Abstract
Some colorectal carcinoma (CRC) invades into vessels and has distal metastasis, largely attributable to the dissemination of tumor cells into circulation as circulating tumor cells (CTCs). Moreover, cancer stem cells (CSCs) within CTCs, which are termed as circulating tumor stem-like cells (CTSCs), are critical for formation of distal metastatic tumors. Although different cell surface markers have been used to characterize and isolate CTCs or CSCs in CRC, no good marker has been identified so far for CTSCs. Here, we show evidence that CD262+ CRC cells appeared to be highly enriched for CTSCs in CRC. CD262+ CRC cells formed more tumor spheres in culture, exhibited higher chemo-resistance, had higher ratio of developing tumors after serial adoptive transplantation in nude mice, and had higher frequency of developing distal metastatic tumor, compared to traditional CD133+ or CD44+ CRC cells. Moreover, tumor cells were significantly more frequently detected in the circulation when CD262+ CRC cells were subcutaneously transplanted. Finally, we detected high CD262 levels in the stage IV CRC specimens, which were associated with poor prognosis of the patients. Together, these data suggest CD262+ may be a novel CTSC markers and selective elimination of CD262+ CRC cells may substantially improve the current CRC therapy.
Keywords: Circulating tumor stem-like cells (CTSCs), colorectal carcinoma (CRC), CD262, CD133, CD44
Introduction
Colorectal carcinoma (CRC) is one of the most common malignant tumors worldwide [1-3]. When CRC cells invade vascular system, they may metastasize through circulation, result into that a number of patients were diagnosed only after these metastases had occurred to miss the optimal time window for surgical resection [1-3]. Therefore, early detection of metastatic CRC may improve its prognosis.
The invasion and metastases of CRC cells are recently associated with the presence of a subtype of cancer cells, called circulating tumor cells (CTCs) that had dissembled from the primary tumor and subsequently invaded into the blood circulation [4-7]. Cancer stem cells (CSCs) are cancer cells with characteristics of stem cells. CSCs are responsible for tumor initiation, progression, relapse, metastasis and chemo-resistance.
Cell surface markers have been generally used for isolation of CSCs by flow cytometry, whereas none of these CSC-markers have been found to be 100% specific, and therefore these markers are actually used to enrich CSCs from a certain tumor. Hence, most characterized “CSCs” are actually CSC-like cells [8,12]. The gold standard to identify CSCs or CSC-like cells is by tumor sphere formation and by tumor formation in serial adoptive transplantation. CD133 has been used to isolate CTCs or CSCs for various cancers, including CRC [8,12]. However, CD133 appears to be lack of cancer specificity and to poorly characterize CSCs in many cases [13]. ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes to be used in identification of stem/progenitor cells or CSCs [14,19]. However, recent evidence suggests the presence of aldefluor-positive cells in other populations, e.g. proliferating pancreatic beta cells [20,21]. CD44 is another CSC maker that has been used in CRC [22,24]. However, it is also suffered from lack of specificity. Hence, searches on novel cancer-specific CTSC markers in CRC are highly needed.
CSCs are not equivalent to CTCs, but share some similarities including cell surface markers. Specifically, only those CTCs that form ectopic metastases have the characteristics of CSCs, and may be termed as circulating tumor stem cells (CTSCs) [4-7]. Identification of CTSCs is critical for cancer therapy [25,28].
Activation of the TNF-related apoptosis-inducing ligand (TRAIL) pathway is a regulatory mechanism of apoptosis by some human cancer cells. CD262, also known as TRAIL-R2, is a receptor for apoptosis ligand TRAIL. Very recently, CD262 has been reported to play a role in the radio-resistance of human neural stem cells and neuroblastoma cells [29]. In addition, crosstalk between TRAIL-R3 and CD262 has been shown to regulate acute myeloid leukemia [30]. However, a role of CD262 in the definition of CTSCs in CRC has not been shown.
Here, we addressed these questions and our findings suggest that CD262+ may be a novel CTSC markers and selective elimination of CD262+ CRC cells may substantially improve the current CRC therapy.
