Abstract
To determine whether the transplantation of bone marrow-derived mesenchymal stem cells (BM-MSCs) can improve the 1,25(OH)2D deficiency-induced rachitic phenotype, 2×106 BM-MSCs from wild-type mice or vehicle were transplanted by tail vein injection into mice deficient in 1,25(OH)2D due to targeted deletion of 1α(OH)ase (1α(OH)ase-/-). Our results show that 1α(OH)ase mRNA was expressed in the BM-MSCs derived from wild-type mice, and was detected in long bone, kidney and intestine from BM-MSC-transplanted 1α(OH)ase-/- recipients. Serum calcium, 1,25(OH)2D3 levels and body weight were significantly increased in BM-MSC-transplanted 1α(OH)ase-/- recipients compared to vehicle-treated 1α(OH)ase-/- mice. Skeletal mineralization improved in 1α(OH)ase-/- recipients as demonstrated by BMD measurement, micro-CT analysis and von Kossa staining of undecalcified sections. Expression levels of type I collagen, osteocalcin, bone sialoprotein and vitronectin and the size of calcified nodules were decreased in BM-MSC cultures from 1α(OH)ase-/- mice compared with those from wild-type mice, however, these parameters were increased in those from BM-MSCs-transplanted 1α(OH)ase-/- recipients compared with those from vehicle-treated 1α(OH)ase-/- mice. This study indicates that donor BM-MSCs cells can relocate to multiple tissues where they synthesize 1α(OH)ase and produce 1,25(OH)2D that contributes to the improvement of serum calcium and skeletal mineralization. Results from this study suggest that BM-MSC transplantation may provide a therapeutic approach to treatment of pseudovitamin D-deficiency rickets.
Keywords: Bone marrow mesenchymal stem cells, transplantation, 1α-hydroxylase, pseudovitamin D-deficiency rickets
Introduction
Vitamin D, as a pluripotent hormone, is involved in a variety of biological actions including calcium homeostasis, cell proliferation and cell differentiation in many target tissues [1]. Vitamin D is absorbed from the diet, and generated in skin by exposure to ultraviolet light. The secosteroid is transported in blood bound to vitamin D-binding protein [2] and hydroxylated in the liver at the 25-position by a vitamin D 25-hydroxylase (CYP2R1) [3]. The metabolite 25-hydroxyvitamin D3 is further hydroxylated at the 1α-position to produce the active moiety, 1,25-dihydroxyvitamin D (1,25(OH)2D) [1]. The enzyme catalyzing the production of 1,25(OH)2D is 25-hydroxyvitamin D3 1α-hydroxylase (1α(OH)ase or CYP27B1). Although 1α(OH)ase is expressed predominantly in kidney, it is also expressed in a variety of extrarenal tissues such as skin, lymph nodes, colon, pancreas, adrenal medulla, dendritic cell, endothelial cells, brain, hypothalamus, placenta and bone cells [4-9]. Recent studies demonstrated that 1α(OH)ase is also expressed in human bone marrow mesenchymal stem cells (BM-MSCs) and the expression levels of 1α(OH)ase in BM-MSCs are associated with osteoblastogenesis [10,11].
Hereditary pseudovitamin D-deficiency rickets, also known as vitamin D-dependent rickets type I, is characterized clinically by hypotonia, weakness, growth failure, and hypocalcemic seizures in early infancy [12]. The patients also have hypocalcemia, radiologic findings typical of rickets, elevated serum parathyroid hormone concentrations, and generalized aminoaciduria. It is inherited as an autosomal recessive trait. In 1997, the cDNA encoding 1α(OH)ase was cloned from several species [13-16]. The mutations of the 1α(OH)ase gene were examined in patients suffering from vitamin D-dependent rickets type I, one of three types of hereditary rickets. Missense mutations of the 1α(OH)ase gene have been identified in the type I rickets patients by several groups [13,17-19]. Thus, these observations establish that inactivating genetic mutations of the human 1α(OH)ase cause type I hereditary rickets. We [20] and others [21] have previously reported a mouse model deficient in 1,25(OH)2D by targeted ablation of the 1α(OH)ase gene (1α(OH)ase-/-). After weaning, mice developed hyperparathyroidism, retarded growth, and the skeletal abnormalities characteristic of rickets. These abnormalities mimic those described in the human genetic disorder vitamin D-dependent rickets type I.
Stem cell therapy holds great promise for treatment of many genetic and acquired diseases, and bone marrow mesenchymal stem cells (BM-MSCs) are one of the most promising candidate stem cell types for this form of therapy due to their availability and the relatively simple requirements for in vitro expansion and genetic manipulation [22]. However, despite extensive in vitro characterization, relatively little has been reported with respect to their in vivo biology and therapeutic potential [23]. Recently we reported that non-adherent bone marrow cells (NA-BMCs) can be expanded in suspension and give rise to multiple mesenchymal phenotypes including fibroblastic, osteoblastic, chondrocytic and adipocytic as well as glial cell lineages in vitro using the “pour-off” BMC culture method [24]. Mesenchymal stem cells (MSCs) derived from NA-BMCs (NA-MSCs) from wild-type mice were transplanted into vitamin D receptor (VDR) gene knockout (VDR-/-) mice that had received a lethal dose of radiation. Results revealed that NA-MSC can be used to rescue lethally irradiated mice and become incorporated into a diverse range of tissues. After lethal dose irradiation, all untransplanted mice died within 2 weeks whereas those transplanted with NA-MSCs were viable for at least 3 months. Transplantation rescued these mice by reconstructing a hematopoietic system and repairing other damaged tissues [24]. This study indicated that adult bone marrow harbors pluripotent NA-MSCs which can migrate in vivo into multiple body organs. In the appropriate microenvironment, they can adhere, proliferate and differentiate into specialized cells of target tissues, and thus function in damaged tissue regeneration and repair. In the current study, we try to determine if transplantation of BM-MSCs could reverse the development of rickets and osteomalacia in 1α(OH)ase-/- mice, as a model for stem cell therapy to treat genetic diseases.
