Abstract
Background: Mesenchymal stromal cells (MSCs) derived extracellular vesicles (EVs) were regarded as a potent medium for kidney injury repair and angiogenesis were regarded as an important step in tissue regeneration. However, the pro-angiogenesis effect of MSC-EVs in ischemia-reperfusion induced kidney injury and its potential mechanisms have yet to be determined. Methods: EVs were isolated from the medium of human umbilical cord-derived MSCs (huMSCs) were injected in rats intravenously after unilateral kidney ischemia. Animals were sacrificed at 24 h and 2 weeks after injury. The renal functions and histology staining were examined to assess the therapeutic effect of the EVs. Moreover, we investigated the pro-angiogenesis effects of EVs in injured kidneys and tested the angiogenesis-related factors to further illuminate the probable mechanisms. Results: It was observed that EVs could reduce cell apoptosis and enhances proliferation 24 h after kidney injury, meanwhile renal function was improved and the histological lesion was mitigated. Moreover, renal VEGF was up-regulated by EVs and HIF-1α was down-regulated. Further, the increase of capillary vessel density and reduce of renal fibrosis was observed after 2 weeks. In vitro, EVs could deliver human VEGF directly to renal tubular epithelial cells (TECs) and increase VEGF levels. Most important, all the beneficial effects of EVs were abrogated by RNase treated except for the delivery of human VEGF. Conclusions: Human MSC-EVs could protect against ischemic/reperfusion injury induced kidney injury through pro-angiogenesis effects in HIF-1α independent manner, and both the delivery of pro-angiogenesis related VEGF and RNAs were involved in this process.
Keywords: Mesenchymal stromal cells, angiogenesis, extracellular vesicles, AKI, CKD
Introduction
Ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) in hospitalized patients. The mortality of AKI is high [1] and the endogenous capacity for kidney repair is insufficient to offset the loss of renal cells [2]. Thus, a potent therapeutic intervention for ischemic AKI is imperative. Up to now, many strategies including mesenchymal stromal cells (MSCs) transplantation have been adopted to treat AKI. However, there are still some restrictions, which including the potential immunological rejection and tumorigenesis. Recently, on the basis of paracrine or endocrine mechanism of MSCs, a novel strategy of “cell-free therapy” which use the cell derived extracellular vesicles (EVs) for tissue repair has been used [3,4]. EVs are membranous structures released by various cells, they can transfer bioactive molecular contents including proteins, mRNAs and miRNAs sequences to target injured tissue cells [5,6]. It has been reported that EVs from MSCs could play a protective role in kidney injury [7-9]. However, the mechanism still remains unclear.
Following AKI, complete tissue regeneration results in normal long-term kidney function. In many cases of severe injury, AKI would lead to the incomplete recovery, as for the injury of tubular epithelium and capillary endothelial cells [10]. Thus, peritubular capillary rarefaction is a typical feature of chronic progressive renal disease [11] and methods that restore the renal microvasculature has been regarded as a promising strategy to treat chronic kidney disease (CKD) [12]. In this case, it has been reported that MSCs could play a vascular trophic role in IRI through paracrine manner [13], and vascular endothelial growth factor (VEGF) which were secreted from MSCs were considered as the reason. However, whether the pro-angiogenesis effects of MSC-EVs would participate in the therapy of renal ischemic injury or not still unknown.
In this context, we checked the density of CD31 positive microvascular in the ischemic kidney after two weeks, and further to illuminated the possible mechanisms of MSC-EVs in ischemic kidney injury repair. We hypothesized that MSC-EVs could enhance the capacity of angiogenesis after renal ischemic injury to prevent microvascular rarefaction in the long term thus decrease the risk of CKD development.
Methods
Ethics statement
In this study, all research involving human participants was approved by the institutional review board of the Chinese Academy of Medical Science and this experiment was approved by the Research Ethics Committee at Shanghai Jiao Tong University First People’s Hospital (Permit number: 2013KY001). Human individuals in this study have been given written informed consent to participate in research and allow us to publish the case details. All works involving animals were in accordance with the animal use protocol enacted by the Institutional Animal Care and Use Committees of School of Medicine, Shanghai Jiao Tong University.
Cell culture
After getting written consent, healthy human umbilical cords were collected and stored in cold Hank’s balanced salt solution (Sigma-Aldrich, USA) and then cellular isolation started within 4h. The huMSCs isolation and identification were performed as previously described [14]. After isolated, MSCs were cultured in Low-glucose Dulbecco, Modified Eagle’s medium (DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) at 37°C in a humidified atmosphere with 5% CO2. Only cells from 3 to 6 passages were used for experiments. Rat tubular epithelial cells (TECs, NRK-52E) were obtained from a commercial source (Shanghai Institutes for Biological Sciences, Shanghai, China) and cultured in Low-glucose DMEM containing 5% FBS and 1.5 g/L of sodium bicarbonate.
