Abstract
Background: The purpose of this work is to explore the correlation between Hsp90-beta level in broncheoalveolar lavage fluid (BALF) and lung cancer. Methods: Hsp90-beta level was measured by immunohistochemistry and enzyme-linked immunosorbent assay. Sensitivity and specificity of Hsp90-beta were calculated by receiver operator characteristic curve. Results: BALF in patients with lung cancer showed a higher expression of Hsp90-beta than those with benign lung disease (P<0.05). Elevated Hsp90-beta was closely related to lymphatic invasion and advanced stage of patients with lung cancer (P<0.05). The sensitivity of BALF Hsp90-beta for discerning lung cancer from patients with benign disease was 82.56% and specificity was 97.56%. Conclusion: Increased BALF Hsp90-beta correlates with lymphatic invasion and advanced stage of patients with lung cancer, suggesting it could be a diagnostic indicator for patients with lung cancer.
Keywords: Lung cancer, bronchoalveolar lavage fluid, BALF, Hsp90-beta diagnosis
Introduction
Lung cancer has been considered as a serious health problem in China due to the development of industrial society and deterioration of natural environment. One recent investigation shows that there will be about 733,000 newly diagnosed lung cancer cases in 2015 in China and about 610,000 Chinese will die from this terrible disease [1]. People believe that early diagnosis of lung cancer play an important role in reducing of morbidity and mortality. Thus, identification of diagnostic and prognostic biomarkers is promising for ameliorating the malignant outcome of lung cancer [2]. And some new methods distinguishing benign lung diseases from lung cancer are increasingly emerged in large numbers so as to avoid patients undergoing surgery for a benign condition.
White light bronchoscopy (WLB) has been used for identification and localization of neoplastic lesions within the bronchus, and specimens acquired from WLB can be used to make the diagnosis such as tissues, bronchoalveolar lavage fluid (BALF) and brush extract [3]. Although this technique is less invasive, sometimes it will fail to get specimens. Moreover, some patients with pulmonary bullae, emphysema and other objective factors possibly increase the biopsy risks. Thus, sometimes the bronchoscope operators take out the BALF for further assay in order to avoid complications. Research shows that protein changes in BALF can reflect biological effects from the pulmonary malignancies in the body. Therefore, detection of some specific proteins in BALF might be a good auxiliary diagnostic tool for differential diagnosis of lung cancer [4].
Heat shock protein 90 (Hsp90) has been shown including two major cytoplasmic isoforms, namely, Hsp90-alpha and Hsp90-beta. Previous studies show that Hsp90-beta is highly expressed in some cancer tissues including lung cancer and breast cancer [5,8]. Formerly, we disclosed Hsp90-beta overexpression in serum and tissues of patients with lung cancer was associated with malignant behavior of lung cancer [5-7]. However, studying on its level in BALF and significance in lung cancer patients remains unclear. Based on the above evidence and problem, we investigate the level of BALF Hsp90-beta and assess its usefulness for discerning lung cancer.
Material and methods
Patient population
From January 2013 to October 2015, 127 patients with pulmonary peripheral lesions were included into this project at Gansu Provincial Hospital and Affiliated Hospital of Jining Medical University (Table 1). This study was approved by the Institutional Review Board (Gansu Provincial Hospital and Affiliated Hospital of Jining Medical University), and written informed consent was obtained from all subjects. All patients were histologically confirmed by WLB biopsy and percutaneous lung biopsy, and 86 of them diagnosed with lung cancer. The blood samples were collected before any therapy from these patients. Patients with lung cancer were classified according to different clinical features. Forty-one of 127 patients were diagnosed with benign lung disease including acute infectious diseases, tuberculosis and interstitial diseases).
Table 1.
Clinico-pathological features of included patients
| Items | Characteristics | Lung cancer (N=86) | Benign lung disease (N=41) |
|---|---|---|---|
| Sex | |||
| Male | 60 (69.8%) | 30 (73.2%) | |
| Female | 26 (30.2%) | 11 (26.8%) | |
| Age | |||
| <60 | 42 (48.8%) | 22 (53.7%) | |
| ≥60 | 44 (51.2%) | 19 (46.3%) | |
| Pack years of smoking | |||
| >40 | 40 (46.5%) | 7 (17.1%) | |
| 20.1-40 | 2 (2.3%) | 6 (14.6%) | |
| 0.1-20 | 8 (9.3%) | 0 (0%) | |
| 0 | 36 (41.9%) | 28 (68.3%) | |
| Histology | |||
| LAC | 28 (32.5%) | ||
| LSCC | 46 (53.5%) | ||
| SCLC | 12 (14%) | ||
| Pathologic grade | |||
| Poorly differentiated | 34 (39.5%) | ||
| Moderately differentiated | 24 (27.9%) | ||
| Well-differentiated | 16 (18.6%) | ||
| Undifferentiated | 12 (14%) | ||
| Clinical staging | |||
| I-II | 21 (24.4%) | ||
| III-IV | 53 (61.6%) | ||
| Unavailable | 12 (14%) | ||
| Lymphatic invasion | |||
| Positive | 53 (61.6%) | ||
| Negative | 23 (26.7%) | ||
| Unavailable | 10 (11.7%) | ||
LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer.
Immunohistochemistry (IHC)
We employed IHC to determine the level of Hsp90-beta expression in lung tissues (Hsp90-beta polyclonal antibody, 1:100 dilution; Bostere Biotech Company, Wuhan, China). We used positive slices provided by Bostere Biotech Company as a positive control. Meanwhile, Taking PBS to replace Hsp90-beta antibody was implemented as a negative control. The IHC for Hsp90-beta was performed complying with a process previously described and the scores of immunostaining was blindly assessed according to a method previously described [5].
Bronchoalveolar lavage (BAL)
BAL was performed according to a method previously described [4]. The bronchoscope operators used 5-10 mL of 2% lidocaine to perform a local anesthesia for local airways that waited for BAL; after that, they used 0.9% saline solution to wash the airways and used a siliconized container to collect bronchoalveolar lavage fluid (BALF). The lavage fluid they gathered was filtered through a nylon filter. The BALF was centrifugated at 3,000 rpm at 4°C for 30 min to isolate the supernatant aliquots. The samples of BLAF were numbered under double-blind conditions, and immediately place supernatant aliquots into a ultra-low temperature freezer and stored at -80°C [4].
