Abstract
Dysregulation of N-acetyltransferase 10 (NAT10) is associated with the development of many types of tumors; however, its role in hepatocellular carcinoma (HCC) has not been fully elucidated. Here, we examined the role of NAT10 during epithelial-to-mesenchymal transition (EMT) in HCC and established its role in metastasis. We evaluated expression of NAT10 expression in four HCC cell lines and determined the effects of knockdown by siRNA or treatment with the NAT10 inhibitor, Remodelin. NAT10 was highly expressed in HCC cell lines with a mesenchymal-like phenotype (SNU387 and SNU449). Knockdown or inhibition of NAT10 resulted in diminished cell invasion and migration. Moreover, decreased levels of NAT10 were correlated with increased E-cadherin expression and down regulation of vimentin, both of which are canonical markers of EMT signaling, suggesting that NAT10-promoted metastasis may be mediated by EMT in HCC. Our data suggests that up regulation of NAT10-promoted metastasis of HCC cells may be mediated by EMT.
Keywords: N-acetyltransferase 10, vimentin, E-cadherin, epithelial-to-mesenchymal transition, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common primary malignant tumors and is the second leading cause of deaths due to cancer globally [1]. Despite significant advancements in HCC treatment in recent years, an effective therapy remains elusive due to a lack of effective clinical strategies to prevent metastasis and relapse [2,3]. Studies have shown that over 50% of patients experience metastasis following resection of HCC, which drastically worsens the prognosis [4]. Intrahepatic and extrahepatic metastases are the main forms of HCC metastasis. The most frequent sites of extrahepatic metastasis are the lungs, lymph nodes, peritoneum, and bone. Intrahepatic recurrence and metastasis of HCC, however, occurs more frequently than dissemination to distal sites [5].
In recent years, the relationship between cancer metastasis and signaling pathways mediating epithelial-to-mesenchymal transition (EMT) has been the subject of several studies. EMT is characterized by the loss of cell-cell interactions and epithelial apico-basal polarity with the concomitant acquisition of mesenchymal markers, which occurs both in physiological and pathological processes [6]. Interestingly, previous studies have reported that EMT can accelerate tumor development and progression by enhancing metastasis [7]. EMT has been extensively studied and validated in HCC [8]. A variety of signaling pathways have been shown to play an important role in EMT. In addition to transforming growth factor-beta (TGFβ) signaling pathways, Wnt pathways are also commonly reported to be involved in EMT. Wnt pathways play a major role in the adhesion and migration of cancer cells [9-11].
A recent study reported redistributed subcellular localization and dysregulation of NAT10 in colorectal cancer tissues. Furthermore, dysregulation was closely connected with Wnt pathway activity [12]. NAT10 is a nucleolar protein consisting of 872 aa with an acetyltransferase domain and a lysine-rich C-terminus, primarily found as a regulator of telomerase activity [13]. NAT10 is also involved in the DNA damage response, cytokinesis, histone and microtubule modification [14-16]. Although dysregulation of NAT10 plays a critical role in carcinogenesis in many different tumors, little information is available regarding the essential role of NAT10 in HCC. Our previous research demonstrated that the elevation of NAT10 expression might contribute to the tumorigenesis and development of HCC [17].
In this study, we further demonstrated that high expression of NAT10 is associated with a mesenchymal phenotype in HCC cell lines. Reduced expression of NAT10 results in diminished cell invasion and migration. Moreover, a decrease in NAT10 is correlated with impaired activation of EMT signaling, suggesting that NAT10-promoted metastasis may be mediated by EMT in HCC.
Materials and methods
Cells lines, culturing conditions and NAT10 inhibitor
The human HCC cell lines Hep3B, SNU387, SNU449 and Huh7 used in this study were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA), containing 10% fetal bovine serum (FBS; Sigma, St. Louis, MO), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma). Remodelin was used as inhibitor of NAT10 (Selleckchem, USA). To analyze the effects of NAT10 on hypoxia-induced metastasis, cell populations were divided into two groups and cultured in normoxia (21% O2) and hypoxia (1% O2) at 37°C in an incubator with a constant humidity.
siRNAs and transfection
The siRNA sequences targeting NAT10 were purchased from Santa Cruz Biotechnology (sc62660, Santa Cruz Biotechnology Inc. USA). Lyophilized oligonucleotides were reconstituted with RNase-free water to obtain a stock solution of 20 μM. The siRNAs were transfected into cells using Lipofectamine 2000 (Invitrogen) and were incubated for 48 h according to the manufacturer’s instructions.
