AD brains (P = 0.047), but negative correlations both in MCI and NCI (P < 0.0001) (Figure 1E, 1F). As transgenic mice is proved to be a valuable AD model system sharing the similar pathological features such as Aβ deposition with AD case, we also determined NEP and GFAP protein levels in different ages of APP/PS1 mice. NEP immunoreactivity increased in 3-month-old APP/PS1 mice (sharing similar pathological features with MCI cases) and then decreased after 6-month-old APP/PS1 mice (similar pathological features with MCI cases), while GFAP increased after 3 months and decreased at 12 months (Figure 1C, 1D), which consisted with the findings in human.
Figure 1.
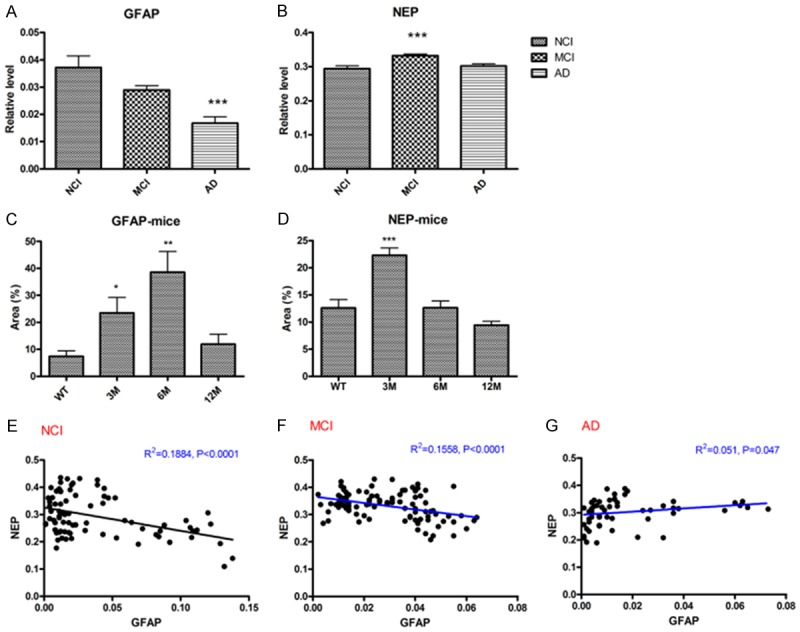
The relationship between NEP and GFAP in AD: (1) correlation analysis of NEP/GFAP pixel density. A significant negative correlation between NEP and GFAP was observed in both NCI (R2 = 0.1884, P < 0.0001) and MCI brains (R2 = 0.1558, P < 0.0001). However, positive correlation between NEP and GFAP was observed in AD brains (R2 = 0.051, P = 0.047). E: NCI; F: MCI; G: AD. (2) The relative levels of NEP and GFAP in NCI, MCI and AD cortex. Brain slides from NCI, MCI and AD cortex were immunostained for NEP and GFAP followed by quantitative analysis using Art Imaging Systems VectraTM. A: GFAP; B: NEP. The data are expressed as mean ± SEM. ***P < 0.01 vs NCI control. (3) Alteration of cortical NEP and GFAP levels in APP/PS1 transgenic mice. Brain slides from C57 WT mice and various ages of transgenic APP/PS1 were immunostained for NEP and GFAP following by quantitative analysis using Art Imaging Systems NuanceTM. Total positive pixel area for NEP and GFAP in mice at 3, 6, 9 and 12 months of age. C: GFAP; D: NEP. The data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs WT control.
We further examined subcellular localization of neprilysin (NEP) in GFAP-positive C6 astrocytoma cell line by double immunostaining of NEP and late endosomal marker proteins Ras-related protein 7 (Rab7). The result showed that neprilysin was expressed on C6 cells and in part colocalized with late endosomal marker proteins Ras-related protein 7 (Rab7) (Figure 2).
Figure 2.
