Abstract
Protein tyrosine kinase 6 (PTK6) is a nonreceptor tyrosine kinase that plays a crucial role in some tumors. However, the role of PTK6 is still unknown in hepatocellular carcinoma (HCC). In this study, we demonstrated that the PTK6 expression was upregulated in HCC tissues compared with adjacent normal tissues. Moreover, PTK6 was upregulated in the HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) compared with the normal liver epithelial cell line (THLE3). Ectopic expression of PTK6 promoted SMMC7721 cell proliferation, colony formation and invasion. Moreover, inhibition PTK6 expression suppressed the SMMC7721 cell proliferation, colony formation and invasion. Overexpression of PTK6 suppressed ERK1/2 phosphorylated expression. These data suggested that PTK6 played an oncogene role in the development of HCC.
Keywords: Hepatocellular carcinoma, PTK6, ERK1/2
Introduction
Hepatocellular carcinoma (HCC) ranks as the third most common cause for cancer-related death worldwide [1-4]. Numerous therapeutic approaches including early detection, surgery and chemotherapy have been made; how-ever, the 5-year survival rate is still about 30% [5-9]. Although a lot of epigenetic and genetic changes have been found in HCC, the detailed molecular pathogenesis about HCC is still not fully elucidated [10-13]. Therefore, it is critical to identify novel diagnosis and therapeutic targets for HCC.
Protein tyrosine kinase 6 (PTK6) (also named as breast tumor kinase) is first found in the metastatic breast tumor patient and human melanocytes [14,15]. Increasing studies have demonstrated that PTK6 expression was upregulated in many tumors such as breast cancer, non-small cell lung cancer and prostate cancer [16-19]. PTK6 played an oncogene role in tumor development and PTK6 promoted tumor cell proliferation, invasion and migration [20,21]. Previous studies also showed that PTK6 interacted with ErbB members such as ErbR2, EGFR, ErbR4 and ErbR3 and promoted ERK1/2 activation [21-24]. However, the expression and role of PTK6 are still unknown.
In this study, we demonstrated that PTK6 expression was higher in HCC tissues than in adjacent normal tissues. Ectopic expression of PTK6 promoted the SMMC7721 cell proliferation, colony formation and invasion.
Materials and methods
Patients and tissue samples
Patient samples were collected at our department following written patient consent. This study was approved by the ethics committee of the Fourth Affiliatted Hospital of Harbin Medical University. Tissues from HCC and adjacent normal tissues were collected from HCC patients during surgery. None of these patients were received radiation therapy or chemotherapy before surgery.
Cell culture and transfection
One normal liver epithelial cell line (THLE3) and four HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) were purchased from ATCC (American Type Culture Collection, Mannasas, USA) and kept in DMEM (Dulbecco’s modified Eagle’s medium) (Invitrogen, Carlsbad, USA) supplemented with 10% FBS (fetal bovine serum) (Invitrogen, USA). PTK6 siRNA and control siRNA were purchased from Genepharma (Shanghai, China). Cell tranfection was performed using LipofectamineTM 2000 (Invitrogen, USA) according to the instruction.
Cell proliferation and colony formation
Cells were cultured in the 96-well plates after transfection. The MTT analysis was performed to determine cell proliferation. The absorbance at 490 nm was detected using the microtiter plate reader (Bio-Rad, CA). 5 × 103 cells were cultured in the 6 well plates for two weeks. The colonies were fixed and stained with crystal violet (Sigma, USA) and counted.
Western blot analysis
Protein was extracted from cells or tissues using RIPA lysis buffer. Total protein was separated using 12% SDS-PAGE and transferred to the PVDF membranes membrane (Millipore, Germany). The membrane was blocked with non-fat dried milk and then incubated with primary antibodies: PTK6, and GAPDH (Abcam, USA). Proteins were measured using enhanced chemiluminescence (PerkinElmer).
Quantitative reverse transcriptase PCR (qRT-PCR) analysis
Total RNA was collected from cells or tissues by TRIzol (Invitrogen, USA) following to the instruction. qRT-PCR assay was performed to measure the PTK6 and GAPDH expression. GAPDH was used as internal control. The special primers were used follows: PTK6 forward, 5’-GCTATGTGCCCCACAACTACC-3’ and reverse, 5’-CCTGCAGAGCGTGAACTCC-3’; GAPDH forward, 5’-TCAACGACCACTTTGTCAAGCTCAGCT-3’ and reverse, 5’-GGTGGTCCAGGGGTCTTAC-3’.
