Abstract
Hepatic encephalopathy (HE) as a severe neuropsychiatric complication is commonly present in the end stage of Hepatocellular Carcinoma (HCC). However, widely accepted biomarkers for diagnosing early HE are still absent. Here, we screened glycosylation patterns of serum proteins from Chinese HCC patients with or without early HE by lectin microarray. Then, phaseolus vulgaris erythroagglutinin (PHA-E) as a lectin binding with bisecting GlcNAc structure which was significantly decreased in sera from Chinese HCC patients with early HE, was chosen to perform lectin affinity chromatography, following by in-gel digestion, Mass Spectrometry (MS) analysis and bioinformatics analysis. Here we found, 13 lectins showed statistically significant reduction suggesting GalNAc, terminal α-1,3 Man, bisecting GlcNAc, (GlcNAc)n, O-GlcNAc, Neu5Ac, tetra-antennary complex-type N-glycan and GalNAc α/β1-3/6 Gal were decreased in serum glycoproteins from Chinese HCC patients with early HE. Furthermore, a total of 141 PHA-E-associated glycoproteins were identified in MS, of which 12 serum glycoproteins only in Chinese HCC patients without early HE and 26 serum glycoproteins only in Chinese HCC patients with early HE. In addition, bioinformatics analysis revealed the PHA-E-associated serum glycoproteins only in Chinese HCC patients with early HE might be related to early HE occurrence through p38 MAPK signaling pathway and MAPK/ERK signaling pathway. Collectively, this was the first glycomics study of serum proteins in HCC patients with early HE and it could provide a database for discovering and developing serum biomarkers to identify and predict early HE in HCC patients.
Keywords: Hepatocellular carcinoma, hepatic encephalopathy, glycoproteomics, lectin microarray, lectin-affinity chromatography, LC-MS/MS
Introduction
Hepatocellular carcinoma (HCC) is a major primary liver cancer, accounting for 70-85% of the known liver cancers [1]. Hepatic encephalopathy (HE) is often described as a prevalent complication of liver insufficiency including portal hypertension and cirrhosis that results in cognitive, psychiatric and motor impairments in these patients after exclusion of other known brain disease. There are several different stages of HE, of which early HE is the stage with abnormal results of established psychometric or neuropsychological tests, but without clinical manifestations [2]. It was reported that the prevalence of early HE in patients with Child B/C liver dysfunction is 50% [3]. Recently, it’s worth noting that HE is commonly present in the end stage of HCC. These HCC patients with HE will follow a course from some cognitive/behavioral decay to null response, and finally slip into coma [4,5]. By this token, HE severely affects the HCC patient’s prognosis. At present, the biological markers in clinical diagnosis for HE still lack, especially for early HE. It is currently not possible to diagnose HE with a high degree of certainty until relatively late in the disease process. Hence, the importance of diagnosis and treatment of early HE in the HCC patients is always neglected in such a clinical context, leading to most HCC patients with early HE missing the best opportunity for intervention in time. To date, the diagnosis of HE dependents mainly on psychometric tests and brain activity measurement [6], and only several molecules have been assumed as possible markers of HE in patients with cirrhosis [7-9], but definitive serologic predictors for early HE in HCC patients are still absent [10].
Glycosylation is an important protein post-translational modification (PTMs), there are more than 50% of proteins are presumed to have undergone glycosylation in nature [11], being involved in many different biological events such as protein folding, transport, cell-cell interactions and antigenicity [12,13]. However, aberrant glycosylation is one of the most common phenomena linked to human diseases either by influencing protein functionalities or as nonfunctional participants [14]. Increasing evidence shows that the alteration in glycosylation could promote invasive behavior of cells and play an important role in cancer metastasis [15-17]. Moreover, most of current serum biomarkers utilized in clinical practice are glycoproteins including alpha fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 12-5 (CA12-5) and carbohydrate antigen 15-3 (CA15-3). Screening aberrant glycan as diseases biomarkers or drug targets has become an attractive field of research, which might have unique strengths in disease prediction compared with proteins themselves. The representative example is Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), an extensively proven disease glycobiomarker. AFP has widely been used for HCC’s surveillance, but AFP-negative HCC is frequently missing diagnosis because of its low specificity for prediction or diagnosis. In contrast, AFP-L3, as a specific marker which could remarkably distinguish HCC and other hepatic disease by the glycoprotein part of AFP, has been a preferred HCC biomarker in early diagnosis of HCC and in predicting prognosis after treatment [18,19]. For all these above, glycosylation and glycoproteins might play superior roles in diagnosis and prediction of early HE in HCC patients. Seeking a novel glycoprotein biomarker of early HE might reduce the diagnostic window between the time of HE occurrence and diagnosis. Therefore, the serum glycosylation pattern and glycoprotein profiling from HCC patients with early HE are urgently needed.
In the present study, we screened glycosylation patterns of serum glycoproteins from Chinese HCC patients with and without early HE, and then, enriched glycoprotein fractions by lectin affinity chromatography with specific lectin, identified the specific lectin related proteins by modern Mass Spectrometry (MS), and further performed bioinformatics analysis (Figure 1). This study aimed to achieve a novel glycoprotein database for digging out potential markers of identifying early HE in Chinese HCC patients.
Figure 1.
Experimental procedure of this study.
Materials and methods
Clinical specimens
Serum samples from 72 Chinese HCC patients (36 HCC patients with early HE, the other 36 HCC patients without early HE were controls) were collected at First Affiliated Hospital of Dalian Medical University (Liaoning, China) in this study. The diagnosis of HCC was determined by liver biopsy with imaging tests, and the diagnosis criteria for the early HE was according to the 2014 Practice Guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases that include the minimal HE and grade I HE [2,20]. The exclusion criteria was as the same as previously reported [21] that included history of autoimmune diseases, infectious diseases, diabetes, immune deficiency disorder, psychiatric illness, severe cognitive impairment, or a systemic or central nervous system infection two weeks before sample collection. All of the samples were stored at -80°C for further analyses. Clinical characteristic of these HCC patients were provided in Table 1. Pooled serum samples from 10 HCC patients without early HE and 10 HCC patients with early HE were used for screening glycosylation patterns and identifying PHA-E-associated glycoprotein profiling by MS. Three biological repeats were measured independently to guarantee the reproducibility of experiment. Other 12 samples were used to re-identifying the results from MS, respectively. The research was carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki) and all of projects in the study were approved by Ethics Committee in the First Affiliated Hospital of Dalian Medical University. Informed consent was obtained from each patient.
Table 1.