Enzyme-linked immunosorbent assay (ELISA)
The samples of BALF and serum all were numbered under double-blind conditions. The ELISA assay of Hsp90-beta in BALF and serum was performed according to the manufacturer’s instructions [7], which was finished at XiTang Biotechnology Co., Shanghai, China (Hsp90-beta product Code: CK-E11190H). The detection kit of Hsp90-beta was developed rabbit anti-human Hsp90-beta antibody as a sandwich-type ELISA pattern.
Statistical analysis
The expression of Hsp90-beta in lung tissues was calculated by the χ2 and Fisher’s exact test. The values of Hsp90-beta in BLAF and serum belong to measurement data, which were analysed through the Student’s T-test and One-WAY ANOVA and Kruskal Wallis Test [7]. We selected the SPSS 17.0 software (SPSS, Chicago, IL, USA) to implement the statistical analyses. To assess the diagnostic potential of Hsp90-beta in BLAF for lung cancer, we performed a receiver operator characteristic curve (ROC) analysis. All tests were two-sided; and we made a determination of having a statistical significance when P value was less than 0.05 [7].
Results
Lung cancer tissues showed a higher expression of Hsp90-beta than benign tissues
Some specimens of biopsies from 127 patients were too short to finish IHC analysis. Eventually, 58 specimens of lung cancer tissues and 28 specimens of benign lung tissues were qualified for IHC. The results showed Lung cancer tissues showed a higher expression of Hsp90-beta (32/58, 55.2%) than benign lung tissues (2/28, 7.1%), indicating Hsp90-beta plays a significant role in progress and development of lung cancer (P<0.05) (Table 2, Figure 1A-F).
Table 2.
Expressions of Hsp90-beta in lung cancer tissues and benign lung tissues
| Items | Expression grade | Benign | Cancerous | χ2 value | P value |
|---|---|---|---|---|---|
|
| |||||
| N=28 | N=58 | ||||
| Hsp90-beta | |||||
| Low (%) | 16 (57.1) | 10 (17.2) | 14.254 | <0.05 | |
| Moderate (%) | 10 (35.8) | 16 (27.6) | |||
| High (%) | 2 (7.1) | 32 (55.2)★ | |||
cancerous tissues compared with benign lung tissues.
Figure 1.
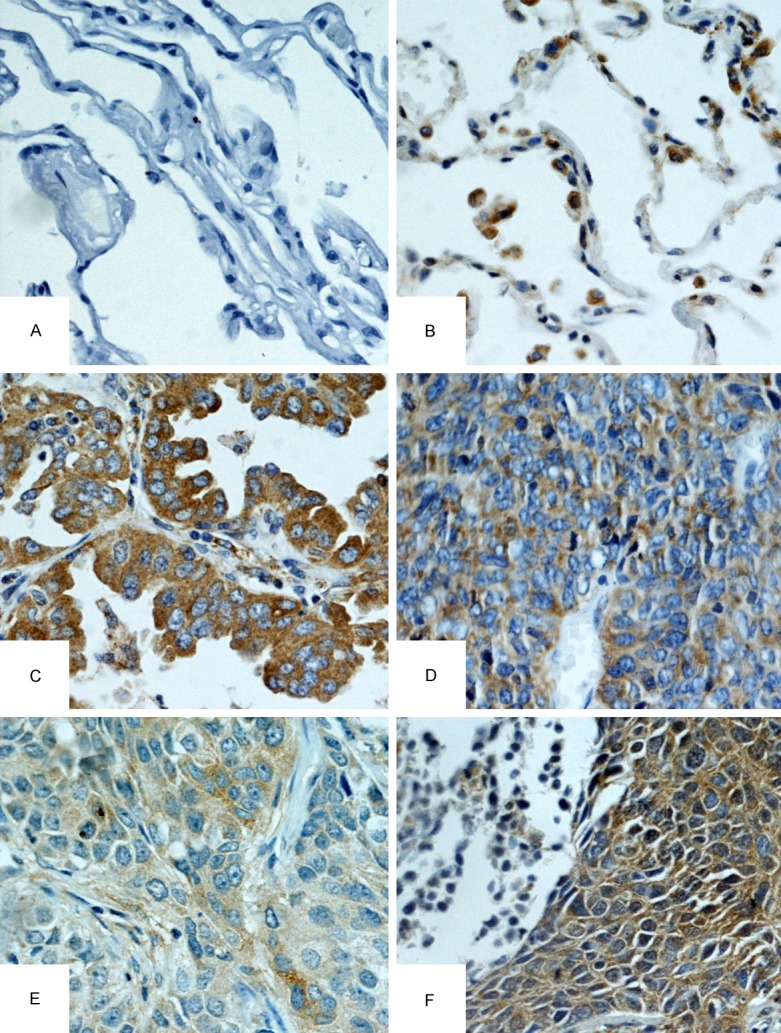
IHC analysis of Hsp90-beta in lung cancer and benign lung disease tissues (IHC×400). A. Low staining of Hsp90-beta in normal tissues; B. High staining of Hsp90-beta in normal tissues; C. High staining of Hsp90-beta in poorly differentiated LAC; D. High staining of Hsp90-beta in SCLC; E. Moderate staining of Hsp90-beta in moderately differentiated LSCC; F. High staining of Hsp90-beta in poorly differentiated LSCC; LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer.
Elevated Hsp90-beta in lung cancer tissues correlated with lymphatic invasion and advanced stage of lung cancer
Hsp90-beta expression did not correlate with histological type of lung cancer (P>0.05) (Table 3, Figure 2A). Poorly differentiated lung cancer tissues displayed a higher Hsp90-beta expression (68.4%) than well-differentiated tissues (7.7%) (P=0.004) (Table 3, Figure 2B). Lung cancer tissues with lymph node metastasis had a overexpression of Hsp90-beta (55.2%), as compared with those without lymph node metastases (15%, p=0.003) (Table 3, Figure 2C). Moreover, Hsp90-beta was highly expressed in lung cancer tissues with stages III-IV (50%), as compared with those of stages I-II (11.8%) (P=0.01) (Table 3, Figure 2D).