Migration and invasion assays
Wound healing assay was performed to detect the cell migration. The cells were cutured in the six-well plates and a wound was generated using a pipette tip. Cells were cultured for 48 hours and woud was photographed under the microscope (Olympus, Japan). Transwell assay was conducted to determine the cell invasion. Cells resuspended in no-serum medium and were cultured on the upper surface chamber coated with Matrigel. FBS medium was put to the lower filter. After 24 hours, the cells attached to lower chamber were counted.
Statistical analysis
Statistical analyses were done using the SPSS software and data was shown as mean ± (SD) standard deviation. Statistical difference about two groups was calculated using an student’s t-test and differences between more than groups. P≤0.05 was considered to statistically significant difference.
Result
PTK6 expression was upregulated in Hepatocellular carcinoma (HCC) tissues
Our data showed that the expression level of PTK6 was higher in the HCC tissues than in these adjacent normal tissues (Figure 1A). In addition, increased PTK6 expression in HCC tissues was found in 24 of the 35 cases (Figure 1B). In addition, we observed that the protein expression of PTK6 was upregulated in the HCC tissues compared with adjacent normal tissues (Figure 1C).
Figure 1.
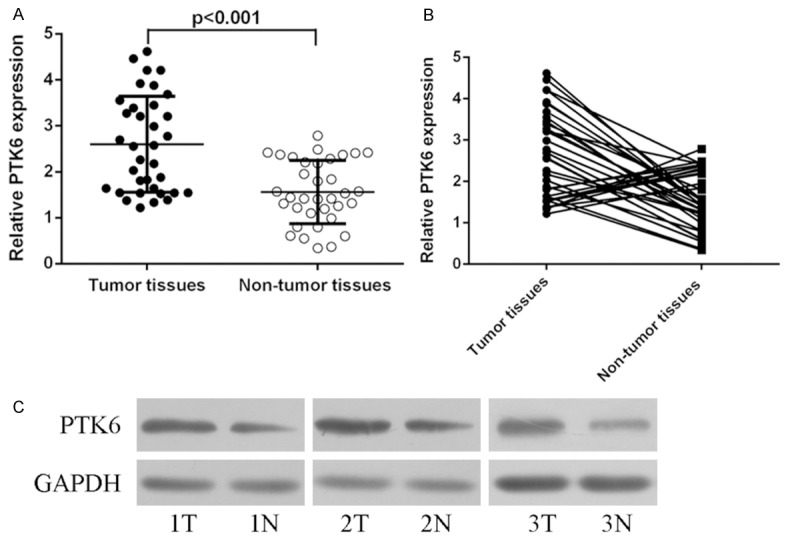
The expression of PTK6 was upregulated in Hepatocellular carcinoma (HCC) tissues. A. qRT-PCR analysis was performed to measure the expression of PTK6 in the HCC tissues and adjacent normal tissues. B. The increased PTK6 expression in HCC tissues was found in 24 of the 35 cases. C. The protein expression of PTK6 was detected in the HCC tissues and adjacent normal tissues using western blot.
PTK6 expression was upregulated in HCC cells lines
The mRNA expression of PTK6 was upregulated in HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) compared with the normal liver epithelial cell line (THLE3) (Figure 2A). The PTK6 protein expression was also upreguated in HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) compared with the normal liver epithelial cell line (THLE3) (Figure 2B).
Figure 2.
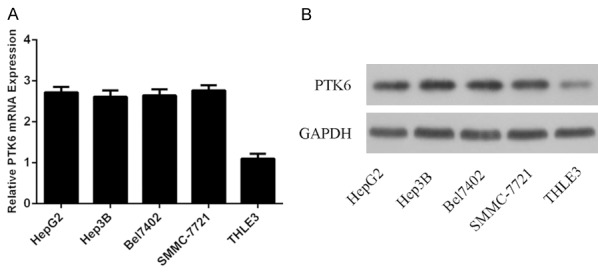
PTK6 expression was also upregulated in HCC cells lines. A. The mRNA expression of PTK6 in the HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) and the normal liver epithelial cell line (THLE3) was measured using qRT-PCR. B. The protein expression of PTK6 in the HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) and THLE3 was measured using western blot.