Materials and methods
Protocol approval
All the experimental methods have been approved by the research committee at Shanghai General Hospital. All animal experiments were approved by the Institutional Animal Care and Use Committee at the First Clinical Medical School of Harbin Medical University (Animal Welfare Assurance). All the experiments have been carried out in accordance with the guidelines from the research committee at the First Clinical Medical School of Harbin Medical University. The methods regarding animals and human specimens were carried out in “accordance” with the approved guidelines. Surgeries were performed in accordance with the Principles of Laboratory Care, supervised by a qualified veterinarian.
Patient specimens
Surgical specimens from 100 CRC (stage IV) patients and paired adjacent non-tumor tissues (NT) were obtained postoperatively in the First Clinical Medical School of Harbin Medical University from 2005 to 2008. All patients gave signed, informed consent for the tissue to be used for scientific research. Ethical approval for the study was obtained from the First Clinical Medical School of Harbin Medical University. All diagnoses were based on pathological and/or cytological evidence. The histological features of the specimens were evaluated by senior pathologists according to the World Health Organization classification criteria. All patients had been followed-up for 60 months. Complete clinical data was electronically recorded.
Cell line culture and treatment
Two human colon epithelial adenocarcinoma cell lines, Caco-2 and T-84 were used in the current study. Caco-2 was originally developed by Dr. Jorgen Fogh, and T-84 was obtained from a 72 year-old male [31]. Both lines were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA), and cultured in RPMI1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA) in a humidified chamber with 5% CO2 at 37°C. Fluorouracil (5-FU, Sigma-Aldrich) was prepared in a stock of 1 mmol/l and applied to the cultured cells at 5 µmol/l. Cisplatin (Sigma-Aldrich) was prepared in a stock of 1 mmol/l and applied to the cultured cells at 20 µmol/l.
Cell transduction
Luciferase (LUC) allows in vivo tracing of the cells. GFP is a green fluorescent protein and RFP is a red fluorescent protein. We used a pcDNA3.1-CAG-GFP plasmid and a pcDNA3.1-CAG-luciferase plasmid as backbones (Clontech, Mountain View, CA, USA). The GFP coding sequence was digested with Xhol and BamHI and subcloned with a 2A into a pcDNA3.1-CAG-luciferase, resulting in a construct named pCAG-luciferase-2A-GFP. The small 2A peptide sequences induce, stoichiometric production of 2 protein products within a single vector. Sequencing was performed to confirm the correct orientation of the new plasmid. To generate lentiviral particles, HEK293T cells were seeded in a 100 mm dish at 50,000 cells/cm2 and co-transfected with 10 µg of pCAG-luciferase-2A-GFP and 5 µg each of packaging plasmids (REV, pMDL and VSV-G) using Lipofectamine-2000 (Invitrogen). The supernatant containing lentiviral particles was collected 48 hours after transfection and filtered through a 0.45 µm syringe filter. The viruses were purified using CsCl density centrifugation and then titered by a quantitative densitometric dot-blot assay. For cell transduction in vitro, CRC cells were seeded in 100 mm plates at 15,000 cells/cm2 one day prior to lentiviral infection. The lentiviral particles were added along with 10 µg/ml polybrene (Sigma-Aldrich) to the cell culture at a multiplicity of infection (MOI) of 100 for 48 hours. Then the cells were washed twice with complete media and purified for transduced bells based on GFP by flow cytometry. Transduced cells were monitored in vivo by their expression of luciferase, and detected by their expression of GFP.
Mouse manipulation
Ten week-old male NOD/SCID mice (SLAC Laboratory Animal Co. Ltd, Shanghai, China) were used for subcutaneous (s.c.) transplantation of tumor cells and serial adoptive transfer. The bioluminescence was monitored 4 weeks after s.c. transplantation. For s.c. transplantation of cancer cells into NOD/SCID mice, 200 cancer cells were implanted s.c. and the tumor formation was examined after 8 weeks by bioluminescence. For serial adoptive transplantation of cancer cells, 30 cancer cells were isolated from implanted tumor and re-transplanted s.c. into NOD/SCID mice. The tumor formation was examined after 6 weeks by bioluminescence. Three rounds of serial adoptive transfer were performed.
