Abstract
Aim: Propofol, an intravenous anesthetic agent, has been found to inhibit invasion and growth of pancreatic cancer cells in vitro. However, the mechanisms underlying these tumor-promoting phenotypes are not known. The microRNA miR-21 has been reported to be overexpressed in pancreatic cancer, and overexpression of miR-21 confers a poor prognosis to patients with pancreatic cancer. Further studies have identified the E-cadherin transcription repressor Slug as a direct target of miR-21. In this study, we assessed whether propofol inhibits invasion and growth of pancreatic cancer cells by regulation of miR-21/Slug signaling. Methods: PANC-1 pancreatic cancer cells were treated with different concentrations of propofol (1, 5 or 10 μg/mL) for 48 h, or 10 μg/mL propofol for 12, 24 or 36 h. Cell survival and apoptosis were detected by LDH release, BrdU cell proliferation and flow cytometry assays; cell invasion and migration were detected by transwell migration assays. miR-21 mimic (miR-21), Slug cDNA, PUMA siRNA and E-cadherin siRNA transfection was used to assess the signaling pathway in which propofol functions in PANC-1 cells. Protein and mRNA expression, respectively, were detected by western blotting and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assays. Results: Propofol inhibited growth and invasion, and induced apoptosis, in a dose- and time-dependent manner in PANC-1 cells. Propofol inhibited miR-21 levels and decreased Slug expression, resulting in an increase in Slug-dependent PUMA and E-cadherin expression in PANC-1 cells. miR-21 overexpression or PUMA or E-cadherin silencing impaired propofol-induced cell apoptosis, growth and invasion. Re-expression of Slug attenuated the expression of PUMA and E-cadherin that was induced by propofol treatment, the reduction of growth and invasion, and the increase in cell apoptosis. Conclusions: Propofol can effectively inhibit invasion and induce apoptosis of PANC-1 cells by regulating miR-21/Slug signals.
Keywords: Propofol, pancreatic cancer, miR-21, slug, PUMA, E-cadherin, apoptosis, invasion
Introduction
Formation of circulating tumor cells (CTCs) is widely accepted to be a key step in the metastatic process [1]. With the growth of a primary tumor, angiogenesis is activated and, subsequently, primary tumor cells enter the bloodstream, leading to the formation of CTCs. CTCs that survive in the vasculature arrest in capillaries distant from the primary tumor site and extravasate into a foreign microenvironment. At this point, such cancer cells are speculated to revert to an epithelial phenotype via a mesenchymal-epithelial transition (MET) and either stay dormant or proliferate into macroscopic secondary tumors [2,3].
Numerous studies have recently demonstrated that tumor cells intravasate, rapidly transit through the circulation, and arrest in the vasculature of a secondary organ during surgery, and that this process generally requires only a few minutes [4-6]. In addition, platelets form aggregates around CTCs or arrest tumor cells during this period. It has recently been reported that 7 to 48 h after tail-vein injection of tumor cells, monocytes/macrophages are also recruited to their vicinity. Extravasation typically takes place within the first 24-72 h after initial arrest. By that time, most tumor cells have exited the bloodstream and seeded into the stroma of the secondary site [7]. The invasion of tumor cells in the circulation may occur very early in tumor development. Therefore, the first hours of metastasis during surgery may be a potential therapeutic window. However, currently therapy is not altered based upon CTCs status. A lack of understanding of the biology of CTCs has served as a barrier to develop rational therapy tailored to these high risk patients.
Propofol, the intravenously administered hypnotic agent, is widely used in all kinds of surgeries due to its short effect and rapid recovery. Patients receiving total intravenous anesthesia (TIVA) with propofol have been shown to experience less postoperative pain. Accumulating clinical evidence indicates that propofol TIVA for cancer surgery reduces the risk of recurrence or metastasis during the initial years of follow-up [8-11], indicating that propofol might kill any cancer cells released into the circulation in the perioperative period.
MicroRNAs (miRs) are endogenous, non-coding RNA molecules that act to regulate nearly every cellular process through inhibition of target messenger RNA expression. The role for miRs in the carcinogenesis, development and progression of pancreatic cancer has been well established [12]. miR-21 is overexpressed in the early stages of pancreatic cancer (PC) [13], and it has been shown that PC cells are addicted to miR-21 [14]. Lentiviral targeting of miR-21 by its antagonists resulted in increasing angiogenesis as well as strong inhibition of proliferation of PC cells both in vitro and in vivo. Furthermore, combination treatment involving miR-21 antagonists and gemcitabine (125 mg/kg) leads to significant regression of tumor growth in vivo.
Recently, it has found that propofol dose- and exposure time-dependently induced significant embryonic stem cell-derived neurons and down-regulated several miRs, including miR-21 [15]. Wang et al. reported that propofol inhibited hydrogen peroxide-induced upregulation of miR-21 and increased its target gene-mediated programmed cell death [4]. Propofol-mediated injury was attenuated by restoringmiR-21 expression [16].