Table 3.
Correlation between clinico-pathological features and the expressions of Hsp90-beta in lung cancer tissues (N=58)
| Parameter | Group | N | Expression of Hsp90-beta | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (%) | Moderate (%) | High (%) | χ2 value | P value | |||
| Gender | |||||||
| Male | 41 | 9 (22) | 11 (26.8) | 21 (51.2) | 5.602 | 0.06 | |
| Female | 17 | 6 (35.3) | 8 (47.1) | 3 (17.6) | |||
| Ages | |||||||
| <60 | 27 | 8 (29.6) | 9 (33.3) | 10 (37) | 0.513 | 0.77 | |
| ≥60 | 31 | 7 (22.6) | 10 (32.3) | 14 (45.2) | |||
| Smoking | |||||||
| 0 | 30 | 8 (26.7) | 10 (33.3) | 12 (40) | 0.864 | 0.93 | |
| 0.1-40 | 4 | 1 (25) | 2 (50) | 1 (25) | |||
| >40 | 24 | 6 (25) | 7 (29.2) | 11 (45.8) | |||
| Histology | |||||||
| LAC | 19 | 6 (31.6) | 7 (36.8) | 6 (31.6) | 1.617 | 0.806 | |
| LSCC | 28 | 7 (25) | 9 (32.1) | 12 (42.9) | |||
| SCLC | 11 | 2 (18.2) | 3 (27.3) | 6 (54.5) | |||
| Pathological grade | |||||||
| Undifferentiated | 11 | 2 (18.2) | 3 (27.3) | 6 (54.5)★ | |||
| Poorly | 19 | 1 (5.3) | 5 (26.3) | 13 (68.4)★ | 15.618 | 0.004 | |
| Moderately | 15 | 5 (33.3) | 6 (40) | 4 (26.7) | |||
| Well | 13 | 7 (53.8) | 5 (38.5) | 1 (7.7) | |||
| Lymphatic invasion | |||||||
| N0 | 20 | 11 (55) | 6 (30) | 3 (15) | 16.367 | 0.003 | |
| N1-N3 | 29 | 2 (6.9) | 11 (37.9) | 16 (55.2)▲ | |||
| Unavailable | 9 | 2 (22.2) | 2 (22.2) | 5 (55.6) | |||
| cTNM | |||||||
| IB-IIB | 17 | 9 (52.9) | 6 (35.3) | 2 (11.8) | 13.23 | 0.01 | |
| IIIA-IV | 30 | 4 (13.3) | 11 (36.7) | 15 (50)● | |||
| Unavailable | 11 | 2 (18.2) | 2 (18.2) | 7 (63.6) | |||
p=0.004, undifferentiated and poorly differentiated compared with moderately and well differentiated, respectively;
p=0.003, N1-N3 compared with N0;
p=0.01, IB-IIB compared with IIIA-IV;
LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; SCLC, small cell lung cancer; Smoking, pack years of smoking.
Figure 2.
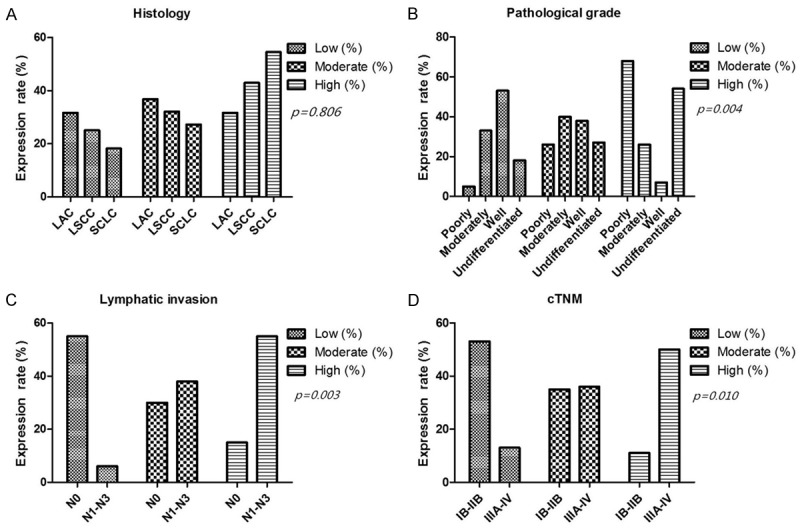
Correlation between clinico-pathological features and the expression of Hsp90-beta in lung cancer tissues. A. The expressions of Hsp90-beta did not correlate with histology of lung cancer; B. Up-regulation of Hsp90-beta was observed in poorly differentiated lung cancer tissues compared with well-differentiated tissues (p=0.004); C. Hsp90-beta expression in lung cancer cases without lymphnode metastasis was lower than that in lung cancer cases with lymph node metastasis (p=0.003); D. Up-regulated Hsp90-beta was found in lung cancer tissues at stages III to IV compared with that at stages I to II (p=0.010); LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer; N, node stage (TNM classification).
BALF of lung cancer patients had a high level of Hsp90-bet compared with benign lung disease patients
The Hsp90-beta of BALF and serum were detected in a series of 86 specimens of lung cancer patients and 41 specimens of benign lung disease patients. Hsp90-beta of lung patients was up-regulated in both BALF (0.98±0.02 ng/ml) and serum (1.63±0.03 ng/ml), as compared with benign lung disease patients (0.27±0.03 ng/ml; 0.36±0.02 ng/ml) (P<0.05) (Table 4, Figure 3A and 3B).
Table 4.