Tumor monitoring by bioluminescence
Formation of tumor at s.c. sites was monitored by luciferin assay, based on luciferase activity of tumor cells. Bioluminescence was measured with the IVIS imaging system (Xenogen Corp., Alameda, CA, USA). All of the images were taken 10 minutes after intraperitoneal injection of luciferin (Sigma-Aldrich) of 150 mg/kg body weight, as a 60-second acquisition and 10 of binning. During image acquisition, mice were sedated continuously via inhalation of 3% isoflurane. Image analysis and bioluminescent quantification was performed using Living Image software (Xenogen Corp.).
Primary tumor sphere culture
Cancer cells were washed, acutely dissociated in oxygenated artificial cerebrospinal fluid and subject to enzymatic dissociation to single cells. Afterward, single cancer cells were re-suspended in tumor sphere media (TSM) consisting of a serum-free DMEM, human recombinant Epidermal growth factor (20 ng/ml; Sigma-Aldrich), bFGF (20 ng/ml; Sigma-Aldrich), leukemia inhibitory factor (10 ng/ml; Sigma-Aldrich) and N-acetylcysteine (60 µg/ml; Sigma-Aldrich), and then plated at a density of 2×104 cells/60 mm plate for examination of tumor sphere formation.
Cell viability assay
The CCK-8 detection kit (Sigma-Aldrich) was used to measure cell viability according to the manufacturer’s instructions. Briefly, cells were seeded in a 96-well microplate at a density of 5×104/ml and then treated with drugs. After 24 h, cells were treated with resveratrol. Subsequently, CCK-8 solution (20 ml/well) was added and the plate was incubated at 37oC for 2 h. The viable cells were counted by absorbance measurements with a monochromator microplate reader at a wavelength of 450 nm. The optical density value was reported as the percentage of cell viability in relation to the control group (set as 100%).
ELISA for CD262
Total protein was used for an ELISA assay, with a human CD262 ELISA kit (Abcam, Cambridge, MA, USA).
Statistical analysis
All of the statistical analyses were performed using the GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Statistical analysis of group differences was carried out using a one-way analysis of variance (ANOVA) test followed by followed by Turkey multiple comparison post-hoc analysis. Patients’ survival was determined by Kaplan-Meier analysis. All values represent the mean ± standard deviation (SD). A value of P<0.05 was considered statistically significant after Bonferroni correction.
Results
Preparation of GFP and luciferase labeled CRC cells
Two human CRC cell lines, Caco-2 and T-84 were used in the current study. To allow tracing tumor formation in living mice and isolation of tumor cells from mice, we transduced the CRC cells with a lentivirus carrying both luciferase and GFP reporter under the control of a CAG promoter (Figure 1A). The transduced cells were purified based on GFP expression by flow cytometry (Figure 1B, 1C).
Figure 1.
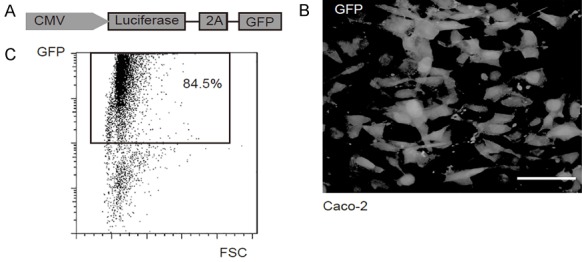
Preparation of GFP and luciferase labeled CRC cells. A. To allow tracing tumor formation in living mice and isolation of tumor cells from mice, we transduced the CRC cells with a lentivirus carrying both luciferase and GFP reporter under the control of a CAG promoter. B. The transduced Caco-2 cells were purified based on GFP expression by flow cytometry. C. The purified GFP+ Caco-2 cells in culture. Scale bar is 20 µm.