Wound healing assay
For wound healing migration assays, 5 × 104 cells were seeded in a 24 well plate and incubated for two days. A wound (0.5 mm) was produced by a pipette tip. Floating cells were removed by three washes with medium, and fresh medium with 1% FBS added. Images were acquired at designated time points. The distance from two different scratched regions was measured with Image J software. The length of the healed scratch and the length of the initial scratch were compared. Three independent assays were performed followed by statistical analysis.
Transwell assay
For transwell invasion assays, transwell chambers (Millipore, MA, USA) with Matrigel-coated membranes (BD Bioscience, CA, USA) were used. Fifty thousand cells were seeded in the upper chamber. The cells on the upper side of the membrane were removed after three days. Cells migrating to the other side of the membrane were fixed and stained using crystal violet. Stained cells were counted in five random fields at a magnification of 100×. Each assay was performed in triplicate.
Real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA from HCC cell lines was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. The RNA was reverse transcribed to cDNA using a kit from TAKARA and the following thermocycling conditions: 15 min at 37°C and 5 s at 85°C (PrimeScript™ RT Master Mix, TAKARA, Japan). Expression of NAT10 was evaluated by qPCR with β-actin serving as a reference gene. The primers were as follows: NAT10 forward primer, 5’-GGGATTGGCCTGCAGCATA-3’, Reverse primer, 5’-GGCTCCATGACCACATCCTT-3’; β-actin forward primer, 5’-ATCAAGGAGAAGCTCTGCTACATC-3’, Reverse primer, 5’-TCAGACTCGGCTGGAAGAGA-3’.
The qRT-PCR reaction performed using the ABI 7500 system with the following cycling parameters: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. These experiments were carried out in triplicate.
Western blot
Expression levels of NAT10, the mesenchymal marker vimentin, the epithelial marker E-cadherin and an internal control (GAPDH) were examined by Western blot. Cells treated as indicated were lysed in lysis buffer with 50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% Nonidet P-40 (NP40), 1 mM EDTA, and 200 mM Na3VO4 supplemented with protease inhibitor cocktail. Protein concentrations were quantified by a BCA protein assay (Applygen Technologies Inc. Beijing, China). Approximately 20 μg of protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA). The PVDF membrane was blocked with 5% nonfat milk then incubated with the designated primary antibodies against NAT10 (Santa Cruz, USA), vimentin (CST, USA), E-cadherin (CST) and GAPDH (CST). The blots were then incubated with an HRP-conjugated secondary antibody (CST) and the chemiluminescence signal intensity analyzed by Image J.
Immunofluorescence staining
Immunofluorescence staining was performed using previously described protocols [18]. Briefly, cells were seeded on a coverslip and fixed with 4% formaldehyde for 30 min. Blocking with 3% BSA was performed for an additional 30 min followed by permeabilization with 0.1% Triton-X 100. Cells were then stained with a primary antibody against E-cadherin or vimentin at a concentration of 1:100. Finally, a secondary antibody (1:200) and DAPI (1:1000) were added. The secondary antibody was removed and the Hep3B, Huh7, SNU387 and SNU449 cells were observed by confocal immunofluorescence microscopy (Zeiss, Oberkochen, Germany).
Statistical analysis
Data are presented as the mean ± SD and statistical analyses were performed with the SPSS software package (Version 18.0, Chicago). Statistical differences between two groups were examined with the Student’s t test and multiple groups were compared using one-way analysis of variance (ANOVA). The significance level was set at P<0.05.