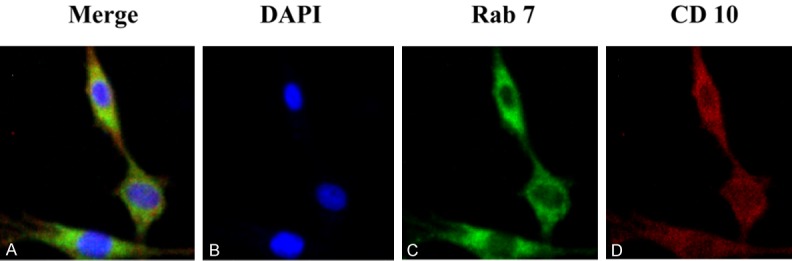
Intracellular distribution of neprilysin (NEP) on C6 cells (A). C6 cells were co-stained with antibodies against rab7 (C), marker of late endosome, and CD10 (D), marker of NEP. Nuclei were stained with DAPI (B).
Astrocytes were capable of clearing oligomeric Aβ involving NEP upregulation
Astrocytes play neuroprotective roles via internalizing and degrading Aβ in brain. We investigated the process of Aβ clearance by cultured mouse astrocytes and examined whether there was involvement of Aβ degrading protease such as NEP. To quantitate the clearance of Aβ by astrocytes, we exposed these cells to oligomeric Aβ1-42 and determined the Aβ content of cell-culture supernatant and the cell fraction by ELISA (Figure 3B) and Western blotting analysis (Figure 3A). After addition of 0.2 µM Aβ1-42 peptides into the medium, the Aβ in supernatant sharply declined at 3 h and then gradually decreased and dropped to minimum at 24 h. The monomer and aggregation of Aβ1-42 were detected in cell pellet by immunoblotting with anti-Aβ antibody (Figure 3A). Interestingly, most of intracellular Aβ in astrocytes are tetramers (as the molecular weight of the brand is about 16 kDa), suggesting that astrocytes can phagocytose oligomeric Aβ. The result is consistent with the previous study which demonstrated that human astrocytes preferably take up Aβ oligo over Aβ fib [2]. And soluble Aβ oligomers are more toxic to cells as compared with aggregates such as Aβ fibrils and amyloid plaques [27,28]. In addition, we also found that the level of neprilysin (NEP) in pellet was increasing with time, indicating that NEP is involved in Aβ clearance by astrocytes. These findings showed a normal function of cultured neonatal astrocytes in degrading Aβ1-42 with the involvement of NEP. Similar results were obtained using standard ELISA procedures to measure Aβ1-42 levels in the same cell cultures (Figure 3B).
Figure 3.
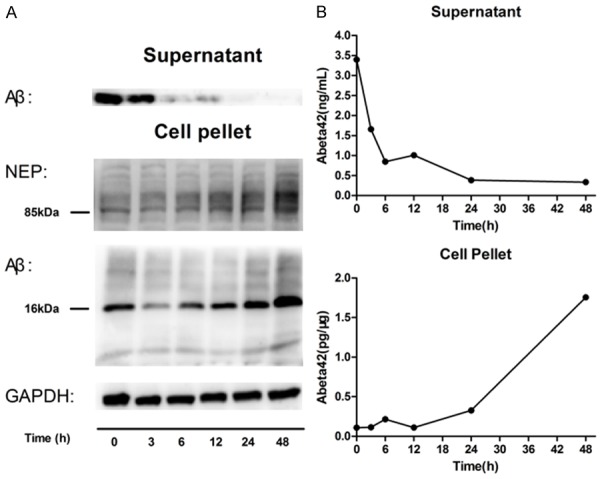
Clearance of Aβ42 by neonatal mouse astrocytes as measured by ELISA (B) and western blot (A). Cultured astrocytes from neonatal mice were incubated with 0.2 μM synthetic Aβ1-42 for 0-48 h, and supernatant or adherent cells (pellet) were collected after the indicated time points. The monomer and aggregation of Aβ1-42, mostly tetramers, were detected in cell pellet when Aβ apparently decreased after 3 h and disappeared at 24 h in supernatant. Besides, with the increasing amount of Aβ1-42 in cell, a key Aβ degrading protease neprilysin (NEP) level rose. One representative experiment is shown (n = 3 experiments).