Extraction and characterization of EVs
EVs were isolated from conditioned medium of huMSCs as described previously [14-16]. Briefly, cells were cultured in medium without fetal bovine serum overnight. The supernatant was collected at the second day. Ultra-centrifugation was carried out at 100,000 g in an SW41 swing rotor (Beckman Coulter Optima L-80K ultracentrifuge; Beckman Coulter, Fulle-rton, CA) for 1 h at 4°C after condition medium (CM) was centrifuged at 2,000 g for 20 minutes to remove debris. EVs were washed once with serum-free M199 containing 25 mM HEPES (PH 7.4) and submitted to a second ultracentrifugation in the same conditions. To quantify the protein content, EVs pellets were suspended in serum-free M199 and estimated by a Bradford assay. After quantified, it’s found that 100 ug EVs could be achieved from about 5×105 cells after serum-free overnight.
Characterization of EVs was performed as previously described [16]. Morphology analysis for EVs was carried out by transmission electron microscopy. Flow cytometry assay was used for determining the surface markers of EVs.
RNAs contained in EVs were degraded by RNase treating as our previously described [17]. Briefly, a portion of EVs were treated with 100 μg/mL RNase (Fermentas, Burlington, ON, Canada) for 3 h at 37°C, and the reaction was stopped by the addition of RNase inhibitor (Fermentas). After ultracentrifugation at 100,000 g for 1 h at 4°C, the EVs were suspended in M199.
Rats AKI model induced by ischemia-reperfusion injury and Hypoxia injury for tubular epithelial cells
8-weeks old male rats (180-200 g) were used. Animals were housed at a constant temperature and humidity, with a 12:12-h light-dark cycle. AKI model was set up as described previously [18]. Briefly, rats were subjected to right nephrectomy after induction of isoflurane anesthesia. Left renal ischemia was induced by non-traumatic vascular clamps over the renal artery for 45 minutes. Reperfusion was established. Shame-treated animals were performed in a similar manner, except that the renal vessels were not clamped. 100 μg EVs (treated with RNase or not) in 1 mL vehicle (M199) or 1 ml vehicle only were administered via caudal vein immediately after reperfusion (n=18 for each group). Rats were sacrificed 24 hour or 2 weeks after injury; blood and left kidney were collected for examination.
Tubular epithelial cells were injured by hypoxia were incubated on chamber slides and exposed to EVs or control medium for 24 hours at 37°C in a humidified atmosphere with 5% CO2.
Detection of EVs in vivo and in-vitro
Tracing of EVs in vivo was conducted as previously described [15]. Briefly, the PKH-26 dye (Sigma) was used to label the EVs. Then, 100 μg of PKH-26 labeled EVs were injected intravenously into AKI rats, and the unlabeled EVs were used as a control. Rats were sacrificed after 24 h and kidneys were acquired for frozen sectioning. The Cytokeratin 19 antibody (Abcam) was used for staining the cytoplasm of tubular epithelial cells; Hoechst 33258 dye (Sigma) was added for nuclear staining.
Detection of EVs fused to TECs was also carried out. Briefly, 100 ug PKH-26 labeled EVs was added into 106 TECs, 24 h later the cells were fixed in 4% paraformaldehyde and the Hoechst 33258 dye (Sigma) was added for nuclear staining.
Histological examinations and renal functions
Part of left kidney was fixed in 4% paraformaldehyde, then dehydrated in ethanol and embedded in paraffin. Kidney tissue blocks were cut into 4μm sections and subjected to Periodic Acid-Schiff (PAS) staining. The sections were viewed by light microscopy. The score was given based on the grading of tubular necrosis, loss of brush border, cast formation and tubular dilatation in 10 randomly chosen. The histological scoring was assessed by the following criteria and in a blind fashion: 0, none; 1, 0-10%; 2, 11-25%; 3, 26-45%; 4, 46-75% and 5, 76-100%.
Renal fibrosis was evaluated by Masson’s trichromatic staining. The degree of interstitial fibrosis was scored semi-quantitatively on a 0 to 3 scale (0, no lesion; 1, <33% of parenchyma affected by the lesion; 2, 33% to 67% of parenchyma affected by the lesion; 3, >67% of parenchyma affected by the lesion). The scores were assessed by a blinded observer in 20 HPFs (magnification 400×) of parenchyma for each rat (n=6 rats, each group). The total score was obtained by the addition of all scores, with a maximum score of 300.