Ectopic expression of PTK6 promoted HCC cell proliferation, colony formation and invasion
The PTK6 expression was upregulated in the SMMC7721 cell after transfected with pcDNA-PTK6 (Figure 3A and 3B). CCK8 analysis demonstrated that overexpression of PTK6 promoted SMMC7721 cell proliferation (Figure 3C). Ectopic expression of PTK6 promoted SMMC7721 cell colony formation (Figure 3D). Overexpression of PTK6 increased SMMC7721 cell invasion (Figure 3E).
Figure 3.
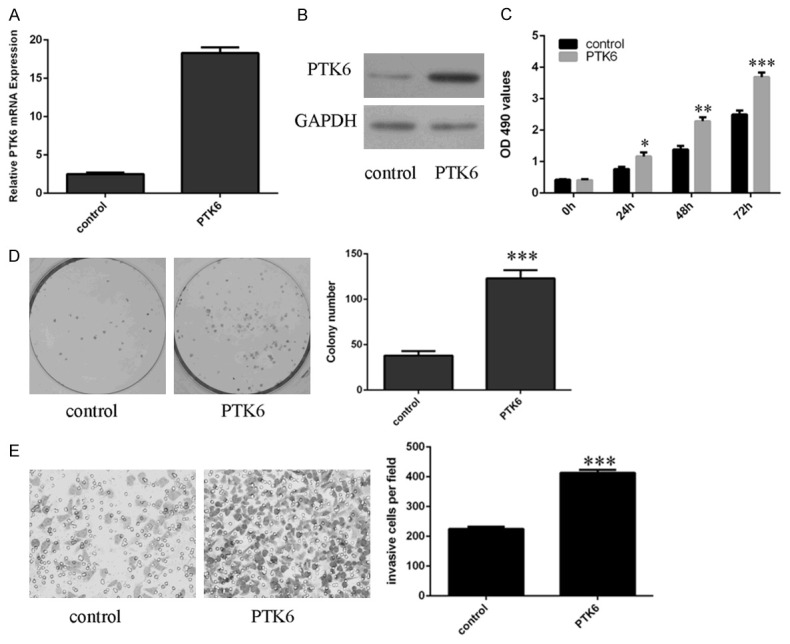
Ecpotic expression of PTK6 promoted HCC cell proliferation, colony formation and invasion. A. The mRNA expression of PTK6 in the SMMC7721 cell was determined by qRT-PCR. B. The protein expression of PTK6 in the SMMC7721 cell was measured by western blot. C. CCK8 analysis was performed to measure the cell proliferation. D. Ecpotic expression of PTK6 promoted SMMC7721 cell colony formation. The relative colony number was shown in the right. E. Ecpotic expression of PTK6 promoted SMMC7721 cell invasion. The relative invasive cell number was shown in the right. *p<0.05, **p<0.01 and ***p<0.001.
Inhibition expression of PTK6 suppressed HCC cell proliferation, colony formation and invasion
We measured the effect of PTK6 inhibition on proliferation, colony formation and invasion. PTK6 expression was downregulated in the SMMC7721 cell after transfected with si-PTK6 (Figure 4A and 4B). CCK8 analysis demonstrated that inhibition of PTK6 suppressed SMMC7721 cell proliferation (Figure 4C). Inhibited expression of PTK6 decreased SMMC7721 cell colony formation (Figure 4D). Suppression of PTK6 decreased SMMC7721 cell invasion (Figure 4E).
Figure 4.