Clinical characteristic of Chinese HCC patients
| HCC patients without early HE | HCC patients with early HE | |
|---|---|---|
| Number of individuals | 36 | 36 |
| Gender (male/female) | 27 (75%)/9 (25%) | 26 (72.2%)/10 (27.8%) |
| Age (years) | 59 ± 10 | 61 ± 11 |
| ALT (IU/L) | 49.8 ± 29.7 | 57.1 ± 42.3 |
| AST (IU/L) | 75.4 ± 56.3 | 88.7 ± 61.5 |
| AFP (IU/ml) | 352.6 ± 378.1 | 410.2 ± 439.4 |
| PT (s) | 14.9 ± 3.6 | 15.2 ± 2.9 |
Mean ± standard deviation; ALT, Alanine aminotransferase; AST, Aspartate transaminase; AFP, alpha fetoprotein; HbsAg, hepatitis B surface antigen; PT, Prothrombin time.
Lectin microarray
Pooled serum samples were processed by ProteoExtract® Albumin/IgG removal kit (Calbiochem, Billerica, MA, USA) to deplete albumin and IgG. Then, Lightning-Link Biotin Labeling Kit (Innova Biosciences, Cambridge, UK) was used to biotinylate serum proteins according to the manufacturer’s description. Glycosylation patterns were screened by a lectin microarray constructed in our previous study [22] including 50 lectins form Vector Laboratories (Burlingame, CA, USA) and Sigma-Aldrich (Castle Hill, NSW, Australia), the protocol was summarized as follows: Firstly, the lectin microarray was blocked by 2% bovine serum albumin (BSA)-TBS solution. Secondly, incubated it with equal biotinylated proteins from pooled serum samples from 10 HCC patients without early HE and 10 HCC patients with early HE, respectively. And then, Cy5 labeled streptavidin (Life technologies, Waltham, MA, USA) was used for combining with the microarray. LuxScan 10K/A scanner system (CapitalBio, Beijing, China) was used to scan the lectin microarray and extract the data, which was analyzed as described previously [23] and three biological repeats were measured independently.
Lectin blotting
Albumin and IgG-depleted serum proteins from 10 HCC patients without early HE and 10 HCC patients with early HE, respectively were separated by SDS-PAGE and analyzed by lectin blotting. In brief, proteins in the gels were transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were incubated with biotinylated jacalin (JAC), phaseolus vulgaris erythroagglutinin (PHA-E), ricinus communis agglutinin I (RCA-I), solanum tuberosum lectin (STL), wisteria floribunda lectin (WFL) and wheat germ agglutinin (WGA, Vector Laboratories, Burlingame, CA, USA), respectively. The membranes were washed with 0.1% TBS-Tween20 (TBST, 50 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.6), and then incubated with Streptavidin Horseradish Peroxidase (HRP) Conjugate (Invitrogen, Waltham, MA, USA). Amersham ECL prime western blotting detection reagents (GE Healthcare, Piscataway, NJ, USA) were used to detect the bands on the membranes.
Lectin affinity chromatography
PHA-E-agarose was washed and supplemented with lectin-binding solution (10 mM Tris-HCl, 0.15 M NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.5). Serum samples depleted albumin and IgG from 10 HCC patients without early HE and 10 HCC patients with early HE, respectively were added into PHA-E-agarose and incubated at 4°C overnight. Then, the agarose was washed by lectin-binding solution and followed by elution of bound fraction with 200 mM N-acetyl-D-(+)-glucosamine. The eluted fraction was separated by SDS-PAGE and stained by PhastGelTM Blue R (GE Healthcare, Piscataway, NJ, USA). Gels containing all bands were cut into slices and processed for in-gel digestion; three biological repeats were performed independently.
In-gel digestion and nanoflow liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
Gel slices were destained, then Tris-(2-carboxy-ethyl)-phosphine hydrochloride (TCEP, Sigma, Castle Hill, NSW, Australia) and iodoacetamide (IAA, Sigma, Castle Hill, NSW, Australia) were used to reduce and alkylate, respectively. Subsequently, after redehydrating with 100% acetonitrile (ACN), those gel slices were digested in trypsin solution at 37°C overnight and extracted by extraction buffer (50% ACN, 0.1% trifluoroacetic acid). The dried solutions were resuspended with 12 μl solvent A (A: water with 0.1% formic acid; B: ACN with 0.1% formic acid) and then separated by nanoLC and analyzed by on-line electrospray tandem mass spectrometry. The experiments were performed on a Nano Aquity UPLC system (Waters Corporation, Milford, MA) connected to a quadrupole-Orbitrap mass spectrometer (Q-Exactive, Thermo Fisher Scientific, Bremen, Germany) equipped with an online nano-electrospray ion source. 8 μl peptide sample was loaded onto the trap column (Thermo Scientific Acclaim PepMap C18, 100 μm×2 cm), with a flow of 10 μl/min for 3 min and subsequently separated on the analytical column (Acclaim PepMap C18, 75 μm×25 cm) with a linear gradient, from 2% B to 45% B in 75 min. The column was re-equilibrated at initial conditions for 15 min. The column flow rate was maintained at 300 nL/min and column temperature was maintained at 40°C. The electrospray voltage of 2.0 kV versus the inlet of the mass spectrometer was used.
The Q-Exactive mass spectrometer was operated in the data-dependent mode to switch automatically between MS and MS/MS acquisition. Survey full-scan MS spectra (m/z 350-1600) were acquired with a mass resolution of 70K, followed by fifteen sequential high energy collisional dissociation (HCD) MS/MS scans with a resolution of 17.5K. For MS, the automatic gain control (AGC) was set to 1,000,000 ions, with maximum accumulation times of 20 ms. For MS/MS, precursor ions were activated using 30% normalized collision energy, an isolation window of 2 m/z and the AGC was set to 200,000 ions, with maximum accumulation time of 120 ms. Single charge state was rejected and dynamic exclusion was used with one microscan and 60 s exclusion duration.
Enriching and identification of PHA-E-associated matrix metalloproteinase-9 (MMP9)
PHA-E-associated MMP9 was enriched in 6 serum samples from Chinese HCC patients without early HE and 6 serum samples from Chinese HCC patients with early HE by lectin affinity chromatography with PHA-E-agarose as described before. Then, the enriched PHA-E-associated MMP9 was separated by a 10% SDS-PAGE and transferred to PVDF membranes. After blocking with 5% Bull Serum Albumin (BSA), membranes were incubated with a polyclonal goat anti-human MMP9 antibody (R & D, Minneapolis, MN, USA) at 4°C overnight. Washed the membranes with 0.1% TBST and incubated them with HRP-conjugated secondary antibodies (KangChen Bio-tech, Shanghai, China), and then the membranes were washed by 0.1% TBST again. Amersham ECL detection (GE Healthcare, Piscataway, NJ, USA) was used to visualize the bands.