Immunohistochemistry was performed as previously described [14]. The primary antibodies used were rabbit antibody to rat HIF-1α (dilution 1:50; CST), rabbit antibody to rat Ki67 (dilution 1:500; Abcam). For analysis of vascular density, CD 31 antibody (dilution 1:1,000; Abcam) was used. Then, the vascular structures were counted under microscope by a blinded observer; 10 random fields in each section were counted. The vascular density was expressed as vessels/field. The vessel density was assessed by counting CD31-positive vessels in each HPF (magnification 200×).
Renal cell apoptosis was assessed by TUNEL staining (Roche Diagnostics, Mannheim, Germany) for different groups. Numbers of TUNEL-positive tubular cells were quantified by counting 10 randomly chosen, non-overlapping fields per slide.
The blood urea nitrogen (BUN) and serum creatinine (SCr) levels were determined by a Biochemistry Auto-analyzer (Olympus, Tokyo, Japan).
Western blot analysis
Western blot was performed similarly to that described previously [15]. The primary antibodies used were rabbit to rat VEGF (dilution 1:200; Bio world), rabbit antibody to rat HIF-1α (dilution 1:1,000; Abcam) or a GAPDH antibody. The secondary antibodies were chicken anti-rabbit antibody (#sc-2953, Santa Cruz) (dilution 1:10000). The density of each band was analyzed by Image-Pro Plus 6.0 software.
Quantitative real-time PCR
Total RNAs from kidney tissues was isolated using Trizol (Invitrogen). Expression levels of mRNAs were quantified in total RNA using real-time PCR with Taqman chemistry (Applied Biosystems, Carlsbad, CA, USA) as described previously [18]. 18 S rRNAs were used as an internal normalizer for mRNAs. Real-time PCR was carried out by using the following primers: VHL, 5-GTACGTTCGCGTCGTTTAC, 3-TTCACAAAACGTAAAACCGA; PHD2, 5-CTGGGACGCCAAGGTGA, 3-CAATGTCAGCAAACTGG, 18 S, 5-GAGGATGAGGTGGAACGTGT, 3-AGAAGTGACGCAGCCCTCTA; One-step qPCR method was used. Each reaction was performed in triplicate in clear 384-well plates at 48°C, 30 min.; 95°C, 10 min.; then 95°C, 15 sec., and 60°C, 1 min., for 40 cycles. Ct numbers (the number of cycles at which fluorescent signals reached a detection threshold that was set within the exponential phase of PCR) were used to calculate the expression levels of genes of interest normalized to endogenous cellular 18S rRNA.
The detection of VEGF levels and delivery of human VEGF in vitro
VEGF expression was evaluated in huMSCs and injured renal tubular epithelial cells by immunofluorescence staining. The slides with incubated cells were fixed in 4% paraformaldehyde and permeabilized with HEPES-250 Triton ×100 buffer (Sigma, St. Louis, MO, USA). Indirect immunofluorescence staining was carried out using rabbit to rat VEGF (dilution 1:200; Bio world), mouse to human VEGF (not reacts with rats; dilution 1:200; Abcam) in blocking buffer at 4°C overnight followed by fluorescein isothiocyanate-conjugated secondary antibody (ZhongShan JinQiao, Beijing, China) at room temperature for 1 hour. Hoechst 33258 dye (Sigma) was added for nuclear staining. The fluorescence was detected by microscope (TE2000, Nikon, Japan) in dark.
Statistical analysis
All the data were expressed as means ± SD. Statistical analysis was performed using SPSS software (Ver. 18.0, Chicago, IL, USA). Statistical significance was assessed by one-way analysis of variance (ANOVA). A value of P<0.05 was considered to be statistically significant.
Results
Characterization of MSC-EVs
EVs were isolated from huMSCs and characterized as our previous described [16]. Purified EVs showed a homogenous pattern of spheroid particles. FACS analysis showed EVs were positive for some surface-expressed molecules typically expressed by huMSCs, such as CD9, CD44, CD63 and CD73 and negative for CD34 and CD45 [15]. All MSC-EVs showed a size ranging from 150 to 350 nm and mean size is 211.4 ± 61.7 nm, which was confirmed by Nanosight analysis (Date not show).