Correlation between the BLAF and serum levels of Hsp90-beta and clinicopathologic factors of lung cancer patients
| Groups | N | BALF level of Hsp90-beta | Serum level of Hsp90-beta | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| M±SD (ng/ml) | df | Statistical value | P | M±SD (ng/ml) | df | Statistical value | P | ||
| Resource | |||||||||
| Benign | 41 | 0.27±0.03 | 125 | T=15.51 | <0.05 | 0.36±0.02 | 125 | T=21.3 | <0.05 |
| Lung cancer | 86 | 0.98±0.02● | 1.63±0.03● | ||||||
| Histology | |||||||||
| LAC | 28 | 0.98±0.23 | 2 | F=3.876 | 0.06 | 1.58±0.2 | 2 | F=0.92 | 0.40 |
| LSCC | 46 | 0.93±0.27 | 1.63±0.44 | ||||||
| SCLC | 12 | 1.14±0.07 | 1.74±0.08 | ||||||
| Pathologic grade | |||||||||
| Undifferentiated | 12 | 1.14±0.08▲ | 3 | F=38.88 | <0.05 | 1.74±0.08▲ | 3 | F=21.77 | <0.05 |
| Poorly | 34 | 1.11±0.13▲ | 1.84±0.35▲ | ||||||
| Moderate | 24 | 0.95±0.13 | 1.55±0.13 | ||||||
| Well | 16 | 0.62±0.24 | 1.22±0.14 | ||||||
| Lymphatic invasion | |||||||||
| N0 | 23 | 0.69±0.25 | 2 | F=48.49 | <0.05 | 1.32±0.36 | 2 | F=21.53 | <0.05 |
| N1-N3 | 53 | 1.07±0.13▲▲ | 1.77±0.26▲▲ | ||||||
| Unavailable | 10 | 1.02±0.19▲▲ | 1.74±0.06▲▲ | ||||||
| TNM stage | |||||||||
| I-II | 21 | 0.67±0.24 | 2 | F=44.38 | <0.05 | 1.26±0.14 | 2 | ||
| III-IV | 53 | 1.06±0.15★ | 1.75±0.31★ | F=22.25 | <0.05 | ||||
| Unavailable | 12 | 1.03±0.17★ | 1.73±0.07★ | ||||||
BALF, bronchoalveolar lavage fluid; LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer;
cancerous compared with benign and non-cancerous;
undifferentiated and poorly compared with moderate and well;
N1-N3 compared with N0;
III-IV compared with I-II;
M±SD, mean±standard deviation; df, degree of freedom.
Figure 3.
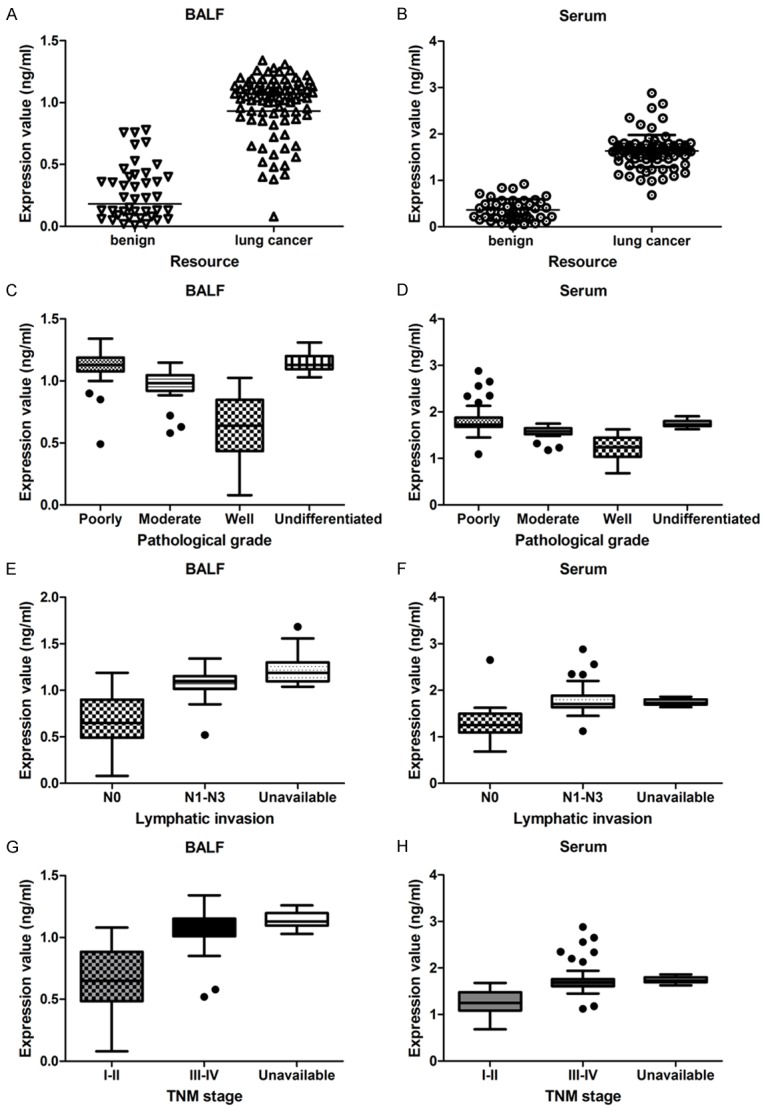
Correlation between clinicopathologic factors of lung cancer patients and the Hsp90-beta level of BALF and serum. A, B. Hsp90-beta of BALF and serum was higher in lung cancer patients than benign control group; C, D. Poorly undifferentiated patients revealed up-regulated level of Hsp90-beta in BALF and serum than moderate and well differentiation; E, F. Lymphatic invasion patients (N1-N3) revealed increased level of Hsp90-beta in BALF and serum than non-lymphatic invasion; G, H. Patients of III-IV stage had higher Hsp90-beta value in BALF and serum than both the I-II stage; LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer; N, node stage (TNM classification).