Astrocytes from AD mice showed deficiency in clearance of extracellular Aβ1-42
Next, we investigated the difference in capacity to remove extracellular Aβ between astrocytes derived from control and AD model mice. The senescence characteristics in astrocyte cultures of WT and AD were evaluated using the β-galactosidase staining kit. Astrocytes with an intense blue stain were only clearly evident in AD model astrocyte cultures (Figure 4C and 4D). β-galactosidase staining displayed more aged astrocytes in APP/PS1 mice (Figure 4D) than that from WT mice (Figure 4C). To quantitate the degradation of Aβ by aged astrocytes, we determined the Aβ content of the cell fraction and cell-culture supernatant by ELISA assay. Aβ levels in supernatant of astrocytes from APP/PS1 markedly decreased after 24 h, whereas Aβ in WT astrocytes’ culture was quickly removed within 3 h (Figure 4A and 4B), indicating that aged astrocytes from AD mice were less capable of degrading Aβ than WT astrocytes. Besides, astrocytes from AD mice expressed more NEP than the WT group, while intracellular NEP protein level gradually rose in the process of Aβ clearance by astrocytes both from two groups (Figure 4E).
Figure 4.
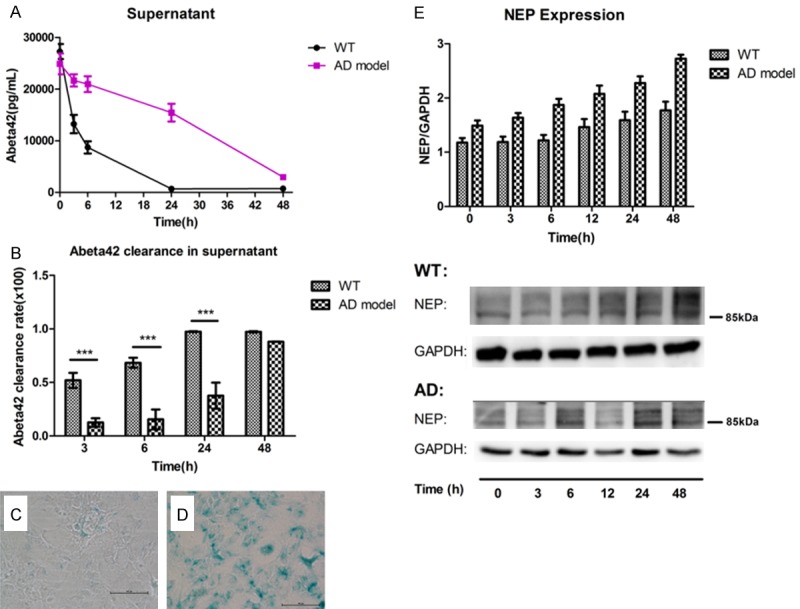
The difference of capacity of extracellular Aβ42 clearance (A, B) and NEP (E) expression in the astrocytes from AD model and WT mice. Cultured astrocytes from 11-month APP/PS1 (AD model) mice and wide-type (WT) mice were incubated with 0.2 μM synthetic Aβ1-42 for 0-48 h, and supernatant or adherent cells (pellet) were collected after the indicated time points. Clearance of Aβ42 in supernatant by WT astrocytes or AD astrocytes was measured by ELISA (A) and the capacity to clear extracellular Aβ42 was valued by extracellular Aβ42 clearance rate (B). Extracellular Aβ42 clearance rate = (the max amount of Aβ42 in supernatant - the present amount of Aβ42 in supernatant) ÷ the max amount of Aβ42 in supernatant. Each value is the mean ± SD of three determinations (*P < 0.05). NEP level in WT astrocytes or AD astrocytes pellet was determined by western blotting and quantification analysis of bands intensity was conducted (E). Data is means ± S.E.M from at least three independent experiments. A representative stain of β-galactosidase in cultured astrocytes from WT mice (C) and AD model mice (D). Blue-stained cells were detected much more in cultured astrocytes from AD model mice than cells from WT mice. (C, D) 200×.
Inflammation contributes more to astrocytic impair capacity of Aβ clearance rather than Aβ itself
As deficits of astrocyte related Aβclearance in AD, we speculated that overexposure of astrocytes to Aβ might cause alteration of Aβ clearance. Therefore we next investigated the process of Aβ clearance by astrocytes continuously exposed to oligomeric Aβ1-42 peptide. Primary cultured astrocytes were treated as illustrated in (Figure 5A). In the first period, Aβ42 level decreased in the supernatant (Figure 5B) without significant increase in the cell-fraction (Figure 5C). In the second period, with the rapid decrease of Aβ in the supernatant (Figure 5B), intracellular Aβ significantly increased and reached a peak at 6 h (Figure 5C). In the third period, however, both supernatant and intracellular Aβ were much lower than that in the second period (Figure 5B, 5C). Similar results were obtained using Western blot analysis (Figure 5D). The result suggested continuous exposure of exogenous Aβ enhanced the ability in astrocytes in Aβ clearance to respond to exogenous Aβ rapidly.