Slug is a zinc finger transcriptional repressor of the Slug/Snail family that promotes carcinoma cell invasion, stemness and survival [17]. Clinical evidence supports a role for Slug in advanced pancreatic malignancies, and high Slug expression in PC is associated with poor prognosis, recurrence and metastasis [18]. Slug is known to inhibit E-cadherin transcription, attenuate the epithelial phenotype and promote malignancy [19]. In addition, targeting Slug induces apoptosis by repressing PUMA (p53 pro-apoptotic target gene) [20]. PUMA (BBC3), or p53-upregulated modulator of apoptosis, is a BH3-only member of the Bcl-2 family and a target of p53-mediated apoptosis [21]. It activates an apoptotic cascade by facilitating Bax activation, causing cytochrome C release from the mitochondria, caspase-3 activation and DNA fragmentation [22].
Accumulating evidence suggests that the miRs/Slug axis regulates mesenchymal tumor development by interfering with metastatic cancer cell programming [23-26]. It has recently found that miR-21 promotes EMT in lung epithelial cells during lung fibrosis [27]. miR-21 substantially promotes the fibroblast-like phenotype arising from fibrogenic EMT, whereas an antagonist that targets miR-21 blocks this effect as assessed by the E-cadherin/α-smooth muscle actin balance, cell viability, matrix activity and cell motility [28].
In the present study, we assessed the effect of propofol on apoptosis, survival and invasion of pancreatic cancer cells in vitro and explored its molecular mechanisms. Our findings demonstrate that propofol induces apoptosis and inhibits survival and invasion of PC cells by regulating the miR-21/Slug/E-cadherin and miR-21/Slug/PUMA signaling pathways.
Materials and methods
Cell line and culture
The human pancreatic cancer PANC-1 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and was routinely maintained at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% heat inactivated (1 h at 58°C) fetal calf serum, 1X L-glutamine, 1 mM sodium pyruvate, 1X nonessential amino acids, 100 units/mL of penicillin, and 0.1 mg/mL of streptomycin (Invitrogen, Hangzhou, China).
miR-21 mimic and siRNA/cDNA transfection
PANC-1 cells were seeded into 24-well plates at 60-70% confluence and kept in an incubator at 37°C and 5% CO2 overnight. miR-21 mimics (miR-21) and miR-21 negative control mimic (NC) were purchased from RiboBio (Guangzhou, China). PUMA, E-cadherin siRNA and control siRNAs were purchased from Santa Cruz Biotechnology (Shanghai, China). pcDNA3.1 Slug cDNA and pcDNA3.1 control were kindly gifted by Dr. Chen (Department of General Surgery, The Affiliated Hospital of Qingdao University). miR-21 or NC, PUMA or E-cadherin siRNA, or pcDNA3.1 Slug cDNA or pcDNA3.1 control were transfected into PANC-1 cells using Lipofectamine 2000 (Invitrogen, Shanghai, China) according to manufacturer instructions. Transfected cells were incubated at 37°C in a 5% CO2 incubator for 24 or 48 h. Total cellular RNA and protein were harvested separately and stored at -80°C until use.
Drug treatment
PANC-1 cells were cultured in 96-well plates (3 × 104 per well) and co-incubated with propofol (1, 5 or 10 μg/mL) for 48 h or 10 μg/mL propofol for 12, 24 or 36 h. To determine the signaling pathways involved in the production of miR-21, PANC-1 cells were transfected with miR-21, PUMA or E-cadherin siRNA, or pcDNA3.1 Slug cDNA or control for 24 h, then exposed to propofol (1, 5 or 10 μg/mL) for 48 h or 10 μg/mL propofol for 12, 24 or 36 h.
Measurement of LDH release
For the LDH release assay, culture medium was collected and LDH activity was assessed using an LDH cytotoxicity assay kit (Guangzhou, China) according to the manufacturer’s protocol. LDH activity was quantified by measuring absorbance at 490 nm with a microplate reader. The ratio of released LDH to total LDH was calculated and presented as relative LDH release compared to non-treated cells. All experiments were performed in triplicate and repeated three times.
BrdU cell proliferation assay
The BrdU assay was performed using a BrdU cell proliferation assay kit from Oncogene (San Diego, CA) according to manufacturer’s instructions. Briefly, PANC-1 cells were treated per the above methods. Ten hs before treatment termination, BrdU 5’-monophosphate (30 μg/ml) was added to culture medium. After allowing 10 h for BrdU labeling, cells were washed three times with sterile PBS, then the monoclonal anti-BrdU (2 μg/ml) was added to the medium, incubated overnight at 4°C, and then incubated for 1 h at room temperature with rhodamine-conjugated goat anti-mouse IgG (Jackson Immuno Research, West Grove, PA, USA; 1:200). The BrdU labeling index, reported as the percentage of cells labeled with BrdU, was determined by counting 10,000 cells from two independent reactions using a Zeiss Axiovert 40 inverted microscope and AxioVision Rel. 4.8.2 software (Carl Zeiss, New York, USA).