Data base searching and bioinformatics analysis
Thermo Scientific Proteome Discoverer software version 1.4 with the MASCOT v2.3.2 search engine was used for all searches of the database. The database was the Human UniProtKB/Swiss-Prot database (Release 2015-03-11, with 20199 sequences). Raw files generated by the Q-Exactive instrument were searched directly using a 10 ppm precursor mass tolerance and a 50 mmu fragment mass tolerance. The enzyme specificity with trypsin was used. Up to two missed cleavages were allowed and peptides with at least 7 amino acids were retained. Carbamidomethyl on cysteine was set as a fixed modification. Oxidation on methionine and Deamidation on asparagine were set as variable modifications. Use the percolator algorithm to control peptide level false discovery rates (FDR) lower than 1%.
Uniport database (http://www.uniport.org) was used to evaluate the identified PHA-E-associated proteins and the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) was used to further analyze functional categories of the different groups of PHA-E-associated glycoproteins. Moreover, we investigated biological interactions by Ingenuity Pathway Analysis (IPA, QIAGEN, Redwood City, CA, USA).
Statistical analysis
In the lectin microarray analysis, the Spot Intensity Median (S) and the Background Intensity Median (B) were extracted by LuxScan 10K/A scanner system and S/B was calculated. S/B≥2 was used as cutoff to define positive lectin binding spots. T-test was used to statistically analyze the S/B of each kind of positive lectin spots binding different samples and the difference was statistically significant for P<0.05.
Statistical analysis was performed by SPSS 16.0 statistical packages (SPSS Inc., Chicago, IL, USA). Quantitative variable was evaluated using Student’s T-test. P<0.05 was considered statistically significance.
Results
Glycosylation patterns of sera from HCC patients with and without early HE
The lectin microarray constructed in our previous study [22], which included 50 different kinds of lectins, 2 blank controls and 2 positive controls in each block (Figure 2A), was applied to screen glycosylation patterns of sera from Chinese HCC patients with and without early HE. Fluorescence intensities were scanned and showed in Figure 2B. Hierarchical clustering of 23 positive lectins in sera from both HCC patients with and without early HE was mapped by the MeV 4.8.1. (Figure 2C).
Figure 2.
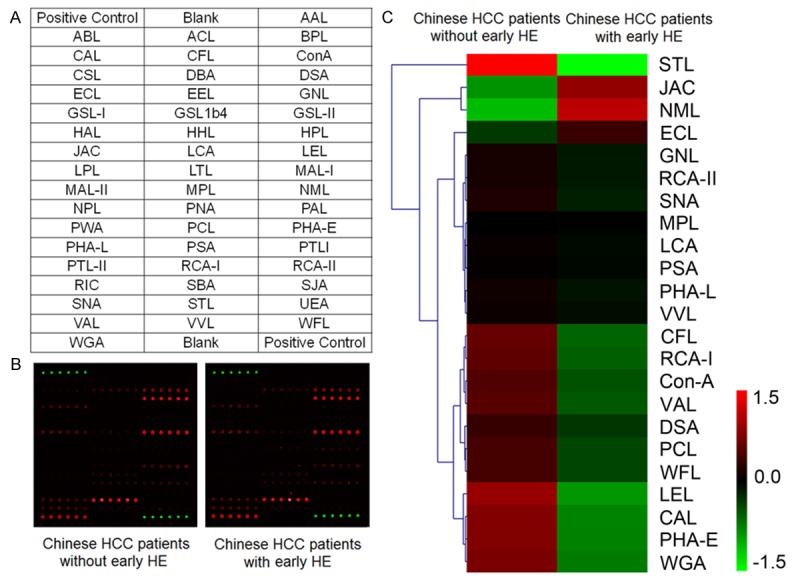
Lectin microarray analysis. A. The arrangement of lectins in the lectin microarray. B. Scan images of the lectin microarray incubated with sera from Chinese HCC patient with and without early HE, respectively. C. Hierarchical clustering of positive lectin binding spots (S/B≥2). Each row represented a lectin, and red represents a lectin with high S/B value, while green represents a lectin with low S/B value.
To facilitate the analysis, statistically significant 13 lectins (lectin signal pattern of sera from Chinese HCC patients with HE vs. pattern of sera from Chinese HCC patients without early HE, P<0.05) were divided into 3 grades according to protein-lectin binding intensities: weak binding (5>S/B≥2), medium binding (8>S/B≥5) and strong binding (S/B≥8). Stronger combining capacity meant more carbohydrate epitopes. In sera from Chinese HCC patients with early HE, caragana arborescens lectin (CAL), galanthus nivalis lectin (GNL), naja mossambica lectin (NML), phaseolus coccineus lectin (PCL), phaseolus vulgaris leucoagglutinin (PHA-L), WFL were weak binding; RCA-I, viscum album lectin (VAL) were medium binding; codium fragile lectin (CFL), JAC, PHA-E, STL and WGA were strong binding. While, in sera from Chinese HCC patients without early HE, CAL, GNL, PCL, PHA-L and WFL were weak binding; CFL, NML, RCA-I and VAL were medium binding; JAC, PHA-E, STL and WGA were strong binding.
Quantitative results of S/B and binding structures of these 13 lectins were shown in Figure 3A. Only 2 lectins: galactosyl (β-1,3) N-acetylg alactosamine binder JAC and branched mannose (branched Man) binder NML, had more increased S/B in sera from Chinese HCC patients with early HE compared to patients without early HE, while S/B of other lectins were all decreased in Chinese HCC patients with early HE, which suggested that specific carbohydrate epitopes like N-acetylgalactosamine (GalNAc, recognized by CAL, CFL, RCA-I and VAL), terminal α-1,3 Man (recognized by GNL), bisecting N-acetylglucosamine (bisecting GlcNAc, recoginzed by PHA-E), (GlcNAc)n (recognized by PCL, STL and WGA), O-GlcNAc (recognized by WGA), N-Acetyl Neuraminic Acid (Neu5Ac, recoginzed by PCL and WGA), tetra-antennary complex-type N-glycan (recoginzed by PHA-L) and GalNAc α/β1-3/6 Gal (recoginzed by WFL) were all decreased in sera from Chinese HCC patients with early HE.