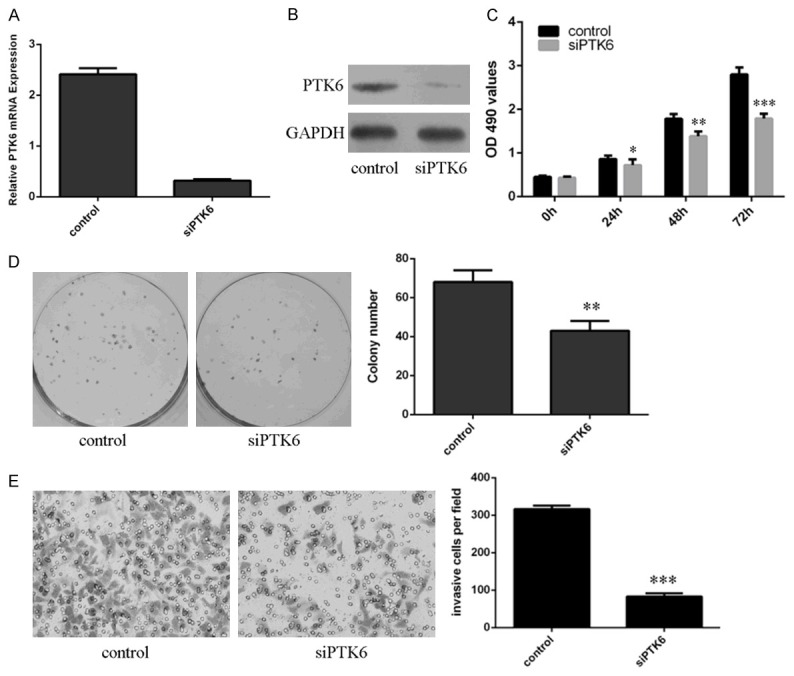
Inhibition expression of PTK6 suppressed HCC cell proliferation, colony formation and invasion. A. The mRNA expression of PTK6 in the SMMC7721 cell was determined by qRT-PCR. B. The protein expression of PTK6 in the SMMC7721 cell was measured by western blot. C. Inhibition of PTK6 expression suppressed the SMMC7721 cell proliferation. D. Inhibition expression of PTK6 suppressed SMMC7721 cell colony formation. The relative colony number was shown in the right. E. Suppression of PTK6 expression inhibited SMMC7721 cell invasion. The relative invasive cell number was shown in the right. *p<0.05, **p<0.01 and ***p<0.001.
Ectopic expression of PTK6 inhibited the ERK1/2 phosphorylated
Overexpression of PTK6 suppressed the ERK1/2 phosphorylated expression in the SMMC7721 cell (Figure 5A). Inhibited expression of PTK6 reduced the ERK1/2 phosphorylated expression in SMMC7721 cell (Figure 5B).
Figure 5.
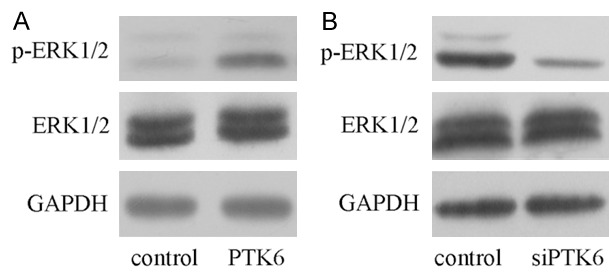
Ecpotic expression of PTK6 inhibited the ERK1/2 phosphorylated. A. The protein expression of ERK1/2 and p-ERK1/2 in the SMMC7721 cell was determined by western blot. B. Inhibition expression of PTK6 inhibited the ERK1/2 phosphorylated expression in the SMMC7721 cell.
Discussion
In this study, we showed that PTK6 expression was upregulated in HCC tissues compared with in these adjacent normal tissues. In addition, increased PTK6 expression in HCC tissues was found in 24 of the 35 cases. Moreover, the expression level of PTK6 was increased in HCC cell lines (Bel7402, Hep3B, SMMC7721 and HepG2) compared with the normal liver epithelial cell line (THLE3). Ectopic expression of PTK6 promoted SMMC7721 cell proliferation, colony formation and invasion. Inhibition of PTK6 suppressed the SMMC7721 cell proliferation, colony formation and invasion. Overexpression of PTK6 suppressed the ERK1/2 phosphorylated expression. These data suggested that PTK6 played an oncogene role in the development of HCC.