Flow cytometer for apoptosis assay
Using an Annexin V-FITC apoptosis detection kit (BD Biosciences, Guangzhou, China), Annexin V-staining followed by analysis using a FACS can flow cytometer was used to detect cell apoptosis according to the manufacturer’s instructions. Cell Quest software was used to analyze the data (Becton-Dickinson, Franklin Lakes, NJ, USA).
qRT-PCR analysis of miR-21
Quantitative real-time PCR (qRT-PCR) was performed on cDNA harvested from PANC-1 cell suspension the Taqman Fast System and reagents (Applied Biosystems, Foster City, CA) per manufacturer’s instructions. Total RNA (1 μg) was reverse-transcribed using random primer and MultiScribe RT (High-Capacity cDNA Archive Kit) (Shanghai, China) for mRNA analysis and miScript Reverse Transcription Kit for miRNA analysis. PCR was performed with the resulting reverse transcription products using specific oligonucleotide primers. Reactions containing miRNA-specific forward primer, TaqMan® probe and reverse primers were loaded into a PCR reaction plate in quadruplicate and incubated in a thermocycler (iCycler iQ, BIO-RAD, Hercules, CA) for 10 min at 95°C followed by 40 cycles of denaturing (15 sec. at 95°C), annealing and extension (60 sec. at 60°C). Experiments were set up in quadruplicate and repeated three times. Mean threshold cycles (CT) were calculated by averaging the technical replicates for each experiment and then averaging the mean replicate CT across the three runs. Quadruplicates with a standard deviation greater than 0.50 were eliminated, and these assays were repeated. miR-21 expression was normalized to that of RNU6B (ΔCT) for each tissue. Relative expression and fold differences were determined by comparing normalized expression levels between tissues (ΔΔCT) using the 2-ΔΔCT method. Statistical significance was determined using a Student’s t-test assuming unequal variance.
Western blotting
Cells were lysed in RIPA lysis buffer with proteinase inhibitor. Total cellular proteins were separated by SDS-PAGE and transferred to PVDF membranes, which were probed with antibodies against Slug (1:200, Abcam, Shanghai, China), PUMA (1:200, Cell Signaling, Shanghai, China), E-cadherin (1:200, Santa Cruz Biotechnology, Shanghai, China) and β-actin (1:10000, Sigma, Shanghai, China) overnight at 4°C followed by horseradish peroxidase-labeled goat-anti-rabbit IgG (1:6000, Abcam, Cambridge, UK) for 1 h. Signals were detected by enhanced chemiluminescence. β-actin was used as a loading control.
Cell migration and invasion assays
Motility in the absence of a chemoattractant was determined in membrane invasion culture system chambers containing a polycarbonate filter with 10-μm pores coated with 0.1% gelatin. Untreated PANC-1 cells (7.5 × 104) or PANC-1 cells transfected with miR-21 or NC, PUMA or E-cadherin siRNA, or pcDNA3.1 Slug cDNA or control pcDNA3.1 for 24 h, then treated with propofol (1, 5 or 10 μg/mL) for 48 h or 10 μg/mL propofol for 12, 24 or 36 h were seeded in each upper well, incubated at 37°C for 6 h in RPMI-1640 medium containing 10% FBS, and subsequently processed by fixation with staining. The number of cells that migrated to the lower side of the membrane was determined by examining five random fields (at 100 × magnification) per test condition. Triplicate samples were measured for each condition and results were averaged. Invasion assays were performed in a similar fashion, using membrane invasion culture system chambers containing a polycarbonate filter with 10-μm pores coated with a collagen-based matrix. For invasion assays, the time frame of incubation was extended to overnight (ie, approximately 20 hours).
Statistical analysis
All data are shown as mean ± SD. Statistical significance was determined by a Student t-test using the SPSS 17.0 software package. P<0.05 was considered statistically significant.
Results
Effect of propofol on survival and apoptosis of PANC-1 cells
PANC-1 cells were first treated with propofol (1, 5 or 10 μg/mL) for 48 h. Cell survival was assessed by lactate dehydrogenase (LDH) cell toxicology assays. As shown in Figure 1A, LDH release increased in a dose-dependent manner with propofol treatment (P<0.05 and P<0.01, respectively), suggesting that propofol decreased cell viability in a dose-dependent way.
Figure 1.
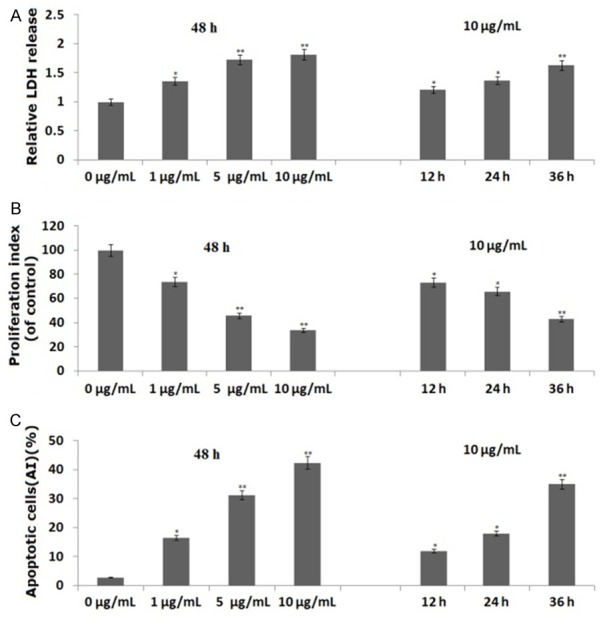
Propofol inhibits cell survival and proliferation, and induces apoptosis, in PANC-1 cells. PANC-1 cells were treated with 5 and 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h. A. Propofol inhibits cell survival by a LDH toxicology assay; B. Propofol inhibits cell proliferation by a BrdU cell proliferation assay; C. Propofol induces cell apoptosis by a flow cytometric assay. Data are represented as the means ± SD of three independent experiments. *P<0.05, **P<0.01 compared with control groups.