CD262+ CRC cells generate more tumor spheres in vitro
We isolated CD44+, CD133+ or CD262+ cells from the two human CRC lines, Caco-2 and T-84, by flow cytometry. The control unsorted cells (CTL), CD44+, CD133+ or CD262+ cells were subjected to tumor sphere formation assay. We found that compared to CTL, CD44+, CD133+ or CD262+ Caco-2 cells generated significantly more tumor spheres. Moreover, the CD262+ Caco-2 cells generated significantly more tumor spheres than CD44+ or CD133+ Caco-2 cells (Figure 2A, 2B). Similarly, compared to CTL, CD44+, CD133+ or CD262+ T-84 cells generated significantly more tumor spheres. Moreover, the CD262+ T-84 cells generated significantly more tumor spheres than CD44+ or CD133+ T-84 cells (Figure 2C, 2D). Hence, CD262+ cells are better enriched CSCs than CD44+ or CD133+ CRC cells.
Figure 2.
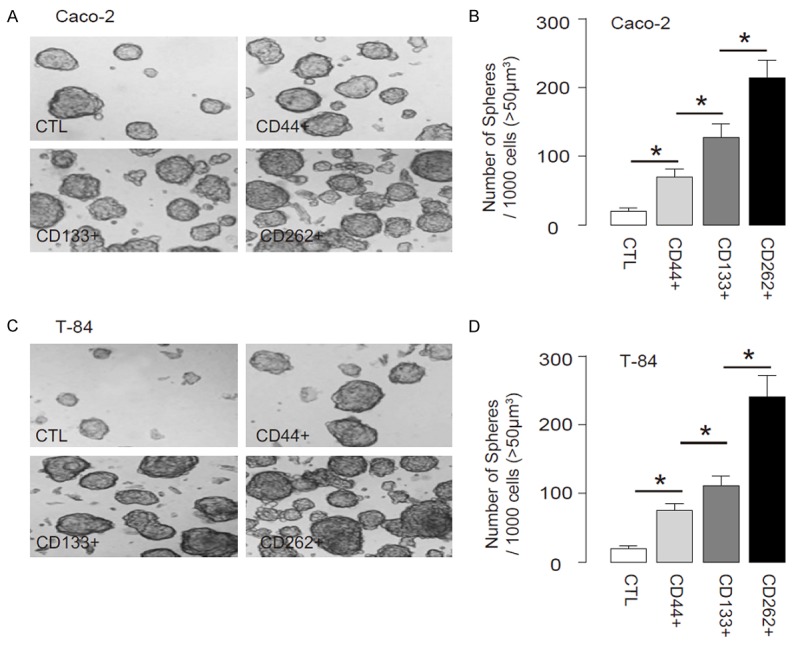
CD262+ CRC cells generate more tumor spheres in vitro. We isolated CD44+, CD133+ or CD262+ cells from the two human CRC lines, Caco-2 and T-84, by flow cytometry. (A, B) The control unsorted cells (CTL), CD44+, CD133+ or CD262+ Caco-2 cells were subjected to tumor sphere formation assay, shown by representative images (A) and by quantification (B). (C, D) The control unsorted cells (CTL), CD44+, CD133+ or CD262+ T-84 cells were subjected to tumor sphere formation assay, shown by representative images (C) and by quantification (D). *P<0.05. N=5.
CD262+ CRC cells are more resistant to chemotherapy
Next, the CTL, CD44+, CD133+ or CD262+ Caco-2 and T-84 cells were subjected to 5-FU or Cisplatin treatment in vitro. We found that compared to CTL, CD44+, CD133+ or CD262+ cells appeared to have higher cell viability, and the CD262+ cells were most resistant to either treatment in a CCK-8 assay (Figure 3A-D). Hence, CD262+ CRC cells are more resistant to chemotherapy.
Figure 3.