Tubular injury was mitigated and renal functions were improved at 24 h after reperfusion by MSC-EVs. Moreover, PKH26-labeled EVs were found in the ischemic-injured kidney
The effects of MSC-EVs in an experimental model of acute renal IRI (removal of the right kidney and occlusion of the left renal pedicle for 45 min) were evaluated. Renal IRI could lead to tubular necrosis, tubular dilatation, and cast formation. In comparison with sham animals, rats subjected to kidney IRI were showed severe tissue lesions and morphological changes at 24 h after injury. When rats were treated with MSC-EVs, significant decreases of tubular lesions were found. However, the therapeutic effects were abolished in RNase-treated EVs group (Figure 1A). The statistics of injury scoring showed the similar outcomes (Figure 1B). PKH26-labeled EVs (red fluorescence) were administrated intravenously, red fluorescence was detected in kidney tissues of AKI rats after 24 hours, while unlabeled EVs could not be detected. The cytoplasm of tubular epithelial cells was stained with cytokeratin (green) and the cell nucleuses were stained blue (Figure 1C). What is more, serum creatinine and blood urea nitrogen were increased after reperfusion and could be reduced by EVs treatment. Yet RNase treated EVs lost these functions (Figure 1D and 1E).
Figure 1.
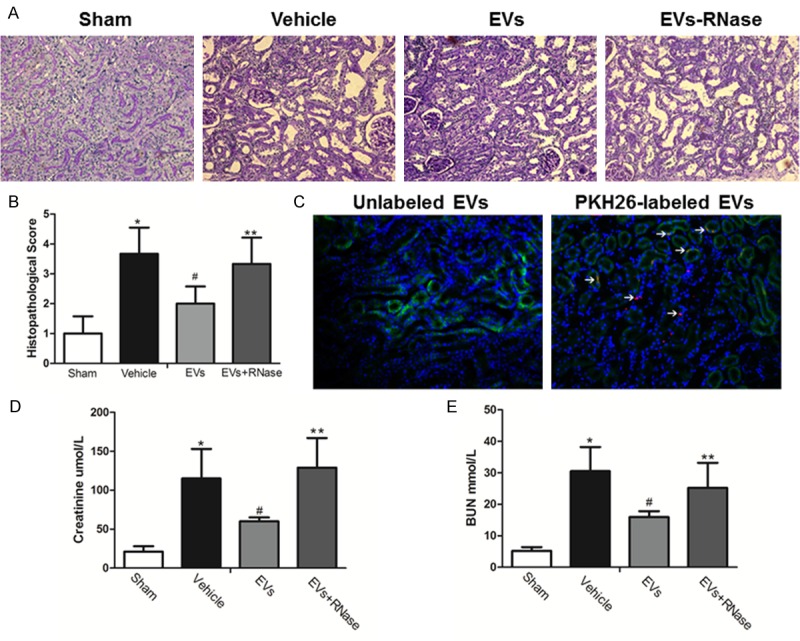
MSCs-EVs entered ischemia-reperfusion injured renal tissues and restored tubular injury. A. Representative renal sections from Sham, Vehicle, EVs and EVs-RNase groups (PAS Original magnification ×200); B. Renal injury scoring and quantitative analysis. (Data are expressed as mean ± SD of 10 different mice for each experimental condition. *P<0.05, versus Sham group, #P<0.05, versus Vehicle group, **P<0.05, versus EVs group). C. PKH26-labeled EVs were detected in kidney tissues 24 h after injection. Tubular epithelial cell cytoplasm and nuclei were stained green (cytokeratin) and blue respectively. Original magnification ×200.D, E. Serum creatinine and blood urea nitrogen levels from Sham, Vehicle, EVs and EVs-RNase groups. (Data are expressed as mean ± SD of 10 different mice for each experimental condition *P<0.01, versus Sham group. #P<0.05, versus Vehicle group, **P<0.05, versus EVs group).
MSC-EVs mitigated the apoptosis and enhanced the proliferation of renal cells in early stage
As shown in Figure 2A, the proliferation, and apoptosis of renal tubular epithelial cells were reflected by Ki67 and TUNEL staining. The number of TUNEL-positive cells in the vehicle, EVs and RNase-EVs groups increased significantly at 24 h after IRI compared to the Sham group, while the kidneys of MSC-EVs treated rats exhibited fewer TUNEL-positive cells than the Vehicle group. There is no difference between RNase-EVs and Vehicle groups. The number of Ki67-positive cells in Vehicle, EVs and RNase-EVs groups increased significantly at 24 h after IRI compared to the Sham group. Meanwhile, the number of proliferating cells in MSC-EVs groups much higher than the Vehicle group. There is no difference between RNase-EVs and Vehicle groups (Figure 2B and 2C).
Figure 2.