Figure 3.
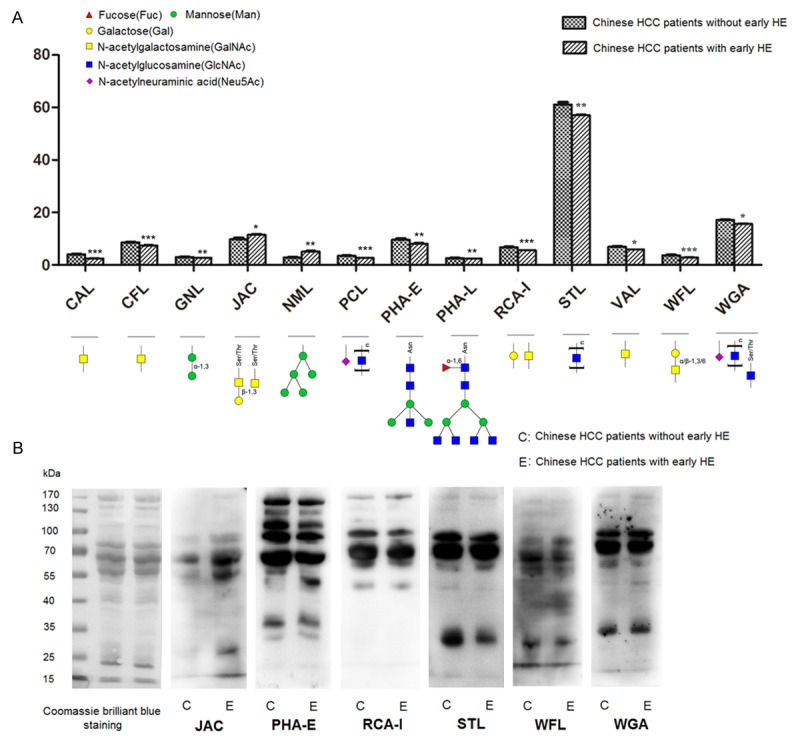
Different glycosylation patterns in sera from Chinese HCC patients with and without early HE. A. Specific carbohydrate epitopes and quantitative results of lectins had statistical significance between sera form Chinese HCC patients with and without early HE. Data was the average ± SD, *P<0.05, **P<0.01, ***P<0.001. B. Validation of different glycosylation patterns by lectin blotting. Coomassie brilliant blue staining was used to show the protein loading. Signal strength of JAC was increased, while signal strengths of PHA-E, RCA-I, STL, WFL and WGA were all decreased in sera from Chinese HCC patients with early HE compared with in sera from Chinese HCC patients without early HE.
Validation of different glycosylation patterns
Lectin blotting was performed by using biotinylated lectin JAC, PHA-E, RCA-I, STL, WFL and WGA to further validate the different glycosylation patterns observed in lectin microarray; meanwhile, coomassie brilliant blue staining of total proteins was also performed to show similar loading quantity of different samples. It was showed that results of lectin blotting analysis by ECL detection reagents were consistent with results of lectin microarray: signal strength of JAC was increased, while signal strengths of PHA-E, RCA-I, STL, WFL and WGA were all decreased, in sera from Chinese HCC patients with early HE (Figure 3B). Among these lectins, PHA-E was chosen to perform subsequent lectin affinity chromatography, based on its strong combining capacity and specific recognition for the bisecting GlcNAc.
Mass spectrometric identification of PHA-E associated glycoproteins
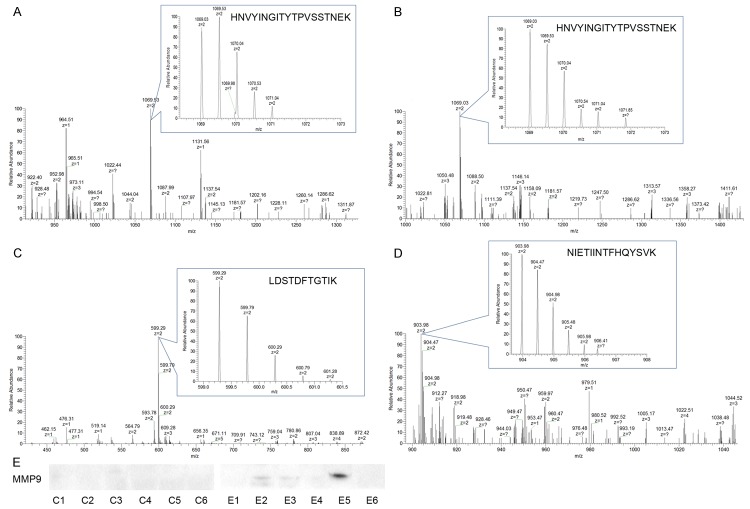
Proteins were enriched by PHA-E affinity chromatography in sera from Chinese HCC patients with and without early HE, and identified by LC-MS/MS. All of identified proteins were searched by the Uniport database and totally 141 glycoproteins were identified in three biological repeats. Among them, 103 glycoproteins like alpha-2-macroglobulin were identified in sera from HCC patients both with and without early HE (Table S1). Figure 4A and 4B showed representative MS spectrum of peptide HNVYINGITYTPVSSTNEK from alpha-2-macroglobulin in sera from Chinese HCC patients without and with early HE, respectively. There were 12 glycoproteins in Table 2 only identified in sera from Chinese HCC patients without early HE. Representative MS spectra of peptide LDSTDFTGTIK form transferrin receptor protein 1 in sera from Chinese HCC patients without early HE was showed in Figure 4C. While, it was worth noting there were still 26 glycoproteins only identified in sera from Chinese HCC patients with early HE, though total bisecting GlcNAc-modified was decreased in sera from Chinese HCC patients with early HE (Table 3). Representative MS spectra of peptide NIETIINTFHQYSVK from MMP9 in sera from Chinese HCC patients with early HE was showed in Figure 4D.
Figure 4.
PHA-E-associated proteins were examined by LC MS/MS. A and B. MS spectra of HNVYINGITYTPVSSTNEK from alpha-1-antichymotrypsin in sera from Chinese HCC patients without and with early HE, respectively. C. MS spectra of LDSTDFTGTIK from transferrin receptor protein 1 which only identified in serum from Chinese HCC patients without early HE. D. MS spectra of NIETIINTFHQYSVK from matrix metalloproteinase-9 which only identified in serum from Chinese HCC patients with early HE. E. PHA-E-associated MMP9 glycoproteins enriched in serum from 6 HCC patients without early HE (C1-C6) and 6 HCC patients with early HE (E1-E6) were subjected to western blotting.