To determine whether the transplantation of BM-MSCs can rescue the rachitic phenotype induced by active vitamin D deficiency in mice, BMC-MSCs from wild-type mice or vehicle (as a control) were transplanted into male 1α(OH)ase-/- mice by tail vein injection. The phenotypes were analyzed at 4 weeks after the transplantation. Serum calcium and 1,25(OH)2D3 levels were examined in BMC-MSC-transplanted 1α(OH)ase-/- recipients and vehicle-treated wild-type and 1α(OH)ase-/- mice; skeletal mineralization was examined by radiography, bone mineral density (BMD) using Piximus in femurs and the von Kossa procedure on undecalcified resin embedded sections. Expression levels of bone matrix proteins and the formation of calcified nodules were examined in ex vivo cultured BMC-MSCs by Western blots and double staining with immunocytochemistry and the Von Kossa method. The percentages of CD4 and CD8 positive cells were examined by double immunoflorescence staining and flow cytometric analysis.
Materials and methods
Animals
The use of animals in this study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval ID 2012-00628). Mutant mice and control littermates were maintained in a virus- and parasite-free barrier facility and exposed to a 12-h/12-h light/dark cycle. The derivation of the parental strain of 1α(OH)ase-/- mice by homologous recombination in embryonic stem cells was previously described by Panda et al [20]. Briefly, a neomycin resistance gene was inserted in place of exon VI, VII and VIII of the mouse 1α(OH)ase gene replacing both the ligand binding and heme binding domains. RT-PCR of renal RNA from homozygous 1α(OH)ase-/- mice confirmed that no 1α(OH)ase mRNA is expressed from this allele [20]. Mice heterozygous for the null 1α(OH)ase allele were fertile [20]. Tail fragment genomic DNA was isolated by standard phenol-chloroform extraction and isopropanol precipitation. The genotyping at the 1α(OH)ase was determined by PCR as described previously [25].
Preparation of donor cells
The donor cells were prepared as described previously [24]. Briefly, tibiae and femurs of 2-4 month old wild-type male C57 BL/6J mice were removed under aseptic conditions and BMCs were flushed out with Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 10% fetal calf serum (FCS) and 50 μg/ml ascorbic acid. A single-cell suspension was achieved by forcefully expelling the cells through a 22 gauge syringe needle. 2×106 bone marrow cells were cultured in 36 cm2 petri dishes in 5 ml of the above mentioned medium in the presence of 10-8 M 1,25-(OH)2D3. After 4 days, the non-adherent cells (4 day pour-offs) were suspended by pipetting gently and then transferred to a fresh petri dish. The medium was changed after 5 days. After 10 days the cells were detached from the substratum by exposure to 2 ml of trypsin (0.05%, w/v)/EDTA (0.02%, w/v) at 37°C for 3-5 minutes, and then washed 3 times with normal saline. Cells were suspended in normal saline at 107 cells/ml and used for transplantation.
Transplantation of BM-MSCs
Forty, 2-month-old, male 1α(OH)ase-/- mice received BM-MSC transplants. The control mice were injected with normal saline and the transplanted group received 2×106 BM-MSCs by tail vein injection [24]. This dose of BM-MSCs was the same as that used in our previous study [24] and has been demonstrated to be effective for regenerating tissue damaged by a lethal dose of radiation. Serum was isolated and serum calcium was determined by autoanalyzer (Beckman Synchron 67; Beckman Instruments). Serum 1,25(OH)2D3 was measured by radioimmunoassay (ImmunoDiagnostic Systems, Bolden, UK).
RT-PCR
RNA was isolated from BM-MSCs or mouse long bones, kidney and intestine, using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The forward and the reverse primers used for amplification of the mouse 1α(OH)ase mRNA were 5’-GCAGAGGCTCCGAAGTCTTC-3’ and 5’-TGTCTGGGACACGGGAATTC-3’. The conditions for 32 cycles of PCRs were 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min.
Skeletal radiography
Tibiae were removed and dissected free of soft tissue. Contact radiographs were taken using a Faxitron model 805 (Faxitron Contact, Faxitron, Germany) radiographic inspection system (22-kV voltage and 4-min exposure time). Eastman Kodak Co. (Kodak, Rochester, NY) X-Omat TL film was used and processed routinely.
Histopathological analysis
Tibiae from vehicle-treated wild-type, 1α(OH)ase-/- mice or BM-MSC-transplanted 1α(OH)ase-/- recipients were removed, fixed, and embedded in LR White acrylic resin (London Resin Company Ltd., London, UK) and 1-μm sections were cut on an ultramicrotome. These sections were stained for mineral with the von Kossa staining procedure and counterstained with toluidine blue.