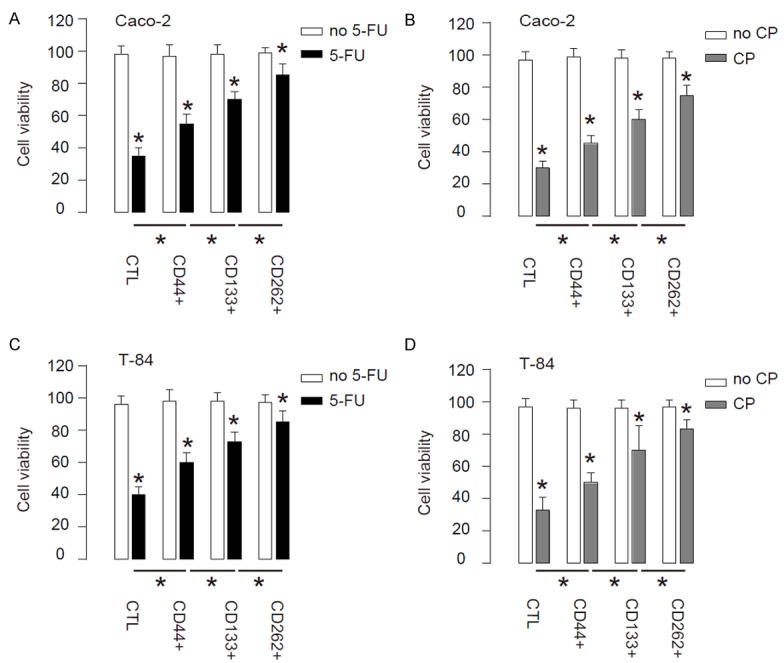
CD262+ CRC cells are more resistant to chemotherapy. (A, B) CTL, CD44+, CD133+ or CD262+ Caco-2 cells were subjected to 5-FU (A) or Cisplatin (CP, B) treatment in vitro. (C, D) CTL,CD44+, CD133+ or CD262+ T-84 cells were subjected to 5-FU (A) or CP (B) treatment in vitro. *P<0.05. N=5.
CD262+ CRC cells generate the greater cancer after s.c. transplantation
Thus, same number of CTL, CD44+, CD133+ or CD262+ Caco-2 cells were s.c. implanted into NOD/SCID mice, and the formed tumor was monitored after 8 weeks. We found that compared to CTL, CD44+, CD133+ or CD262+ Caco-2 cells generated significantly larger tumor, and the CD262+ Caco-2 cells generated the highest tumor mass among all, based on bioluminescence examination, shown by quantification (Figure 4A), and by representative images (Figure 4B).
Figure 4.
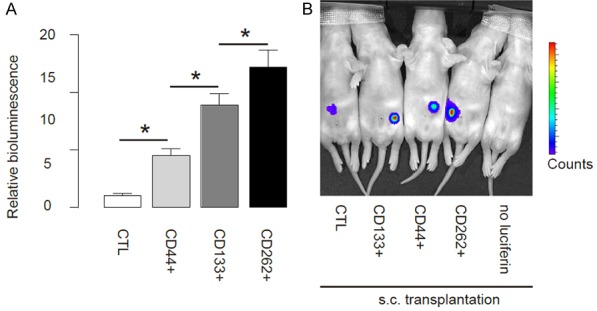
CD262+ CRC cells generate the greater cancer after s.c. transplantation. Thus, same number of CTL, CD44+, CD133+ or CD262+ Caco-2 cells were s.c. implanted into NOD/SCID mice, and the formed tumor was monitored after 8 weeks. (A, B) The luciferase activity was determined in mice, shown by quantification (A) and by representative images (B). *P<0.05. N=10.
CD262+ CRC cells induce the highest occurrence of tumor formation in serial adoptive transplantation
Another gold standard for determining CSC-like cells is potent of tumor formation after serial adoptive transplantation. Thus, 30 tumor cells were isolated from s.c. tumor developed from either CTL, CD44+, CD133+ or CD262+ Caco-2 cells, and were transplanted s.c. to new NOD/SCID mice. The formation of tumor was examined by bioluminescence after 6 weeks. The formation of the tumor was first verified by bioluminescence and then confirmed by histology of the dissected out. The newly formed tumors were then dissected out and used for isolation of 30 tumor cells for the next round of transplantation. Three rounds of transplantation were performed (Figure 5A). We found that CD262+ Caco-2 cells had significantly higher rate of developing tumor, compared to others, based on bioluminescence examination (Figure 5B). Moreover, the tumor mass formed by CD262+ cells was significantly greater, compared to others (Figure 5C). These data suggest that CD262+ cells are highly enriched for CSCs in CRC, and are better markers for sorting CSC-like cells, compared to CD44 or CD133.