Table 2.
PHA-E-associated glycoproteins only identified in sera from Chinese HCC patients without early HE
| Uniprot Number | Protein Name | Gene Name |
|---|---|---|
| P01596 | Ig kappa chain V-I region CAR | KV104 |
| P0C0L4 | Complement C4A | CO4A |
| A6NMB1 | Sialic acid-binding Ig-like lectin 16 | SIG16 |
| Q15485 | Ficolin-2 | FCN2 |
| P04070 | Vitamin K-dependent protein C | PROC |
| P02786 | Transferrin receptor protein 1 | TFR1 |
| Q8NBJ4 | Golgi membrane protein 1 | GOLM1 |
| O75144 | ICOS ligand | ICOSL |
| Q9Y6R7 | IgGFc-binding protein | FCGBP |
| Q10588 | ADP-ribosyl cyclase 2 | BST1 |
| Q9NPR2 | Semaphorin-4B | SEM4B |
| P04003 | C4b-binding protein alpha chain | C4BPA |
Table 3.
PHA-E-associated glycoproteins only identified in sera from Chinese HCC patients with early HE
| Uniprot Number | Protein Name | Gene Name |
|---|---|---|
| A0M8Q6 | Ig lambda-7 chain C region | LAC7 |
| O00391 | Sulfhydryl oxidase 1 | QSOX1 |
| O75015 | Low affinity immunoglobulin gamma Fc region receptor III-B | FCG3B |
| O95445 | Coagulation factor XII | FA12 |
| P01854 | Ig epsilon chain C region | IGHE |
| P02766 | Transthyretin | TTHY |
| P02788 | Lactotransferrin | TRFL |
| P02790 | Hemopexin | HEMO |
| P05160 | Coagulation factor XIII B chain | F13B |
| P05164 | Myeloperoxidase | PERM |
| P06276 | Cholinesterase | CHLE |
| P08294 | Extracellular superoxide dismutase [Cu-Zn] | SODE |
| P0C0L5 | Complement C4B | CO4B |
| P10721 | Mast/stem cell growth factor receptor Kit | KIT |
| P12259 | Coagulation factor V | FA5 |
| P13671 | Complement component C6 | CO6 |
| P14780 | Matrix metalloproteinase-9 | MMP9 |
| P17813 | Endoglin | EGLN |
| P19827 | Inter-alpha-trypsin inhibitor heavy chain H1 | ITIH1 |
| P27169 | Serum paraoxonase/arylesterase 1 | PON1 |
| P35916 | Vascular endothelial growth factor receptor 3 | VGFR3 |
| P54289 | Voltage-dependent calcium channel subunit alpha-2/delta-1 | CA2D1 |
| P55058 | Phospholipid transfer protein | PLTP |
| Q02413 | Desmoglein-1 | DSG1 |
| Q12913 | Receptor-type tyrosine-protein phosphatase eta | PTPRJ |
| P00748 | Coagulation factor XII | FA12 |
Western blotting analysis of the enriched PHA-E-associated MMP9 in sera from Chinese HCC patients with and without early HE by lectin affinity chromatography was showed in Figure 4E. There was no band in sera from Chinese HCC patients without early HE (C1-C6); but three positive bands in 6 serum samples from Chinese HCC patients with early HE (E1-E6).
Functional annotation analysis
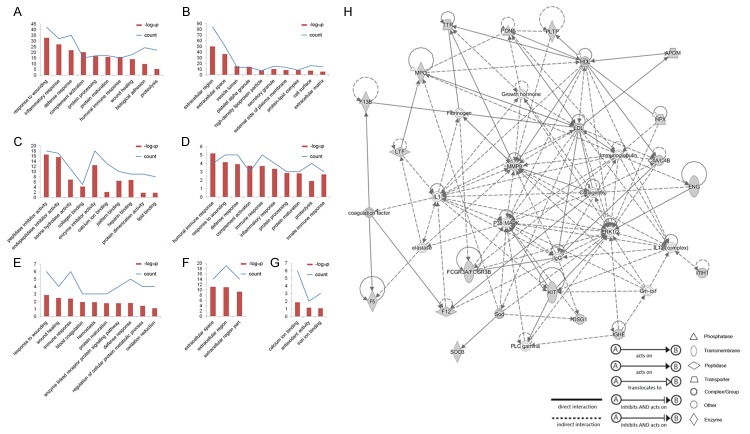
To clearly understand these identified PHA-E-associated glycoproteins, we carried out functional annotation analysis by DAVID. The group of glycoproteins identified in sera from Chinese HCC patients both with and without early HE were classified according to biological process (BP), cellular component (CC) and molecular function (MF). Figure 5A showed that most of these glycoproteins identified in sera from Chinese HCC patients both with and without early HE were involved in response to wounding, inflammatory response, defense response, and so on. Cellular components of them were focus on extracellular region, extracellular space, vesicle lumen and platelet alpha granule (Figure 5B). Furthermore, these glycoproteins were involved in many different kinds of molecular function: inhibitor activity, like peptidase inhibitor activity and endopeptidase inhibitor activity; binding activity, like collagen binding, calcium ion binding, pattern binding, heparin binding and lipid binding (Figure 5C). On the other hand, the above-mentioned BP, CC and MF were not only implicated in Chinese HCC patients with early HE but also in Chinese HCC patients with early HE, suggesting that they might not play key roles in the occurrence of early HE in HCC patients.
Figure 5.
Functional annotation by bioinformatics analysis. A-C. the biological processes, cellular components and molecular function of the PHA-E-associated glycoproteins identified in sera from Chinese HCC patients both with and without early HE. D. The biological processes functional annotation of the PHA-E-associated glycoproteins only identified in serum from Chinese HCC patients without early HE. E-G. the biological processes, cellular components and molecular function of the PHA-E-associated glycoproteins only identified in serum from Chinese HCC patients with early HE. H. Corresponding network of the PHA-E-associated glycoproteins only identified in sera from Chinese HCC patients with early HE by IPA analysis.