Bone marrow mesenchymal stem cell cultures
Tibiae and femurs of wild-type mice, 1α(OH)ase-/- mice and BMC-MSC-transplanted 1α(OH)ase-/- recipients were removed under aseptic conditions, and bone marrow cells (BMC) were flushed out with DMEM containing 15% FCS, 50 mg/ml ascorbic acid, 10 mM β-glycerophosphate, and 10-8 M dexamethasone. Cells were dispersed by repeated pipetting, and a single cell suspension was achieved by forcefully expelling the cells through a 22-gauge syringe needle. Total bone marrow cells (106) were cultured in 36-cm2 petri dishes in 5 ml of the above-mentioned medium. The medium was changed every 4 days, and cultures were maintained for 18 days. At the end of the culture period cells were washed with PBS, fixed, and double stained by immunocytochemistry and by the Von Kossa method for calcified nodules. Proteins were extracted for Western blotting, as described below.
Double staining by immunocytochemistry and by the von kossa method
Cultured cells in petri dishes were stained immunocytochemically for bone matrix proteins using the avidin-biotin-peroxidase complex (ABC) technique [26]. Primary antibody was applied to cells overnight at room temperature. As a negative control, the preimmune serum or TBS (50 mM Tris-HCl, 150 mM NaCl, and 0.01% Tween-20, pH 7.6) was substituted for the primary antibody. After washing with high salt buffer (50 mm Tris-HCl, 2.5% NaCl, and 0.05% Tween-20, pH 7.6) for 10 min at room temperature followed by two 10-min washes with TBS, the cells were incubated with secondary antibody [biotinylated rabbit antigoat IgG, biotinylated goat antirabbit IgG (Sigma)]. Cells were then washed as before and incubated with the Vectastain ABC-AP kit (Vector Laboratories, Inc. Ontario, Canada) for 45 min. After washing as before, red pigmentation to demarcate regions of immunostaining was produced by a 10- to 15-min treatment with Fast Red TR/Naphthol AS-MX phosphate (Sigma; containing 1 mM levamisole as endogenous ALP inhibitor). After washing with distilled water, the cells were stained for mineral with the von Kossa staining procedure and mounted with Kaiser’s glycerol jelly.
Western blot analysis
Western blots were performed as described previously by us. Proteins were extracted from 18-day cultures and quantitated by the protein assay kit (Bio-Rad Laboratories, Inc., Mississauga, Canada). Protein samples (30 mg) were fractionated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Immunoblotting was carried out using the antibodies described previously [26]. Bands were visualized using the ECL chemiluminescence detection method (Amersham Pharmacia Biotech, Aylesbury, UK). Western products were quantified by image analysis using NIH 1.61 image software.
Fluorescence-activated cell sorter (FACS) lymphocyte phenotyping
Freshly isolated peripheral blood lymphocytes were pretreated with antibodies to mouse CD16/CD32 to minimize nonspecific binding. Cells were then incubated on ice for 30 min with FITC-conjugated anti-CD4 and phycoerythrin (PE)-conjugated anti-CD8. The cells were washed, treated with erythrolysis buffer, rewashed, and resuspended in 1ґ Hanks’ buffered saline solution (HBSS), 2% FBS, and 0.1% NaN2 and analyzed by FACScan (Becton Dickinson).
Statistical analysis
Data are presented as means ± SEM. For each analysis, statistical comparisons were made using a two-way ANOVA, with P<0.05 being considered significant.
Results
Donor BM-MSCs expressed the 1α(OH)ase gene
To determine whether the donor BM-MSCs expressed the 1α(OH)ase gene, the expression of 1α(OH)ase mRNA was examined in the BM-MSCs derived from wild-type or 1α(OH)ase-/- mice by RT-PCR. Results revealed that 1α(OH)ase mRNA was expressed in the BM-MSCs derived from wild-type mice, but not from 1α(OH)ase-/- mice (Figure 1A). Thus BM-MSCs derived from wild-type mice that were used as donor cells for transplantation, carried the 1α(OH)ase gene.
Figure 1.
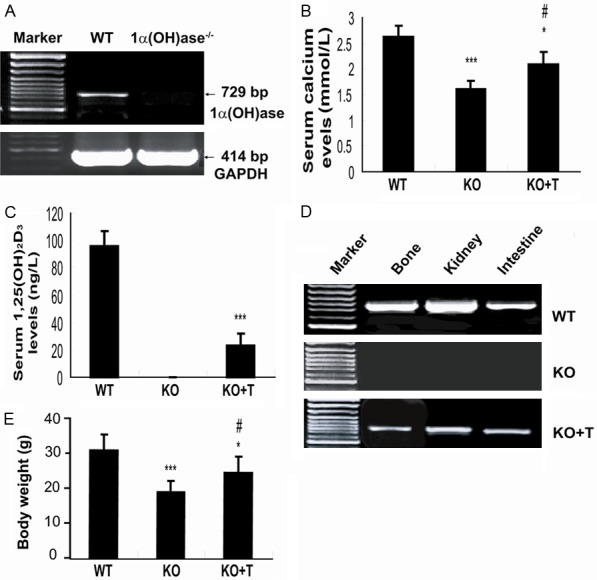
Transplantation of BM-MSCs improved serum calcium through the production of 1,25(OH)2D. (A) 1α(OH)ase mRNA expression assessed by RT-PCR in BM-MSCs derived from wild-type (WT) and 1α(OH)ase-/- mice. Serum calcium (B) and 1,25(OH)2D3 (C) levels in BM-MSC-transplanted 1α(OH)ase-/- mice (KO+T) and vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice. (D) 1α(OH)ase mRNA expression assessed by RT-PCR in bone, kidney and intestine of BM-MSCs-transplanted 1α(OH)ase-/- recipients (KO+T) and vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice. (E) Body weight of the vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice and of the BM-MSC-transplanted 1α(OH)ase-/- mice (KO+T). Each value is the mean ± SEM of determinations in five mice of each group. *, P<0.05; ***, P<0.001 relative to WT mice. #, P<0.05 relative to KO mice.