Figure 5.
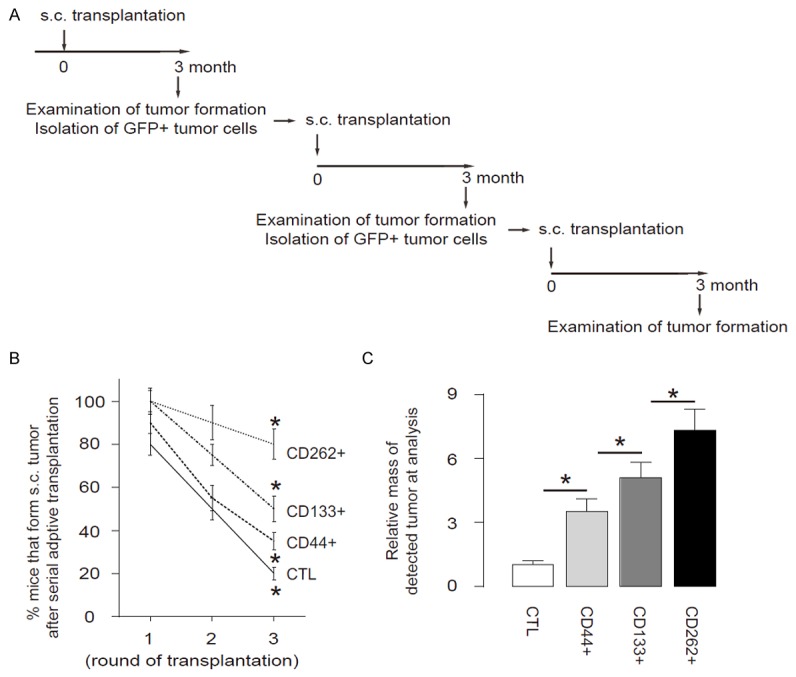
CD262+ CRC cells induce the highest occurrence of tumor formation in serial adoptive transplantation. A. Schematic of the experiment: 30 tumor cells were isolated from s.c. tumor developed from either CTL, CD44+, CD133+ or CD262+ Caco-2 cells, and were transplanted s.c. to new NOD/SCID mice. The formation of tumor was examined by bioluminescence after 6 weeks. The formation of the tumor was first verified by bioluminescence and then confirmed by histology of the dissected out. The newly formed tumors were then dissected out and used for isolation of 30 tumor cells for the next round of transplantation. Three rounds of transplantation were performed. B. Frequency of developing tumor. C. The tumor mass. *P<0.05. N=10.
High CD262 levels in CRC specimens are associated with poor prognosis
We examined CD262 levels by ELISA in 100 resected CRC (stage IV) specimens, compared to the paired adjacent normal tissue (NT). All the patients underwent routine surgery to remove the original tumor and no metastatic lesion was resected. We found that CRC tissue expressed higher levels of CD262, compared to NT (Figure 6A). To examine the clinical significance of CD262 levels in CRC, the 100 CRC patients were followed-up for 60 months after resection of the primary cancer. Overall survival, which was defined as the time from randomization to death, and preset as 5 years, was evaluated. The median value of all 100 cases was chosen as the cutoff point for separating CD262-high cases (n=50) from CD262-low cases (n=50). Kaplan-Meier curves indicated that CRC patients with high CD262 levels had a significantly worse 5-year survival than those with low Lgr5 levels (Figure 6B). Together, these data suggest that high CD262 levels in CRC specimens may be associated with poor prognosis.
Figure 6.