Results
NAT10 expression in human HCC cell lines
To determine whether expression levels of NAT10 were associated with HCC, we analyzed the expression of NAT10 in a panel of HCC cell lines by qRT-PCR and Western blot. As shown in Figure 1A and 1B, differential expression of NAT10 in all HCC cell lines was observed. Furthermore, epithelial HCC cell lines, such as Hep3B and Huh7, had high expression of NAT10, whereas mesenchymal cell lines, such as SNU449 and SNU387, demonstrated low expression of NAT10 (Figure 1A, 1B). These data suggest that NAT10 levels may be associated with EMT in HCC.
Figure 1.

Differential expression of NAT10 in human epithelial (Hep3B and Huh7) and mesenchymal (SNU449 and SNU387) HCC cell lines. A. Quantification by qRT-PCR of NAT10 levels in HCC cell lines. Expression of NAT10 mRNA is clearly lower in the epithelial cell lines. β-actin served as a reference gene. B. Western blot showing NAT10 expression levels in HCC cell lines. GAPDH served as a loading control. Values represent the mean ± SD of three independent experiments.
Down regulation of NAT10 inhibits invasion and migration of HCC cell lines
Metastasis is a main factor predicting poor prognosis in HCC. We, therefore, studied the function of NAT10 in HCC metastasis. The effect of NAT10 silencing on metastasis was examined by transwell invasion and wound-healing migration assays. Transwell invasion assays demonstrated that, following the 48 h incubation, fewer NAT10-siRNA or Remodelin-treated HCC cells passed through the transwell membranes as compared to controls (Figure 2A). NAT10-siRNA and Remodelin treatment reduced the levels of protein expression compared with the mock treated HCC cells (Figure 2B). Similarly, the wound-healing migration assay showed that the wound was much wider in NAT10-siRNA HCC cells and Remodelin-treated cells than in the control HCC cells (Figure 3A). The siRNA knockdown efficiency was confirmed by qRT-PCR and Western blot. NAT10-siRNA reduced the level of mRNA expression compared with the mock-transfected HCC cells (Figure 3B).
Figure 2.

Down regulation of NAT10 inhibits invasion of HCC cells. A. Images and quantification of transwell migration assays of HCC cell lines following transfection with NAT10-siRNA, treatment with Remodelin, or controls. *P < 0.05 vs. Control. Images are at 200× magnification. B. Western blot confirming knockdown of NAT10 following transfection with NAT10-siRNA or treatment with Remodelin. GAPDH was used as a loading control. Values represent the mean ± SD of thee independent experiments. *P < 0.05 vs. 0 h; #P < 0.05 vs. 24 h.
Figure 3.
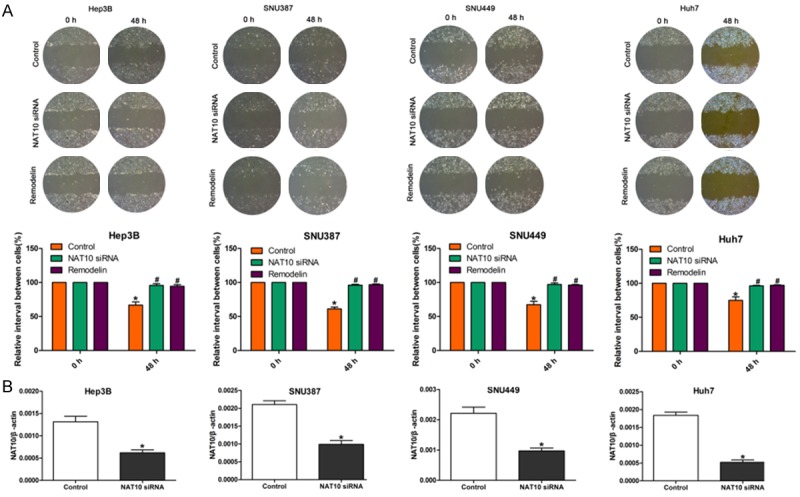
Down regulation of NAT10 inhibits migration of HCC cells. A. Wound healing assay showing the effects of NAT10-siRNA, mock or Remodelin treatment on the migration ability of HCC cells. B. Quantification of knockdown of NAT10 following NAT10-siRNA transfection as determined by qRT-PCR. β-actin was used as the internal reference.