Transplantation of BM-MSCs improved serum calcium through the production of 1,25(OH)2D
To determine whether the transplantation of BM-MSCs can rescue the phenotype of 1α(OH)ase deficient mice, 2×106 BMC-MSCs from wild-type mice or vehicle (as a control) were transplanted into male 1α(OH)ase-/- mice by tail vein injection. At 4 weeks after transplantation, serum calcium and 1,25(OH)2D3 levels and body weight were examined in BM-MSC-transplanted 1α(OH)ase-/- recipients and vehicle-treated wild-type and 1α(OH)ase-/- mice. Serum calcium levels rose to 2.08±0.25 mmol/L in BM-MSCs-transplanted 1α(OH)ase-/- recipients from 1.6±0.18 mmol/L in vehicle-treated 1α(OH)ase-/- mice, however, they did not reach the normal levels seen in the wild-type mice (Figure 1B). Serum 1,25(OH)2D levels were undetectable in vehicle-treated 1α(OH)ase-/- mice and rose to 24% of vehicle-treated wild-type mice (96±11.3 ng/L) in 1α(OH)ase-/- transplant recipients (23±8.5 ng/L) (Figure 1C). To identify the possible origin of 1,25(OH)2D in 1α(OH)ase-/- transplant recipients, the expression of the 1α(OH)ase gene was examined in long bone, kidney and intestine by RT-PCR. 1α(OH)ase mRNA in these tissues was undetectable in vehicle-treated 1α(OH)ase-/- mice, but was detected in BM-MSC-transplanted 1α(OH)ase-/- recipients, although levels were lower when compared to levels in wild-type tissues (Figure 1D). Body weight was decreased significantly in vehicle-treated 1α(OH)ase-/- mice, and was increased significantly in BM-MSC-transplanted 1α(OH)ase-/- recipients compared with vehicle-treated 1α(OH)ase-/- mice, although it did not reach the wild-type level (Figure 1E).
Transplantation of BM-MSCs improved skeletal mineralization in 1α(OH)ase-/- recipients
To determine whether transplantation of BM-MSCs can rescue the rachitic skeletal lesions in 1α(OH)ase-/- mice, skeletal mineralization was examined by radiography, bone mineral density (BMD) measurement using Piximus in femurs and von Kossa staining of undecalcified resin embedded sections. Results revealed that the BMD was improved in BM-MSC-transplanted 1α(OH)ase-/- mice compared to vehicle-treated 1α(OH)ase-/- mice, although it did not reach levels seen in vehicle-treated wild-type mice (Figure 2A and 2B). 3D reconstructed longitudinal sections demonstrated that the mineralized epiphyseal volume and trabecular bone volume were increased and the width of the unmineralized growth plate become narrower in BM-MSC-transplanted 1α(OH)ase-/- mice compared to vehicle-treated 1α(OH)ase-/- mice (Figure 2C). Von Kossa stained sections showed that mineralization in the hypertrophic zone of the growth plate and in trabeculae were improved in BM-MSC-transplanted 1α(OH)ase-/- mice compared to vehicle-treated 1α(OH)ase-/- mice, although mineralization did not recover to normal levels (Figure 2D and 2E).
Figure 2.
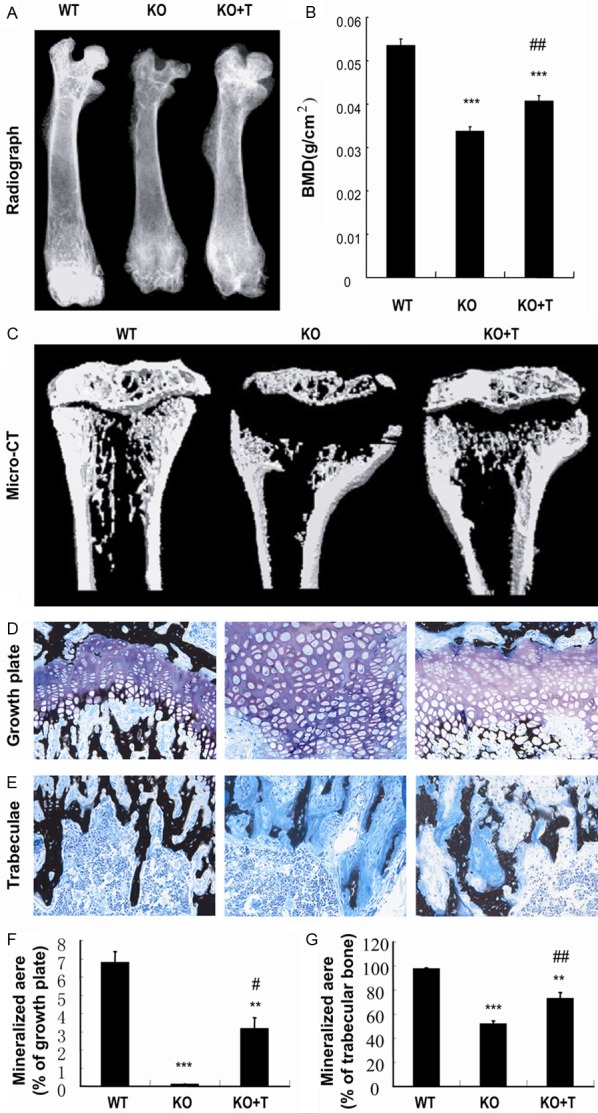
Transplantation of BM-MSCs improved skeletal mineralization in 1a(OH)ase-/- recipients. (A) Representative contact radiographs and (B) bone mineral density (BMD) of the femurs of vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice and BM-MSCs-transplanted 1α(OH)ase-/- mice (KO+T). (C) Representative longitudinal sections of the proximal end of tibiae by micro-CT scan and 3D reconstruction. Representative micrographs of (D) the growth plates and (E) trabeculae of tibiae in undecalcified sections stained by the von Kossa procedure as described in Materials and Methods and photographed at a magnification of 400×. Quantitation of mineralized areas of (F) growth plates and (G) trabecular bone. Each value is the mean ± SEM of determinations in five mice of each group. **, P<0.01; ***, P<0.001 relative to WT mice. #, P<0.05; ##, P<0.01 relative to KO mice.
