Abstract
Growing evidence indicates that long non-coding RNAs (lncRNAs) play key roles in cancer initiation and progression. However, little is known about the therapeutic significance of lncRNAs in glioma. In this study, we explored the tumorigenic role of a classical lncRNA, FOXD3 antisense RNA 1 (FOXD3-AS1) in glioma. Systemic analysis of the patient specimens and clinical data showed that FOXD3-AS1 was markedly up-regulated in high-grade glioma tissues (WHO grade III-IV) compared with that in low-grade glioma (WHO grade I-II) and normal brain tissues (both P<0.01), and patients with low FOXD3-AS1 expression had grater survival probability. Multivariate regression analysis showed that increased FOXD3-AS1 expression was a significant independent indicator of poor prognosis in glioma patients (P=0.034). To understand the tumorigenic mechanism of FOXD3-AS1, the expression pattern and functional role of FOXD3-AS1 in glioma were detected using real-time PCR and Smart Silencer-mediated knockdown study. In related cell biological assays, we discovered that FOXD3-AS1 knockdown significantly inhibited cell proliferation, induced cell cycle S-phase arrest, and impaired cell migration and invasion in malignant glioma cells. As expected, we also found that the expression of FOXD3-AS1 was positively correlated with FOXD3 mRNA. Knockdown of FOXD3-AS1 reduced the protein level of FOXD3 in cultured U251 and A172 cell lines. These results suggest that FOXD3-AS1 is an oncogenic lncRNA, which may promote the occurrence and development of glioma through transcriptional regulation of FOXD3.
Keywords: Long non-coding RNA, FOXD3-AS1, glioma, FOXD3
Introduction
Glioma is the most common primary malignant tumor in the central nervous system (CNS) [1]. According to the World Health Organization (WHO) glioma grading criteria, gliomas can be classified as low-grade (WHO I-II) and high-grade (WHO III-IV) gliomas according to their degree of malignancy. Despite major advances made in the conventional treatment of glioma including surgery, chemotherapy and radiotherapy in the past decades, overall survival (OS) of patients with high-grade glioma remains poor [2,3]. The progression of glioma is a complex process which can be affected by manu factors [4]. Although alterations in oncogenes and tumor suppressors have been reported in glioma, the precise molecular mechanisms remain largely unknown. Therefore, it is necessary to explore the specific molecular mechanisms underlying glioma for the sake of selecting suitable predictive biomarkers and seeking new therapeutic strategies for the treatment of glioma.
Long non-coding RNAs (lncRNAs), which were initially argued to be spurious transcriptional noise, are now recognized as a class of RNAs with transcripts longer than 200 nucleotides without the function of encoding proteins [5-7]. Studies have found that lncRNAs play a critical regulatory role in many human diseases, including cancer [8,9]. Unlike their shorter counterparts including miRNAs and other smaller noncoding RNAs, lncRNAs can regulate downstream target genes by multiple means via cis- and trans- regulatory effects [10,11]. Recent studies have reported that a growing number of lncRNAs can cooperate with neighbor genes to form “lncRNA-mRNA” pairs to affect their function [12-18]. Close relationships are often found between these lncRNAs and their nearby mRNAs in expression or function.
In our previous study with microarrays of glioma specimens [19], we found that FOXD3-AS1 was aberrantly expressed in glioma. FOXD3-AS1 (ENST00000449386/RP4-792G4.2) is an lncRNA whose function has never been described. FOXD3-AS1 is the antisense transcript of a protein coding gene FOXD3. In addition, transcript factors chip-seq data from encode/analysis (http://genome.ucsc.edu/) show that FOXD3-AS1 shares its mRNA partner FOXD3 the promoter region, meaning that FOXD3-AS1 belongs to a category of lncRNA called promoter upstream transcripts (PROMPTs) [20,21]. The expression level and functional role of PROMPTs are often related to the adjacent protein-coding transcripts. Studies have demonstrated that FOXD3 is a tumor suppressor of melanoma, neuroblastoma and gastric cancer [22-24]. FOXD3 was found to be upregulated in renal and endometrial cancers and might play tumor suppressive and oncogenic roles in cervical and renal cancers [23]. Xu et al. reported that 32D cells with overexpressed FOXD3 failed to differentiate regularly when they were stimulated with G-CSF but maintained a primitive phenotype and continued to proliferate [25]. Therefore, FOXD3 may exhibit tissue-specific expression patterns and functions in human tumors. However, whether FOXD3-AS1 is associated with cancer remains unknown.
The purpose of this study was to confirm the oncogenic role of FOXD3-AS1 in glioma and explore the underlying mechanism by detecting the expression patterns of FOXD3-AS1 in glioma tissues and normal brain tissues, evaluating the functional role of FOXD3-AS1 in malignant glioma cell lines, and preliminarily analyzing the interaction of FOXD3-AS1 and FOXD3.
Materials and methods
Patient tissue samples
Included in this study were 44 patients with glioma who underwent initial surgery in Changzheng Hospital (Shanghai, China) between 2010 and 2013. Normal brain tissue samples obtained from six patients with severe head trauma for which partial resection of the normal brain tissues was required for decompression were used as control. No patients had received chemotherapy or radiotherapy before resection. Each patient provided written informed consent before participating in the study, and the use of the tumor samples for research was approved by the Specialty Committee on the Ethics of Biomedicine Research of the Second Military Medical University (Shanghai, China). All tumors were classified according to the WHO criteria for tumors of the CNS and immediately frozen after surgery until analysis. The treatment was carried out according to the National Comprehensive Cancer Network (NCCN) guideline in all glioma patients included in this study. Clinical follow-up was available for all patients. OS of the patients was calculated from the date of initial surgery to the date of patient death.
Cell lines and cell culture
Human glioma cell lines U87, A172 and U251 (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco, USA), 100 mg/ml penicillin and 50 μg/ml streptomycin (Gibco, USA) at 37°C with 5% CO2.