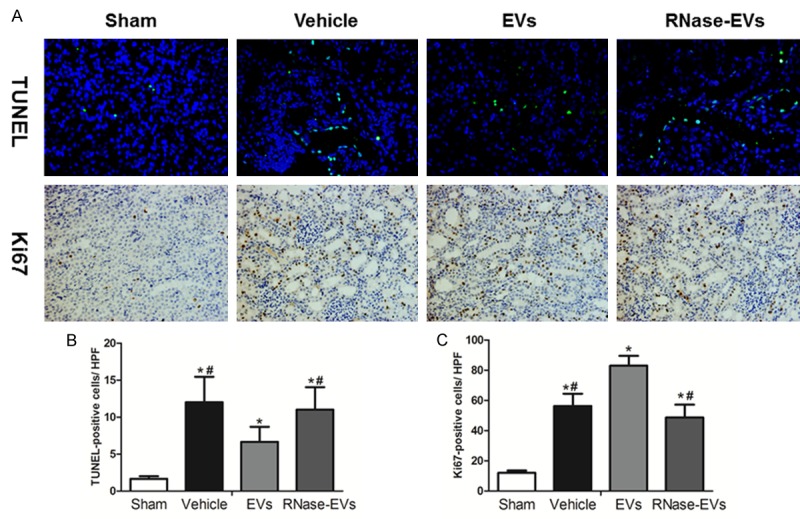
MSCs-EVs enhanced cell proliferation and reduced apoptosis at 24 h after reperfusion. A. Representative micrograph of TUNEL staining (×200) and Ki67 staining (IHC, ×200) from Sham, Vehicle, EVs and EVs-RNase groups. B, C. Quantitative analysis of tubular cells apoptosis and proliferation. (Data are expressed as mean ± SD of 10 different mice for each experimental condition. *P<0.05, versus Sham group, #P<0.05, versus EVs group).
MSC-EVs elevated VEGF level in kidney tissues independent of HIF-1α pathway
As shown in Figure 3, the suppression effect of MSC-EVs on HIF-1α was observed by IHC staining and western blot analysis. The HIF-1α protein in the injured kidney was up-regulated compared with the Sham group, and the down-regulation of HIF-1α was observed after MSC-EVs treatment. There is no difference between RNase-EVs and Vehicle groups, and there has a similar observation with IHC results. In contrast, VEGF, the downstream transcriptional factor of HIF-1α, was increased in EVs group compared with the Vehicle. What is more, RNase treated EVs could not increase VEGF or decrease HIF-1α in the injured kidney (Figure 3C). After examining some regulators of HIF-1α, we found that mRNA expression of von Hippel-Lindau (VHL) and hydroxylases domain (PHD2), which were negative regulators of HIF-1α, were suppressed by EVs under hypoxia condition. RNase treating could also abrogate the effects mentioned above (Figure 3D).
Figure 3.
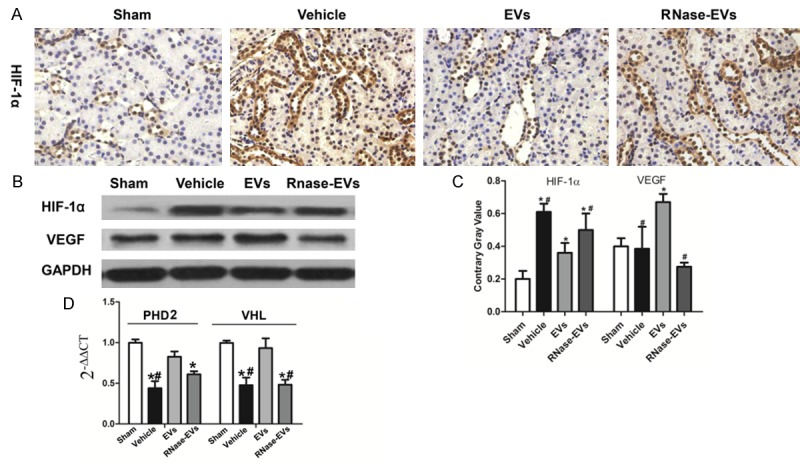
VEGF was up-regulated by MSC-EVs and HIF-1α was downregulated. A. Representative micrograph of HIF-1α staining (IHC, ×200) from Sham, Vehicle, EVs and EVs-RNase groups. B. Protein levels of HIF-1α and VEGF in renal tissues from different groups. C. Quantitative analysis of HIF-1α and VEGF expression level in renal tissues from different groups. D. Gene expression of prolyl hydroxylases domain (PHD2) and von Hippel-Lindau (VHL) in kidneys. (Experiments are performed in triplicate. *P<0.05, versus Sham group, #P<0.05, versus EVs group).