Transplantation of wild-type BM-MSCs stimulated osteoblast differentiation and calcified nodule formation in BM-MSC cultures from 1α(OH)ase-/- recipients
To determine whether the improvement of skeletal mineralization in BM-MSC-transplanted 1α(OH)ase-/- recipients was associated with stimulating osteoblast differentiation and matrix calcification, bone marrow cells isolated from wild-type and 1α(OH)ase-/- mice and 1α(OH)ase-/- transplant recipients were cultured for 18 days and analyzed by double staining with immunocytochemistry and Von Kossa, and by Western blotting. The areas of calcified nodule formation and immunodetectable type I collagen, osteocalcin, bone sialoprotein and vitronectin were smaller in 1α(OH)ase-/- mouse cultures relative to wild-type mouse cultures, however they were larger in BM-MSC-transplanted 1α(OH)ase-/- mice compared to vehicle-treated 1α(OH)ase-/- mice; nevertheless they did not reach the normal levels seen in the wild-type cultures (Figure 3A-D). Western blot analysis showed that type I collagen, osteocalcin, bone sialoprotein and vitronectin were down-regulated in cells from 1α(OH)ase-/- mouse BM-BMC cultures compared with wild-type mouse BM-BMC cultures, however, they were up-regulated significantly in cells from BM-MSC-transplanted 1α(OH)ase-/- mouse BM-BMC cultures compared with cells from vehicle-treated 1α(OH)ase-/- mouse BM-BMC cultures (Figure 3E and 3F).
Figure 3.
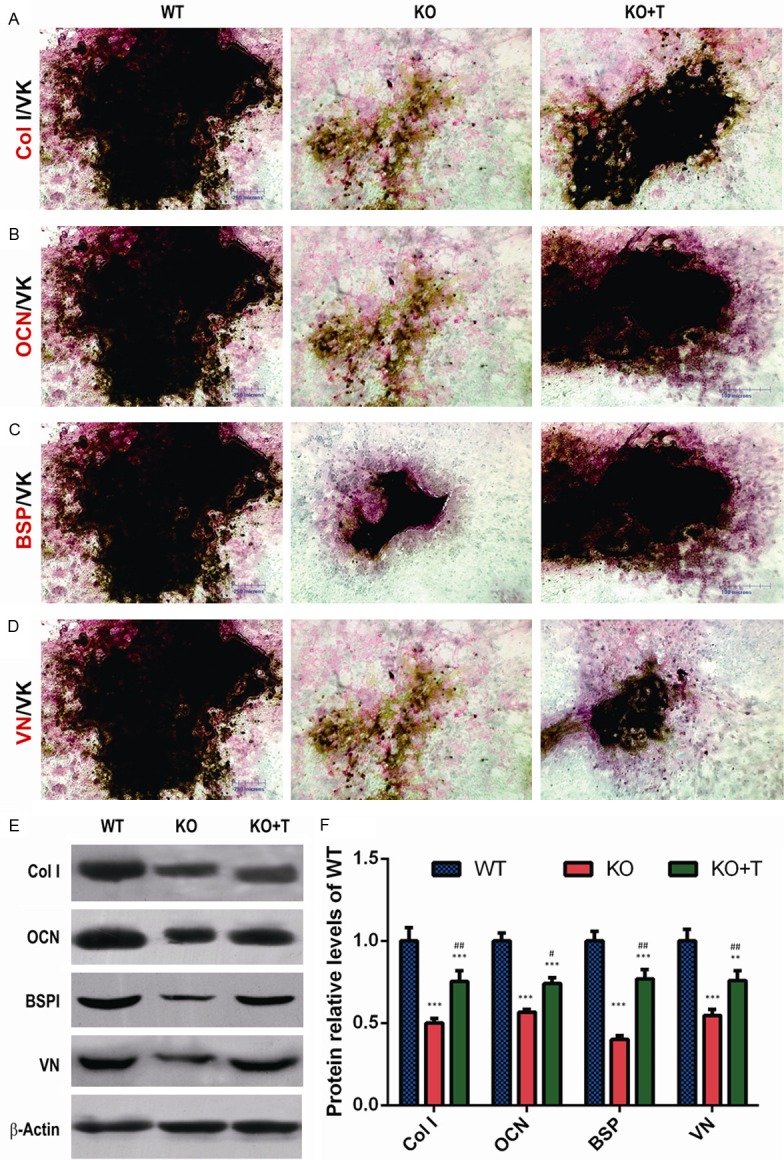
Transplantation of wild-type BM-MSCs stimulated bone matrix protein synthesis and calcified nodule formation in BM-MSC cultures from 1α(OH)ase-/- recipients. (A) Representative micrographs of the resulting cells of bone marrow cell cultures derived from vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice and BM-MSCs-transplanted 1α(OH)ase-/- mice (KO+T) with double staining with immunocytochemistry and Von Kossa for (A) type I collagen and von Kossa (Col I/VK), (B) osteocalcin and von Kossa (OCN/VK), (C) bone sialoprotein and von Kossa (BSP/VK), and (D) vitronectin and von Kossa (VN/VK). (E) Western blots of the cell extracts for expression of Col I, OCN, BSP and VN. β-actin was used as loading control for Western blots. (F) Protein levels relative to β-actin protein level were assessed by densitometric analysis and expressed relative to levels of WT mice. Each value is the mean ± SEM of determinations in 5 mice of each genotype. **: P<0.01; ***: P<0.001 relative to WT mice. #: P<0.05; ##: P<0.01, relative to KO mice.