Previous studies showed that PTK6 expression was upregulated in the prostate cancer, breast cancer, and ovarian tumor [20,25,26]. For example, Zheng et al [20]. showed that PTK6 overexpression enhanced prostate cancer cell EMT (epithelial-mesenchymal transition) partly through enhancing AKT expression and promoted the cell metastases and migration in vivo. Irie et al [25]. demonstrated that PTK6 expression was upregulated in the Her2+ breast cancer and grade ER+, Luminal B cancers. Downregulation of PTK6 induced ovarian and breast cancer cell apoptosis. Zhao et al [19]. showed that PTK6 expression level was higher expression in non-small cell lung cancer (NSCLC) than in normal pulmonary tissues. Higher PTK6 expression was correlated with shorter OS (overall survival) time. Higher expression of PTK6 was a potential independent prognostic factor for patients with NSCLC. However, Liu et al [27] found that the expression level of PTK6 was lower in laryngeal squamous cell carcinoma (LSCC) tissues than in the adjacent non-tumor tissues. In our study, we found that PTK6 expression was upregulated in the HCC tissues compared with these adjacent normal tissues. In addition, increased PTK6 expression in HCC tissues was found in 24 of the 35 cases. Moreover, the expression level of PTK6 was also increased in HCC cell lines compared to the normal liver epithelial cell line. Overexpression of PTK6 promoted SMMC7721 cell proliferation, colony formation and invasion. Inhibited PTK6 expression suppressed SMMC7721 cell proliferation, colony formation and invasion. To our knowledge, this was the first report investigating the expression and the function of PTK6 in HCC.
Many downstream effector genes such as ErbB family members (ErbR2, EGFR, ErbR3, and ErbR4) and ERK1/2 have been proved to be associated with PTK6 [25,28-30]. PTK6 can activate the STAT and PI3k/Akt pathway [21,31-33]. In addition, more signaling molecules including p190RhoGAP, paxillin and KAP3A also were found in many human tumor cells [34-36]. In our study, we demonstrated that overexpression of PTK6 activated the ERK1/2 phosphorylated and inhibition expression of PTK6 inhibited the ERK1/2 phosphorylated in the SMMC7721 cell. ERK1/2 is a member of the MAPK (mitogen-activated protein kinase) family and plays a regulatory role in cellular proliferation, invasion and migration in many cancers including HCC.
In conclusion, we demonstrated that PTK6 expression was upregulated in HCC tissues and cell lines. Ectopic expression of PTK6 promoted SMMC7721 cell proliferation, colony formation and invasion. Overexpression of PTK6 suppressed the ERK1/2 phosphorylated expression in the SMMC7721 cell. These data suggested that PTK6 played an oncogene role in the development of HCC.
Acknowledgements
This study was supported by Science and technology Research projects of the Education Department of Heilongjiang province (11551301) and grant of Natural science Foundation of Heilongjiang, (H201383) and The Presidential Foundation of the Fourth Affiliated Hospital of Harbin Medical University (HYDSYYZPY201502).
References
- 1.Yang XW, Zhang LJ, Huang XH, Chen LZ, Su Q, Zeng WT, Li W, Wang Q. miR-145 suppresses cell invasion in hepatocellular carcinoma cells: miR-145 targets ADAM17. Hepatol Res. 2014;44:551–559. doi: 10.1111/hepr.12152. [DOI] [PubMed] [Google Scholar]
- 2.Furuta M, Kozaki K, Tanimoto K, Tanaka S, Arii S, Shimamura T, Niida A, Miyano S, Inazawa J. The tumor-suppressive miR-497-195 cluster targets multiple cell-cycle regulators in hepatocellular carcinoma. PLoS One. 2013;8:e60155. doi: 10.1371/journal.pone.0060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung TM, Ho CM, Liu YC, Lee JL, Liao YR, Wu YM, Ho MC, Chen CH, Lai HS, Lee PH. Up-regulation of microRNA-190b plays a role for decreased IGF-1 that induces insulin resistance in human hepatocellular carcinoma. PLoS One. 2014;9:e89446. doi: 10.1371/journal.pone.0089446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SC, Lin XL, Li J, Zhang TT, Wang HY, Shi JW, Yang S, Zhao WT, Xie RY, Wei F, Qin YJ, Chen L, Yang J, Yao KT, Xiao D. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One. 2014;9:e101330. doi: 10.1371/journal.pone.0101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Yan J, Zhou C, Ma Q, Jin Q, Yang Z. miR-1285-3p acts as a potential tumor suppressor miRNA via downregulating JUN expression in hepatocellular carcinoma. Tumour Biol. 2015;36:219–25. doi: 10.1007/s13277-014-2622-5. [DOI] [PubMed] [Google Scholar]