Enhanced renal angiogenesis and mitigated fibrosis were found in MSC-EVs treated rats 2 weeks after IRI
Renal angiogenesis was determined by CD31 staining, the biomarker of endothelial cells. As shown in Figure 4A, it can be seen that there are more vessels in Vehicle, EVs and RNase-EVs group than the Sham group at two weeks post injury. By semiquantitative assay, blood capillary density in EVs group is significant higher than vehicle groups, which indicates the enhanced angiogenesis effects of EVs in injured kidney and there is no difference between RNase-EVs and Vehicle groups (Figure 4B). What is more, the exposure to IRI led to the development of significant fibrotic lesions in the renal interstitial area, as indicated by Masson’s trichrome staining. After MSC-EVs’ administration, the fibrosis was mitigated significantly compared to the vehicle. In line with this, α-SMA expression on tubular cells, and an indicator of epithelial-mesenchymal transition (EMT), occurred mostly in Vehicle treated animals and slightly in MSC-EVs infusion rats. All these beneficial effects were abrogated after RNase treated (Figure 4C).
Figure 4.
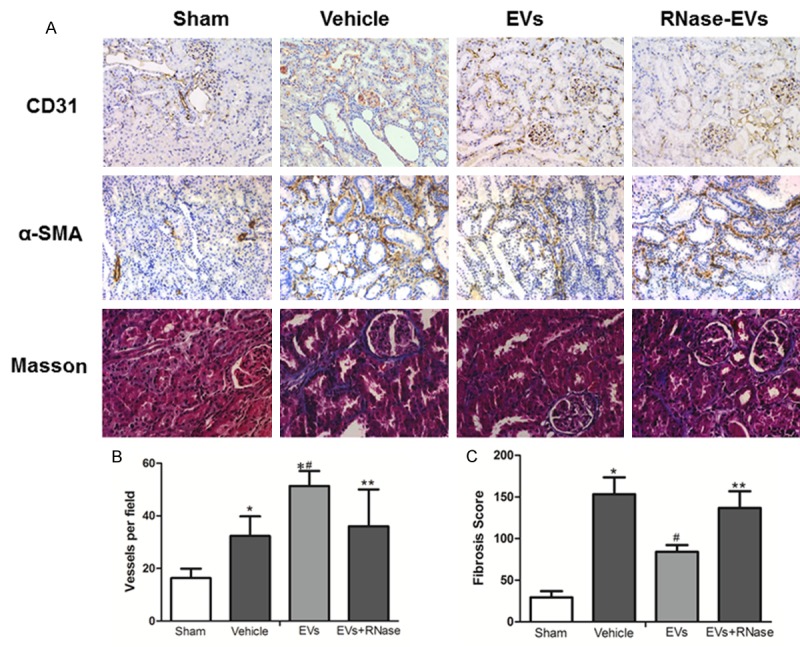
Enhanced angiogenesis and reduced renal fibrosis were found after 2 weeks of MSC-EVs’ administration. A. Representative micrograph of CD31 staining (IHC, ×200), α-SMA staining (IHC, ×200) and Masson’s staining (×400) from Sham, Vehicle, EVs and EVs-RNase groups. B, C. Quantitative analysis of renal vessel density and fibrosis score of different groups. (Data are expressed as mean + SD of 10 different mice for each experimental condition *P<0.05, versus Sham group, #P<0.05, versus Vehicle group, **P<0.05, versus EVs group).
huMSC derived EVs fused and deliver human VEGF directly to renal tubular epithelial cells (TECs), and increased VEGF expression in vitro
As was shown in Figure 5A. Human MSCs exists a strong expression of human VEGF and there is no human VEGF expression in rat TECs under hypoxia conditions. However, human VEGF emerged when administrated with EVs after 24 hours. Interestingly, after RNase-treated the human VEGF still can be delivered to TECs. This indicated that huMSC-EVs could deliver VEGF protein directly to rat TECs and this process is not reliable RNAs. Meanwhile, the PHK26 labeled EVs were found fused to TECs after 24 hours (Figure 5B). Further, we found that VEGF level in TECs was elevated with the administration of MSC-EVs and this effect was abolished after RNase treated (Figure 5C and 5D).
Figure 5.