Transplantation of BM-MSCs increased the percentage of CD4 and CD8 positive cells in 1α(OH)ase-/- recipients
To determine whether the transplantation of BM-MSCs can normalize the abnormality in the percentage of CD4 and CD8 positive cells occurring in 1α(OH)ase-/- mice [20], CD4 and CD8 positive cells were examined by double immunoflorescence staining and flow cytometry analysis. Our results showed that the percentage of CD4 and CD8 positive cells were decreased in the vehicle-treated 1α(OH)ase-/- mice, and were increased significantly in viable BM-MSCs-transplanted 1α(OH)ase-/- mice compared to vehicle-treated 1α(OH)ase-/- mice, although they did not recover to normal levels (Figure 4A, 4B).
Figure 4.
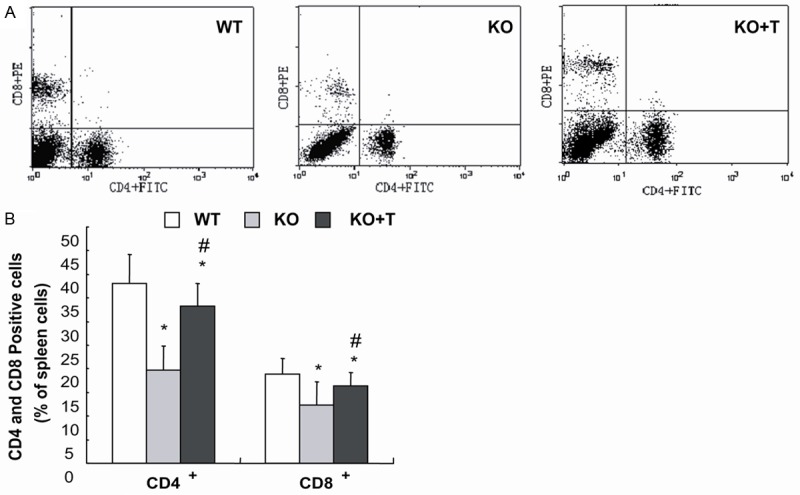
Transplantation of BM-MSCs increased the percentage of CD4 and CD8 positive cells in 1α(OH)ase-/- recipients. Comparison of the distribution of CD4+ and CD8+ peripheral T lymphocytes in vehicle-treated wild-type (WT) and 1α(OH)ase-/- (KO) mice and BM-MSCs-transplanted 1α(OH)ase-/- recipients (KO+T) by fluorescence-activated cell sorter (FACS) analysis. The data shown are (A) representative results and (B) quantitation for five individual mice of each group. Each value is the mean ± SEM of determinations in five mice of each group. *, P<0.05; ***, P<0.001 relative to WT mice. #, P<0.05 relative to KO mice.
Discussion
In the present study, we demonstrated that BM-MSCs derived from wild-type mice express 1α(OH)ase mRNA. When the BM-MSCs carrying 1α(OH)ase mRNA were injected into the circulation of 1α(OH)ase-/- mice, they partially rescued the rachitic phenotype of 1α(OH)ase-/- mice, by improving serum calcium and skeletal mineralization through the production of 1,25(OH)2D3 and enhancing osteoblast differentiation. Furthermore, we found that the transplantation of BM-MSCs partially rescued decreased CD4 and CD8 positive cells which were reduced due to 1,25(OH)2D3 deficiency.
Previous studies have demonstrated that human MSCs have the capacity to enzymatically activate 25(OH)D to 1,25(OH)2D via 1α(OH)ase (CYP27B1) [11]. In the current study, the expression of 1α(OH)ase mRNA was examined in the BM-MSCs derived from either wild-type or 1α(OH)ase-/- mice using RT-PCR. Our results demonstrated that 1α(OH)ase mRNA was detected in the BM-MSCs derived from wild-type mice, but not from 1α(OH)ase-/- mice. Therefore, such BM-MSCs carrying the 1α(OH)ase gene when used as donor cells for transplantation into 1,25(OH)2D3 deficient mice appeared to be potentially helpful to rescue the rachitic phenotype.