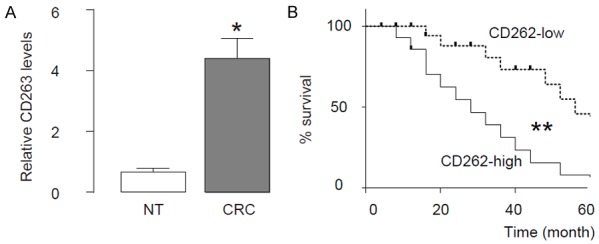
High CD262 levels in CRC specimens are associated with poor prognosis. We examined CD262 levels by ELISA in 100 resected CRC (stage IV) specimens, compared to the paired adjacent normal tissue (NT). All the patients underwent routine surgery to remove the original tumor and no metastatic lesion was resected. A. The levels of CD262 by ELISA in CRC specimens, compared to NT. B. To examine the clinical significance of CD262 levels in CRC, the 100 CRC patients were followed-up for 60 months after resection of the primary cancer. Overall survival, which was defined as the time from randomization to death, and preset as 5 years, was evaluated. The median value of all 100 cases was chosen as the cutoff point for separating CD262-high cases (n=50) from CD262-low cases (n=50). Kaplan-Meier curves were performed. *P<0.05. **P<0.01. N=100.
Discussion
CTSCs are critical for tumor initiation, growth, progression and metastasis. Identification of CSCs has been largely relied on flow-cytometry-based examination of surface markers, e.g. CD133 and CD44. However, the presently used markers have significant shortcoming of lack of specificity [13].
Here, we analyzed CD262 as a novel CTSC marker for CRC. Our study was coming from the inspiration by a recent study in neural science, which shows that CD262 plays a role in the radio-resistance of human neural stem cells and neuroblastoma cells [29].
Ivanov el al. show that a constitutive high-level expression of TRAIL-R2 in human NSC is detected after ionizing radiation through generation of reactive oxygen species targeted cell signaling pathways. A significant increase of endogenous expression and secretion of TRAIL could induce autocrine/paracrine stimulation of the TRAIL-R2-mediated signaling cascade with activation of caspase-3-driven apoptosis. Subsequently, TRAIL production through elimination of bystander TRAIL-R-positive human neural stem cells might substantially restrict a final yield of differentiating young neurons. This mechanism is also used to explain the maintenance of radio-resistance of neuroblastoma cells by constitutive PI3K-AKT over-activation and endogenous synthesis of TGF-beta1 [29].
In the current study, we purified CD262+ CRC cells, and compared to CD44+ or CD133+ cells. We found that these CD262+ cells showed higher tumor formation potential in vitro and in vivo, more resistant to chemotherapy, and greater ability to generate tumor after serial adoptive transplantation. These are gold standards for determining CTSC and CSC properties. Here, all these assays were performed to support CD262+ cells as a highly purified CTSC population, and its advantage over CD44 or CD133 is very important for identification of the true CTSC population in CRC to promote development of innovative targeting therapy.
Although here we provide direct data to support the usefulness of using CD262 for characterizing CTSC in CRC, the clinical data that show association of High CD262 levels in CRC with poor prognosis of the patients support this possibility. Additional efforts are needed to further validate it, e.g. a large samples may be analyzed to detect the CD262, and to compare with CD44 and CD133 expression, not only in primary CRCs, but also in the metastatic tumors. Moreover, a combination of CD262 and CD44 or CD262 and CD133 can be examined to see whether it could further improve the purification of CTSC in CRC.
Disclosure of conflict of interest
None.