We further analyzed the other group of glycoproteins. Firstly, the group of 12 glycoproteins was only identified in sera from Chinese HCC patients without early HE, which were involved in humoral immune response, response to wounding, defense response and so on (Figure 5D). Besides, these glycoproteins were mainly localized on extracellular region, rather than the abundant cellular components of glycoproteins identified in sera from Chinese HCC patients both with and without early HE (not shown here). Then, the 26 glycoproteins only identified in sera from Chinese HCC patients with early HE were analyzed. In addition to the biological processes mentioned above (just like response to wounding, wound healing and so on), these 26 glycoproteins showed special processes and might be closely linked with blood coagulation, hemostasis, enzyme linked receptor protein signaling pathway, regulation of cellular protein metabolic process and oxidation reduction (Figure 5E) and mainly localized on extracellular space and extracellular region (Figure 5F). Among these 26 glycoproteins, voltage-dependent calcium channel subunit alpha-2/delta-1, coagulation factor V, desmoglein-1, inter-alpha-trypsin inhibitor heavy chain H1, MMP9 and myeloperoxidase were involved in calcium ion binding; myeloperoxidase and extracellular superoxide dismutase in antioxidant activity; hemopexin, lactotransferrin and myeloperoxidase in iron ion binding (Figure 5G). Figure 5H showed the top corresponding network of 19 glycoproteins among the 26 glycoproteins only identified in sera from Chinese HCC patients with early HE by IPA analysis, which is based on the Ingenuity Knowledge database to collect and generate information about molecule-to-molecule interactions, biological networks and canonical pathways. Seven molecules were enzyme, transcription, complex/group, peptidase, phosphatase, transporter, and other, respectively. Nobatly, MMP9, Low affinity immunoglobulin gamma Fc region receptor III-B among these 19 glycoproteins, and other molecules like p38 MAPK, ERK1/2, IL1 and IL12 (complex) were enriched in the network.
Discussion
Glycosylation is one of the most common and complex forms of post-translational modification of proteins and regulates a lot of critical cellular processes. Nowadays, impressive progress in exploring the structure and roles of glycans in various diseases, especially in cancer, has contributed to the discovery of glycans as promising biomarkers and highlighted their application in the clinical setting as appealing targets [24]. We had previously reported the alterations of cell surface glycan in HCC EMT [22], and a series of glycoprotein biomarkers of HCC occurrence and metastasis like paraoxonase 1 (PON1) and expression of alpha-1-antitrypsin (SERPINA1) [25,26]. Glycoproteomics is a focus in the glycobiology, however, research in this field is difficult because of limited techniques exploring the vast number of glycan motifs and potential glycosylation sites of glycoproteins. There are various glycoproteins secreted or shed from cell surfaces or released from tissue into serum, which enables serum as the most available sample to be the primary clinical specimen in disease diagnosis and biomarker discovery [27]. Here, we paid our attention to glycoproteins in sera from Chinese HCC patients with and without early HE based on an integrated strategy of lectin-related techniques like lectin microarray, lectin bolting, lectin affinity chromatography combines with LC-MS/MS to provide early HE related glycosylation pattern and glycoprotein profiling, and tried to provide the database for early HE biomarker discovery in HCC patients.
Our results from lectin microarray analysis demonstrated specific carbohydrate epitopes: GalNAc, terminal α-1,3 Man, bisecting GlcNAc, (GlcNAc) n, O-GlcNAc, Neu5Ac, tetra-antennary complex-type N-glycan and GalNAc α/β1-3/6 Gal were all decreased in sera from Chinese HCC patients with early HE. Although there are not plenty of researches focused on glycoproteomics in HE, results from other researches of neurodegenerative diseases could be used as references. O-GlcNAc-modification, which plays key roles in maintenance and function of the synapse, could be recognized by WGA [28]. Mounting evidence shows O-GlcNAc-modified proteins were decreased in Alzheimer’s disease (AD) brain, and the disruption of glucose metabolism was not only a consequence of the disease but also a causative agent for AD [29,30]. In addition, O-GlcNAc can reduce protein phosphorylation at specific sites by modify competitively, which was associated with amyotrophic lateral sclerosis (ALS) [31]. PHA-L reactive structure, β1,6-GlcNAc branched N-glycan, was catalyzed by glycosyltransferase GnT-V. GnT-V deficiency in T cells could negatively regulate experimental autoimmune encephalomyelitis (EAE) and spontaneous inflammatory demyelination [32]. In this study, most glycosylation modification were decreased in HCC patients with early HE, suggesting that the occurrence of early HE in HCC patients might be accompanied by deglycosylation. This is in line with previously published data, showing that inhibiting deglycosylation of glycoprotein might be an innovative way to suppress abnormal phosphorylation in neurodegenerative diseases [33].
As a plant lectin, which has strong combining capacity and could specifically recognize the bisecting GlcNAc structure, PHA-E was chosen for subsequent lectin affinity chromatography. In the lists of PHA-E-associated glycoprotein profiling of sera from Chinese HCC patients, there were some interesting findings. PHA-E-associated complement C4A and C4B, although they were both as isoforms of C4 [34], they belonged to different PHA-E-associated glycoprotein groups. Complement C4A was only identified in sera from Chinese HCC patients without early HE, while complement C4B was only identified in sera from Chinese HCC patients with early HE. According to the covalent linkage with the target cells or immune complexes through the highly reactive thioester carbonyl group, C4A and C4B are distinguished: activated C4A tends to form an amide bond with amino groups on antigens, while activated C4B is strongly reactive towards hydroxyl groups on glycerols or glycosylated antigens [35]. Increased frequency of complement C4A deficiency could be identified in various diseases, like subacute sclerosing panencephalitis, a chronic neurodegenerative infection of the central nervous system [36]. Furthermore, PHA-E-associated MMP9 was only identified in sera from Chinese HCC patients with early HE. Previous researches had shown that upregulated MMP9 could induce the blood-brain barrier (BBB) permeability during the later stages of HE [37,38]. On the other hand, in this study we could not detect PHA-E-associated MMP9 in sera from Chinese HCC patients without early HE, suggesting that serum PHA-E-associated MMP9 showed a promising diagnostic accuracy in Chinese HCC patients with early HE and might be a potential biomarker to identify HCC patients with early HE. It was noting that the roles of MMP9 in tumor invasion and progression were prominent; down-regulation of MMP9 could inhibit the metastasis of HCC cells [39-41]. According to previous studies, higher expression of MMP9 always was associated with tumor invasion and metastasis. While bisecting GlcNAc modification, which is recognized by PHA-E, could be a suppressor in the tumor metastasis [42]. That might be the reason why PHA-E-associated MMP9 was so few in HCC without early HE, in contrast, detecting PHA-E-associated MMP9 might identify specifically early HE patients in Chinese HCC patients.