The effect of propofol on proliferation of PANC-1 cells was detected by BrdU cell proliferation assay. As shown in Figure 1B, treatment of PANC-1 cells with propofol (1, 5 or 10 μg/mL) for 48 h resulted in dose-dependent cell proliferation inhibition, which agreed with the results of the LDH assay (P<0.05 and P<0.01, respectively).
Next, we examined whether the inhibition of cell survival by propofol was accompanied by the induction of apoptosis. PANC-1 cells were treated with propofol (1, 5 or 10 μg/mL) for 48 h, and cell apoptosis was measured by flow cytometry assay. The induction of apoptosis was found to be dose-dependent (P<0.05 and P<0.01; Figure 1C), suggesting that propofol could induce apoptosis of PANC-1 cells.
We also treated PANC-1 cells with 10 μg/mL propofol for 12, 24 or 36 h. Propofol treatment also inhibited proliferation and induced apoptosis in a time-dependent manner (Figure 1A-C).
Effect of propofol on invasion and migration of PANC-1 cells
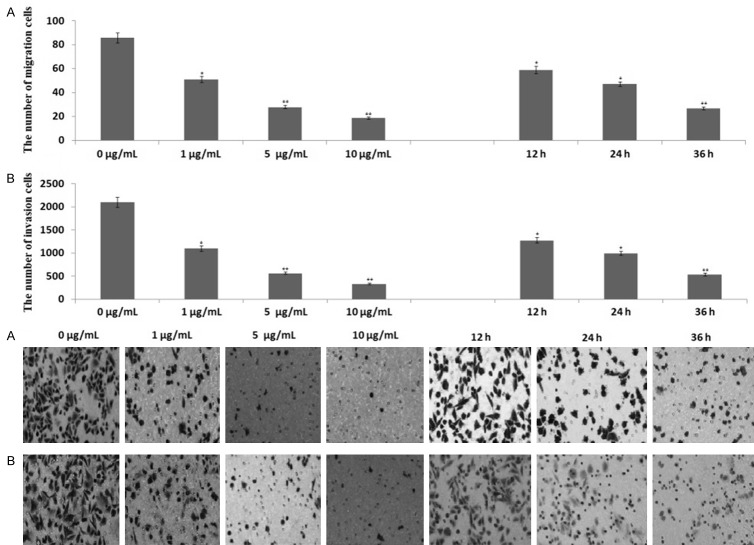
To determine the effect of propofol on the migration and invasion of PANC-1 cells, PANC-1 cells were treated with 1, 5 or 10 μg/mL propofol for 48 h, and then transwell migration and matrigel invasion assays were performed. Cells in the lower chamber of the transwell were obviously reduced when PANC-1 cells were treated with propofol as compared to untreated cells (Figure 2A, 2B), indicating that propofol inhibited both the migration and invasion of PANC-1 cells in a dose-dependent manner.
Figure 2.
Propofol inhibits migration and invasion of PANC-1 cells. PANC-1 cells were treated with 5 and 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h. A. Transwell migration assays; B. Matrigel invasion assays. Migrated or invaded cells were counted in five random fields of each filter under a microscope using 200 × magnification. The scale bar indicates 50 μm. Bars represent the average number of migrated or invaded cells. *P<0.05, **P<0.01 compared with control groups.
To determine whether propofol inhibits migration and invasion of PANC-1 cells in a time-dependent manner, PANC-1 cells were treated with 10 μg/mL propofol for 12, 24 or 36 h, respectively. Propofol treatment inhibited migration and invasion of PANC-1 cells in a time-dependent fashion (Figure 2A, 2B).
Upregulation of PUMA by propofol correlates with apoptosis induction in PANC-1 cells
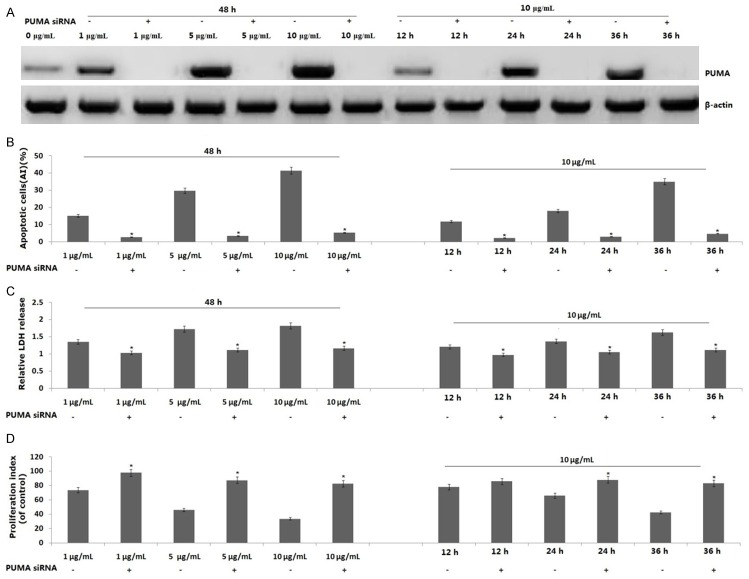
To determine the role of PUMA in propofol-induced apoptosis, PANC-1 cells were treated with 1, 5 or 10 μg/mL propofol for 48 h. PUMA protein was induced by propofol in a dose-dependent manner (Figure 3A). When the PANC-1 cells were treated with 10 μg/mL propofol for 12, 24 or 36 h, expression of PUMA protein was increased in a time-dependent fashion (Figure 3A).