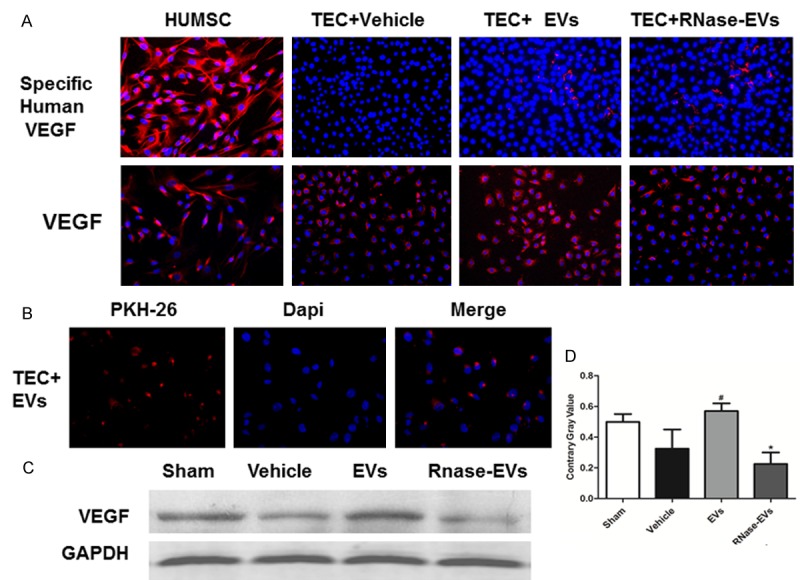
MSC-EVs which fused to TECs could deliver human VEGF to rat TECs and elevate TEC VEGF level in vitro. A. Representative micrograph of specific human VEGF staining and total VEGF staining (Immunofluorescence ×200) in huMSCs and TECs. B. PKH26 labeled EVs were found fusing to TECs after EVs treated. C. Western-Blot analysis of total VEGF in TECs from different groups. D. Quantitative analysis of total VEGF expression level in TECs from different groups. (Experiments are performed in triplicate. *P<0.05, versus Sham group, #P<0.05, versus Vehicle group).
Discussion
AKI in clinical patients have a high mortality and recent therapeutic options are limited, so the development of new potential regenerative approaches is highly desirable. In the present study, we observed that a single administration of human MSC-EVs could alleviate renal IRI induced AKI and mitigate renal fibrosis in later stage. Meanwhile, the increase of capillary vessel density and the up-regulation of vasculotrophic cytokine VEGF were demonstrated, and the down-regulation of HIF-1α was observed in this process. What’s more, all these effects were abrogated by RNase except for the delivery of human VEGF.
In recent years, mesenchymal stromal cell transplantation has been regarded as a promising strategy for various types of tissue injury including kidney IRI [18,19]. However, there were still limitations for MSCs transplantation, such as immune-mediated rejection, senescence-induced genetic instability or loss of function, which limited cell survival and malignancy transforming [3]. Therefore, “cell-free therapy” which uses extracellular vesicles from MSCs has been suggested basic to endocrine or paracrine mechanisms. In the present study, we demonstrated the therapeutic potential of MSC-EVs for AKI in an early stage which is in line with some previous studies [7,8,20]. What is more, the renal restoration reflected by enhanced angiogenesis and reduced fibrosis after 2 weeks by MSC-EVs. Moreover, the angiogenesis of ischemic kidney after reperfusion was also observed after 2 weeks. Under the IRI conditions, the expression of VEGF in the injured kidney is variation under the different time after reperfusion and the decrease of microvasculature in injured kidney was observed in the long term [21,22]. Some CD31 positive microvascular is immature and may increase temporary following the ischemic kidney injury. The sustained change of some molecular signals following AKI is necessary to CKD progression has also been reported [23]. MSC-EVs treatment could enhance angiogenesis effects after 2 weeks may help sustain capillary density to prevent microvascular rarefaction thus decrease the risk of CKD developing. However, as for the limited observed time point here, we cannot fully testify this hypothesis in this study and more detailed should be addressed in future.
By tracing PKH-26 labeled EVs, it suggested that administrated EVs could locate in injured kidney and play a role. Actually, the mechanism for the reno-protective role of MSC-EVs has been studied extensively [5,24,25]. Huang et.al found the majority of nucleic acids contained in EVs of plasma is microRNA by deep sequencing, and RNase could catalyze all RNAs [26]. Also, Eirin et. al demonstrated EVs released by adipose MSCs contained miRNAs and mRNAs related to angiogenesis and other pathway modulation [27]. In addition, it has been also reported that AKI recovery by MSC-EVs was attributed to RNAs that contained in the EVs [8,9]. In our study, RNase treating could abrogate the therapeutic potential of MSC-EVs, which implies that RNAs contained in EVs played a major role.
As focused on the angiogenesis as well as reduced fibrosis which may be subsequence of kidney repair in AKI by EVs, VEGF expression was evaluated. It was found that VEGF was upregulated after MSC-EVs treated. At odds, it is interesting that HIF-1α was down-regulated by EVs, which means the elevation of VEGF was independent of the increase of HIF-1α. The HIF-1α regulators expression analysis showed that EVs could suppress von Hippel-Lindau (VHL) and hydroxylases domain (PHD2). We speculate that the reason might be MSC-EVs changed the hypoxia micro-environment and modulated the HIF-1α regulators. HIF-1α is common regarded as the upstream transcriptional factor for VEGF. So other pathways were involved in the modulation of VEGF or MSC-EVs could directly modulate the VEGF levels [28,29]. In a previous study of our lab, we suggested that heterogeneous mRNA might be transferred by EVs to injured renal cells and transcript to corresponding protein [17]. As the abrogation of RNAs by RNase in this study, we found EVs lost their functions for evaluating VEGF level, which also supported the RNA-dependent regulation roles in tissue repair.