Increased Hsp90-beta of BALF correlated with lymphatic invasion and advanced stage of lung cancer
As shown in Table 4, patients with poorly differentiated tissues (1.11±0.13 ng/ml in BALF; 1.84±0.35 ng/ml in serum) revealed a up-regulated level of Hsp90-beta, as compared with those of well differentiated tissues (0.62±0.24 ng/ml in BALF; 1.22±0.14 ng/ml in serum; P<0.05) (Figure 3C and 3D). In addition, patients with lymphatic invasion (N1-N3) displayed an increased level of Hsp90-beta (1.07±0.13 ng/ml in BALF; 1.77±0.26 ng/ml in serum), as compared with those of without lymphatic invasion (P<0.05) (Figure 3E and 3F). And patients with III-IV stage had a higher Hsp90-beta (1.06±0.15 ng/ml in BALF; 1.75±0.31 ng/ml in serum) than those of I and II stage (0.67±0.24 ng/ml in BALF; 1.26±0.14 ng/ml in serum; P<0.05) (Figure 3G and 3H).
Cut-off of Hsp90-beta for discerning lung cancer patients
Our results showed that the cut-off values for discerning lung cancer mainly ranged from 0.6 to 0.8 ng/ml (for BALF) and 0.5-1.0 ng/ml (for serum) respective (Table 5). The 95% confidence interval were 0.213 to 2.396 (for BALF) and 0.0383 to 0.441 (for serum) respectively, which indicated that their thresholds must be in these areas mentioned above (Figure 4A, 4B and Figure 5A, 5B). We further calculated thresholds of Hsp90-beta for discerning lung cancer, the BALF threshold was 0.76 ng/ml, and serum threshold was 0.92 ng/ml (Table 6).
Table 5.
NLR and PLR of Hsp90-beta level in BALF and serum
| NLR and PLR of Hsp90-beta BALF concentration in lung cancer patients and benign lung diseases patients | ||||
|
| ||||
| Interval | Positive | Negative | Likelihood ratio | 95% CI |
|
| ||||
| 0.0-0.2 | 1 | 19 | 0.0251 | 0.00348 to 0.181 |
| 0.2-0.4 | 2 | 12 | 0.0795 | 0.0186 to 0.339 |
| 0.4-0.6 | 6 | 5 | 0.572 | 0.185 to 1.766 |
| 0.6-0.8 | 6 | 4 | 0.715 | 0.213 to 2.396 |
| 0.8-1.0 | 16 | 1 | 7.628 | 1.047 to 55.567 |
| 1.0-1.2 | 46 | 0 | ∞ | 2.771 to ∞ |
| 1.2-1.4 | 9 | 0 | ∞ | 0.510 to ∞ |
| Total | 86 | 41 | ||
|
| ||||
| NLR and PLR of Hsp90-beta serum concentration in lung cancer patients and benign lung diseases patients | ||||
|
| ||||
| Interval | Positive | Negative | Likelihood ratio | 95% CI |
|
| ||||
| 0.0-0.5 | 0 | 27 | 0.000 | 0.000 to 0.141 |
| 0.5-1.0 | 3 | 11 | 0.130 | 0.0383 to 0.441 |
| 1.0-1.5 | 19 | 1 | 9.058 | 1.255 to 65.358 |
| 1.5-2.0 | 57 | 0 | ∞ | 3.443 to ∞ |
| 2.0-2.5 | 4 | 2 | 0.953 | 0.182 to 4.995 |
| 2.5-3.0 | 3 | 0 | ∞ | 0.147 to ∞ |
| Total | 86 | 41 | ||
NLR, negative likelihood ratio; PLR, positive likelihood ratio; 95% CI, 95% confidence interval; BALF, bronchoalveolar lavage fluid.
Figure 4.
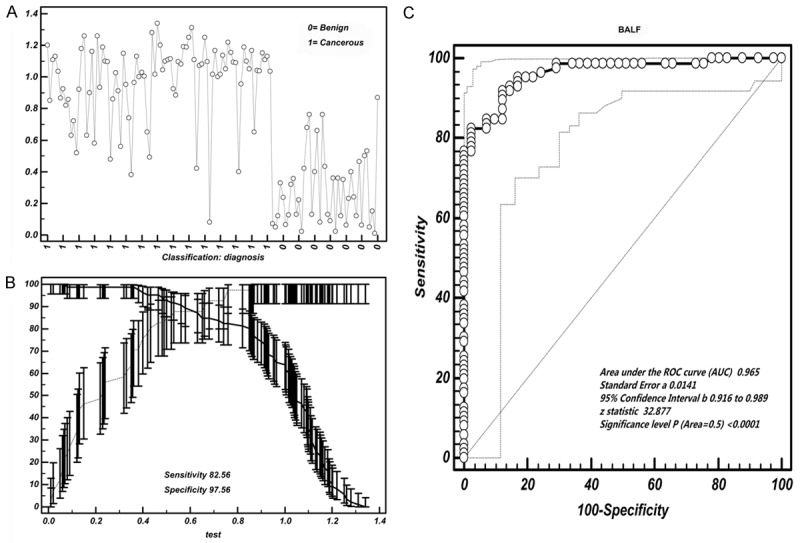
Cut-off selection of BALF Hsp90-beta by ROC curve analysis. A. Data distribution of BALF value of Hsp90-beta in distinguishing lung cancer patients from benign group; B. Selection of cut-off for BALF value of Hsp90-beta in distinguishing lung cancer patients from benign group; C. Receiver operating characteristic curves for BALF [area under the curve (AUC): 0.965]; ROC, receiver operating characteristic curve.
Figure 5.