Figure 3.
Effect of PUMA on propofol-induced apoptosis in PANC-1 cells. PANC-1 cells were treated 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h or transfected with PUMA siRNA for 24 h, then treated propofol according to the above. A. PUMA protein expression was detected by western blot assay; B. Propofol induces cell apoptosis by a flow cytometric assay; C. Propofol inhibits cell viability by a LDH toxicology assay; D. Propofol inhibits cell proliferation by a BrdU cell proliferation assay. Data are represented as the means ± SD of three independent experiments. *P<0.05 compared with control groups.
To determine whether PUMA is essential for propofol-induced apoptosis and growth inhibition, PANC-1 cells were transfected with PUMA siRNA for 24 h, then treated with 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h. As shown in Figure 3A, expression of PUMA protein in PANC-1 cells was inhibited by PUMA siRNA transfection followed by propofol treatment. In PUMA siRNA transfected PANC-1 cells, the cell apoptosis rate was dose- and time-dependent decreased with propofol treatment (Figure 3B), suggesting that propofol induced PUMA-mediated apoptosis. In addition, cell viability and proliferation were significantly decreased in PUMA siRNA transfected PANC-1 cells as compared to PANC-1 cells treated with propofol alone (Figure 3C, 3D).
Propofol-induced E-cadherin inhibits the migration and invasion of PANC-1 cells
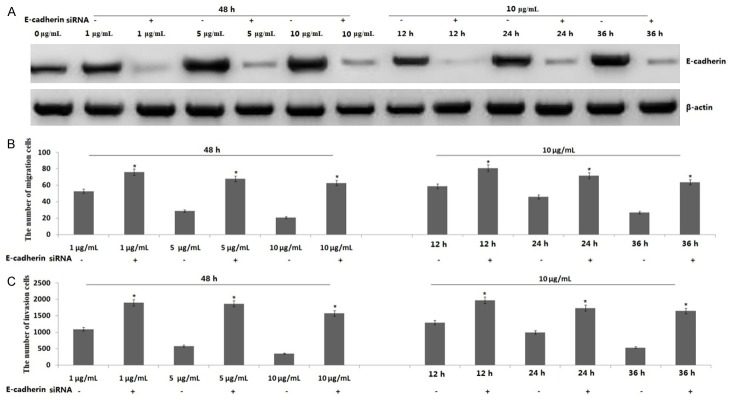
To determine the role of E-cadherin in propofol-induced inhibition of migration and invasion of PANC-1 cells, PANC-1 cells were transfected with E-cadherin siRNA for 24 h, then treated with 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h. As shown in Figure 4A, E-cadherin protein expression was inhibited in PANC-1 cells by E-cadherin siRNA transfection followed by propofol treatment. In addition, knockdown of E-cadherin reversed propofol-induced migration and invasion of PANC-1 cells (Figure 4B, 4C). Propofol inhibited the migration and invasion of PANC-1 cells via E-cadherin upregulation.
Figure 4.
Effect of E-cadherin on propofol-induced migration and invasion of PANC-1 cells. PANC-1 cells were treated 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h or transfected with E-cadherin siRNA for 24 h, then treated propofol according to the above. A. E-cadherin protein expression was detected by a western blot assay; B. Transwell migration assays; C. Matrigel invasion assays. Bars represent the average number of migrated or invaded cells. *P<0.05 compared with control groups.
Both PUMA and E-cadherin activation by propofol is mediated by Slug
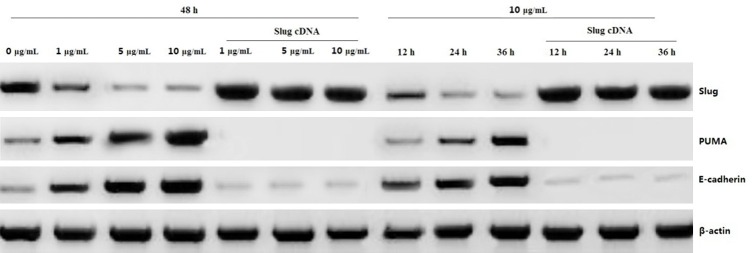
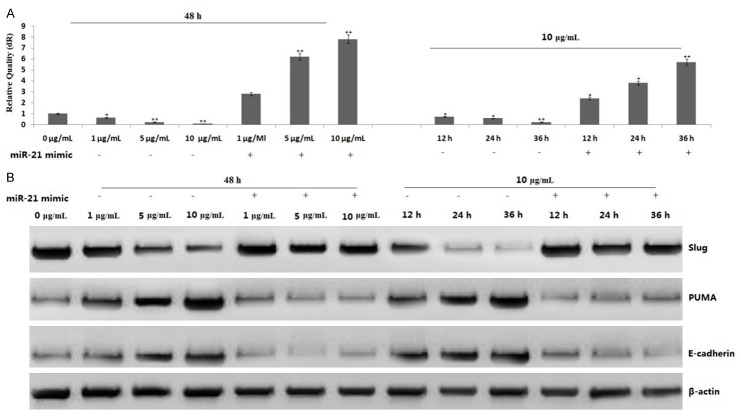
We then analyzed the mechanism of PUMA and E-cadherin induction by propofol in PANC-1 cells. Treatment with 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h downregulated Slug expression in a dose- and time-dependent manner, followed by increasing PUMA and E-cadherin expression (Figure 5). However, Slug overexpression by Slug cDNA transfection led to decreasing expression of PUMA and E-cadherin (Figure 5), as well as decreasing apoptosis and increasing invasion ability (data not shown), suggesting that PUMA and E-cadherin induction by propofol is due to Slug inhibition.