Figure 5.
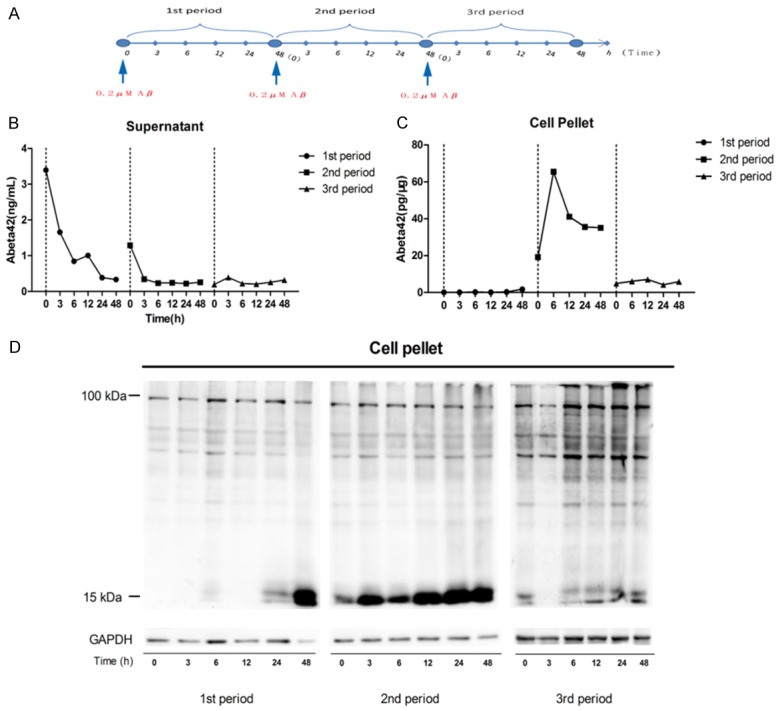
Clearance of Aβ42 by continuous Aβ oligo stimulated cultured astrocytes. Astrocytes were incubated with 0.2 μM synthetic Aβ1-42 for different period of time as illustrated in (A), and supernatant or adherent cells (pellet) were collected after the indicated time points. The amount of Aβ was measured by ELISA (B, C) and western blot (D).
Meanwhile, neuroinflammation has been increasingly demonstrated as a prominent hallmark in various neurodegenerative diseases, which may contributes to the exacerbation of the disease. We therefore examined whether the capacity of astrocytes to clear Aβ was altered when incubated with lipopolysaccharide (LPS), and anti-inflammatory agents retrieved the ability of astrocyte in Aβ clearance. After a 24 h pre-treatment of LPS, synthetic Aβ1-42 was added to the medium and the cell fractions and supernatants was determined by ELISA at different time points (Figure 6). As shown in Figure 6A, the extracellular Aβ decreased rapidly and undetected after 24 h in control astrocytes (no LPS pre-treatment). The Aβ in supernatant of LPS-treated astrocyte, however, was still detectable after 48 h, indicating Aβ scavenging of LPS-treated astrocytes was much slower than untreated astrocytes. So it is supposed that LPS inhibited astrocytes to clear Aβ peptide from the cultures.
Figure 6.
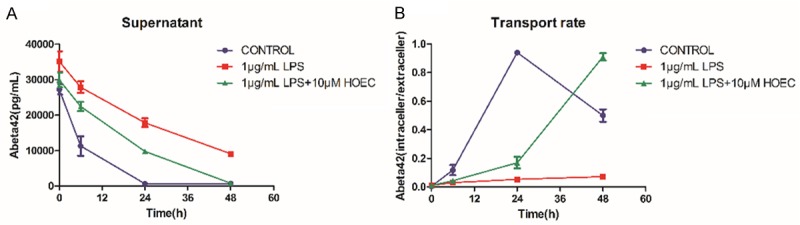
Protective effect of HOEC on LPS-induced decrease of Aβ clearance in primary cultured mouse astrocytes in vitro. Three groups of astrocytes were treated differently before assay of Aβ clearance: (i) cells with no treatment as control, (ii) cells treated with 1 µg/mL LPS for 24 h alone, (iii) cells pretreated with 10 μM HOEC for 2 h and then treated with 1 µg/mL LPS. Later cells were incubated with 0.2 μM synthetic Aβ1-42 for 0-48 h, and supernatant or adherent cells (pellet) were collected after the indicated time points. The amount of Aβ42 in supernatant was determined by ELISA (A). And transport rate was caculated (B). Transport rate = intracellular Aβ42 level/extracellular Aβ42 level. Values are means ± SEM (n = 3).
