Abstract
Catalpol, isolated from the roots of Rehmanniaglutinosa, Chinese foxglove, is an iridoid glycoside with antioxidant, anti-inflammatory and anti-hyperglycemic agent. The present study was to investigate the effects of catalpol on diabetic atherosclerosis in alloxan-induced diabetic rabbits. Diabetes was induced in rabbits by a hyperlipidemic diet and intravenous injection of alloxan (100 mg/kg). Rabbits were treated for 12 weeks. The fasting blood glucose, insulin, homeostasis model of insulin resistance, total cholesterol and triglyceride were measured. The thoracic aorta was excised for histology. The plasma and vascular changes including some markers of oxidative stress, inflammatory cytokines and fibrosis factors were examined. Plasma levels of fasting blood glucose, insulin and homeostasis model of insulin resistance were significantly decreased in catalpol group. Catalpol treatment ameliorated diabetic atherosclerosis in diabetic rabbits as demonstrated by significantly inhibited neointimal hyperplasia and macrophages recruitment. Catalpol treatment also enhanced the activities of superoxide dismutase, glutathione peroxidase, and increased the plasma levels of total antioxidant status, meanwhile reduced the levels of malondialdehyde, protein carbonyl groups and advanced glycation end product. Furthermore, catalpol also reduced circulating levels of tumor necrosis factor-α, monocyte chemotactic protein-1 and vascular cell adhesion molecule-1. Catalpol also decreased transforming growth factor-β1 and collagen IV mRNA and protein expressions in the vessels. Catalpol exerts an ameliorative effect on atherosclerotic lesion in alloxan-induced diabetic rabbits. The possible mechanisms may be related to inhibition of oxidative stress inflammatory response and anti-fibrosis and reduced aggregation of extracellular matrix.
Keywords: Catalpol, diabetic atherosclerosis, oxidative stress, inflammatory response
Introduction
Diabetes mellitus (DM) is the most common metabolic disease characterized by chronic hyperglycemia and is associated with long-term complications. The most common and life-threatening complication is cardiovascular diseases (CVD) [1,2]. Atherosclerosis is accelerated in diabetes’, leads to the development of CVD. Although the precise mechanisms of the accelerated atherosclerosis are still unclear, many factors attributed to the development of atherosclerosis in diabetes have been reported, such as hyperglycemia, insulin resistance, dyslipidemia, oxidative stress, inflammation and increased generation of advanced glycation end products (AGEs) [3-5].
Oxidative stress and inflammation play fundamental roles in the pathogenesis of atherosclerosis in DM [6,7]. It is possible that oxidative stress induced by hyperglycemia and hyperlipidemia, along with products of glycation and lipid peroxidation, plays critical roles in inducing inflammatory cytokines expression [8]. Alongside the increased reactive oxygen species (ROS) generated in diabetes [9], ROS productions are markedly increased along with an obvious decline in the antioxidant defense systems [10,11]. In conjunction with oxidative stress, the levels of the inflammatory cytokines including TNF-α, MCP-1 and VCAM-1 are elevated in diabetic individuals [7,12,13]. Esposito K et al. reported that the release of TNF-α induced by high glucose in vitro experiments may be mediated by ROS and oxidative stress be implicated in promoting systemic inflammation in diabetic patients [14]. Therefore, antioxidant and anti-inflammatory therapy may prove to be effective therapeutic tools in preventing diabetic atherosclerosis.
Catalpol, the most abundant bioactive component in the roots of Rehmanniaglutinosa, is an iridoid glucoside. It has been reported that catalpol can ameliorate hyperglycemia [15,16], neurotoxicity [17,18], and diabetic encephalopathy [19]. In addition, catalpol has anti-oxidative and anti-inflammatory effects [20]. However, there is no report about the effects of catalpol on diabetic atherosclerosis in alloxan-induced diabetic rabbits.
Based on the above background, we aimed to evaluate the protective effects of catalpol in diabetic atherosclerosis in alloxan-induced diabetic rabbits. And also to investigate the possible mechanisms involved in ameliorating oxidative stress and inflammation.
Materials and methods
Drug preparation
Catalpol (purity >98%, molecular formula: C15H22O10, molecular weight: 362.33) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Catalpol was dissolved in normal saline solution as a vehicle for its administration as previously described [21]. Catalpol was administered 5 mg/kg once a day. The dosage of catalpol used in this study was chosen as indicated by our prior preliminary experiments [20].