To test our hypothesis, we transplanted BM-MSCs derived from wild-type mice to 1,25(OH)2D deficient mice. Four weeks after the transplantation of BM-MSCs, phenotypes were compared between the BM-MSCs-transplanted 1α(OH)ase-/- recipients and vehicle-treated 1α(OH)ase-/- mice. We indeed found that the rachitic phenotype was substantially improved by the transplantation of BM-MSCs, including improvements in both skeletal growth and mineralization. We also found that serum calcium, 1,25(OH)2D3 levels and body weight were increased significantly in the BM-MSCs-transplanted 1α(OH)ase-/- recipients. Our previous study demonstrated that transplanted donor cells could migrate to multiple organs via the circulation, including heart, spleen, liver, kidney, lung, intestine, peripheral blood and bone marrow [24]. In this study, we demonstrated that following transplantation, 1α(OH)ase mRNA was detected by RT-PCR in bone, kidney and intestine from BM-MSCs-transplanted 1α(OH)ase-/- recipients, but not from vehicle-treated 1α(OH)ase-/- mice. These results suggest that donor BM-MSCs cells can relocate to multiple tissues where they synthesize 1α(OH)ase and produce 1,25(OH)2D3 that contributes to the improvements of the 1,25(OH)2D-deficient state, including increases in growth.
1,25(OH)2D has been shown to stimulate bone formation and mineralization in studies using human osteoblasts and to stimulate osteogenic differentiation from human mesenchymal stem/stromal cells [6,27]. 1,25(OH)2D enhanced mineralization through its effects on human osteoblasts before the onset of mineralization [28]. To further investigate whether the improvement of skeletal mineralization in BM-MSC-transplanted 1α(OH)ase-/- recipients was associated with stimulating osteoblast differentiation, bone matrix protein synthesis and matrix calcification, we performed ex vivo BM-MSC cultures and examined the alterations of osteoblast differentiation parameters and calcified nodule forming efficiency. Our results demonstrated that bone matrix protein expression levels, including type I collagen, osteocalcin, bone sialoprotein and vitronectin, and calcified nodule forming efficiency were reduced in vehicle-treated 1α(OH)ase-/- mice, whereas the transplantation of wild-type BM-MSCs to 1α(OH)ase-/- mice can significantly up-regulate these bone matrix protein expression levels and increase calcified nodule forming efficiency. 1,25(OH)2D has been described to stimulate the synthesis of type I collagen [29] and the production of osteocalcin in human osteoblasts [6,30]. Bone sialoprotein is present in the matrix of osteoblasts and may have a role as a nucleus of hydroxyapatite crystal formation and cell attachment [31]. In human bone marrow stromal cells, addition of 1,25(OH)2D alone had no significant effect on bone sialoprotein mRNA expression, but high levels of BSP were observed in dexamethasone-treated cultures to which 1,25 (OH)2D3 had been added [32]. Vitronectin is a multifunctional extracellular matrix glycoprotein that contains an RGD sequence and is involved in cell adhesion, spreading, and migration by interaction with integrins [33]. Our previous study has shown that the expression levels of osteocalcin, bone sialoprotein and vitronectin at both mRNA and protein levels were down-regulated in BM-MSCs from Phex deficient mice [26]. Osteocalcin, bone sialoprotein and vitronectin are all localized in mineralized bone matrix in wild-type mice, but not in defective mineralized bone matrix in Phex deficient mice [26]. Other previous studies also demonstrated that BM-MSCs could differentiate into osteoblasts in transplanted recipients [34]. Clinical studies have reported successful treatment of vitamin D-dependent rickets, type I with physiologic doses of 1,25(OH)2D3 administered on a daily basis [18,35]. Therefore, the transplantation of BM-MSCs rescues rachitic phenotypes both via providing donor cells that produce 1,25(OH)2D3 and by stimulating BM-MSC differentiation into osteoblasts, that subsequently enhance bone matrix protein synthesis and mineralization.
Although transplantation of MSCs can attenuate or possibly correct genetic disorders of bone and cartilage, clinical application of MSC transplantation for the treatment of genetic disorders of bone and cartilage is uncommon. Studies in a mouse model of osteogenesis imperfecta, a genetic disorder in which osteoblasts produce defective type I collagen, showed that infusion of BM-MSCs resulted in a significant increase in collagen production [36]. Additionally other studies have also demonstrated that normal murine MSCs migrate and incorporate into the developing neonatal heterozygous and homozygous osteogenesis imperfecta mouse, differentiate into osteoblasts and appear to participate in the bone formation of the recipient mouse in vivo [37,38]. Clinical studies in humans have demonstrated that mesenchymal progenitors in normal transplanted marrow can migrate to bone in children with osteogenesis imperfecta, and then give rise to osteoblasts whose presence correlates with an improvement in bone structure and function [39]. However, until now there has been no report that has examined BM-MSC transplantation to treat pseudovitamin D-deficiency rickets. This may be because replacement therapy with exogenous 1,25(OH)2D3, administered on a daily basis, has been considered the standard of care for vitamin D-dependent rickets, type I [18]. Our study has demonstrated that transplantation of normal BM-MSCs can partially rescue the phenotype of pseudovitamin D-deficiency rickets. Furthermore, partial expression of both renal and extra-renal 1α(OH)ase was restored. To the extent that locally produced 1,25(OH)2D3 is required to regulate skeletal metabolism [40] and immune function [41] by an autocrine/paracrine mechanism, this restoration of local 1α(OH)ase may provide an advantage over standard therapy. Consequently results from this study suggest that BM-MSC transplantation may provide an alternative or adjunct to treatment of pseudovitamin D-deficiency rickets. Nevertheless in our study, when 2×106 BM-MSCs derived from wild-type mice were transplanted to 1,25(OH)2D deficient mice, the rachitic phenotype was only partially rescued. To obtain better therapeutic effects, it may be necessary to increase the donor cell dose or examine increase durations after transplantion with the same donor cell dose. Alternatively overexpression of 1α(OH)ase in donor BM-MSCs might be more potent in improving the rachitic phenotype induced by 1,25-Dihydroxyvitamin D deficiency. Clearly therefore more preclinical studies are required to optimize the application of BM-MSC transplantation to treatment of pseudovitamin D-deficiency rickets.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81230009), from the National Basic Research Program of China (2012CB966902 and 2014CB942900), from and the Basic Research Program of Chongqing (CSTC2013jcyjC00009) to D.M. and from the Canadian Institutes of Health Research (CIHR) to D.G.