References
- 1.Labianca R, Beretta GD, Mosconi S, Pessi MA, Milesi L. The development of clinical research in CRC. Ann Oncol. 2005;16(Suppl 4):iv37–43. doi: 10.1093/annonc/mdi906. [DOI] [PubMed] [Google Scholar]
- 2.Van Schaeybroeck S, Allen WL, Turkington RC, Johnston PG. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 2011;8:222–232. doi: 10.1038/nrclinonc.2011.15. [DOI] [PubMed] [Google Scholar]
- 3.East JE, Dekker E. Colorectal cancer diagnosis in 2012: A new focus for CRC prevention--more serration, less inflammation. Nat Rev Gastroenterol Hepatol. 2013;10:69–70. doi: 10.1038/nrgastro.2012.245. [DOI] [PubMed] [Google Scholar]
- 4.Sainz B Jr, Heeschen C. Standing out from the crowd: cancer stem cells in hepatocellular carcinoma. Cancer Cell. 2013;23:431–433. doi: 10.1016/j.ccr.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Chiba T, Kamiya A, Yokosuka O, Iwama A. Cancer stem cells in hepatocellular carcinoma: Recent progress and perspective. Cancer Lett. 2009;286:145–153. doi: 10.1016/j.canlet.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Chiba T, Kanai F, Iwama A, Yokosuka O. Circulating cancer stem cells: a novel prognostic predictor of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2013;2:4–6. doi: 10.3978/j.issn.2304-3881.2012.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagano H, Ishii H, Marubashi S, Haraguchi N, Eguchi H, Doki Y, Mori M. Novel therapeutic target for cancer stem cells in hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:600–605. doi: 10.1007/s00534-012-0543-5. [DOI] [PubMed] [Google Scholar]
- 8.Nagata T, Sakakura C, Komiyama S, Miyashita A, Nishio M, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Ochiai T, Kokuba Y, Sonoyama T, Otsuji E. Expression of cancer stem cell markers CD133 and CD44 in locoregional recurrence of rectal cancer. Anticancer Res. 2011;31:495–500. [PubMed] [Google Scholar]
- 9.Fang DD, Kim YJ, Lee CN, Aggarwal S, McKinnon K, Mesmer D, Norton J, Birse CE, He T, Ruben SM, Moore PA. Expansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br J Cancer. 2010;102:1265–1275. doi: 10.1038/sj.bjc.6605610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Tian R, Wang M, Wang X, Jiang J, Zhang Z, Li X, He Z, Gong W, Qin R. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10:1182–1190. doi: 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- 11.Ottaiano A. Finding markers for cancer stem cells in renal cell carcinoma: looking beyond CD133. Cell Cycle. 2010;9:4431. doi: 10.4161/cc.9.22.13823. [DOI] [PubMed] [Google Scholar]
- 12.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N, Lako M. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 15.Hess DA, Craft TP, Wirthlin L, Hohm S, Zhou P, Eades WC, Creer MH, Sands MS, Nolta JA. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–620. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 17.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, Eades WC, Creer MH, Nolta JA. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, Buckanovich RJ. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Jiang X, Zeng Y, Zhou H, Yang J, Cao R. Proliferating pancreatic beta-cells upregulate ALDH. Histochem Cell Biol. 2014;142:685–91. doi: 10.1007/s00418-014-1248-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Wang L, Liu X, Zheng D, Liu S, Liu C. ALDH expression characterizes G1-phase proliferating beta cells during pregnancy. PLoS One. 2014;9:e96204. doi: 10.1371/journal.pone.0096204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou JY, Chen M, Ma L, Wang X, Chen YG, Liu SL. Role of CD44(high)/CD133(high) HCT-116 cells in the tumorigenesis of colon cancer. Oncotarget. 2016;7:7657–7666. doi: 10.18632/oncotarget.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+ CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 25.Petersson M, Niemann C. Stem cell dynamics and heterogeneity: implications for epidermal regeneration and skin cancer. Curr Med Chem. 2012;19:5984–5992. [PubMed] [Google Scholar]
- 26.Perez-Losada J, Balmain A. Stem-cell hierarchy in skin cancer. Nat Rev Cancer. 2003;3:434–443. doi: 10.1038/nrc1095. [DOI] [PubMed] [Google Scholar]
- 27.Singh SR. Stem cell niche in tissue homeostasis, aging and cancer. Curr Med Chem. 2012;19:5965–5974. doi: 10.2174/092986712804485917. [DOI] [PubMed] [Google Scholar]
- 28.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 29.Ivanov VN, Hei TK. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014;19:399–413. doi: 10.1007/s10495-013-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamuleau ME, Ossenkoppele GJ, van Rhenen A, van Dreunen L, Jirka SM, Zevenbergen A, Schuurhuis GJ, van de Loosdrecht AA. High TRAIL-R3 expression on leukemic blasts is associated with poor outcome and induces apoptosis-resistance which can be overcome by targeting TRAIL-R2. Leuk Res. 2011;35:741–749. doi: 10.1016/j.leukres.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Murakami H, Masui H. Hormonal control of human colon carcinoma cell growth in serum-free medium. Proc Natl Acad Sci U S A. 1980;77:3464–3468. doi: 10.1073/pnas.77.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]