Animals and treatment protocols
The Guidelines for the Care and Use of Laboratory Animals published by the Chinese National Institutes of Health were followed while using live animals. The protocol was approved by the Animal Ethics Committee of Weifang Medical University (WYLY 2013-006, Jun 12, 2013). Twenty-eight male 4-month-old New Zealand White rabbits (2.5-2.8 kg), obtained from Experimental Animal Center of Weifang Medical University, were housed in individual cages under controlled temperature and a 12 h light/dark cycles. All rabbits were provided with standard diet and water ad libitum. After a week of acclimation to the experimental area, the diabetic rabbit model was developed using a hyperlipidemic diet plus alloxan similar to that employed in previously studies [22]. The hyperlipidemic diet consisted of 1% cholesterol and 10% lard. The NC group was given a standard diet. After 4 weeks of dietary intervention, the diabetic rabbit models were injected intravenously with alloxan monohydrate (dissolved in saline solution, 100 mg/kg, Sigma, USA) to induce diabetes. The rabbits with fasting blood glucose levels above 11.1 mmol/l were considered diabetic and were fed with the hyperlipidemic diet continually. All the rabbits were randomly divided into 4 groups with seven in each group: the NC group was treated with saline in a matched volume, the DM group had diabetic rabbits treated with saline in a matched volume, the metformin group had diabetic rabbits administered with metformin (Shi Guibao Co. Ltd, Shanghai, China) 50 mg/kg/d, and the catalpol group had diabetic rabbits administered with catalpol 5 mg/kg/d. All medicines were administered by oral gavage for 12 weeks. Body weight, fasting blood glucose, insulin and HOMA-IR were measured every 4 weeks. At the end of 12 weeks, fasting blood samples were obtained from the marginal ear vein for plasma separation. Plasma samples obtained from centrifugation at 4000×g for 10 min and were stored at -70°C until laboratory analysis. To obtain thoracic aortas, all rabbits were sacrificed humanely with an overdose of pentobarbital intravenously. Thoracic aortas were rapidly excised and fixed in 10% formaldehyde solution for subsequent histological analysis.
Determination of glucose, insulin and lipid levels
The plasma levels of glucose and insulin were determined respectively by glucose oxidase and radioimmunoassay method using commercial diagnostic kits (Nanjing Jiancheng Biotechnology Institute, China). Plasma total cholesterol (TC) and triglyceride (TG) were detected by an automatic blood chemical analyzer (Olympus, AU 600, Japan).
Determination of homeostasis model of insulin resistance
Insulin resistance was evaluated by homeostasis model assessment estimate of insulin resistance (HOMA-IR) [23] as follows: HOMA-IR = Fasting insulin level (μU/ml)× Fasting blood glucose (mmol)/22.5.
Oxidative stress status analysis in plasma
The plasma concentrations of MDA, PCG and AGEs as well as the activities of SOD, GSH-Px and TAS were all determined by using commercially available kits (Nanjing Jiancheng Biotechnology Institute, China). Concentrations of plasma MDA, PCG and AGEs in the present study were used to indicate the oxidation state. MDA content was measured as thiobarbituric acid reactive substances (TBARS) according to the manufacturer’s instructions [24]. PCG concentration was assayed by the modified method of Levine et al. reported [25] according to the manufacturer’s instructions. AGEs concentration was measured by specific ELISA kit according to the manufacturer’s instructions. Plasma SOD, GSH-PX activities and TAS were used to evaluate the antioxidant status in rabbits. SOD activity was assayed using the hydroxylamine oxidation assay method of Kakkar et al. reported [26]. One unit (U) of SOD activity was defined as the amount of enzyme necessary to reduce hydroxylamine by 50%. GSH-PX activity was evaluated by the 5-5’-dithiobis (2-nitrobenzoic acid) (DTNB) method [27]. One U of GSH-PX activity was defined as 1 μmol/l GSH consumption per minute at pH 7.0 and 37°C. TAS was assessed using colorimetry according to the manufacturer’s instructions [28].
Inflammatory cytokines in plasma and transforming growth factor-β1 in vascular
Plasma concentrations of TNF-α, MCP-1 and VCAM-1 and transforming growth factor-β1 (TGF-β1) in vascular were measured by specific ELISA kits (Boster Biotechnology Co., Ltd, Wuhan, China) according to the manufacturer’s instructions. Optical density was read at 450 nm using THERMO microplate reader. Standard curve was generated by correlating the known concentration and the corresponding optical densities.