Reverse transcription and real-time quantitative PCR assays
Total RNA was extracted from the normal brain tissues, glioma tumor tissues and glioma cell lines by using the Trizol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. Complementary DNA (cDNA) synthesis was conducted with 1 μg total RNA using the Prime Script RT Master Mix (Takara, Japan). The primers were obtained from Sangon (Shanghai, China) and the sequences were designed as follows: For FOXD3-AS1, the forward primer was 5’-GGTGGAGGAGGCGAGGATG-3’ and the reverse primer was 5’-AGCGGACAGACAGGGATTGG-3’. For FOXD3, the forward primer was 5’-GACGACGGGCTGGAAGAGAA-3’ and the reverse primer was 5’-GCCTCCTTGGGCAATGTCA-3’. For GAPDH, the forward primer was 5’-GGGAAACTGTGGCGTGAT-3’ and the reverse primer was 5’-GAGTGGGTGTCGCTGTTGA-3’. Quantitative PCR was performed using the SYBR Premix Ex TaqTM II (Takara, Japan) on 7900HT (Applied Biosystems, USA). The reaction mixtures were incubated at 95°C for 60 s, followed by 45 amplification cycles at 95°C for 15 s and 60°C for 31 s. Change in expression level was calculated by quantitative analysis in triplicate using the comparative cycle threshold method. The raw data of target gene were normalized to GAPDH.
Transfection of lncRNA Smart Silencer
LncRNA Smart Silencer, synthesized from RiboBio (Guangzhou, China), was used to knock down the expression of FOXD3-AS1. FOXD3-AS1 Smart Silencer is a mixture of three siRNAs and three antisense oligonucleotides (ASOs). The target sequences of siRNAs are as follows: 5’-CTCCAAGATTTAACTTCCA-3’, 5’-GGAGTTCCGAGAGGAAATA-3’, 5’-GATGCTGGGATGTGGATTT-3’. The target sequences of ASOs are as follows: 5’-CAGAGGAAGGAGCACGAGGG-3’, 5’-GGTGGAGGAGGCGAGGATGT-3’, 5’-AGAAGATGCTGGGATGTGGA-3’. The negative control (NC) Smart Silencer does not contain domains homologous to humans, mice and rats. LncRNA Smart Silencer transfection was performed with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Approximately 5% U251 and A172 cells were plated into each well of the 12-well plate at least 24 h before transfection to achieve 30-50% confluency. Cells were collected 24 h after transfection for RNA isolation, Cell Counting Kit-8 (CCK-8) cell proliferation assay, cell cycle distribution analysis, migration and invasion assay.
Cell proliferation assay
24 h after Smart Silencer transfection, treated and untreated cells were plated at a density of 2×103 cells per well into 96-well plates. At indicated time points, CCK-8 solution (Dojindo Lab, Kumamoto, Japan) was added to each well and then incubated at 37°C for 1.5 h. Optical density (OD) was measured at 450 nm using a micro-plate reader (KHB ST-360, Shanghai, China). All experiments were performed in triplicate and repeated three times.
Cell cycle analysis
After 24-h treatment with Smart Silencer, cells were trypsinized, harvested, washed with phosphate-buffered saline (PBS) and fixed in 70% ethanol at -20°C overnight, and incubated with RNase A (0.10 mg/ml, Sigma) and propidium iodide (PI) (0.05 mg/ml, Sigma) at 37°C in the dark for 30 min, and then analyzed by a FACScan flow cytometry (BD Biosciences, USA). The cell-cycle profiles were analyzed by ModFit 3.0 software (BD Biosciences, USA). The assay was carried out in triplicate.
Cell migration and invasion assays
Quantitative cell motility and invasiveness assays were carried out using 24-well Transwell (Millipore Inc., USA) and Matrigel (Coring Inc., USA) chamber plates, respectively. Treated and untreated cells (5×104) were plated onto Transwell or Matrigel insert membranes with a pore size of 8 μm on day 2 following transfection. Growth medium containing 10% FBS in the lower chamber served as the chemoattractant. After 24-h incubation at 37°C, cells that migrated or invaded through the filters were stained and counted. The migrating and invading cells were counted in three random fields for each condition under a Leica inverted microscope. The experiments were repeated three times.
Immunohistochemistry
The normal brain and glioma specimens were formalin-fixed, paraffin-embedded, and sliced into 3 μm thick sections for FOXD3 immunohistochemistry. Then, the sections were deparaffinized to eliminate endogenous peroxidase activity by incubation with 1% H2O2. After antigen retrieval, FOXD3 primary antibody (Abcam, USA) with 1:50 dilution was applied at 4°C overnight. After rinsing with PBS, the biotinylated secondary IgG antibody was applied at room temperature for 30 min. Immunoperoxidase staining was conducted using an ABC kit (Santa Cruz Biotechnology, USA), and sections were counterstained with hematoxylin.
Antibodies and western blot assay
Anti-FOXD3 was purchased from Abcam (USA). Antibodies against cyclin-dependent kinase 2 (CDK2), Cyclin A, P21, and GAPDH were purchased from Proteintech (USA). The glioma tumor tissue, normal brain tissue, and the treated and untreated glioma cell lines were lysed using a total protein extraction kit (KeyGen Biotech, Nanjing, China). Protein isolates were then separated by 10% sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to the polyvinylidene difluoride (PVDF) membranes. After 1-h blocking with 5% skimmed milk at room temperature, the membranes were incubated with primary antibodies at 4°C overnight followed by incubation with appropriate correlated HRP-conjugated secondary antibodies (Proteintech, USA) for 1 h at room temperature. Immunoblots were visualized using enhanced chemiluminescence (ECL) (Thermo Fisher Scientific, USA) and scanned by Image-Pro Plus 6.0 software (Media Cybernetics Inc., USA). Band density of target proteins was standardized to that of GAPDH.
Statistical analysis
Experimental data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, USA). Data are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to test for differences between the glioma and normal brain tissues in all groups, and a least significant difference post-hoc test was used to obtain individual P values followed by ANOVA. The chi-square test was used to examine the relationship between FOXD3-AS1 expression level and the clinicopathologic features. Survival analysis was performed using the Kaplan-Meier method and compared using the logrank test. The Cox multivariate proportional hazards model was performed to analyze the significance of survival variables. Correlation between gene expressions was studied by using Pearson’s correlation. A value of P<0.05 was considered statistically significant.