HOEC retrieved the capacity of Aβ clearance in injured or aged astrocytes
To our knowledge, restoring the function of astrocytes via neuroprotective and anti-inflammatory treatments might be a potential approach to treat AD [29]. (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate (HOEC), a caffeic ester, is a natural product isolated from lncarvilleamairei var. granditlora (Wehrhahn) Grierson and has been reported a neuroprotective and anti-inflammatory effects [21-23]. Here we examined the effect of HOEC on LPS-induced changes of astrocytes in Aβ clearance. We found HOEC could retrieved the ability of injured astrocytes to clear Aβ, when cells were pretreated with 10 µM HOEC for 2 h prior LPS-stimulation (Figure 6). Furthermore, pretreated with 10 µM HOEC, astrocytes from 11 months of APP/PS1 mice could significantly increase the clearance rate of removing extracellular Aβ, suggesting that HOEC could improve the aged astrocytes of AD-model on removing Aβ from the medium (Figure 7).
Figure 7.
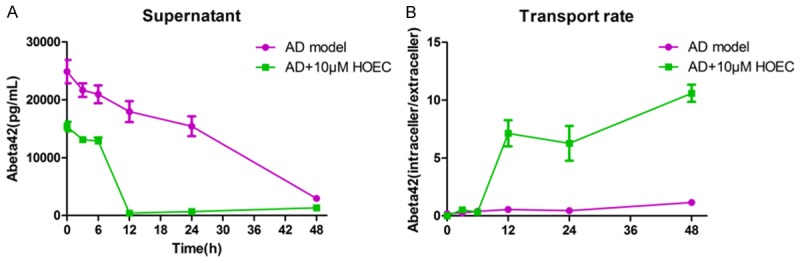
HOEC retrieved the capacity of AD model astrocyte to clear Aβ42 in vitro. Cultured astrocytes from 11-month of APP/PS1 (AD model) mice were treated with 10 μM HOEC for 2 h. Then cells were incubated with 0.2 μM synthetic Aβ1-42 for 0-48 h. Supernatants or adherent cells (pellet) were collected at the indicated time points. The amount of Aβ42 in supernatant was detected by ELISA (A). And transport rate was caculated (B). Transport rate = intracellular Aβ42 level/extracellular Aβ42 level. Values are means ± SEM representing 3 independent experiments (n = 3).
Discussion
As the most abundant type of cells in the CNS, astrocytes with important metabolic and supportive functions play a crucial role in AD pathogenesis. Upon exposure to Aβ, the glial was activated, and the activation has been considered as an endogenous defensive mechanism against plaque deposition. On the other hand, the persistent activation and associated inflammation may also contribute to the progression of AD [30]. Exposure of astrocytes to Aβ could cause detrimental consequences by upregulating inflammatory cytokines, increasing the release of nitric oxide in cultured astrocytes [15], and even inducing death of astrocytic cell [31].
Our data showed, either in human cases or animal models, cortical GFAP immunoreactivity was significantly regulated in the process of AD development, suggesting the involvement of astrocyte in AD progression. Increased cortical GFAP immunoreactivity during 3 to 6 months’ APP/PS1 mice indicated that astrocytes were activated or stimulated in response to Aβ accumulation in early stage of AD. However, GFAP immunoreactivity declined both in AD cases and 12 months’ APP/PS1 mice, suggested that astrocytes impaired and lost in late stage of AD. We also observed a significant increase of NEP immunoreactivity in the cortex of MCI and AD mice at 3 month, which is agree with the previous reports [32]. Meanwhile, correlation analysis showed a positive correlation of immunoreactivity level between NEP and GFAP was observed in the present study. While the correlation between NEP and GFAP was negative in NCI and MCI. Since there is no correlation between NeuN and NEP indicated by our previous report [32], we speculate astrocyte-related NEP might play more important roles in AD, especially in the late stage.