Histological and immunohistochemical staining
To determine the effect of catalpol on diabetic atherosclerosis, hematoxylin-eosin (HE) and immunohistochemical staining were performed on 5 mm-thin paraffin sections from thoracic aortas. Intima-media thickness was evaluated by HE staining according to the method of Zhang et al. [29]. The expression of macrophages and vascular smooth muscle cells (VSMCs) was evaluated by immunohistochemical method. After routine deparaffinization and rehydration, 5 mm-thin paraffin sections were incubated with 3% hydrogen peroxide for 10 minutes, and then with 5% BSA serum for 20 minutes. Subsequently, they were immunostained with macrophages, monoclonal antibody (mAb) RAM-11 (DAKO, dilution 1:200) and smooth muscle cells, mAb HHF-35 (DAKO, dilution 1:200) overnight at 4°C, and then with horseradish peroxidase complex system (1:200, Santa Cruz, USA) as a secondary antibody for 30 minutes at 37°C. Visualisation of a positive reaction was developed with the use of a DAB Peroxidase Substrate Kit (Vector Laboratories) to display the reaction product with a brown color and the sections were then counterstained with hematoxylin. All the photographs were analyzed using Image-Pro Plus6.0 (Media Cybernetics, USA). Incubation with PBS instead of the primary antibody was used as a negative control.
Quantification of mRNA levels
Vascular tissues were lysed in TRIZOL reagent and total RNA was isolated. The protocol of synthesis of cDNA is previously described [30]. For quantification of TGF-β1 and Collagen IV transcripts, conventional real-time RT-PCR was carried out with total RNA samples extracted from the blood vessel.
Western blot analyses
The protein samples were extracted from aorta, following essentially the same procedure as described in detail elsewhere [30]. Protein samples (50 µg) were fractionated by SDS-PAGE (7.5-10% polyacrylamide gels). The primary antibodies against Collagen IV (Abcam), with β-actin (Abcam) used as an internal control.
Statistical analysis
All the data were analysed using SPSS (version 18.0) for Windows. Data was expressed as mean ± SE. Statistical analysis was performed using one-way analysis of variance (ANOVA). Bartlett’s box-test was used to test the homogeneity of variance. Individual differences among groups were analyzed by Dunnett-t test. A significance level of P < 0.05 was required for all tests.
Results
Effects of catalpol on body weight, blood glucose, insulin, HOMA-IR and lipid
All the rabbits completed the experimental process. There were no significant differences in mean body weight at baseline. With the progress of the experiment, the plasma levels of blood glucose, HOMA-IR, TC and TG were significantly increased, but the plasma insulin and body weight were significantly reduced in diabetic rabbits compared with normal rabbits. Catalpol and metformin treatment could both significantly improved plasma insulin level and body weight, and decreased the blood glucose and HOMA-IR in diabetic rabbits. Catalpol and metformin could both reduced plasma levels of TG and TC compared to the diabetic rabbits, however the result for catalpol was not statistically significant (Figure 1). These findings suggest that catalpol improved hyperglycemia and hypoinsulinemia, but had no effect on plasma lipids profiles in diabetic rabbits.
Figure 1.
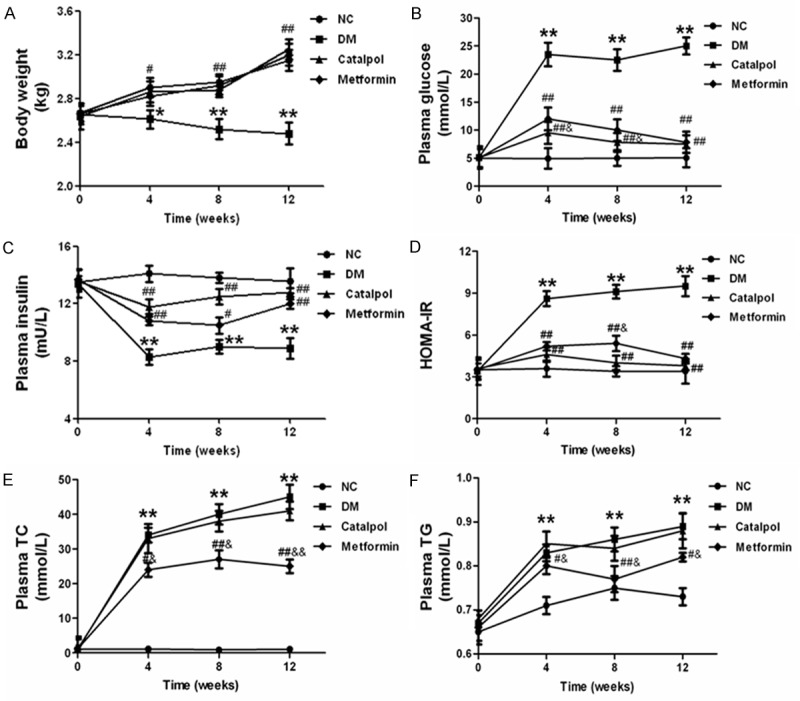
Effects of catalpol on body weight, blood glucose, insulin, HOMA-IR and lipid. A: Effects of catalpol on Body weight. B: Effects of catalpol on plasma blood glucose. C: Effects of catalpol on plasma insulin. D: Effects of catalpol on HOMA-IR. E: Effects of catalpol on plasma TC. F: Effects of catalpol on plasma TG. *P < 0.05 and **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs DM; &P < 0.05 vs catalpol.