Results
Overexpression of FOXD3-AS1 associates with poor prognosis in glioma
FOXD3-AS1 expression was assessed in 44 glioma tissues and 6 normal brain tissues by real-time PCR. The results showed that FOXD3-AS1 expression was significantly higher in the high-grade glioma tissues compared with that in the normal brain tissues and low-grade glioma tissues (both P<0.01) (Figure 1A), while there was no significant difference in FOXD3-AS1 expression between the low-grade glioma and normal brain tissues. We then measured the correlation between FOXD3-AS1 expression and clinicopathological characteristics of glioma. Glioma tissues were divided into the high expression group (n=22) and the low expression group (n=22), based on the median expression level of all gliomas (mean expression value 215.87). As summarized in Table 1, FOXD3-AS1 was significantly associated with WHO grade (I-II vs. III-IV, P=0.003). However, no significant association between FOXD3-AS1 expression and other clinicopathological parameters was identified, including age (<50 vs. ≥50, P=0.112), gender (male vs. female, P=0.623), tumor size (<5 cm vs. ≥5 cm, P=0.268), and KPS (<70 vs. ≥70, P=0.697). Besides, Kaplan-Meier analysis and log-rank tests were performed to investigate the association between FOXD3-AS1 expression and the prognosis of the glioma patients. It was found that glioma patients with high FOXD3-AS1 expression had significantly shorter OS (Figure 1B) than patients with low FOXD3-AS1 expression. Our stratified analysis indicated that low FOXD3-AS1 expression was significantly correlated with greater OS in patients with high-grade glioma (Figure 1C) but not in patients with low-grade glioma (Figure 1D), probably because of the small sample size of this subpopulation. Univariate analysis identified three prognostic factors: age (<50 or ≥50), WHO grade (I-II or III-IV), and FOXD3-AS1 expression. Multivariate regression analysis of the prognosis factors confirmed that increased FOXD3-AS1 expression was an independent indicator of poor survival in glioma patients (P=0.013), in addition to age (P=0.019) and WHO grade (P=0.005) (Table 2).
Figure 1.
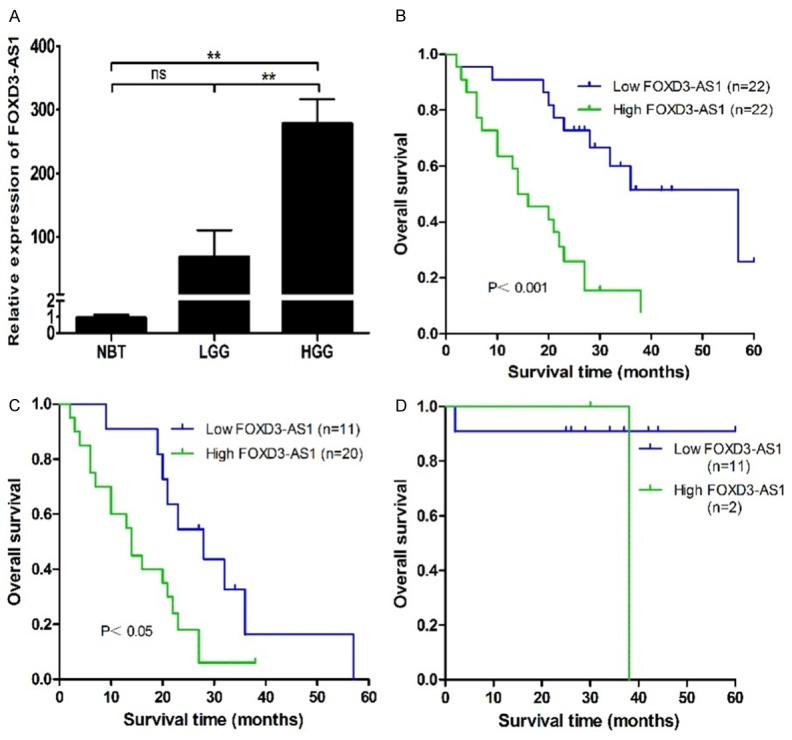
Increased FOXD3-AS1 expression confers poor prognosis in glioma patients. A. FOXD3-AS1 expression was significantly higher in the high-grade glioma tissues compared with that in the normal brain tissues and low-grade glioma tissues. B. Kaplan-Meier overall survival curves according to FOXD3-AS1 expression level. Glioma patients with high FOXD3-AS1 expression had significantly shorter overall survival than patients with low FOXD3-AS1 expression (P<0.001). C and D. Low FOXD3-AS1 expression was significantly with better overall survival in patients with high-grade glioma (P<0.05) but not in patients with low-grade glioma. NBT, normal brain tissue; LGG, low-grade glioma; HGG, high-grade glioma. ns P>0.05, **P<0.01.
Table 1.
FOXD3-AS1 expression and clinicopathological features of human gliomas
| Characteristics | Patients, n | FOXD3-AS1 expression, n | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age (years) | ||||
| <50 | 19 | 7 | 12 | 0.112 |
| ≥50 | 25 | 15 | 10 | |
| Gender | ||||
| Male | 28 | 14 | 14 | 0.623 |
| Female | 16 | 8 | 8 | |
| WHO grade | ||||
| I-II | 13 | 2 | 11 | 0.003 |
| III-IV | 31 | 20 | 11 | |
| Tumor size (cm) | ||||
| <5 | 17 | 7 | 10 | 0.268 |
| ≥5 | 27 | 15 | 12 | |
| KPS | ||||
| <70 | 4 | 2 | 2 | 0.697 |
| ≥70 | 40 | 20 | 20 | |
KPS, karnofsky performance score.
Table 2.