- 6.Yin H, Peng X, Ren P, Cheng B, Li S, Qin C. MicroRNAs as a novel class of diagnostic biomarkers in detection of hepatocellular carcinoma: a meta-analysis. Tumour Biol. 2014;35:12317–26. doi: 10.1007/s13277-014-2544-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Zhao H, Zhao X, Wan J, Wang D, Bi W, Jiang X, Gao Y. Association between an insertion/deletion polymorphism within 3’UTR of SGSM3 and risk of hepatocellular carcinoma. Tumour Biol. 2014;35:295–301. doi: 10.1007/s13277-013-1039-x. [DOI] [PubMed] [Google Scholar]
- 8.Li QJ, Zhou L, Yang F, Wang GX, Zheng H, Wang DS, He Y, Dou KF. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumour Biol. 2012;33:1455–1465. doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zheng D, Xiong Y, Xue C, Chen G, Yan B, Ye Q. miR-202 suppresses cell proliferation in human hepatocellular carcinoma by downregulating LRP6 post-transcriptionally. FEBS Lett. 2014;588:1913–1920. doi: 10.1016/j.febslet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Ohno M, Otsuka M, Kishikawa T, Shibata C, Yoshikawa T, Takata A, Muroyama R, Kowatari N, Sato M, Kato N, Kuroda S, Koike K. Specific delivery of microRNA93 into HBV-replicating hepatocytes downregulates protein expression of liver cancer susceptible gene MICA. Oncotarget. 2014;5:5581–5590. doi: 10.18632/oncotarget.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Z, Zhang C, Cui J, Song Q, Wang L, Kang J, Li P, Hu X, Song H, Yang J, Sun Y. Bioinformatic analysis of the membrane cofactor protein CD46 and microRNA expression in hepatocellular carcinoma. Oncol Rep. 2014;31:557–564. doi: 10.3892/or.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fesler A, Zhai H, Ju J. miR-129 as a novel therapeutic target and biomarker in gastrointestinal cancer. Onco Targets Ther. 2014;7:1481–1485. doi: 10.2147/OTT.S65548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaira V, Roncalli M, Carnaghi C, Faversani A, Maggioni M, Augello C, Rimassa L, Pressiani T, Spagnuolo G, Di Tommaso L, Fagiuoli S, Rota Caremoli E, Barberis M, Labianca R, Santoro A, Bosari S. MicroRNA-425-3p predicts response to sorafenib therapy in patients with hepatocellular carcinoma. Liver Int. 2015;35:1077–86. doi: 10.1111/liv.12636. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–2390. [PubMed] [Google Scholar]
- 15.Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–1065. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Aubele M, Walch AK, Ludyga N, Braselmann H, Atkinson MJ, Luber B, Auer G, Tapio S, Cooke T, Bartlett JM. Prognostic value of protein tyrosine kinase 6 (PTK6) for long-term survival of breast cancer patients. Br J Cancer. 2008;99:1089–1095. doi: 10.1038/sj.bjc.6604660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan Anderson TM, Peacock DL, Daniel AR, Hubbard GK, Lofgren KA, Girard BJ, Schorg A, Hoogewijs D, Wenger RH, Seagroves TN, Lange CA. Breast tumor kinase (Brk/PTK6) is a mediator of hypoxia-associated breast cancer progression. Cancer Res. 2013;73:5810–5820. doi: 10.1158/0008-5472.CAN-13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Tyner AL. Context-specific protein tyrosine kinase 6 (PTK6) signalling in prostate cancer. Eur J Clin Invest. 2013;43:397–404. doi: 10.1111/eci.