Effects of catalpol on oxidative stress
MDA, PCG and AGEs are widely recognized as presumptive biomarkers of enhanced oxidative stress. The generations of MDA, PCG and AGEs were increased significantly in the DM group compared with the NC group (P < 0.05) (Figure 2). Catalpol treatment markedly decreased the plasma concentration of MDA, PCG and AGEs compared with the DM and metformin group (P < 0.05) (Figure 2). The activities of SOD, GSH-Px and plasma level of TAS were used to evaluate the antioxidant status in rabbits. The reduction of SOD, GSH-Px activity and TAS level in the DM group were remarkably preserved after catalpol and metformin treatment. There were significant differences between catalpol and metformin group (Figure 2). These data suggest that catalpol attenuates oxidative stress in alloxan-induced diabetic rabbits by inhibiting oxidation and promoting the antioxidant capacity.
Figure 2.
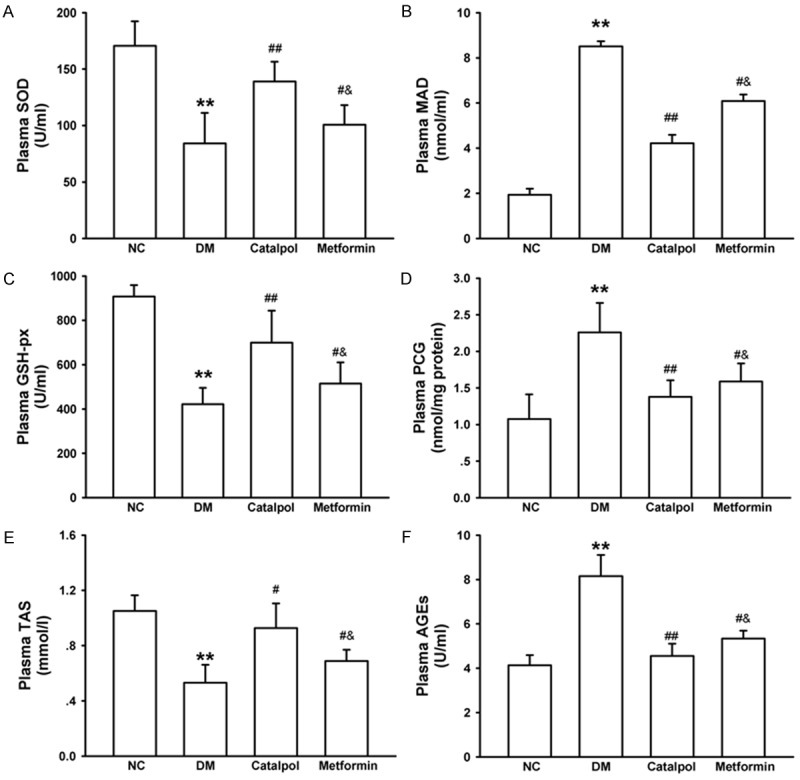
Effects of catalpol on oxidative stress. A: Effects of catalpol on plasma SOD. B: Effects of catalpol on plasma GSH-px. C: Effects of catalpol on plasma TAS. D: Effects of catalpol on plasma MDA. E: Effects of catalpol on plasma PCG. F: Effects of catalpol on plasma AGEs. *P < 0.05 and **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs DM; &P < 0.05 vs catalpol.
Effects of catalpol on inflammatory cytokines
Inflammation plays a pivotal role in atherosclerosis. Therefore, we assessed inflammatory cytokines expression in plasma. Plasma levels of TNF-α, MCP-1 and VCAM-1 were significantly increased in the DM group compared with the NC group as the experiment time progressed (P < 0.05). In contrast, catalpol treatment markedly reduced the levels of TNF-α, MCP-1 and VCAM-1 compared with DM group and metformin group (P < 0.05). There were significant differences between the DM and metformin groups (Figure 3). These data suggested that catalpol might suppress inflammation in alloxan-induced diabetic rabbits through reducing the over-expression of TNF-α, MCP-1 and VCAM-1 in circulation.
Figure 3.
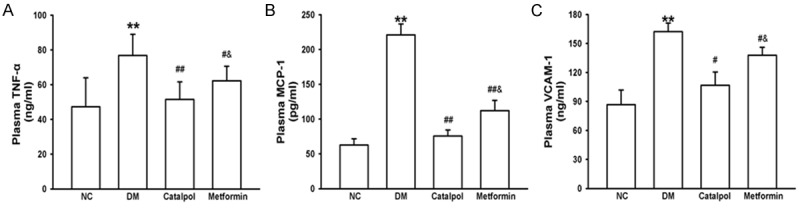
Effects of catalpol on inflammatory cytokines. A: Effects of catalpol on plasma TNF-α. B: Effects of catalpol on plasma MCP-1. C: Effects of catalpol on plasma VCAM-1. *P < 0.05 and **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs DM; &P < 0.05 vs catalpol.