NAT10 is associated with an EMT phenotype in human HCC cell lines
We investigated the expression of EMT-associated proteins in a panel of HCC cell lines by Western blot. We observed high expression of E-cadherin and low expression of vimentin in both Hep3B and Huh7 cell lines, while SNU449 and SNU387 cells showed low expression of E-cadherin and high expression of vimentin (Figure 4A, 4D). This data suggests that Hep3B and Huh7 display an epithelial phenotype, while SNU449 and SNU387 display mesenchymal phenotypes.
Figure 4.

NAT10 is associated with an EMT phenotype in human HCC cell lines. A. Expression of E-cadherin and vimentin in HCC cell lines as determined by Western blot. GAPDH served as a loading control. Decreased levels of NAT10 were associated with an epithelial phenotype in HCC cells, while increased NAT10 levels were associated with a mesenchymal phenotype. B. Western blot of the EMT markers E-cadherin and vimentin expression following knockdown of NAT10 with NAT10-siRNA. C. Expression of E-cadherin and vimentin following treatment with the NAT10 inhibitor Remodelin as determined by Western blot. GAPDH was used as an internal reference. D. Immunofluorescence staining of HCC cell lines for E-cadherin or vimentin. Magnification is 200×. Data represent three independent experiments.
Next, all four HCC cell lines were transfected with NAT10-siRNA or treated with Remodelin. Following silencing of NAT10, significant changes consistent with the phenomenon of mesenchymal-to-epithelial transition (MET) were apparent (Figure 4B, 4D). The epithelial biomarker E-cadherin was up regulated, while the mesenchymal biomarker vimentin was down regulated. Furthermore, the MET phenomenon was particularly apparent in SNU449 and SNU387, the HCC cell lines with a mesenchymal phenotype.
Remodelin, an inhibitor of NAT10, blocks invasion and migration of HCC cells in hypoxic conditions
To analyze the effects of NAT10 on hypoxia-induced metastasis, HCC cells were cultured for 48 h with or without Remodelin in hypoxic conditions and then assessed for metastasis capacity by transwell invasion assays and wound-healing migration assays. As depicted in Figure 5A, transwell invasion assays showed that Remodelin could reverse hypoxia-induced invasion. There was no difference between hypoxic cells treated with Remodelin and the control group. In addition, wound-healing migration assays also revealed that Remodelin could reverse hypoxia-induced cell migration ability (Figure 5B). These results suggest that NAT10 may play an important role in the process of hypoxia-induced metastasis of HCC cells.
Figure 5.

Remodelin blocks invasion and migration of HCC cells in hypoxic conditions (A) Images and quantification of migration of HCC cells in hypoxic conditions with or without Remodelin treatment, or controls. Migrated cells stained with crystal violet and counted. (B) Wound-healing assay of HCC cells in hypoxic conditions with or without Remodelin treatment, or controls. The data represent the mean ± SD of three independent experiments. Student’s t-test was used for statistical analysis. *P < 0.05 vs. Control; #P < 0.05 vs. hypoxia.
Down regulation of NAT10 reduces the expression of EMT markers in hypoxic HCC cells
In recent years, multiple studies have focused on signaling pathways associated with hypoxia-induced EMT. In a previous study from our group, we demonstrated that hypoxia promotes cell metastasis in epithelial HCC cell lines such as Hep3B and Huh7. To further investigate whether EMT plays a crucial role in the process of hypoxia-induced metastasis, we tested the EMT markers E-cadherin and vimentin under different conditions in HCC cell lines by Western blot. As shown in Figure 6, the HCC cell lines Hep3B and Huh7 displayed higher expression of the epithelial marker E-cadherin in normoxic conditions, while higher expression of the mesenchymal marker vimentin was observed under hypoxic conditions. This study demonstrated that down regulation of NAT10 by siRNA or Remodelin could effectively inhibit hypoxia-induced metastasis of HCC cells in vitro. Interestingly, down regulation of NAT10 in HCC cells by siRNA or Remodelin induced the reversal of hypoxia-induced EMT characteristics, which was confirmed by the expression changes of the EMT-associated markers E-cadherin and vimentin. These data demonstrate that down regulation of NAT10 may be a potential strategy for the suppression of metastasis via the reversal of EMT in HCC cells.