In AD, Aβ is considered to be the principle factor responsible for inducing and chronically stimulating glial cell activation. To explore the changes of astrocyte on Aβ clearance in the process of AD, we next studied how astrocytes act to multi- or persistent Aβ burden, and how different that astrocytes from WT and late stage of AD response to Aβ burden in vitro. Our results demonstrated that cultured astrocytes of wild-type mouse are capable of clearing extracellular Aβ. This Aβ clearance is associated with internalization of oligomeric Aβ and upregulation of Aβ degrading protease NEP in astrocytes. Moreover, it is reported that adult human astrocytes prefer to take up oligomeric Aβ rather than fibrillar Aβ [2,3], since oligomeric Aβ are more toxic to cells than fibrillar Aβ [27,28,33]. Because astrocytes greatly outnumber microglia in the brain [34] and there is no difference in uptake of Aβ oligo between human astrocytes and microglia [35], astrocytes could play more critical role in Aβ removal than ever thought. On the other hand, it should be noted that most Aβ we found in pellets are trimers and tetramers which were previously identified to be higher toxic than monomers and dimers [33,36,37]. Therefore, it is possible that the intracellular oligomeric Aβ is toxic to astrocytes, and the astrocytes engulfing Aβ got injured gradually and eventually died, which is in accordance with our findings that GFAP immunoreactivity decreased in AD brains.
In addition, we investigated subcellular localization profiles of NEP in astrocyte and found NEP was abundantly present in late endosomes of astrocytes, a place involving the generation [12], accumulation [13] and secretion [14] of Aβ, which was an important compartment in Aβ metabolism. The expression of Aβ-degrading proteases specifically in endosomes possibly indicates their involvement in endosomal/lysosomal degradation of internalized Aβ peptides, which was thought to be advantageous for efficient degradation of Aβ oligomers. It’s been proposed that the expression of endosomes specific NEP possibly indicated the involvement of endosomal/lysosomal degradation of internalized Aβ peptides, and to be beneficial to efficient degradation of Aβ oligomers [38]. And the uptake of Aβ42 oligomers and their subsequent proteolytic degradation in astrocytes area key course of extracellular Aβ clearance [38]. Thus, these results indicate that NEP may play crucial roles in the clearance of Aβ in astrocytes.
Although astrocytes in normal condition with no stimuli have effective phagocytic and proteolytic activity, little was known about the function of astrocytes in AD cases. Since phenotypes of activated astrocytes from aging rat cerebral cortex persisted in primary cultures [39], we cultured the astrocytes from aged APP/PS1 mice and found these cells became senescent. Pihlaja, Koistinaho et al. reported that endogenous astrocytes in the transgenic APdE9 mouse brain do not able to reduce Aβ to the same extent as the transplanted cells [6]. Coincidentally, our date shows that cultured astrocytes from AD model mice were less capable of removing extracellular Aβ, which supports our assumption that the ability of astrocytes in AD cases to internalize Aβ may be impaired or saturated. They raise the possibility that astrocytic dysfunction in Aβ clearance may contribute to the accumulation of Aβ in AD. Besides, the deficiency of astrocytes on Aβ clearance in AD is uncorrelated to the expression of NEP, suggesting the NEP activity may decrease or other non-peptidolytic removal pathway of Aβ may be impaired, which is consistent with our previous report [32].
Zhao et al. hypothesized the ability of astrocytes to clear Aβ may be impaired in aged AD astrocytes, especially when extracellular Aβ levels increase continuously or remain exceptionally high. However, by continuous exposure of cultured astrocyte to exogenous oligomeric Aβ, we did not observe the impairment in astrocytic ability to clear Aβ but a faster response to exogenous Aβ. An environment where Aβ continue to be “produced” could lead to intracellular Aβ accumulation and APP expression increase, since oligomeric Aβ42 is reported to increase levels of astrocytic BACE1, APP, and beta-secretase processing [40]. Overall, Aβ alone may not able to reduce the ability of astrocytes to clear Aβ both intracellular and extracellular.