Univariate and multivariate Cox regression analyses of overall survival
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | ||||||
| Female | 1 | |||||
| Male | 1.467 | 0.677-3.182 | 0.332 | |||
| Tumor size (cm) | ||||||
| ≥5 | 1 | |||||
| <5 | 0.988 | 0.459-2.126 | 0.975 | |||
| KPS | ||||||
| ≥70 | 1 | |||||
| <70 | 1.52 | 0.446-5.182 | 0.504 | |||
| Age (years) | ||||||
| ≥50 | 1 | 1 | ||||
| <50 | 0.281 | 0.132-0.599 | 0.001 | 0.319 | 0.123-0.832 | 0.019 |
| WHO grade | ||||||
| III-IV | 1 | 1 | ||||
| I-II | 0.208 | 0.097-0.448 | <0.001 | 0.12 | 0.027-0.535 | 0.005 |
| FOXD3-AS1 | ||||||
| High | 1 | 1 | ||||
| Low | 0.236 | 0.107-0.522 | <0.001 | 0.406 | 0.176-0.936 | 0.034 |
HR, hazard ratio; 95% CI, 95% confidence interval; KPS, karnofsky performance score.
Silencing of FOXD3-AS1 expression in glioma cells
We then measured the expression of FOXD3-AS1 in three glioma cell lines by real-time PCR. The expression level of FOXD3-AS1 was higher in U251 and A172 cells than that in U87 cells (Figure 2A).
Figure 2.
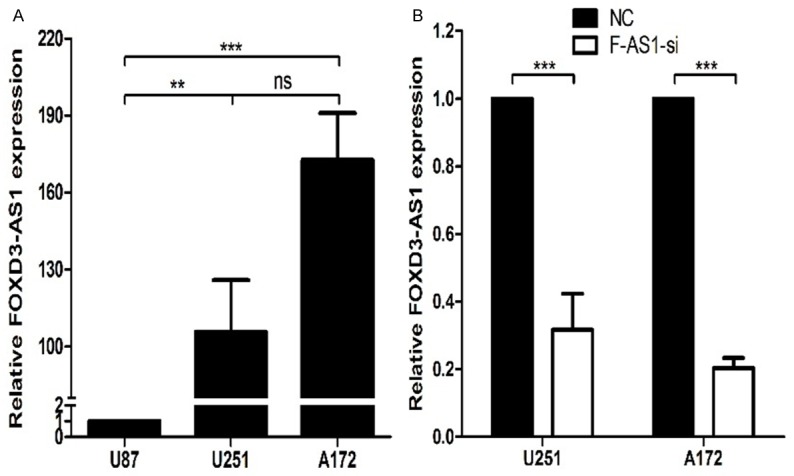
The expression levels of FOXD3-AS1 in U251 and A172 cell lines after smart silencer transfection. A. The expression levels of FOXD3-AS1 in three glioma cell lines were analyzed by real-time PCR. B. The expression levels of FOXD3-AS1 in U251 and A172 cell lines were analyzed by real-time PCR. NC, NC Smart Silencer; F-AS1-si, FOXD3-AS1 Smart Silencer. ns P>0.05, **P<0.01, ***P<0.001.
To investigate the effect of FOXD3-AS1 overexpression on cell biological behaviors, we used Smart Sliencer-mediated knockdown strategy to inhibit its expression in glioma cells. LncRNA Smart Silencer targeting FOXD3-AS1 (FOXD3-AS1 Smart Silencer) and negative control (NC Smart Silencer) were used to transfect U251 and A172 cells. As shown in Figure 2B, the FOXD3-AS1 expression level was down-regulated in FOXD3-AS1 Smart Silencer group compared with NC Smart Silencer group.
Knockdown of FOXD3-AS1 inhibits cell proliferation in glioma
We used CCK-8 assay to investigate the effect of FOXD3-AS1 knockdown on cell proliferation in glioma. FOXD3-AS1 Smart Silencer notably inhibited cell proliferation of U251 and A172 cells compared with NC Smart Silencer cells (Figure 3A), suggesting that FOXD3-AS1 played a role in promoting proliferation of glioma cells.
Figure 3.
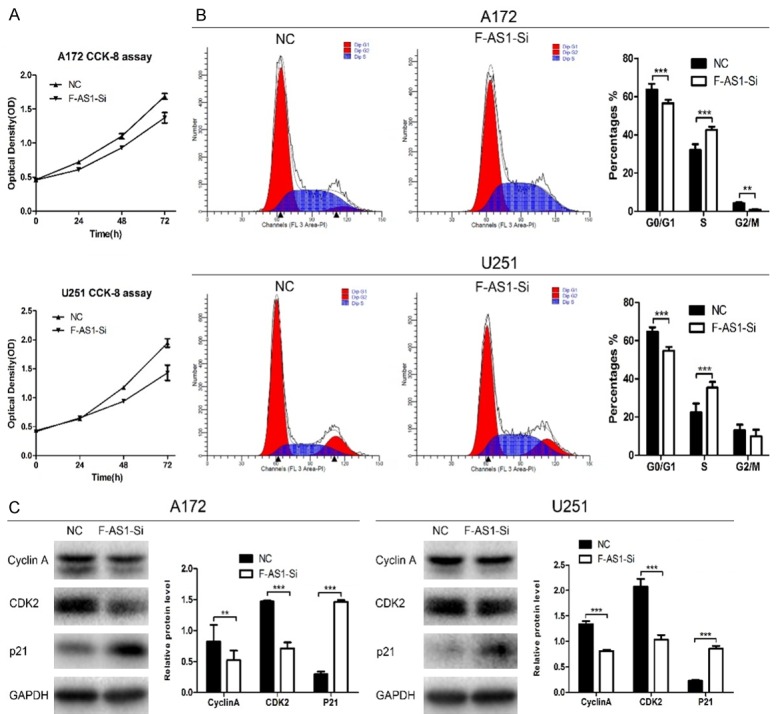
Knockdown of FOXD3-AS1 induced S-phase arrest in glioma cells. A. FOXD3-AS1 Smart Silencer reduced the A172 and U251 cells growth compared with NC Smart Silencer group in CCK-8 assay. B. A172 and U251 cells were transfected with indicated Smart Silencer, and 24 h later, cells were collected. Cell cycle profiling was analyzed using flow cytometry. C. Protein levels of cell cycle genes (cyclin A, CDK2, p21) in A172 and U251 cells were detected by Western blot analysis. GAPDH was used as an endogenous normalizer. Data were based on at least three independent experiments and shown as mean ± SD. **P<0.01, ***P<0.001.