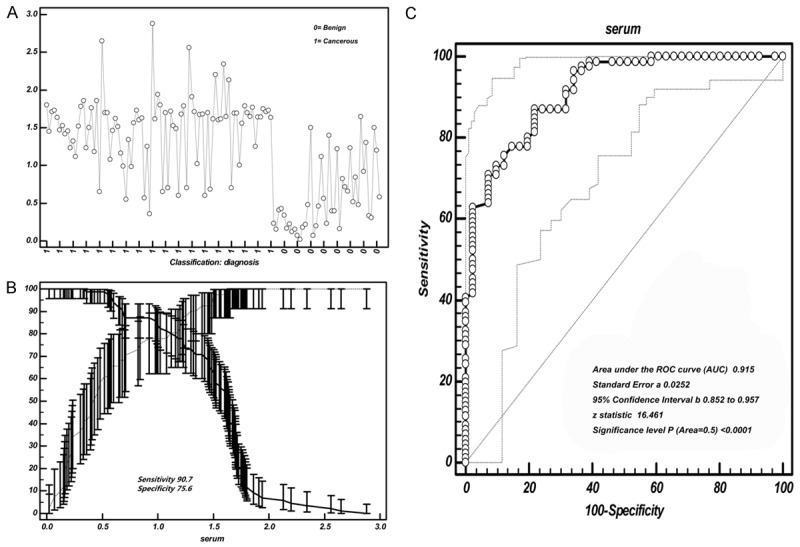
Cut-off selection of serum Hsp90-beta by ROC curve analysis. A. Data distribution of serum value of Hsp90-beta in distinguishing lung cancer patients from benign group; B. Selection of cut-off for serum value of Hsp90-beta in distinguishing lung cancer patients from benign group; C. Receiver operating characteristic curves for serum [area under the curve (AUC): 0.915]; ROC, receiver operating characteristic curve.
Table 6.
Cut-off score of Hsp90-beta level in specimens of BALF and serum for differentiating lung cancer patients
| Selection of cut-off score for BALF value of Hsp90-beta in lung cancer patients and benign lung disease patients | ||||||
|
| ||||||
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | -LR |
|
| ||||||
| >0.07 | 100.00 | 95.8-100.0 | 21.95 | 10.6-37.6 | 1.28 | 0.00 |
| >0.356 | 98.84 | 93.7-100.0 | 65.85 | 49.4-79.9 | 2.89 | 0.018 |
| >0.53 | 91.86 | 83.9-96.7 | 87.80 | 73.8-95.9 | 7.53 | 0.093 |
| >0.65 | 84.88 | 75.5-91.7 | 87.80 | 73.8-95.9 | 6.96 | 0.17 |
| >0.76* | 82.56 | 72.9-89.9 | 97.56 | 87.1-99.9 | 33.85 | 0.18 |
| >0.82 | 81.40 | 71.6-89.0 | 97.56 | 87.1-99.9 | 33.37 | 0.19 |
| >0.85 | 80.23 | 70.2-88.0 | 97.56 | 87.199.9 | 32.90 | 0.20 |
| >0.86 | 77.91 | 67.7-86.1 | 97.56 | 87.199.9 | 31.94 | 0.23 |
| >0.87 | 76.74 | 66.4-85.2 | 100.00 | 91.4-100.0 | 0.23 | |
|
| ||||||
| Selection of cut-off score for serum value of Hsp90-beta in lung cancer patients and benign lung disease patients | ||||||
|
| ||||||
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | -LR |
|
| ||||||
| >0.336 | 100.00 | 95.8-100.0 | 41.46 | 26.3-57.9 | 1.71 | 0.00 |
| >0.48 | 98.84 | 93.7-100.0 | 58.54 | 42.1-73.7 | 2.38 | 0.020 |
| >0.6 | 94.19 | 87.0-98.1 | 65.85 | 49.4-79.9 | 2.76 | 0.088 |
| >0.82 | 91.21 | 83.7-94.3 | 78.17 | 57.1-85.8 | 3.25 | 0.17 |
| >0.92* | 90.7 | 78.3-93.4 | 75.61 | 62.4-89.4 | 3.97 | 0.21 |
| >1.2 | 77.91 | 67.7-86.1 | 82.93 | 67.9-92.8 | 4.56 | 0.27 |
| >1.46 | 66.28 | 55.3-76.1 | 92.68 | 80.1-98.5 | 9.06 | 0.36 |
| >1.565 | 55.81 | 44.7-66.5 | 97.56 | 87.1-99.9 | 22.88 | 0.45 |
| >1.65 | 40.70 | 30.2-51.8 | 100.00 | 91.4-100.0 | 0.59 | |
BALF, bronchoalveolar lavage fluid;
cut-off value of Hsp90-beta level for differentiating lung cancer;
95% CI, 95% confidence.
Accuracy of Hsp90-beta in BALF for discerning lung cancer
When the 0.76 ng/ml was considered as a BALF threshold of Hsp90-beta, the sensitivity and specificity were 82.56% and 97.56% respectively. As to serum, a threshold of 0.92 ng/ml responded a sensitivity of 90.7% and a specificity of 75.6%, respectively. We noticed that the sensitivity of BALF was lower than that of serum, but the specificity increased significantly (Figure 4C and Figure 5C). To discern lung cancer from benign lung disease, the area under the curve (AUC) of BALF Hsp90-beta was 0.965, Z value was 32.877 (P<0.0001); and the AUC of serum Hsp90-beta was 0.915, Z value was 16.461 (P<0.0001) (Figure 4C and Figure 5C).
Comparison of diagnosis value of Hsp90-beta for lung cancer between BALF and serum
Linear correlation model indicated there was strongly linear correlation between BALF Hsp90-beta and serum Hsp90-beta, which showed that the correlation coefficient of regression model was 0.72 (95% confidence interval: 0.6209 to 0.7927) (P<0.0001; Figure 6A). We found that the Yonden index of BALF Hsp90-beta is more close to 1, suggesting the diagnostic value of BALF Hsp90-beta is better than serum (Figure 6B). The sensitivity and specificity of combined detection BALF with serum were higher than single detection. With benign disease used as the controls, the AUC of BALF was up to 0.965 (95% confidence interval: 0.916 to 0.989). Difference areas between BALF and serum was 0.0696, Z statistic was 1.894 (P=0.046) (Figure 6C).
Figure 6.