Figure 5.
Effect of Slug on propofol-induced PUMA and E-cadherin expression. PANC-1 cells were treated 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h or transfected with Slug cDNA for 24 h, then treated propofol according to the above. Slug, PUMA and E-cadherin protein expression was detected by a western blot assay.
miR-21-dependent Slug inactivation mediates PUMA and E-cadherin induction by propofol
miR-21 mRNA was overexpressed in PANC-1 cells detected by qRT-PCR (Figure 6A). Treatment with 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h strongly suppressed miR-21 mRNA expression in a dose- and time-dependent manner (Figure 6A), followed by decreased Slug expression and increased PUMA and E-cadherin expression (Figure 6B). However, treatment of miR-21 rescued miR-21 mRNA expression (Figure 6B), promoted Slug expression and inhibited PUMA and E-cadherin expression (Figure 6B), and reversed propofol-induced apoptosis and invasion inhibition (data not shown). These results suggest that miR-21 inhibition mediates the inactivation of Slug and activation of PUMA and E-cadherin by propofol. Together, these results demonstrate that PUMA and E-cadherin induction by propofol is mediated by miR-21 inhibition and subsequent Slug inactivation.
Figure 6.
Effect of miR-21 on propofol induced PUMA and E-cadherin expression. PANC-1 cells were treated with 1, 5 or 10 μg/mL propofol for 48 h or 10 μg/mL propofol for 12, 24 or 36 h or transfected with miR-21 for 24 h, then treated propofol according to the above. A. Relative expression of miR-21 as determined by qRT- PCR. B. Slug, PUMA and E-cadherin protein expression was detected by western blot assay. *P<0.05 compared with control groups.
Discussion
Propofol is an intravenous sedative-hypnotic agent administered to induce and maintain anesthesia. It has been recently revealed to have anticancer properties including direct and indirect suppression of the viability and proliferation of cancer cells by promoting apoptosis in some cancer cell lines [29-31]. Some studies have found that propofol increased migration of breast cancer cells [32]. However, clinically relevant concentrations of propofol decreased the invasion ability of some human cancer cells [33,34]. Thus, we set a concentration range of propofol (1, 5 or 10 μm) to test its effect on the behavior of PANC-1 cells. Treatment with propofol (1-10 μM) reduced cell death and leakage in a dose- and time-dependent manner, which accords with reports that propofol has a cytoprotective effect in myocardial cells [35]. In addition, propofol treatment inhibited invasion and migration of PANC-1 cells in vitro. The mechanisms by which propofol induces apoptosis and inhibits invasion of cancer cells are not well understood.
One previous study has demonstrated that whether inhibition of p53-upregulated modulator of apoptosis (PUMA) induction by propofol contributes to neuroprotection from cerebral ischemic cell death through both autophagic and apoptotic mechanisms [36]. Li et al. have found that propofol inhibits H2O2-induced injury in cardiac H9c2 cells via decreasing NF-κB activation and PUMA expression [37], suggesting that propofol has as anti-apoptotic effect by inhibiting PUMA expression. However, propofol was also found to induce apoptosis by activation of caspase-8 and caspase-9 in hepatocellular carcinoma cells [38]. In pancreatic cancer cells, treatment of cells with propofol induced apoptosis and potentiated gemcitabine-induced killing by downregulation of nuclear factor-κB [39]. In addition, propofol also inhibited proliferation and induced apoptosis of lung cancer H460 cells in vitro and in vivo [40]. In this study, we tested the role of PUMA in propofol-induced apoptosis and growth inhibition. Propofol increased PUMA expression in a dose- and time-dependent manner. Targeting PUMA by siRNA transfection inhibited propofol-induced apoptosis and growth inhibition, suggesting that PUMA upregulation contributed to propofol-induced apoptosis.
E-Cadherin is a tight junction protein that is differentially expressed in compact and diffused tumors [41,42] and plays a critical role in tumor metastasis. Accumulating bodies of evidence indicate that E-cadherin acts as an invasion suppressor of various epithelial malignancies. Loss of E-cadherin expression is emerging as one of the most common indicators of EMT onset. Furthermore, reduced expression of E-cadherin has been reported in various cancers and is associated with tumor progression and metastasis [43]. In this study, we test the role of E-cadherin on propofol-induced invasion and migration of PANC-1 cells. Propofol induced E-cadherin expressionin a dose- and time-dependent manner. Targeting E-cadherin by siRNA transfection inhibited propofol-induced invasion and migration, suggesting that E-cadherin upregulation contributed to propofol-induced invasion and migration of PANC-1 cells.