Knockdown of FOXD3-AS1 induces S-phase arrest in glioma cells
We next analyzed the effect of FOXD3-AS1 knockdown on cell cycle distribution by PI staining and flow cytometry. It was found that the number of S-phase cells was increased and the number of G1- and G2/M-phase cells was decreased after silencing of FOXD3-AS1 in U251 or A172 cells (Figure 3B), indicating that silencing of FOXD3-AS1 prevented S-phase glioma cells from entering G2/M phase. To explore the molecular mechanisms of FOXD3-AS1-induced S-phase arrest, the expression of S phase-specific cell cycle regulatory proteins was investigated. As shown in Figure 3C, FOXD3-AS1 Smart Silencer treatment in U251 and A172 cells decreased cyclin A and CDK2 protein expression markedly compared with NC group, while p21 was increased.
Downregulation of FOXD-AS1 inhibits glioma cell migration and invasion
To further investigate the function of FOXD3-AS1, transwell assays were performed to measure the effect of FOXD3-AS1 knockdown on cell migration. The results showed a strong inhibitory motility in FOXD3-AS1 Smart Silencer group compared with that in NC Smart Silencer group (Figure 4A). We also investigated whether FOXD3-AS1 affected the invasiveness of glioma cells. As shown in Figure 4B, silencing of FOXD3-AS1 dramatically impaired cell invasion in U251 and A172 cell lines compared with NC group. These results indicate that FOXD3-AS1 promoted glioma cell migration and invasion.
Figure 4.
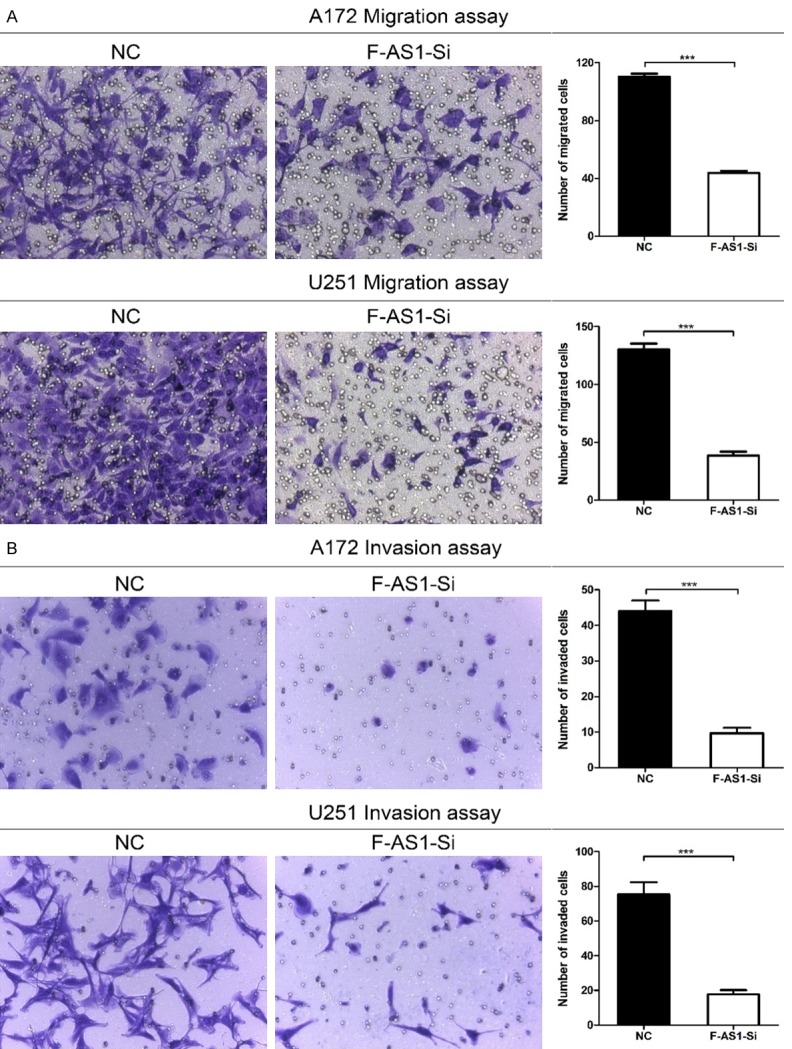
Silencing of FOXD3-AS1 inhibited cell migration and invasion in glioma cells. A. The capacity of cell migration in FOXD3-AS1 Smart Silencer group was impaired markedly. B. The capacity of cell invasion in FOXD3-AS1 Smart Silencer groups was impaired markedly. ***P<0.001.
Overexpression of FOXD3 associates with poor prognosis in glioma
To confirm that FOXD3 was aberrantly expressed in glioma, it was detected in the aforementioned specimens by quantitative real-time PCR. The expression level of FOXD3 was also significantly up-regulated in the high-grade glioma tissues compared with that in the normal brain tissues and low-grade glioma tissues (both P<0.05) (Figure 5A). We next identified the correlation between FOXD3 expression and clinicopathological characteristics of glioma. Glioma tissues were divided into the high expression group (n=18) and the low expression group (n=26), based on the median expression level of all gliomas (mean expression value 3154.955). As summarized in Table 3, FOXD3 was significantly associated with WHO grade (I-II vs. III-IV, P=0.026). However, no significant association between FOXD3 expression and other clinicopathological parameters was identified, including age (<50 vs. ≥50, P=0.434), gender (male vs. female, P=0.509), tumor size (<5 cm vs. ≥5 cm, P=0.18), and KPS (<70 vs. ≥70, P=0.545). In addition, Kaplan-Meier analysis showed that glioma patients with high FOXD3 expression had significantly shorter OS than patients with low FOXD3 expression (P<0.01, Figure 5B). The overexpression of FOXD3 in glioma was further confirmed by immunohistochemical staining and Western blot assay (Figure 5D and 5E).
Figure 5.