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Chen Y, Zhang W, Zhang J, Xu Y, Li W, Chen S, Deng A. Expression of protein tyrosine kinase 6 (PTK6) in nonsmall cell lung cancer and their clinical and prognostic significance. Onco Targets Ther. 2013;6:183–188. doi: 10.2147/OTT.S41283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Wang Z, Bie W, Brauer PM, Perez White BE, Li J, Nogueira V, Raychaudhuri P, Hay N, Tonetti DA, Macias V, Kajdacsy-Balla A, Tyner AL. PTK6 activation at the membrane regulates epithelial-mesenchymal transition in prostate cancer. Cancer Res. 2013;73:5426–5437. doi: 10.1158/0008-5472.CAN-13-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono H, Basson MD, Ito H. PTK6 promotes cancer migration and invasion in pancreatic cancer cells dependent on ERK signaling. PLoS One. 2014;9:e96060. doi: 10.1371/journal.pone.0096060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, Hung MC, Fan Z. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012;31:4372–4383. doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuguchi Y, Specht S, Isse K, Sasatomi E, Lunz JG 3rd, Takizawa T, Demetris AJ. Breast tumor kinase/protein tyrosine kinase 6 (Brk/PTK6) activity in normal and neoplastic biliary epithelia. J Hepatol. 2015;63:399–407. doi: 10.1016/j.jhep.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Aubele M, Spears M, Ludyga N, Braselmann H, Feuchtinger A, Taylor KJ, Lindner K, Auer G, Stering K, Hofler H, Schmitt M, Bartlett JM. In situ quantification of HER2-protein tyrosine kinase 6 (PTK6) protein-protein complexes in paraffin sections from breast cancer tissues. Br J Cancer. 2010;103:663–667. doi: 10.1038/sj.bjc.6605836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, Zou L, Yao J, Lu Y, Epstein CB, Natesan S, Richardson AL, Polyak K, Mills GB, Hahn WC, Brugge JS. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One. 2010;5:e11729. doi: 10.1371/journal.pone.0011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan G, Lin G, Lucito R, Tonks NK. Protein-tyrosine phosphatase 1B antagonized signaling by insulin-like growth factor-1 receptor and kinase BRK/PTK6 in ovarian cancer cells. J Biol Chem. 2013;288:24923–24934. doi: 10.1074/jbc.M113.482737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XK, Zhang XR, Zhong Q, Li MZ, Liu ZM, Lin ZR, Wu D, Zeng MS. Low expression of PTK6/Brk predicts poor prognosis in patients with laryngeal squamous cell carcinoma. J Transl Med. 2013;11:59. doi: 10.1186/1479-5876-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Jung J, Lee ES, Kim YC, Lee W, Lee ST. Molecular dissection of the interaction between the SH3 domain and the SH2-Kinase Linker region in PTK6. Biochem Biophys Res Commun. 2007;362:829–834. doi: 10.1016/j.bbrc.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Kang SA, Lee ST. PTK6 promotes degradation of c-Cbl through PTK6-mediated phosphorylation. Biochem Biophys Res Commun. 2013;431:734–739. doi: 10.1016/j.bbrc.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Ai M, Liang K, Lu Y, Qiu S, Fan Z. Brk/PTK6 cooperates with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition. Cancer Biol Ther. 2013;14:237–245. doi: 10.4161/cbt.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou O, Huppi K, Chakka S, Gehlhaus K, Dubois W, Patel J, Chen J, Mackiewicz M, Jones TL, Pitt JJ, Martin SE, Goldsmith P, Simmons JK, Mock BA, Caplen NJ. Loss-of-function RNAi screens in breast cancer cells identify AURKB, PLK1, PIK3R1, MAPK12, PRKD2, and PTK6 as sensitizing targets of rapamycin activity. Cancer Lett. 2014;354:336–347. doi: 10.1016/j.canlet.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma S, Bao JY, Kwan PS, Chan YP, Tong CM, Fu L, Zhang N, Tong AH, Qin YR, Tsao SW, Chan KW, Lok S, Guan XY. Identification of PTK6, via RNA sequencing analysis, as a suppressor of esophageal squamous cell carcinoma. Gastroenterology. 2012;143:675–686. e671–612. doi: 10.1053/j.gastro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Brauer PM, Tyner AL. Building a better understanding of the intracellular tyrosine kinase PTK6-BRK by BRK. Biochim Biophys Acta. 2010;1806:66–73. doi: 10.1016/j.bbcan.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML, Settleman J, Chen RH. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 35.Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukong KE, Richard S. Breast tumor kinase BRK requires kinesin-2 subunit KAP3A in modulation of cell migration. Cell Signal. 2008;20:432–442. doi: 10.1016/j.cellsig.2007.11.003. [DOI] [PubMed] [Google Scholar]