To confirm the VEGF induction, we investigated rat renal tubular epithelial cells (TECs) under hypoxia injury model in vitro. As it was observed that PKH-26 labeled EVs fused to TECs, it’s believed that EVs entered target cells and delivered their contents after administrated. Moreover, the up-regulation of VEGF by EVs was found and the effects were abrogated by RNase as well, which was in accordance with the outcome in vivo. Intriguingly, the most notable observation is that human VEGF emerged after human MSC-EVs treated and RNase could not exterminate this, which implied that heterogeneous VEGF induction was not depending on RNA transferring. It was known that EVs membranous structures containing RNA, DNA and protein, so we believe the emerged VEGF might be delivered by EVs directly. Even though, there was not obvious therapeutic effects was seen after RNA erasing in the present study. It doesn’t mean the protein cargo cannot take effects at all. There has been reported the transfer of functional proteins between different cells through EVs to regulate cell biological activity [30]. In the present study, the heterogeneous human VEGF proteins were transferred to the rat TECs directly and this effect was not depending on the RNA. Moreover, the protective effects of EVs were abolished after the RNase treated. In this case, we preferred to believe that human proteins may have limited functions in heterogeneous rat cells and the amounts of functional proteins are also limited. However, it would be more meaningful if we use human MSC-EVs as the vehicle to transfer bioactive proteins in human clinical treatment in future. Thus, RNA transferring was attributed as the main mechanism of pro-angiogenesis effects in human MSC-EVs treated rat and human MSC-EVs increase VEGF levels in ischemic kidney through a HIF-1α independent manner. Moreover, human MSC-EVs also could transfer the human VEGF proteins directly to rat cells.
In summary, MSC-EVs exerted strikingly beneficial effects on recovery of IRI induced AKI and it could promote angiogenesis by inducing VEGF elevation through HIF-1α independent manner. It was also proved that both RNA delivery and protein cargo involved in the modulation of VEGF. This work provides a new insight into mechanisms of MSC-EVs’ bio-function, which may also pave the way for clinical AKI therapy for EVs.
Acknowledgements
This study is supported by grants from the National Key Research and Development Program of China (2016YFC1101400), the National Natural Science Foundation of China (81170642, 81470919 and 81670632) and Health and Family Planning Commission of Jiangsu Province Foundation (Z201609).
Disclosure of conflict of interest
None.
Authors’ contribution
Conceived and designed the experiments: XYZ, YJZ and GYZ. Performed the experiments: XYZ, DG, ZLC, GYZ, XYX and DLG. Analyzed the data: XYZ and GYZ. Wrote the paper: XYZ. All authors read and approved the final manuscript.
References
- 1.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 2.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG University of Toronto Acute Kidney Injury Research Group. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 3.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 6.Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 8.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol. 2015;26:2349–60. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long DA, Norman JT, Fine LG. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 13.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of Micro-vesicles derived from human WhartonJelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9:e92129. doi: 10.1371/journal.pone.0092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Ju GQ, Du T, Zhu YJ, Liu GH. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8:e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY, Gu D, Miao S, Zhu YJ, Sun J, Du T. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One. 2015;10:e0121534. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du T, Cheng J, Zhong L, Zhao XF, Zhu J, Zhu YJ, Liu GH. The alleviation of acute and chronic kidney injury by human Wharton’s jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy. 2012;14:1215–1227. doi: 10.3109/14653249.2012.711471. [DOI] [PubMed] [Google Scholar]
- 19.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33:1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294:F928–936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 22.Logue OC, McGowan JW, George EM, Bidwell GL 3rd. Therapeutic angiogenesis by vascular endothelial growth factor supplementation for treatment of renal disease. Curr Opin Nephrol Hypertens. 2016;25:404–9. doi: 10.1097/MNH.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. J Am Soc Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno S, Camussi G. Exploring mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Methods Mol Biol. 2014;1213:139–145. doi: 10.1007/978-1-4939-1453-1_12. [DOI] [PubMed] [Google Scholar]
- 25.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novotny NM, Markel TA, Crisostomo PR, Meldrum DR. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NFkB. Cytokine. 2008;43:215–219. doi: 10.1016/j.cyto.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 2011;6:e29055. doi: 10.1371/journal.pone.0029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]