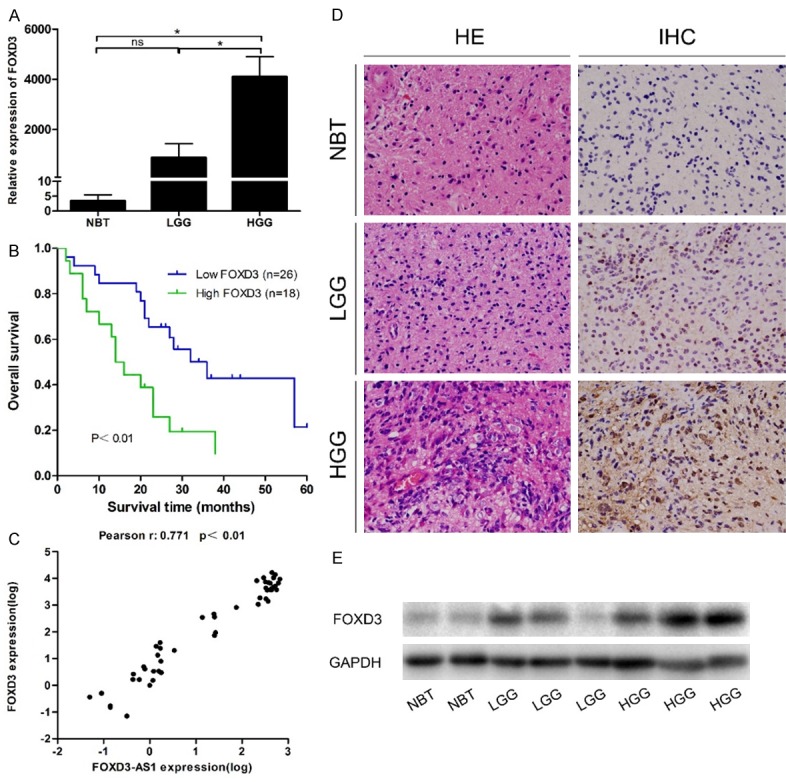
The expression levels of FOXD3 in the aforementioned specimens. A. Real-time PCR revealed that FOXD3 expression was significantly higher in the high-grade glioma tissues compared with that in normal brain tissues and low-grade glioma tissues. B. Overall survival of patients with glioma. The survival time of patients with high FOXD3 expression was significantly shorter than that in patients with low FOXD3 expression (P<0.01). C. There was a positive correlation between FOXD3-AS1 expression and FOXD3 expression in normal brain tissues and glioma tissues. D. Hematoxylin eosin (HE) staining was used to observe glioma morphology, and immunohistochemistry (IHC) staining was used to evaluate FOXD3 expression in glioma tissues and normal brain tissues. E. Western blot indicated higher protein levels of FOXD3 in high-grade glioma tissues than those in normal brain tissues and low-grade glioma tissues. ns P>0.05, *P<0.05.
Table 3.
FOXD3 expression and clinicopathological features of human gliomas
| Characteristics | Patients, n | FOXD3 expression, n | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age (years) | ||||
| <50 | 19 | 7 | 12 | 0.434 |
| ≥50 | 25 | 11 | 14 | |
| Gender | ||||
| Male | 28 | 11 | 17 | 0.509 |
| Female | 16 | 7 | 9 | |
| WHO grade | ||||
| I-II | 13 | 2 | 11 | 0.026 |
| III-IV | 31 | 16 | 15 | |
| Tumor size (cm) | ||||
| <5 | 17 | 5 | 12 | 0.18 |
| ≥5 | 27 | 13 | 14 | |
| KPS | ||||
| <70 | 4 | 2 | 2 | 0.545 |
| ≥70 | 40 | 16 | 24 | |
KPS, karnofsky performance score.
Down-regulation of FOXD3-AS1 by Smart Silencer reduces the protein level of FOXD3 in glioma cells
To preliminarily clarify the relationship between FOXD3-AS1 and FOXD3 in glioma, we assessed the correlation of their expression levels using Pearson’s correlation. Then we detected the FOXD3 expression in glioma cell lines, and examined the protein level of FOXD3 after FOXD3-AS1 knockdown. The results indicated that FOXD3-AS1 expression was positively correlated with FOXD3 expression (R=0.771, P<0.01) (Figure 5C). Real-time PCR and Western blot analysis showed that the expression of FOXD3 was highest in A172 and the lowest in U87 (Figure 6A and 6B). By Western blot, we found that the FOXD3 expression was significantly downregulated in FOXD3-AS1 Smart Silencer group compared with NC Smart Silencer group (Figure 6C). The results indicated that the FOXD3 expression may be modulated by FOXD3-AS1.
Figure 6.
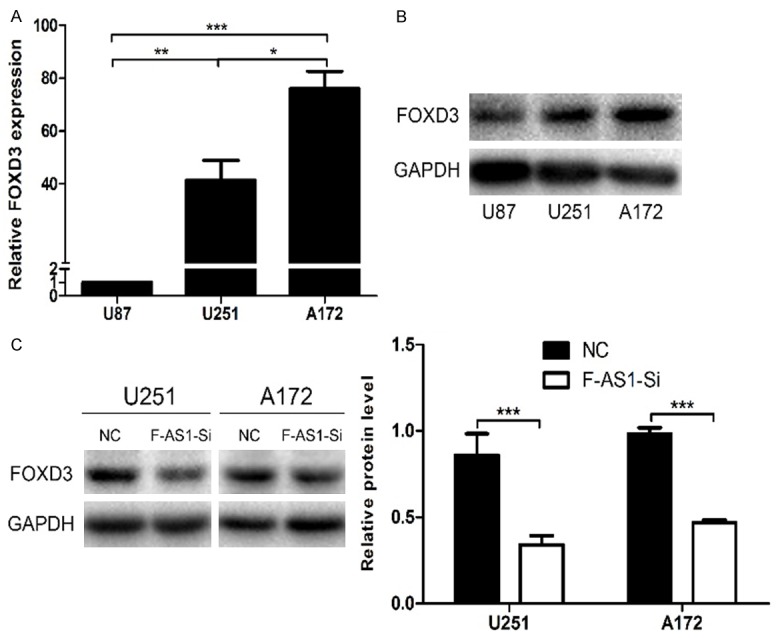
The FOXD3 expression was significantly downregulated in FOXD3-AS1 Smart Silencer group compared with the NC Smart Silencer group. A and B. FOXD3 mRNA and protein expressions in U87, U251 and A172 cells. C. Western blot analysis showed that the expression of FOXD3 was apparently downregulated in U251 and A172 cells after transfection with FOXD3-AS1 Smart Silencer. GAPDH was used as an endogenous normalizer. Data were based on at least three independent experiments and shown as mean ± SD. ns P>0.05, *P<0.05, **P<0.01, ***P<0.001.