In addition to the glycoprotein MMP9 discussed above, there were other two significant nodes, p38 MAPK and ERK1/2, only identified in Chinese HCC patients with early HE in the IPA network of PHA-E-associated glycoproteins. It was reported that rats with early HE due to portacaval shunt (PCS) presented increased phosphorylation (activity) of p38 [43]. p38 is a therapeutic target for HE, inhibitors of p38 have been reported to improve or reverse neuropsychiatric manifestations and prevent the onset and progression of HE in patients and animal models [44,45]. Further analysis showed that ammonia could induce ERK1/2 phosphorylation through the activity of EGF receptor and Src [46]. These results suggest that those PHA-E-associated glycoproteins only identified in sera from Chinese HCC patients with early HE might closely be bound up with the occurrence of early HE in HCC patients and involved in the occurrence of early HE in HCC patients through p38 MAPK signaling pathway and MAPK/ERK signaling pathway.
However, one limitation of this study is that we performed a non-quantitative MS analysis. As mentioned above, proteins identification in our study could be associated with the precision of mass spectrometry system. Thus, future studies performing the quantitative determination by MS could be needed in order to confirm and optimize our results. Currently, our study just presented the difference in glycosylation patterns and PHA-E-associated glycoprotein profiling between sera from Chinese HCC patients with and without early HE, further studies are needed to further explore the relationship between these differences and pathogenic mechanism of early HE in HCC patients.
In conclusion, we performed an integrated strategy of lectin-related techniques and obtained the different glycosylation patterns and PHA-E-associated glycoprotein profiling of sera from Chinese HCC patients with and without early HE. Further bioinformatics analysis showed specific PHA-E-associated glycoproteins might be involved in the occurrence of early HE through p38 MAPK signaling pathway and MAPK/ERK signaling pathway. As we know, this is the first comprehensive glycomics analysis which presented the alteration of glycoproteomics in sera from Chinese HCC patients with and without early HE. Hence, we believe this research will provide potential biomarkers and potential targets in the prophylaxis and treatment of early HE in HCC patients.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81001057), Shanghai Pujiang Program (15PJD007), Shanghai Natural Science Fund (15ZR1406300) and Proteome information research techniques and analysis (2014DFB30010). We thanked Mr. Zhang Yang and Ms. Sun Yiwen (Institute of Biomedical Science, Fudan University) for their helps to perform the IPA analysis. We thanked Mr. Yan Guoquan (Department of Chemistry, Fudan University) for his helps in MS analysis and database searching.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 3.Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748–753. doi: 10.1016/s0168-8278(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama K, Nebashi Y, Kiuchi Y, Shibata M, Mitamura K. Prognostic index of cirrhotic patients with hepatic encephalopathy with and without hepatocellular carcinoma. Dig Dis Sci. 2004;49:1174–1180. doi: 10.1023/b:ddas.0000037808.44897.8a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Xu D, Wang R, Zhu P, Mei B, Wei G, Xiao H, Chen X. Perioperative antiviral therapy improves safety in patients with hepatitis B related HCC following hepatectomy. Int J Surg. 2015;15:1–5. doi: 10.1016/j.ijsu.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Grover VP, Tognarelli JM, Massie N, Crossey MM, Cook NA, Taylor-Robinson SD. The why and wherefore of hepatic encephalopathy. Int J Gen Med. 2015;8:381–390. doi: 10.2147/IJGM.S86854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Li N, Wang R, Li Q, Wu H. Interferon gamma, interleukin-6, and -17a levels were correlated with minimal hepatic encephalopathy in HBV patients. Hepatol Int. 2015;9:218–223. doi: 10.1007/s12072-015-9610-8. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CF, Chu CJ, Huang YH, Wang YP, Liu PY, Lin HC, Lee FY, Lu CL. Detecting minimal hepatic encephalopathy in an endemic country for hepatitis B: the role of psychometrics and serum IL-6. PLoS One. 2015;10:e0128437. doi: 10.1371/journal.pone.0128437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte-Rojo A, Ruiz-Margain A, Macias-Rodriguez RU, Cubero FJ, Estradas-Trujillo J, Munoz-Fuentes RM, Torre A. Clinical scenarios for the use of S100beta as a marker of hepatic encephalopathy. World J Gastroenterol. 2016;22:4397–4402. doi: 10.3748/wjg.v22.i17.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciecko-Michalska I, Szczepanek M, Slowik A, Mach T. Pathogenesis of hepatic encephalopathy. Gastroenterol Res Pract. 2012;2012:642108. doi: 10.1155/2012/642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 12.Seitz O. Glycopeptide synthesis and the effects of glycosylation on protein structure and activity. Chembiochem. 2000;1:214–246. doi: 10.1002/1439-7633(20001117)1:4<214::AID-CBIC214>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Hamby SE, Hirst JD. Prediction of glycosylation sites using random forests. BMC Bioinformatics. 2008;9:500. doi: 10.1186/1471-2105-9-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Cho CH, Kim SH, Kang JG, Yoo JS, Chang CL, Ko JH, Kim YS. Semi-quantitative measurement of a specific glycoform using a DNA-tagged antibody and lectin affinity chromatography for glyco-biomarker development. Mol Cell Proteomics. 2015;14:782–795. doi: 10.1074/mcp.O114.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 16.Pochec E, Bubka M, Rydlewska M, Janik M, Pokrywka M, Litynska A. Aberrant glycosylation of alphavbeta3 integrin is associated with melanoma progression. Anticancer Res. 2015;35:2093–2103. [PubMed] [Google Scholar]
- 17.Lin WL, Lin YS, Shi GY, Chang CF, Wu HL. Lewisy promotes migration of oral cancer cells by glycosylation of epidermal growth factor receptor. PLoS One. 2015;10:e0120162. doi: 10.1371/journal.pone.0120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Yan ZL, Xi T, Wang K, Li J, Shi LH, Wu MC, Shen F. A case-control study of correlation between preoperative serum AFP and recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology. 2012;59:2248–2254. doi: 10.5754/hge11978. [DOI] [PubMed] [Google Scholar]