Effects of catalpol on atherosclerotic lesions
The lipid content of the plaques was determined by HE staining of the thoracic aorta sections. Intima-media thickness was significantly increased in the DM group compared with the NC group (P < 0.05). However, catalpol treatment markedly inhibited neointimal hyperplasia compared with the DM and metformin group (P < 0.05). There was significant difference between the DM and metformin groups (Figure 4). Plaque content of the VSMCs and macrophage infiltration was determined by immunohistochemical staining of the thoracic aorta sections, as shown in Figure 4. Our results showed that the content of VSMCs in plaque was higher in the DM group than that of the NC group, whereas it was lower in catalpol and metformin groups. Meanwhile, rabbits in the DM group showed extensive macrophage infiltration into the wall of aorta. However, the macrophage content was lower in catalpol and metformin groups. Significant difference between the catalpol and metformin groups was observed. These findings suggest that catalpol can attenuate atherosclerotic lesions and delay atherosclerosis progression in alloxan-induced diabetic rabbits.
Figure 4.
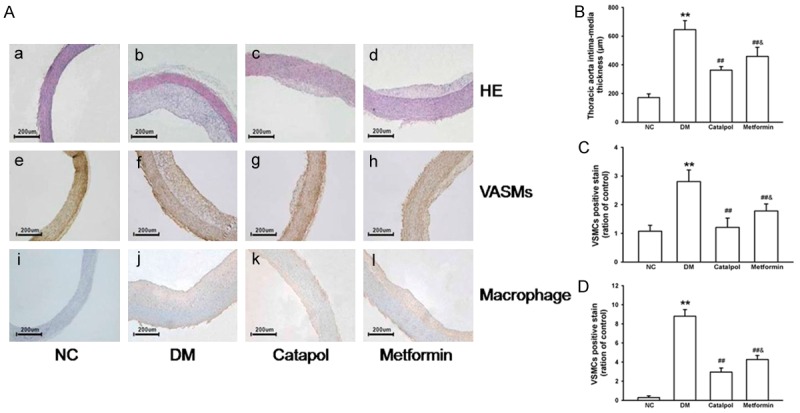
Effects of catalpol on atherosclerotic lesions. A(a-d) and B: Histology illustrations and effect of catalpol on intima-media thickness of atherosclerotic lesions by HE staining. A(e-i) and C: Histology illustrations and effect of catalpol on VSMCs of atherosclerotic lesions by immunohis to chemical staining. A(j-m) and D: Histology illustrations and effect of catalpol on macrophage infiltration of atherosclerotic lesions by immunohis to chemical staining. *P < 0.05 and **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs DM; &P < 0.05 vs catalpol.
Effects of catalpol on TGF-β1 and collagen IV
Fibrosis and aggregation of extracellular matrix can also be seen in atherosclerosis. Therefore, we assessed TGF-β1 and collagen IV expression in vessel with and without atherosclerotic plagues. Vascular expressions of TGF-β1 and collagen IV mRNA and protein were significantly increased in the DM group compared with the NC group (P < 0.05, Figure 5). In contrast, catalpol treatment signifcantly decreased the expressions of TGF-β1 and collagen IV mRNA and protein compared with DM group and metformin group (P < 0.05). There was also similar significant difference between the DM and metformin group (Figure 5). These data suggested that catalpol might suppress fibrosis and aggregation of extracellular matrix through reducing the over-expression of TGF-β1 and collagen IV in vessel.
Figure 5.
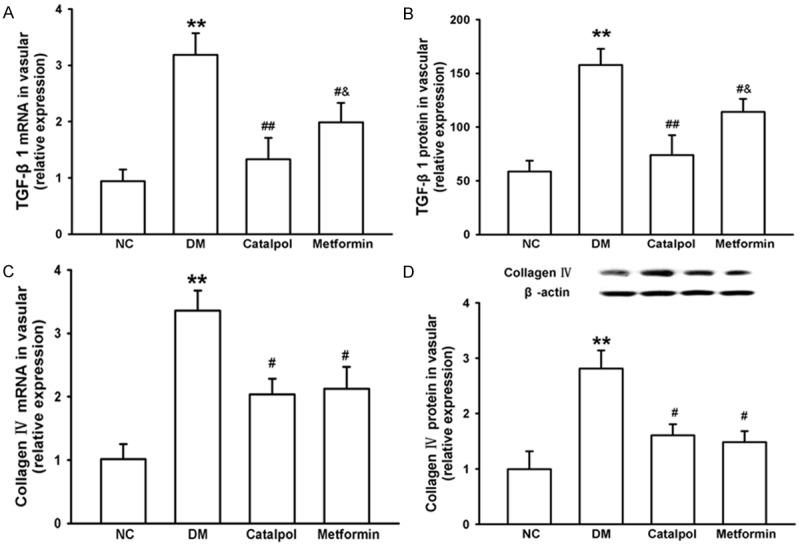
Effects of catalpol on TGF-β1 and collagen IV. A: Effects of catalpol on vascular TGF-β1 mRNA. B: Effects of catalpol on vascular TGF-β1 protein. C: Effects of catalpol on vascular collagen IV mRNA. D: Effects of catalpol on vascular collagen IV protein. *P < 0.05 and **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs DM; &P < 0.05 vs catalpol.