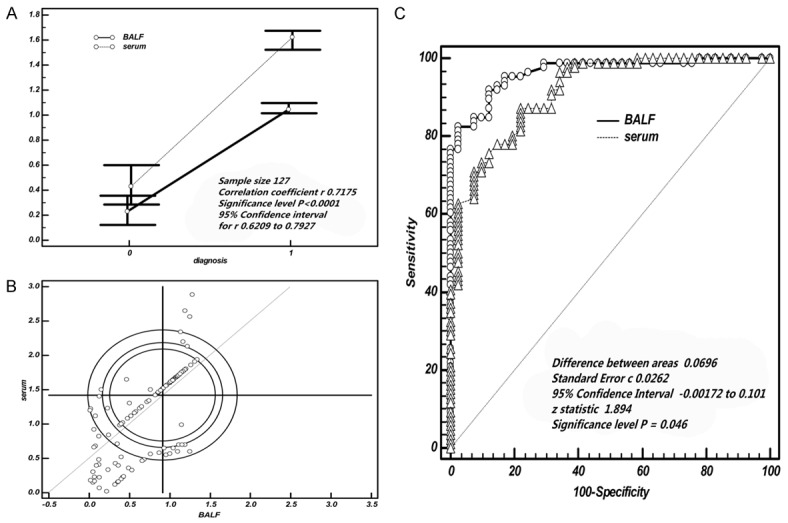
Comparison of diagnosis value of Hsp90-beta for lung cancer between BALF and serum. A. The expression of Hsp90-beta in serum had a better correlation with the level of BALF; B. The Yonden index of BALF Hsp90-beta more close to 1, suggesting the diagnostics value of BALF Hsp90-beta is better than serum; C. With benign disease used as the controls, the diagnostic value of BALF Hsp90-beta surpassed the serum, especially about specificity; ROC, receiver operating characteristic curve.
Discussion
For the diagnosis of respiratory diseases, electronic bronchoscope has been successfully used to diagnose respiratory diseases, which can observe lesions directly, take out living tissue for pathological diagnosis. Electron bronchoscopy has become a common means for routine examinations in respiratory disease department. Newly, BALF is showed to be useful for diagnosis of lung disease such as infectious diseases, immune diseases, even malignant tumor. Thus, patients suspected of malignancies have been recommended to accept this examination, and commonly perform a bronchial washing to collect BALF for improving lung diagnosis [9,10]. Proteomic studies on human body fluids such as bronchoalveolar lavage fluid (BALF) have become essential methods for biomarker discovery, examination of tumor pathways and investigation of potential treatments [11]. Detection of biomarkers in BALF might serve as an important method for differential diagnosis of lung cancer [4,12]. Scientific study has identified that specific tumor biomarker can help discern specific malignant tumor, which has been confirmed extensively through great progress of new proteomic approaches. Now, more and more abnormal protein molecules and their changes in different tumors have been testified, suggesting that such biomarkers had definite value to promote tumor diagnosis [13]. Previously, we employed mass spectrometry to identify abnormal protein expressions between lung cancer cells and normal bronchial epithelial cells. We found lung cancer cells showed a higher expression of Hsp90-beta, and overexpression of lung cancer tissues is closely related to the malignant behavior of lung cancer, including lymphatic metastasis and advanced stages of lung cancer. This present study is a elongation of our previous work [5]. Overexpression of Hsp90 has been disclosed to intimately correlate with the development and progress of malignant tumors, and has been considered as an important molecular chaperone in molecular reaction of cancer. Hsp90α and Hsp90-beta are two main isoforms of Hsp90; research shows that Hsp90-beta is more likely to be involved in the adaptation of cancer cells to the surrounding environment. The sufficient evidence of this inclusion is that Hsp90-beta is highly expressed in some specific cancers, including breast cancers [6,14]. However, it is not clear whether the changes of Hsp90-beta in BALF can reflect some information of lung cancer in body. In this study, we perform a series of studies to disclose whether we can use the changes of Hsp90-beta in BALF to discern lung caner from benign lung disease.
We found that lung cancer tissues had a higher expression of Hsp90-beta as compare to benign lung disease tissues, especially in poorly differentiated lung cancer tissues; but expression of Hsp90-beta did not correlate with histological classification of lung cancer. However, this was important clue in our study that overexpression of Hsp90-beta seems to be closely related to lymphatic metastasis and advanced stage of lung cancer. The results indicate that Hsp90-beta plays a significant role in development and progress of lung cancer, which may be used as lung cancer biomarker for the diagnosis and prognosis of lung cancer. Many studies have shown that a higher expression of Hsp90-beta in malignant tumors is implicated in a worse prognosis, as compared with those individuals who show a lower expression of Hsp90-beta [15-17], which suggests that overexpression of Hsp90-beta promotes the progress of malignant tumors and may be a significant prognosis indicator of cancers.
In our study, BALF Hsp90-beta in patients with lung cancer was found to be significantly higher than those of benign lung disease. More importantly, increased BALF Hsp90-beta intimately correlates with lymphatic invasion and advanced stage of patients with lung cancer. The results suggested that the high BALF level of Hsp90-beta correlates with lung cancer, which indicated that overexpression of Hsp90-beta was a risk factor of patients with lung cancer. Further, we tested and determined whether Hsp90-beta expression of matched BALF and serum had a consistency or not. The results verified that the expression of Hsp90-beta had a better linear correlation between the level of BALF and serum. Cumulative probability plots indicated that most of detection points of two methods were close to the line, or located on the line, which means there is a strong correlation between BALF serum. Based on the evidence above, the high Hsp90-beta level of BALF is a viable and reliable means to reflect the phenotype and development of lung cancer, and may be useful for diagnosis of lung cancer.
From the above, we deduced that the detection of BALF Hsp90-beta might be a potential biomarker for diagnosis and prognosis of lung cancer in future. In order to testify this conclusion, we determined the cutoff of BALF Hsp90-beta for discerning lung cancer in our study using ROC-derived cutoff value. We got that the diagnostic threshold afforded by the ROC analysis for Hsp90-beta in BALF was 0.76 ng/ml. With this cutoff value, BALF Hsp90-beta for discerning lung cancer had a sensitivity of 82.56% and a specificity of 97.56%. We excitedly noticed that the sensitivity of BALF to identify lung cancer was lower than serum, but the specificity promoted to 97.56%, which means the diagnostic accuracy of BALF is better than serum. With benign disease used as the controls, the specificity of BALF surpassed that of serum. Based on the results, Hsp90-beta detection in BALF may be a new tool with a reliable diagnostic value for discerning lung cancer patients who are noticed with pulmonary occupancy lesions by imaging examination. For example, a higher presence of Hsp90-beta in BALF may imply a higher probability of lung cancer for those pulmonary mass. The results indicates that each patient underwent a bronchoscope should collect the BALF for Hsp90-beta detection, which might serve for improving the diagnosis of lung cancer. However, patients with low expression of Hsp90-beta in BALF and serum should not decidedly mean the exclusion of lung cancer. Those patients should undergo further invasive procedures to ascertain the diagnosis of lung cancer.