Apoptosis provoked by propofol requires activation of PUMA, and invasion inhibition by propofol requires activation of E-cadherin, but how propofol regulates PUMA and E-cadherin remains largely unknown. Slug is a zinc finger transcriptional repressor that promotes carcinoma cell invasion, stemness, and survival. It is well known that Slug negatively regulates PUMA and E-cadherin expression [19,20]. In this study, we found that propofol inhibited Slug expression in PANC-1 cells in a dose- and time-dependent manner. However, overexpression of Slug by Slug cDNA transfection reversed propofol-induced PUMA and E-cadherin expression, suggesting that Slug downregulation by propofol treatment contributes to Slug-dependent PUMA and E-cadherin upregulation of PANC-1 cells.
Previous studies have demonstrated that miRs play important roles in cancer development and progression by acting as activators or inhibitors [44]. More recently, several specialized miRs termed metastamirs have been implicated in the regulation of tumor metastasis and proliferation. Slug, E-cadherin and PUMA arereported to be regulated by a subset of miRs, such as miR-9, miR-203, miR-128, miR-128, miR-296, and miR-21 [45-53]. In the present study, propofol inhibited miR-21 mRNA expression in PANC-1 cells in a dose- and time-dependent manner. miR-21 mRNA re-expression by miR-21 transfection reversed Slug expression and downregulated PUMA and E-cadherin in PANC-1 cells. Taken together, these observations indicate that miR-21-dependent Slug inactivation mediates PUMA and E-cadherin in duction by propofol, indicating that propofol targets the miR-21-Slug pathway to inhibit invasion and induce apoptosis in pancreatic cancer.
In conclusion, we found that propofol downregulated miR-21 expression in PANC-1 cells, which inhibited Slug activation and increased downstream PUMA and E-cadherin expression, leading to the inhibition of cell growth and invasion of PANC-1 cells.
Disclosure of conflict of interest
None.
References
- 1.Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15:149–157. doi: 10.1016/j.jmoldx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 4.Rolle A, Günzel R, Pachmann U, Willen B, Höffken K, Pachmann K. Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: A preliminary report. World J Surg Oncol. 2005;3:18. doi: 10.1186/1477-7819-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massagué J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. Bernards R, Weinberg RA. A progression puzzle. Nature 2002; 418: 823. [DOI] [PubMed] [Google Scholar]
- 6.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigmore TJ, Mohammed K, Jhanji S. Long-term Survival for Patients undergoing Volatile versus IV anesthesia for Cancer Surgery: A Retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 9.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 11.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 12.Vorvis C, Koutsioumpa M, Iliopoulos D. Developments in miRNA gene signaling pathways in pancreatic cancer. Future Oncol. 2016;12:1135–1150. doi: 10.2217/fon-2015-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 15.Twaroski DM, Yan Y, Olson JM, Bosnjak ZJ, Bai X. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology. 2014;121:786–800. doi: 10.1097/ALN.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Cheng Y, Liu X, Yang J, Munoz D, Zhang C. Unexpected pro-injury effect of propofol on vascular smooth muscle cells with increased oxidative stress. Crit Care Med. 2011;39:738–745. doi: 10.1097/CCM.0b013e318206bd86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 18.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist inpancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 19.Cates JM, Byrd RH, Fohn LE, Tatsas AD, Washington MK, Black CC. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas. 2009;38:e1–6. doi: 10.1097/MPA.0b013e3181878b7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Cai J, Yu S, Chen S, Li F, Fan C. Accumulating evidence suggests that the deregulation of some microRNAs (miRNAs), is implicated in this process. MiR-630 Inhibits Endothelial-Mesenchymal Transition by Targeting Slug in Traumatic Heterotopic Ossification. Sci Rep. 2016;6:22729. doi: 10.1038/srep22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CW, Yu JC, Hsieh YH, Yao CC, Chao JI, Chen PM, Hsieh HY, Hsiung CN, Chu HW, Shen CY, Cheng CW. MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer. Oncotarget. 2016;24:435–442. doi: 10.18632/oncotarget.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu YH, Wei YP, Shen NJ, Wang ZC, Kan T, Yu WL, Yi B, Zhang YJ. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013;32:1331–1341. doi: 10.1159/000354531. [DOI] [PubMed] [Google Scholar]