Discussion
The present study, observed for the first time, that catalpol attenuates atherosclerotic lesion and delays progression of atherosclerosis progression in alloxan-induced diabetic rabbits. The protective effects of catalpol were associated with the reduction of blood glucose and inhibition of oxidative stress and inflammation, limiting the progression of diabetic atherosclerosis.
The loss of body weight is one of the threats associated with DM. We observed that alloxan-induced diabetic rabbits also tend to lose weight. Catalpol and metformin treatments reversed weight loss in the test rabbits. Hyperglycemia and insulin resistance generates adverse metabolic events in endothelial cells leading to endothelial dysfunction, oxidative stress augmented, inflammation, and thrombosis [31,32]. Elevated TG and TC levels also increase the risk of cardiovascular diseases in diabetes [33].
In the present study, rabbits in the DM group exhibit hyperglycemia, insulin resistance and hyperlipidemia, the hallmarks of diabetic atherosclerosis, setting stage for a more sensitive evaluation on the effects of catalpol on diabetic atherosclerosis. Our study showed that oral supplementation of catalpol significantly decreased the blood glucose level and ameliorated insulin resistance, as well as, reduced intima-media thickness. These results suggested that catalpol has an excellent anti-atherosclerotic effect in diabetic rabbits. Hyperplasia of VSMCs and the infiltration of macrophages in the arteries were early events in the development of atherosclerosis [34]. We also noted a reduction in the expression of α-SM actin and RAM-11. These observations could explain the reduction of plaque extension and retardation of atherosclerosis in the aorta of diabetic rabbits treated with catalpol.
Diabetes, insulin resistance and hyperlipidemia accelerated atherosclerosis by inducing vascular and endothelial dysfunction, enhancing oxidative stress and increasing the inflammatory response [6,35]. Elevated serum glucose level stimulates the formation and accumulation of AGEs, which induce a continuous oxidative stress as well as an increased synthesis of ROS [36]. MDA, as a product of lipid peroxidation, is considered to be a strong predictor of CVD and found to be increased in diabetic rats [37]. Among various oxidative modifications of proteins, formation of PCG perhaps is an early marker of protein oxidation and was reported to be increased in diabetic rabbits [38].
Antioxidants such as SOD, GSH-Px and TAS were reported to be reduced in diabetic rabbits [39,40]. Consistent with previous reports, the activities of SOD, GSH-Px and TAS significantly reduced coupling with a marked increase in the level of MDA, AGEs and PCG in alloxan-induced diabetic rabbits. Treatment with catalpol markedly inhibited the concentration of MDA, AGEs, PCG, and promoted the activity of SOD, GSH-Px and TAS in the serum of diabetic rabbits with atherosclerosis. The effects of catalpol on oxidative stress were obvious, consistent with previous studies [19,41-43]. These effects demonstrated that catalpol confers antioxidant effects via inhibiting the oxidation and enhancing the antioxidant capacity, which is an essential to promote the anti-atherosclerosis. It’s interesting to note that, the antioxidant effects of catalpol were better than metformin.
Atherosclerosis is an inflammatory process, initiated by the adhesion of monocytes to arterial endothelial cells [34], followed by migration into the sub-endothelial space triggered by chemotactic activation processes [44]. Subsequently, monocytes differentiate into intimal macrophages, taking up lipids and become foam cells, leading to the formation of fatty streak lesions, fibrosis and aggregation of extracellular matrix in vascular walls [45]. Type 2 DM is increasingly being recognized as resulting from chronic low grade inflammation [46]. Numerous researchers reported that the circulating TNF-α, MCP-1, VCAM-1 are usually elevated in established type 2 DM [30,47]. Inflammatory cytokines are associated with enhanced macrophage adhesion to vascular endothelium, resulting in neointimal hyperplasia and endothelial dysfunction. Levels of TNF-α, MCP-1, VCAM-1, TGF-β1 and collagen IV were significantly increased in alloxan-induced diabetic rabbits in the present study, consistent with the above reports. Treatment with catalpol drastically decreased the circulating levels of these inflammatory cytokines and vascular pro- fibrotic factors and reduced aggregation of extracellular matrix compared with DM group and metformin group. The anti-inflammatory, anti-fibrosis and reducing extracellular matrix aggregation effects of catalpol were better if not similar to metformin.