Discussion
It has been shown in recent years that the lncRNAs are closely related to the development of cancer. Differential expression of lncRNAs or cancer-specific lncRNAs profiles can be used as new molecular biomarkers for cancer diagnosis and treatment [26]. There are several prior microarray data on the relationship between glioma and lncRNAs [27-29]. Besides, it has been illustrated that some well-known cancer related lncRNAs, such as MEG3 [30], HOTAIR [31], H19 [32] and CRNDE [33], are also involved in glioma progression. The emerging role of lncRNAs in glioma prompted us to conduct the current study.
It was found in our previous study [19] that FOXD3-AS1 was aberrantly expressed in glioma. We therefore picked it out and validated it in 44 glioma tissues and 6 normal brain tissues. The result of real-time PCR analysis showed that the overexpression of FOXD3-AS1 was closely associated with histologic tumor grade and OS of glioma patients. The result of multivariate regression analysis showed that FOXD3-AS1 expression was an independent indicator for OS of glioma patients. The result also showed that silencing the FOXD3-AS1 expression inhibited cell growth and metastasis markedly. Cell cycle analysis showed that silencing of FOXD3-AS1 in glioma cells inhibited cell proliferation by preventing S/G2 cell cycle transition. The cyclin A/CDK2 complex plays an important role as a S-phase regulator, in particular during priming of DNA synthesis and its progression [34]. The kinase activity of this cyclin/CDK complex is negatively regulated by CDK inhibitory proteins, including p21 [35]. As expected, our results showed that the protein levels of cyclin A and CDK2 were decreased in FOXD3-AS1 knockdown cells, while p21 was increased at the same time. To the best of our knowledge, this is the first study about the expression pattern and functional role of lncRNA FOXD3-AS1 in cancer.
As described above, FOXD3-AS1 belongs to a class of lncRNA called PROMPTs, whose expression and function are often correlated with the adjacent protein-coding transcripts. LncRNA FOXD3-AS1 is a 547 bp transcript with 3 exons, and it locates in the chromosome 1p31.3 on the reverse strand. FOXD3-AS1 is the antisense partner of protein coding gene FOXD3, a member of the Forkhead box (FOX) family. FOX transcription factors, a family of proteins containing a monomeric DNA-binding domain known as the forkhead box or winged helix domain, mediate a wide spectrum of biologic processes such as metabolism, differentiation, proliferation, migration and apoptosis [36]. As a transcription factor, FOXD3 (also known as AIS1, HFH2, VAMAS2, and Genesis) was originally identified in embryonic stem cells and serves numerous indispensable roles in neural crest development [37,38]. With the further research of FOXD3 in recent years, its role in the tumorigenesis has attracted great attention in cancer research. It has been reported that FOXD3 is abnormally expressed in tumor cells and participates in tumor onset and progression [36]. Based on these findings, we speculated that FOXD3-AS1 may regulate the cancer cell growth characteristics of glioma by regulating FOXD3.
We then investigated the expression of FOXD3 in the aforementioned specimens. Our data showed that the overexpression of FOXD3 in glioma was also closely associated with histologic tumor grade and OS of glioma patients. The overexpression of FOXD3 in glioma was further confirmed by immunohistochemical staining and Western blot. Besides, the expression of FOXD3-AS1 was positively correlated with FOXD3 mRNA. Then, to further assess the regulatory role of lncRNA FOXD3-AS1 to the protein coding gene FOXD3, a FOXD3-AS1 knockdown experiment was conducted. The result showed that the protein level of FOXD3 was decreased concomitantly with the FOXD3-AS1 downregulation in cultured U251 and A172 cell lines. Based on these findings, we speculated that lncRNA FOXD3-AS1 may fulfill its oncogenic function partly by modulating the FOXD3 expression. However, Du et al. reported that FOXD3 was under-expressed in high-grade glioma tissues, and could exert tumor suppressor properties by inhibiting glioma cell proliferation [39], which is different from the result obtained in the present study. The possible reasons are as follows. First, the sample size in our study is relatively small and the proportion of histopathological types of glioma is different. In addition, there are some methodological differences that might account for this variation. Therefore, the specific role of FOXD3 in glioma needs further validation in future.
In conclusion, this study provides the first evidence that increased expression of lncRNA FOXD3-AS1 is associated with the malignant status and poor prognosis in glioma. The functions of FOXD3-AS1 on cell proliferation, cell cycle regulation, migration, and invasion suggest that it may play a role in promoting tumorigenesis in glioma partly by regulating the protein coding gene FOXD3. However, the specific molecular mechanism underlying this regulatory effect has not been fully studied in this study, and the particular mechanism by which FOXD3-AS1 is up-regulated in glioma is not clear. More studies are needed to verify the role of FOXD3-AS1 as a reliable clinical predictor of the outcome for glioma patients in the future.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81270038).