Neuroinflammation has been increasingly demonstrated as a prominent hallmark in various neurodegenerative diseases such as AD. The astrocytes surrounding Aβ deposition acquire a reactive phenotype and ultimately triggers the neuroinflammatory process [41] which may injure themselves in turn. Lipopolysaccharide (LPS) is commonly accepted as a potent pro-inflammatory agent and has been used as an inflammatory stimulus to examine effects of age on the activation of glial cultures from adult rat brain [42]. And lipopolysaccharide receptor CD14 who interacts with fibrils of Alzheimer amyloid peptide may significantly contribute to the overall neuroinflammatory response to amyloid peptide [43]. It makes sense to presume that the LPS-induced inflammation in astrocytes may affect their neuroprotective function against Aβ in brain. Our data showed that astrocytes under a LPS-induced inflammatory condition possess a less effective ability to remove Aβ from extracellular space than cells in normal condition. Interestingly, HOEC which owns neuroprotective and anti-inflammatory effects [21-23] can restore the ability of astrocytes in an inflammatory condition to clear the Aβ and enhance the internalization of extracellular Aβ. Since there are functional differences between cultured astrocytes from wide-type and AD model mice, HOEC can also rescue the capacity of AD model astrocytes in Aβ clearance possibly by increasing the astrocytic phagocytosis of Aβ. Therefore, our data supports the assumption that early treatment with neuroprotective and anti-inflammatory drugs aimed at restoring astrocyte functions may represent an appropriate approach to treat AD [29].
Acknowledgements
This project was supported by the grants from National Natural Science Foundation of China 81072627 and 81230090; the 111 Project (Grant No. B07023) from Ministry of Education; Key project from Shanghai Science and Technology Committee (12431900901). The human samples used in this study are from NIH grants to David Bennett (P30AG10161, R01AG15819). We are grateful to Dr. Wei Huang (Department of Pathology and Laboratory Medicine, School of Medicine and Public Health, University of Wisconsin) for the technical support and valuable comments on the manuscript writing.
Disclosure of conflict of interest
None.
References
- 1.Cuello AC. Intracellular and extracellular Abeta, a tale of two neuropathologies. Brain Pathol. 2005;15:66–71. doi: 10.1111/j.1750-3639.2005.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen HM, Mulder SD, Belien JA, Musters RJ, Eikelenboom P, Veerhuis R. Astrocytic A beta 1-42 uptake is determined by A beta-aggregation state and the presence of amyloid-associated proteins. Glia. 2010;58:1235–1246. doi: 10.1002/glia.21004. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen HM, Veerhuis R, Holmqvist B, Janciauskiene S. Binding and uptake of A beta1-42 by primary human astrocytes in vitro. Glia. 2009;57:978–988. doi: 10.1002/glia.20822. [DOI] [PubMed] [Google Scholar]
- 4.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 5.Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E. Amyloid beta-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol. 2000;100:608–617. doi: 10.1007/s004010000242. [DOI] [PubMed] [Google Scholar]
- 6.Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M. Multiple cellular and molecular mechanisms are involved in human Abeta clearance by transplanted adult astrocytes. Glia. 2011;59:1643–1657. doi: 10.1002/glia.21212. [DOI] [PubMed] [Google Scholar]
- 7.Leissring MA. The AβCs of Aβ-cleaving Proteases. J Biol Chem. 2008;283:29645–29649. doi: 10.1074/jbc.R800022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfman VB, Pasquini L, Riudavets M, Lopez-Costa JJ, Villegas A, Troncoso JC, Lopera F, Castano EM, Morelli L. Differential cerebral deposition of IDE and NEP in sporadic and familial Alzheimer’s disease. Neurobiol Aging. 2010;31:1743–1757. doi: 10.1016/j.neurobiolaging.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata N, Tsubuki S, Takaki Y, Shirotani K. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 11.Miller BC, Eckman EA, Sambamurti K, Dobbs N, Chow KM, Eckman CB, Hersh LB, Thiele DL. Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci U S A. 2003;100:6221–6226. doi: 10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer’s disease. J Mol Neurosci. 2001;5:137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- 13.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Aβ42 Accumulation in Human Brain. Nat Rev Neurosci. 2007;5:499–509. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Akama KT, Krafft GA, Chromy BA, van Eldik LJ. Amyloid-β peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 16.Yan LJ, Xiao M, Chen R, Cai Z. Metabolic Dysfunction of Astrocyte An Initiating Factor in Beta-amyloid Pathology. Aging Neurodegener. 2013;1:7–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Quintanilla RA, Orellana JA, von Bernhardi R. Understanding risk factors for Alzheimer’s disease: interplay of neuroinflammation, connexin-based communication and oxidative stress. Arch Med Res. 2012;43:632–644. doi: 10.1016/j.arcmed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 2010;16:2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 19.Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis. 2004;6:147–157. doi: 10.3233/jad-2004-6206. [DOI] [PubMed] [Google Scholar]
- 20.Sastre M, Gentleman SM. NSAIDs: How they Work and their Prospects as Therapeutics in Alzheimer’s Disease. Front Aging Neurosci. 2010;2:20. doi: 10.3389/fnagi.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Zeng HW, Liu F, Zhang JG, Yue RC, Lu W, Yuan X, Dai WX, Yuan H, Sun QY. Target identification and validation of (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate, an anti-inflammatory natural product. Eur J Inflam. 2012;10:297–309. [Google Scholar]
- 22.Chen B, Yue R, Yang Y, Zeng H, Chang W, Gao N, Yuan X, Zhang W, Shan L. Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem Res. 2015;40:531–541. doi: 10.1007/s11064-014-1498-5. [DOI] [PubMed] [Google Scholar]
- 23.Shen JN, Xu LX, Shan L, Zhang WD, Li HL, Wang R. Neuroprotection of (+)-2-(1-Hydroxyl-4-Oxocyclohexyl) Ethyl Caffeate Against Hydrogen Peroxide and Lipopolysaccharide Induced Injury via Modulating Arachidonic Acid Network and p38-MAPK Signaling. Curr Alzheimer Res. 2015;12:892–902. doi: 10.2174/156720501209151019111244. [DOI] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juurlink BH, Chen Y, Hertz L. Use of cell cultures to differentiate among effects of various ischemia factors on astrocytic cell volume. Can J Physiol Pharmacol. 1992;70(Suppl):S344–349. doi: 10.1139/y92-281. [DOI] [PubMed] [Google Scholar]
- 27.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 28.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 29.Steardo LJ, Bronzuoli MR, Iacomino A, Esposito G, Steardo L, Scuderi C. Does neuroinflammation turn on the flame in Alzheimer’s disease? Focus on astrocytes. Front Neurosci. 2015;9:259. doi: 10.3389/fnins.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 31.Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY. Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Wei CS, Huang W, Bennett DA, Dickson DW, Wang R, Wang DS. Distinct subcellular patterns of neprilysin protein and activity in the brains of Alzheimer’s disease patients, transgenic mice and cultured human neuronal cells. Am J Transl Res. 2013;5:608–621. [PMC free article] [PubMed] [Google Scholar]
- 33.Larson ME, Lesne SE. Soluble Abeta oligomer production and toxicity. J Neurochem. 2012;120(Suppl 1):125–139. doi: 10.1111/j.1471-4159.2011.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96:195–203. doi: 10.1016/s0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- 35.Mulder SD, Nielsen HM, Blankenstein MA, Eikelenboom P, Veerhuis R. Apolipoproteins E and J interfere with amyloid-beta uptake by primary human astrocytes and microglia in vitro. Glia. 2014;62:493–503. doi: 10.1002/glia.22619. [DOI] [PubMed] [Google Scholar]
- 36.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmeier A, Wozny C, Rost BR, Munter LM, Hua H, Georgiev O, Beyermann M, Hildebrand PW, Weise C, Schaffner W, Schmitz D, Multhaup G. Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity. J Neurosci. 2009;29:7582–7590. doi: 10.1523/JNEUROSCI.1336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Cheng D, Cheng R, Zhu X, Wan T, Liu J, Zhang R. Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-beta proteins. PLoS One. 2014;9:e99939. doi: 10.1371/journal.pone.0099939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rozovsky I, Finch CE, Morgan TE. Age-Related Activation of Microglia and Astrocytes In Vitro Studies Show Persistent Phenotypes of Aging, Increased Proliferation, and Resistance to Down-Regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Abeta production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verkhratsky A, Rodríguez JJ, Steardo L. Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist. 2014;20:576–588. doi: 10.1177/1073858413510208. [DOI] [PubMed] [Google Scholar]
- 42.Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 43.Fassbender K, Walter S, Kühl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]