In conclusion, our findings demonstrate that catalpol has protective effects in alloxan-induced diabetic rabbits, attenuated oxidative stress, suppressed inflammatory responses, has anti-fibrosis effects and reduced aggregation of extracellular matrix in vascular walls. We for the first time report catalpol as an alternate anti-hyperglycemic and anti-atherosclerotic drug in DM with promising effects. Despite the limitations in our study, the extant results provided supporting evidence and important novel pharmacological effect of catalpol, which may have potential therapeutic value for the treatment and/or prevention of atherosclerosis in diabetic patients.
Acknowledgements
This work was supported by the Program of Shandong Natural Science Foundation [ZR2015HL126], Shandong Province Traditional Pharmaceutical Technology Development Project, China (2013-237). Natural Science Foundation of Shanghai [15ZR1434400], Shanghai Municipal Commission of Health and Family Planning [20144Y0196].
Disclosure of conflict of interest
None.
Authors’ contribution
All authors actively participated in the study and in the review and approval of the manuscript. JYL, CZZ, XPH, DJZ and PY contributed to drafting of the manuscript and to study concept and design; JYL, CZZ, XPH and DJZ contributed to data acquisition; JYL, CZZ, XPH, AWM and DJZ contributed to data analysis and interpretation; AWM and PY contributed to critical revision of the manuscript for important intellectual content; JYL, DJZ and PY contributed to study supervision; and JYL and PY contributed to acquisition of funding.
References
- 1.Chen F, Guo Z, Wu M, Zhou Z, Luo W. [Impact of dynamic changes of waist circumference and body mass index on type 2 diabetes mellitus risk] . Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49:1092–1097. [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes C Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Pugliese G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia. 2015;58:845–853. doi: 10.1007/s00125-014-3467-6. [DOI] [PubMed] [Google Scholar]
- 4.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 5.Mark L, Dani G. [Diabetic dyslipidaemia and the atherosclerosis] . Orv Hetil. 2016;157:746–752. doi: 10.1556/650.2016.30441. [DOI] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Ye S, Chen Y, Li X, Yang GW, Fan A, Wang Y. The effect of simvastatin on the serum monocyte chemoattractant protein-1 and intracellular adhesion molecule-1 levels in diabetic rats. J Diabetes Complications. 2009;23:214–218. doi: 10.1016/j.jdiacomp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu S, Zhang Z, Xu Q, Xie F, Wang J, Ping S, Li C, Wang Z, Zhang M, Huang J, Chen D, Hu L, Li C. RAGE mediates accelerated diabetic vein graft atherosclerosis induced by combined mechanical stress and AGEs via synergistic ERK activation. PLoS One. 2012;7:e35016. doi: 10.1371/journal.pone.0035016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar G, Banu S, Murugesan AG. Influence of Helicteres isora administration for diabetes mellitus: Its effect on erythrocyte membrane and antioxidant status. Food Chem Toxicol. 2009;47:1803–1809. doi: 10.1016/j.fct.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Gumieniczek A, Hopkala H, Rolinski J, Bojarska-Junak A. Interleukin-6 and oxidative stress in plasma of alloxan-induced diabetic rabbits after pioglitazone treatment. Immunopharmacol Immunotoxicol. 2006;28:81–91. doi: 10.1080/08923970600625785. [DOI] [PubMed] [Google Scholar]
- 11.Santilli F, D’Ardes D, Davi G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul Pharmacol. 2015;74:23–37. doi: 10.1016/j.vph.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 12.He B, Yu C, Du R, Wang Y, Han P. Roux-en-Y esophagojejunostomy reduces serum and aortic inflammatory biomarkers in type 2 diabetic rats. Obes Surg. 2014;24:916–926. doi: 10.1007/s11695-014-1195-0. [DOI] [PubMed] [Google Scholar]
- 13.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 15.Huang WJ, Niu HS, Lin MH, Cheng JT, Hsu FL. Antihyperglycemic effect of catalpol in streptozotocin-induced diabetic rats. J Nat Prod. 2010;73:1170–1172. doi: 10.1021/np9008317. [DOI] [PubMed] [Google Scholar]
- 16.Shieh JP, Cheng KC, Chung HH, Kerh YF, Yeh CH, Cheng JT. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J Agric Food Chem. 2011;59:3747–3753. doi: 10.1021/jf200069t. [DOI] [PubMed] [Google Scholar]