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor LP. Diagnosis, treatment, and prognosis of glioma: five new things. Neurology. 2010;75:S28–32. doi: 10.1212/WNL.0b013e3181fb3661. [DOI] [PubMed] [Google Scholar]
- 3.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 4.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 5.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Caley DP, Pink RC, Trujillano D, Carter DR. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 2010;10:90–102. doi: 10.1100/tsw.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Kong R, Yin DD, Zhang EB, Xu TP, De W, Shu YQ. Low expression of long noncoding RNA GAS6-AS1 predicts a poor prognosis in patients with NSCLC. Med Oncol. 2013;30:694. [Google Scholar]
- 14.Takayama K, Horie-Inoue K, Katayama S, Suzuki T, Tsutsumi S, Ikeda K, Urano T, Fujimura T, Takagi K, Takahashi S, Homma Y, Ouchi Y, Aburatani H, Hayashizaki Y, Inoue S. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32:1665–1680. doi: 10.1038/emboj.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Song JH, Cheng Y, Wu W, Bhagat T, Yu Y, Abraham JM, Ibrahim S, Ravich W, Roland BC, Khashab M, Singh VK, Shin EJ, Yang X, Verma AK, Meltzer SJ, Mori Y. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin XY, Yao J, Geng PL, Fu XP, Xue JH, Zhang ZW. LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma. Int J Clin Exp Pathol. 2014;7:3065–3072. [PMC free article] [PubMed] [Google Scholar]
- 17.Kong XP, Yao J, Luo W, Feng FK, Ma JT, Ren YP, Wang DL, Bu RF. The expression and functional role of a FOXC1 related mRNA-lncRNA pair in oral squamous cell carcinoma. Mol Cell Biochem. 2014;394:177–186. doi: 10.1007/s11010-014-2093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H, Wu L, Yang Q, Ye M, Zhu X. Functional linc-POU3F3 is overexpressed and contributes to tumorigenesis in glioma. Gene. 2015;554:114–119. doi: 10.1016/j.gene.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Wu JJ, Lin XB, Bao Y, Chen ZH, Zhang CR, Cai Z, Zhou JY, Ding MH, Wu XJ, Sun W, Qian J, Zhang L, Jiang L, Hu GH. Differential lncRNA expression profiles in recurrent gliomas compared with primary gliomas identified by microarray analysis. Int J Clin Exp Med. 2015;8:5033–5043. [PMC free article] [PubMed] [Google Scholar]
- 20.Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, Mapendano CK, Schierup MH, Jensen TH. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- 21.Preker P, Almvig K, Christensen MS, Valen E, Mapendano CK, Sandelin A, Jensen TH. PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res. 2011;39:7179–7193. doi: 10.1093/nar/gkr370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel EV, Aplin AE. FOXD3 is a mutant B-RAF-regulated inhibitor of G(1)-S progression in melanoma cells. Cancer Res. 2010;70:2891–2900. doi: 10.1158/0008-5472.CAN-09-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Mei H, Qi M, Yang D, Zhao X, Xiang X, Pu J, Huang K, Zheng L, Tong Q. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. 2013;4:2021–2044. doi: 10.18632/oncotarget.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng AS, Li MS, Kang W, Cheng VY, Chou JL, Lau SS, Go MY, Lee CC, Ling TK, Ng EK, Yu J, Huang TH, To KF, Chan MW, Sung JJ, Chan FK. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology. 2013;144:122–133. e129. doi: 10.1053/j.gastro.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Xu D, Yoder M, Sutton J, Hromas R. Forced expression of Genesis, a winged helix transcriptional repressor isolated from embryonic stem cells, blocks granulocytic differentiation of 32D myeloid cells. Leukemia. 1998;12:207–212. doi: 10.1038/sj.leu.2400948. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Xu Y, He C, Guo X, Zhang J, He C, Zhang L, Kong M, Chen B, Zhu C. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2015;111:834–839. doi: 10.1002/jso.23888. [DOI] [PubMed] [Google Scholar]
- 27.Han L, Zhang K, Shi Z, Zhang J, Zhu J, Zhu S, Zhang A, Jia Z, Wang G, Yu S, Pu P, Dong L, Kang C. LncRNA pro fi le of glioblastoma reveals the potential role of lncRNAs in contributing to glioblastoma pathogenesis. Int J Oncol. 2012;40:2004–2012. doi: 10.3892/ijo.2012.1413. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Qian J, Wang YY, Zhang JX, You YP. Long noncoding RNA profiles reveal three molecular subtypes in glioma. CNS Neurosci Ther. 2014;20:339–343. doi: 10.1111/cns.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Zhang L, Jiang Y, Xu T, Mei Q, Wang H, Qin R, Zou Y, Hu G, Chen J, Lu Y. LncRNA and mRNA interaction study based on transcriptome profiles reveals potential core genes in the pathogenesis of human glioblastoma multiforme. J Cancer Res Clin Oncol. 2015;141:827–838. doi: 10.1007/s00432-014-1861-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113:1868–1874. doi: 10.1002/jcb.24055. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JX, Han L, Bao ZS, Wang YY, Chen LY, Yan W, Yu SZ, Pu PY, Liu N, You YP, Jiang T, Kang CS Chinese Glioma Cooperative Group. HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol. 2013;15:1595–1603. doi: 10.1093/neuonc/not131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Wang Y, Luan W, Wang P, Tao T, Zhang J, Qian J, Liu N, You Y. Long non-coding RNA H19 promotes glioma cell invasion by deriving miR-675. PLoS One. 2014;9:e86295. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Wang Y, Li J, Zhang Y, Yin H, Han B. CRNDE, a long-noncoding RNA, promotes glioma cell growth and invasion through mTOR signaling. Cancer Lett. 2015;367:122–128. doi: 10.1016/j.canlet.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Yang TY, Chang GC, Chen KC, Hung HW, Hsu KH, Sheu GT, Hsu SL. Sustained activation of ERK and Cdk2/cyclin-A signaling pathway by pemetrexed leading to S-phase arrest and apoptosis in human non-small cell lung cancer A549 cells. Eur J Pharmacol. 2011;663:17–26. doi: 10.1016/j.ejphar.2011.04.057. [DOI] [PubMed] [Google Scholar]
- 35.Li YG, Ji DF, Zhong S, Liu PG, Lv ZQ, Zhu JX, Chen JE, Chen HP. Polysaccharide from Phellinus linteus induces S-phase arrest in HepG2 cells by decreasing calreticulin expression and activating the P27kip1-cyclin A/D1/E-CDK2 pathway. J Ethnopharmacol. 2013;150:187–195. doi: 10.1016/j.jep.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Yan JH, Zhao CL, Ding LB, Zhou X. FOXD3 suppresses tumor growth and angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;466:111–116. doi: 10.1016/j.bbrc.2015.08.116. [DOI] [PubMed] [Google Scholar]
- 37.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 38.Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 39.Du W, Pang C, Wang D, Zhang Q, Xue Y, Jiao H, Zhan L, Ma Q, Wei X. Decreased FOXD3 Expression Is Associated with Poor Prognosis in Patients with High-Grade Gliomas. PLoS One. 2015;10:e0127976. doi: 10.1371/journal.pone.0127976. [DOI] [PMC free article] [PubMed] [Google Scholar]