Figure 6.

Hypoxia potentiates EMT while inhibition of NAT10 reverses these effects. Western blot showing expression of the EMT associated genes E-cadherin and vimentin in HCC cell lines under hypoxic and normoxic conditions. GAPDH served as an internal control. Expression levels of the mesenchymal marker, vimentin, increased under hypoxic conditions as compared to normoxia. E-cadherin showed an opposite trend. Decreased expression of NAT10 as induced by siRNA or Remodelin treatment reversed these effects. The data represent mean ± SD of three independent experiments. Student’s t-test was used for statistical analysis. *P < 0.05 vs. Control.
Discussion
The poor prognosis of HCC patients is due to not only to drug resistance of cancer cells, but also their metastatic behavior, which leads to frequent recurrence [19]. Morphological changes to metastatic cancer cells enhance the migration and invasion behaviors of these cells. Previous studies demonstrated a crucial role for EMT in promoting cancer metastasis through phenotypic changes of metastasis cells [20]. In recent years, a large body of research has demonstrated that activation of Wnt/β-catenin signaling can promote the EMT process [21]. Zhang and colleagues reported that nuclear accumulation of β-catenin correlates with high expression of NAT10, a recently reported factor identified as a coordinator of telomerase activity [12,22]. Importantly, deregulation of NAT10 is especially prominent at the invasive front of colorectal carcinomas, and is correlated with cancer cell migration and invasion [12]. Our previous data showed that NAT10 expression was significantly higher in HCC tissues than peritumoral tissues, which also implied that NAT10 might be a promising prognostic marker and potential therapeutic target in HCC [17]. The relationship between NAT10 and EMT, however, has not been previously examined. Our study demonstrates that NAT10 is involved in the EMT process and plays a vital role in the recurrence and metastasis of HCC.
NAT10 deregulation has been reported in human cancers, and, based on work from our group, elevated expression of NAT10 protein is associated with a poor prognosis in patients (unpublished results). Those findings are consistent with the findings obtained in the present study, which showed that increased expression of NAT10 is associated with a more aggressive phenotype in HCC cell lines. We observed differential expression of NAT10 in all HCC cell lines studied. Since metastasis is a main factor indicating poor prognosis for HCC patients, we studied the function of NAT10 in HCC metastasis. Knockdown of NAT10 clearly decreased cellular migration and invasion of HCC cells. Furthermore, we identified a regulatory role for NAT10 in inducing EMT through expression of EMT markers. As one of the most important cell adhesion proteins, E-cadherin plays a key role in regulation of EMT by maintaining the intercellular junction of epithelial cancer cells [23]. Vimentin is also an important effector for EMT and is considered as a marker for EMT [24]. In our work, we showed up regulation of E-cadherin and down regulation of vimentin upon inhibition of NAT10 by siRNA or Remodelin treatment.
Previous studies have shown that EMT-promoted cancer metastasis is potentiated by hypoxia in the cancer microenvironment [25]. In the current study, we used two epithelial HCC cell lines, Hep3B and Huh7 to analyze the effects of Remodelin, a NAT10 inhibitor, on hypoxia-induced EMT. Our data indicated that hypoxia can induce invasion and migration of HCC cells. When hypoxia-induced HCC cells were treated with Remodelin, an antagonistic activity on cellular invasion and migration was observed. Surprisingly, hypoxia-induced HCC cell lysates showed higher levels of vimentin and lower levels of E-cadherin compared to HCC cells incubated under normoxic conditions, suggesting that hypoxia-induced metastasis is involved in the EMT process. We hypothesized that certain hypoxia-activated factors were associated with the EMT process. Indeed, our studies identified NAT10 as one such factor. According to our results, down regulation of NAT10 could inhibit the hypoxia-related expression of the EMT marker vimentin. Furthermore, NAT10-siRNA transfection or Remodelin treatment rescued E-cadherin expression, and the EMT properties of HCC cells in hypoxic conditions.
A limitation of the current research is the absence of a precise mechanism underlying the regulation of EMT by NAT10 in HCC cells. Therefore, further studies are necessary to establish how NAT10 regulates the EMT process and promotes metastasis of HCC. Even so, our findings contribute to the understanding of the function and regulation of NAT10, especially during EMT in HCC.