- 26.Liang YJ, Wang QY, Zhou CX, Yin QQ, He M, Yu XT, Cao DX, Chen GQ, He JR, Zhao Q. MiR-124 targets Slug to regulate epithelial-mesenchymal transition and metastasis of breast cancer. Carcinogenesis. 2013;34:713–722. doi: 10.1093/carcin/bgs383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada M, Kubo H, Ota C, Takahashi T, Tando Y, Suzuki T, Fujino N, Makiguchi T, Takagi K, Suzuki T, Ichinose M. The increase of microRNA-21 during lung fibrosis andits contribution toepithelial-mesenchymaltransition in pulmonary epithelial cells. Respir Res. 2013;14:95. doi: 10.1186/1465-9921-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brønnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, Kalluri R, Sheikh SP. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cellsinvolving Programmed Cell Death 4 and Sprouty-1. PLoS One. 2013;8:e56280. doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, Brown R, Ma D. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–1349. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, Kishi Y, Nakamura H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–170. doi: 10.1016/s0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 31.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measure. Anesth Analg. 2003;97:1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 32.Garib V, Lang K, Niggemann B, Zänker KS, Brandt L, Dittmar T. Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells. Eur J Anaesthesiol. 2005;22:609–615. doi: 10.1017/s026502150500102x. [DOI] [PubMed] [Google Scholar]
- 33.Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, Kishi Y, Nakamura H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–170. doi: 10.1016/s0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 34.Miao Y, Zhang Y, Wan H, Chen L, Wang F. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother. 2010;64:583–588. doi: 10.1016/j.biopha.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Jin YC, Kim W, Ha YM, Shin IW, Sohn JT, Kim HJ, Seo HG, Lee JH, Chang KC. Propofol limits rat myocardial ischemia and reperfusion injury with an associated reduction in apoptotic cell death in vivo. Vascul Pharmacol. 2009;50:71–77. doi: 10.1016/j.vph.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Cui DR, Wang L, Jiang W, Qi AH, Zhou QH, Zhang XL. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-κB/p53 signaling pathway. Neuroscience. 2013;246:117–132. doi: 10.1016/j.neuroscience.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Liu YJ, Lv G, Zhang DL, Zhang L, Li D. Propofol protects against hydrogen peroxide-induced apoptosis in cardiac H9c2 cells is associated with the NF-κB activation and PUMA expression. Eur Rev Med Pharmacol Sci. 2014;18:1517–1524. [PubMed] [Google Scholar]
- 38.Zhang J, Wu GQ, Zhang Y, Feng ZY, Zhu SM. Propofol induces apoptosis of hepatocellular carcinoma cells by upregulation of microRNA-199a expression. Cell Biol Int. 2013;37:227–232. doi: 10.1002/cbin.10034. [DOI] [PubMed] [Google Scholar]
- 39.Du QH, Xu YB, Zhang MY, Yun P, He CY. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells in vitro by inhibition of nuclear factor-κB activity. World J Gastroenterol. 2013;19:5485–5492. doi: 10.3748/wjg.v19.i33.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui WY, Liu Y, Zhu YQ, Song T, Wang QS. Propofol induces endoplasmic reticulum (ER) stress and apoptosis in lung cancer cell H460. Tumour Biol. 2014;35:5213–5217. doi: 10.1007/s13277-014-1677-7. [DOI] [PubMed] [Google Scholar]
- 41.Weinel RJ, Neumann K, Kisker O, Rosendahl A. Expression and potential role of E-cadherin in pancreatic carcinoma. Int J Pancreatol. 1996;19:25–30. doi: 10.1007/BF02788372. [DOI] [PubMed] [Google Scholar]
- 42.Brinck U, Jacobs S, Neuss M, Tory K, Rath W, Kulle B, Füzesi L. Diffuse growth pattern affects E-cadherin expression in invasive breast cancer. Anticancer Res. 2004;24:2237–2242. [PubMed] [Google Scholar]
- 43.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2015;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Kumar SM, Lu H, Liu A, Yang R, Pushparajan A, Guo W, Xu X. MicroRNA-9 up-regulates E-cadherin through inhibition of NF-κB1-Snail1 pathway in melanoma. J Pathol. 2012;226:61–72. doi: 10.1002/path.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funamizu N, Lacy CR, Kamada M, Yanaga K, Manome Y. MicroRNA-203 induces apoptosis by upregulating Puma expression in colon and lung cancer cells. Int J Oncol. 2015;47:1981–1988. doi: 10.3892/ijo.2015.3178. [DOI] [PubMed] [Google Scholar]
- 48.Fu B, Wang Y, Zhang X, Lang B, Zhou X, Xu X, Zeng T, Liu W, Zhang X, Guo J, Wang G. MiR-221-induced PUMA silencing mediates immune evasion of bladder cancer cells. Int J Oncol. 2015;46:1169–1180. doi: 10.3892/ijo.2015.2837. [DOI] [PubMed] [Google Scholar]
- 49.Adlakha YK, Saini N. miR-128 exerts pro-apoptotic effect in a p53 transcription-dependent and -independent manner via PUMA-Bak axis. Cell Death Dis. 2013;4:e542. doi: 10.1038/cddis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, Gores GJ. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J Lipid Res. 2011;52:1517–1525. doi: 10.1194/jlr.M014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Liang H, Wang Y, Gao S, Yin K, Liu Z, Zheng X, Lv Y, Wang L, Zhang CY, Chen X, Xu G, Zhang W, Zou X. MiRNA-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell. 2016;7:383–387. doi: 10.1007/s13238-016-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, Chen H, Zhang P, Wang F, Han H, Wu W, Gao R, Gasche C, Qin H, Ma Y, Goel A. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2015;20:30845–30855. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 53.Grunder E, D’Ambrosio R, Fiaschetti G, Abela L, Arcaro A, Zuzak T, Ohgaki H, Lv SQ, Shalaby T, Grotzer M. MicroRNA-21 suppression impedes medulloblastoma cell migration. Eur J Cancer. 2011;47:2479–2490. doi: 10.1016/j.ejca.2011.06.041. [DOI] [PubMed] [Google Scholar]