Disclosure of conflict of interest
None.
References
- 1.Christakos S, Hewison M, Gardner DG, Wagner CL, Sergeev IN, Rutten E, Pittas AG, Boland R, Ferrucci L, Bikle DD. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287:45–58. doi: 10.1111/nyas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 3.Thacher TD, Fischer PR, Singh RJ, Roizen J, Levine MA. CYP2R1 Mutations Impair Generation of 25-hydroxyvitamin D and Cause an Atypical Form of Vitamin D Deficiency. J Clin Endocrinol Metab. 2015;100:E1005–1013. doi: 10.1210/jc.2015-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 5.Turner RT, Puzas JE, Forte MD, Lester GE, Gray TK, Howard GA, Baylink DJ. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci U S A. 1980;77:5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 7.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 8.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 9.Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–114. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prader A, Illig R, Heierli E. [An unusual form of primary vitamin D-resistant rickets with hypocalcemia and autosomal-dominant hereditary transmission: hereditary pseudo-deficiency rickets] . Helv Paediatr Acta. 1961;16:452–468. [PubMed] [Google Scholar]
- 13.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 14.Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, DeLuca HF, Suda T. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc Natl Acad Sci U S A. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- 16.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 17.Cui N, Xia W, Su H, Pang L, Jiang Y, Sun Y, Nie M, Xing X, Li M, Wang O, Yuan T, Chi Y, Hu Y, Liu H, Meng X, Zhou X. Novel mutations of CYP27B1 gene lead to reduced activity of 1alpha-hydroxylase in Chinese patients. Bone. 2012;51:563–569. doi: 10.1016/j.bone.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med. 1998;338:653–661. doi: 10.1056/NEJM199803053381004. [DOI] [PubMed] [Google Scholar]
- 19.Wang JT, Lin CJ, Burridge SM, Fu GK, Labuda M, Portale AA, Miller WL. Genetics of vitamin D 1alpha-hydroxylase deficiency in 17 families. Am J Hum Genet. 1998;63:1694–1702. doi: 10.1086/302156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 22.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 23.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZL, Tong J, Lu RN, Scutt AM, Goltzman D, Miao DS. Therapeutic potential of non-adherent BM-derived mesenchymal stem cells in tissue regeneration. Bone Marrow Transplant. 2009;43:69–81. doi: 10.1038/bmt.2008.260. [DOI] [PubMed] [Google Scholar]
- 25.Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 26.Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–939. doi: 10.1210/endo.142.2.7976. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Glowacki J, Kim SW, Hahne J, Geng S, Mueller SM, Shen L, Bleiberg I, LeBoff MS. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J Bone Miner Res. 2012;27:1992–2000. doi: 10.1002/jbmr.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woeckel VJ, Alves RD, Swagemakers SM, Eijken M, Chiba H, van der Eerden BC, van Leeuwen JP. 1Alpha,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J Cell Physiol. 2010;225:593–600. doi: 10.1002/jcp.22244. [DOI] [PubMed] [Google Scholar]
- 29.Thomas GP, Bourne A, Eisman JA, Gardiner EM. Species-divergent regulation of human and mouse osteocalcin genes by calciotropic hormones. Exp Cell Res. 2000;258:395–402. doi: 10.1006/excr.2000.4912. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Chan M, Aubin JE. Pleiotropic effects of the steroid hormone 1,25-dihydroxyvitamin D3 on the recruitment of mesenchymal lineage progenitors in fetal rat calvaria cell populations. J Mol Endocrinol. 2006;36:425–433. doi: 10.1677/jme.1.01900. [DOI] [PubMed] [Google Scholar]
- 31.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 32.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–947. doi: 10.1016/0003-9969(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 33.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539–544. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y, Chen XD, Gronthos S, Shi S. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012;3:40. doi: 10.1186/scrt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delvin EE, Glorieux FH, Marie PJ, Pettifor JM. Vitamin D dependency: replacement therapy with calcitriol? J Pediatr. 1981;99:26–34. doi: 10.1016/s0022-3476(81)80952-3. [DOI] [PubMed] [Google Scholar]
- 36.Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Wang X, Niyibizi C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007;25:3183–3193. doi: 10.1634/stemcells.2007-0466. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Li F, Niyibizi C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: implications of cell therapy for skeletal diseases. Stem Cells. 2006;24:1869–1878. doi: 10.1634/stemcells.2005-0430. [DOI] [PubMed] [Google Scholar]
- 39.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 40.Sapir-Koren R, Livshits G. Bone mineralization is regulated by signaling cross talk between molecular factors of local and systemic origin: the role of fibroblast growth factor 23. Biofactors. 2014;40:555–568. doi: 10.1002/biof.1186. [DOI] [PubMed] [Google Scholar]
- 41.Hewison M. Vitamin D and innate immunity. Curr Opin Investig Drugs. 2008;9:485–490. [PubMed] [Google Scholar]