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LY15H030011).
Disclosure of conflict of interest
None.
Abbreviations
- HCC
hepatocellular carcinoma
- NAT10
N-acetyltransferase 10
- EMT
Epithelial mesenchymal transition
- qRT-PCR
Real-time reverse transcription polymerase chain reaction
- TGFβ
Transforming growth factor-beta
- IHC
Immunohistochemical staining
- PBS
phosphate-buffered saline
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- MET
Mesenchymal-to-epithelial transition
- DAB
3, 3-diaminobenzidine
- RT
Reverse transcription
References
- 1.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 2.Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23:289–311. doi: 10.1016/j.soc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Giannelli G, Rani B, Dituri F, Cao Y, Palasciano G. Moving towards personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut. 2014;63:1668–1676. doi: 10.1136/gutjnl-2014-307323. [DOI] [PubMed] [Google Scholar]
- 4.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 6.Nieto MA, Cano A. The epithelial-mesenchymal transition under control. global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H, Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169–1179. doi: 10.2217/fon.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Yun F, Shi L, Li ZH, Luo NR, Jia YF. Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer. Asian Pac J Cancer Prev. 2015;16:6201–6206. doi: 10.7314/apjcp.2015.16.15.6201. [DOI] [PubMed] [Google Scholar]
- 10.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Hou W, Wang HL, Liu HJ, Jia XY, Zheng XZ, Zou YX, Li X, Hou L, McNutt MA, Zhang B. GSK-3 beta-Regulated N-Acetyltransferase 10 Is Involved in Colorectal Cancer Invasion. Clinical Cancer Research. 2014;20:4717–4729. doi: 10.1158/1078-0432.CCR-13-3477. [DOI] [PubMed] [Google Scholar]
- 13.Lv J, Liu H, Wang Q, Tang Z, Hou L, Zhang B. Molecular cloning of a novel human gene encoding histone acetyltransferase-like protein involved in transcriptional activation of hTERT. Biochem Biophys Res Commun. 2003;311:506–513. doi: 10.1016/j.bbrc.2003.09.235. [DOI] [PubMed] [Google Scholar]
- 14.Liu HJ, Ling Y, Gong YL, Sun Y, Hou L, Zhang B. DNA damage induces N-acetyltransferase NAT10 gene expression through transcriptional activation. Mol Cell Biochem. 2007;300:249–258. doi: 10.1007/s11010-006-9390-5. [DOI] [PubMed] [Google Scholar]
- 15.Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344:527–532. doi: 10.1126/science.1252651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Q, Zheng X, McNutt MA, Guang L, Sun Y, Wang J, Gong Y, Hou L, Zhang B. NAT10, a nucleolar protein, localizes to the midbody and regulates cytokinesis and acetylation of microtubules. Exp Cell Res. 2009;315:1653–1667. doi: 10.1016/j.yexcr.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Liu J, Yan S, Huang K, Bai Y, Zheng S. High expression of N-acetyltransferase 10: a novel independent prognostic marker of worse outcome in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:14765–14771. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, He C, Zhou T, Huang Z, Zhou L, Liu X. NGF increases VEGF expression and promotes cell proliferation via ERK1/2 and AKT signaling in Muller cells. Mol Vis. 2016;22:254–263. [PMC free article] [PubMed] [Google Scholar]
- 19.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginnebaugh KR, Ahmad A, Sarkar FH. The therapeutic potential of targeting the epithelial-mesenchymal transition in cancer. Expert Opin Ther Targets. 2014;18:731–745. doi: 10.1517/14728222.2014.909807. [DOI] [PubMed] [Google Scholar]
- 21.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–1649. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol Cell. 2007;28:773–785. doi: 10.1016/j.molcel.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 24.Cervantes-Arias A, Pang LY, Argyle DJ. Epithelial-mesenchymal transition as a fundamental mechanism underlying the cancer phenotype. Vet Comp Oncol. 2013;11:169–184. doi: 10.1111/j.1476-5829.2011.00313.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11:714–723. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]