The following are the limitations of this study. First, the study was conducted only at two centers, which may limit the strength of the evidence and could not be recommended in the present. Second, the samples of this study was relatively smaller, thus was not adequate to determine the differences effectually in marker level between early stage and advanced stage of lung cancer (I and II stage of lung cancer). Future, we plan to carry out a prospective and large cohort study in multiple centers to clarify these questions. In addition, the studies on functional and molecular mechanism of Hsp90-beta in lung cancer are also required, which will help understand deeply the correlation between Hsp90-beta and lung cancer.
Conclusion
We showed that lung cancer patients had a higher level of BALF Hsp90-beta, and higher level of Hsp90-beta was significantly associated with lymphatic metastasis and malignant progression of lung cancer. In addition, increased BALF Hsp90-beta level had a better sensitivity and specificity for discerning lung cancer, and the major advantage of BALF Hsp90-beta was to improve the specificity of diagnosis. The findings suggested that BALF Hsp90-beta could be a useful tool as a biomarker and predictor for improving the diagnosis of lung cancer.
Acknowledgements
This study was supported by technology Research and Development Plan of Gansu Province (0912TCYA016), China. We wish to thank Drs. WU Dian-lei and Wang Xin-hong for their helpful discussions, critical comments provided during the preparation of this manuscript and also wish to thank all authors of references.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Kim B, Lee HJ, Choi HY, Shin Y, Nam S, Seo G, Son DS, Jo J, Kim J, Lee J, Kim K, Lee S. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007;67:7431–7438. doi: 10.1158/0008-5472.CAN-07-0003. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Q, Feng J, Wu Q. Comparison of autofluorescence imaging bronchoscopy and white light bronchoscopy for detection of lung cancers and precancerous lesions. Patient Prefer Adherence. 2013;7:621. doi: 10.2147/PPA.S46749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao C, Chen ZB, Sun SF, Yu YM, Ding QL, Deng ZC. Evaluation of VEGF-C and Tumor Markers in Bronchoalveolar Lavage Fluid for Lung Cancer Diagnosis. Sci Rep. 2013;3:3473. doi: 10.1038/srep03473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. doi: 10.1186/1756-9966-31-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biaoxue R, Shuanying Y, Wei L, Zongjuan M, Xiguang C, Qiuhong Z. Co-overexpression of Hsp90-beta and annexin A1 with a significantly positive correlation contributes to the diagnosis of lung cancer. Expert Rev Mol Diagn. 2014;14:1067–1079. doi: 10.1586/14737159.2014.960517. [DOI] [PubMed] [Google Scholar]
- 7.Biaoxue R, Chongchong Z, Hua L, Zongjuan M, Xiguang C, Wenglong G, Shuanying Y. Identification and verification of Hsp90-beta as a potential serum biomarker for lung cancer. Am J Cancer Res. 2014;4:874–885. [PMC free article] [PubMed] [Google Scholar]
- 8.Yan W, Xiao J, Liu T, Huang W, Yang X, Wu Z, Huang Q, Qian M. The effects of Hsp90 expression alteration on spinal metastases of breast carcinoma. Tumour Biol. 2013;34:1391–1397. doi: 10.1007/s13277-012-0584-z. [DOI] [PubMed] [Google Scholar]
- 9.Jakubowska K, Naumnik W, Niklińska W, Chyczewska E. Respiratory Carcinogenesis. Springer; 2015. Clinical Significance of HMGB-1 and TGF-β Level in Serum and BALF of Advanced Non-Small Cell Lung Cancer; pp. 49–58. [DOI] [PubMed] [Google Scholar]
- 10.Kim JO, Gazala S, Razzak R, Guo L, Ghosh S, Roa WH, Bedard EL. Non-small cell lung cancer detection using microRNA expression profiling of bronchoalveolar lavage fluid and sputum. Anticancer Res. 2015;35:1873–1880. [PubMed] [Google Scholar]
- 11.Almatroodi SA, McDonald CF, Collins AL, Darby IA, Pouniotis DS. Quantitative proteomics of bronchoalveolar lavage fluid in lung adenocarcinoma. Cancer Genomics-Proteomics. 2015;12:39–48. [PubMed] [Google Scholar]
- 12.Chen Z, Xu Z, Sun S, Yu Y, Lv D, Cao C, Deng Z. TGF-β1, IL-6, and TNF-α in Bronchoalveolar Lavage Fluid: Useful Markers for Lung Cancer? Sci Rep. 2014;4:5595. doi: 10.1038/srep05595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung HJ, Cho JY. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008;41:615–625. doi: 10.5483/bmbrep.2008.41.9.615. [DOI] [PubMed] [Google Scholar]
- 14.Whitesell L, Santagata S, Lin N. Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med. 2012;12:1108–1124. doi: 10.2174/156652412803306657. [DOI] [PubMed] [Google Scholar]
- 15.Jahns F, Wilhelm A, Greulich KO, Mothes H, Radeva M, Wölfert A, Glei M. Impact of butyrate on PKM2 and HSP90β expression in human colon tissues of different transformation stages: a comparison of gene and protein data. Genes Nutri. 2012;7:235–46. doi: 10.1007/s12263-011-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myung JK, Afjehi-Sadat L, Felizardo-Cabatic M, Slavc I, Lubec G. Expressional patterns of chaperones in ten human tumor cell lines. Proteome Sci. 2004;2:8. doi: 10.1186/1477-5956-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDowell CL, Sutton RB, Obermann WM. Expression of Hsp90 chaperome proteins in human tumor tissue. Int J Biol Macromol. 2009;45:310–314. doi: 10.1016/j.ijbiomac.2009.06.012. [DOI] [PubMed] [Google Scholar]