- 17.Tian YY, An LJ, Jiang L, Duan YL, Chen J, Jiang B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006;80:193–199. doi: 10.1016/j.lfs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Jiang B, Du J, Liu JH, Bao YM, An LJ. Catalpol attenuates the neurotoxicity induced by beta-amyloid(1-42) in cortical neuron-glia cultures. Brain Res. 2008;1188:139–147. doi: 10.1016/j.brainres.2007.07.105. [DOI] [PubMed] [Google Scholar]
- 19.Wang CF, Li DQ, Xue HY, Hu B. Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res. 2010;1307:158–165. doi: 10.1016/j.brainres.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Liu JY, Zhang DJ. Amelioration by catalpol of atherosclerotic lesions in hypercholesterolemic rabbits. Planta Med. 2015;81:175–184. doi: 10.1055/s-0034-1396240. [DOI] [PubMed] [Google Scholar]
- 21.Bi J, Jiang B, Liu JH, Lei C, Zhang XL, An LJ. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci Lett. 2008;442:224–227. doi: 10.1016/j.neulet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Ragazzi E, Costa CV, Comai S, Bertazzo A, Caparrotta L, Allegri G. Cloricromene effect on the enzyme activities of the tryptophan-nicotinic acid pathway in diabetic/hyperlipidemic rabbits. Life Sci. 2006;78:785–794. doi: 10.1016/j.lfs.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 23.Tuepker J. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2003;107:e109. doi: 10.1161/01.CIR.0000067693.46108.2D. author reply e109. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Martinez FJ, Pinas-Garcia P, Lleo-Perez AV, Zanon-Moreno VC, Bendala-Tufanisco E, Garcia-Medina JJ, Vinuesa-Silva I, Pinazo-Duran MD. Biomarkers of lipid peroxidation in the aqueous humor of primary open-angle glaucoma patients. Arch Soc Esp Oftalmol. 2016;91:357–62. doi: 10.1016/j.oftal.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 26.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D, Liu J, Wang L, Wang J, Li W, Zhuang B, Hou J, Liu T. Effects of 3,4-dihydroxyacetophenone on the hypercholesterolemia-induced atherosclerotic rabbits. Biol Pharm Bull. 2013;36:733–740. doi: 10.1248/bpb.b12-00710. [DOI] [PubMed] [Google Scholar]
- 30.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 31.Blair M. Diabetes Mellitus Review. Urol Nurs. 2016;36:27–36. [PubMed] [Google Scholar]
- 32.Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 34.Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Wen W, Qi CL, Zhao RX, Lu JH, Zhong CY, Chen YY. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine. 2012;19:712–718. doi: 10.1016/j.phymed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Sharma SB, Singh UR, Bansal SK. Salutary effect of Cassia auriculata L. Leaves on hyperglycemia-induced atherosclerotic environment in streptozotocin rats. Cardiovasc Toxicol. 2011;11:308–315. doi: 10.1007/s12012-011-9120-4. [DOI] [PubMed] [Google Scholar]
- 37.Tetik S, Kilic A, Aksoy H, Rizaner N, Ahmad S, Yardimci T. Oxidative stress causes plasma protein modification. Indian J Exp Biol. 2015;53:25–30. [PubMed] [Google Scholar]
- 38.Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004;26:43–53. doi: 10.1081/ceh-120027330. [DOI] [PubMed] [Google Scholar]
- 39.De Mattia G, Laurenti O, Fava D. Diabetic endothelial dysfunction: effect of free radical scavenging in Type 2 diabetic patients. J Diabetes Complications. 2003;17:30–35. doi: 10.1016/s1056-8727(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Zhang A, Jiang B, Bao Y, Wang J, An L. Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa. Phytomedicine. 2008;15:484–490. doi: 10.1016/j.phymed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Chen Z, Xu F, Zhu C, Fang F, Shu S, Li M, Ling C. Radio-protective effect of catalpol in cultured cells and mice. J Radiat Res. 2013;54:76–82. doi: 10.1093/jrr/rrs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Cui Y, Ji L, Zhang W, Li R, Ma L, Xing W, Zhou H, Chen B, Yu J, Zhang H. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm Biol. 2013;51:463–473. doi: 10.3109/13880209.2012.740052. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan M, Aviram M, Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther. 2012;136:175–185. doi: 10.1016/j.pharmthera.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Khismatullin DB. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS One. 2015;10:e0123088. doi: 10.1371/journal.pone.0123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hazman O, Ovali S. Investigation of the anti-inflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Inflammation. 2015;38:1012–1019. doi: 10.1007/s10753-014-0065-1. [DOI] [PubMed] [Google Scholar]