- 19.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tanaka J, Kagebayashi C, Satomura S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555–563. doi: 10.1007/s00535-013-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissenborn K, Heidenreich S, Giewekemeyer K, Ruckert N, Hecker H. Memory function in early hepatic encephalopathy. J Hepatol. 2003;39:320–325. doi: 10.1016/s0168-8278(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Li N, Jin R, Meng Q, Chen P, Zhao G, Wang R, Li L, Li W. Cytokine levels contribute to the pathogenesis of minimal hepatic encephalopathy in patients with hepatocellular carcinoma via STAT3 activation. Sci Rep. 2016;6:18528. doi: 10.1038/srep18528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Mo C, Peng Q, Kang X, Sun C, Jiang K, Huang L, Lu Y, Sui J, Qin X, Liu Y. Cell surface glycan alterations in epithelial mesenchymal transition process of Huh7 hepatocellular carcinoma cell. PLoS One. 2013;8:e71273. doi: 10.1371/journal.pone.0071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin AJ, Cheng L, Diao H, Wang P, Gu YH, Wu B, Wu YC, Chen GW, Zhou SM, Guo SJ, Shi HJ, Tao SC. Comprehensive profiling of accessible surface glycans of mammalian sperm using a lectin microarray. Clin Proteomics. 2014;11:10. doi: 10.1186/1559-0275-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 25.Qin X, Chen Q, Sun C, Wang C, Peng Q, Xie L, Liu Y, Li S. High-throughput screening of tumor metastatic-related differential glycoprotein in hepatocellular carcinoma by iTRAQ combines lectin-related techniques. Med Oncol. 2013;30:420. doi: 10.1007/s12032-012-0420-8. [DOI] [PubMed] [Google Scholar]
- 26.Sun C, Chen P, Chen Q, Sun L, Kang X, Qin X, Liu Y. Serum paraoxonase 1 heteroplasmon, a fucosylated, and sialylated glycoprotein in distinguishing early hepatocellular carcinoma from liver cirrhosis patients. Acta Biochim Biophys Sin (Shanghai) 2012;44:765–773. doi: 10.1093/abbs/gms055. [DOI] [PubMed] [Google Scholar]
- 27.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 28.Skorobogatko YV, Deuso J, Adolf-Bryfogle J, Nowak MG, Gong Y, Lippa CF, Vosseller K. Human Alzheimer’s disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids. 2011;40:765–779. doi: 10.1007/s00726-010-0645-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Shi J, Tanimukai H, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butterfield DA, Owen JB. Lectin-affinity chromatography brain glycoproteomics and Alzheimer disease: insights into protein alterations consistent with the pathology and progression of this dementing disorder. Proteomics Clin Appl. 2011;5:50–56. doi: 10.1002/prca.201000070. [DOI] [PubMed] [Google Scholar]
- 31.Shan X, Vocadlo DJ, Krieger C. Reduced protein O-glycosylation in the nervous system of the mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2012;516:296–301. doi: 10.1016/j.neulet.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Grigorian A, Demetriou M. Mgat5 deficiency in T cells and experimental autoimmune encephalomyelitis. ISRN Neurol. 2011;2011:374314. doi: 10.5402/2011/374314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer PM. Turning down tau phosphorylation. Nat Chem Biol. 2008;4:448–449. doi: 10.1038/nchembio0808-448. [DOI] [PubMed] [Google Scholar]
- 34.Pereira KM, Faria AG, Liphaus BL, Jesus AA, Silva CA, Carneiro-Sampaio M, Andrade LE. Low C4, C4A and C4B gene copy numbers are stronger risk factors for juvenile-onset than for adult-onset systemic lupus erythematosus. Rheumatology (Oxford) 2016;55:869–873. doi: 10.1093/rheumatology/kev436. [DOI] [PubMed] [Google Scholar]
- 35.Rigby WF, Wu YL, Zan M, Zhou B, Rosengren S, Carlson C, Hilton W, Yu CY. Increased frequency of complement C4B deficiency in rheumatoid arthritis. Arthritis Rheum. 2012;64:1338–1344. doi: 10.1002/art.33472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittner C, Meier EM, Stradmann B, Giles CM, Kochling R, Mollenhauer E, Kreth HW. Partial C4 deficiency in subacute sclerosing panencephalitis. Immunogenetics. 1984;20:407–415. doi: 10.1007/BF00345615. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen JH, Yamamoto S, Steers J, Sevlever D, Lin W, Shimojima N, Castanedes-Casey M, Genco P, Golde T, Richelson E, Dickson D, McKinney M, Eckman CB. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105–1114. doi: 10.1016/j.jhep.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMillin MA, Frampton GA, Seiwell AP, Patel NS, Jacobs AN, DeMorrow S. TGFbeta1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Invest. 2015;95:903–913. doi: 10.1038/labinvest.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Liang J, Zhou J, Mi J, Ma K, Fan Y, Ning J, Wang C, Wei X, Li E. Knockdown of RHOC by shRNA suppresses invasion and migration of cholangiocellular carcinoma cells via inhibition of MMP2, MMP3, MMP9 and epithelial-mesenchymal transition. Mol Med Rep. 2016;13:5255–5261. doi: 10.3892/mmr.2016.5170. [DOI] [PubMed] [Google Scholar]
- 40.Dou CY, Cao CJ, Wang Z, Zhang RH, Huang LL, Lian JY, Xie WL, Wang LT. EFEMP1 inhibits migration of hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2 activity. Oncol Rep. 2016;35:3489–3495. doi: 10.3892/or.2016.4733. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Wen X, Liu B, Zhang Q, Zhang J, Miao H, Zhu R. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP2 and MMP9. Mol Med Rep. 2016;13:2401–2408. doi: 10.3892/mmr.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Y, Aglipay JA, Bernstein JD, Goswami S, Stanley P. The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res. 2010;70:3361–3371. doi: 10.1158/0008-5472.CAN-09-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez-Rabaza V, Agusti A, Cabrera-Pastor A, Fustero S, Delgado O, Taoro-Gonzalez L, Montoliu C, Llansola M, Felipo V. Sildenafil reduces neuroinflammation and restores spatial learning in rats with hepatic encephalopathy: underlying mechanisms. J Neuroinflammation. 2015;12:195. doi: 10.1186/s12974-015-0420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agusti A, Cauli O, Rodrigo R, Llansola M, Hernandez-Rabaza V, Felipo V. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut. 2011;60:1572–1579. doi: 10.1136/gut.2010.236083. [DOI] [PubMed] [Google Scholar]
- 45.Luo M, Liu H, Hu SJ, Bai FH. Potential targeted therapies for the inflammatory pathogenesis of hepatic encephalopathy. Clin Res Hepatol Gastroenterol. 2015;39:665–673. doi: 10.1016/j.clinre.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Dai H, Song D, Xu J, Li B, Hertz L, Peng L. Ammonia-induced Na,K-ATPase/ouabain-mediated EGF receptor transactivation, MAPK/ERK and PI3K/AKT signaling and ROS formation cause astrocyte swelling. Neurochem Int. 2013;63:610–625. doi: 10.1016/j.neuint.2013.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.