Abstract
Lung ischemia/reperfusion injury (LIRI) usually occurs during in lung transplantation and extracorporeal circulation operation and may develop into pulmonary infections, acute rejection and bronchiolitis obliterans syndrome. Recent studies have discovered the protective effect of heat shock protein 70 (HSP70) on various types of injuries. In the present study, we firstly explore the role of over-expressed HSP70 on the protection against LIRI. Lung Wet/Dry (W/D) ratio, biomarkers in the bronchoalveolar lavage fluid (BALF), lung histological changes and apoptosis markers, oxidative products and proinflammatory cytokines in the lung tissues were analyzed. Next, the expression of eNOS, SIRT1 and AMPK were measured. Finally, the changes of the lung W/D ratio and biomarkers in the BALF using the inhibitors of SIRT1/AMPK/eNOS pathway were evaluated. Mice exposed to LIRI procedure had significant increases in lung W/D ratio and biomarkers (protein level, LDH level, leukocytes and total cells) in BALF. LIRI also caused histological injury, demonstrated by hemorrhage, alveolar septal thickening and fibrin deposition. Apoptosis, oxidative products and proinflammatory cytokines in lung tissue were also induced by LIRI. The over-expression of HSP70 antagonized the impacts of LIRI by attenuating these parameters. It significantly increased the expression of eNOS, SIRT1 and AMPK, while the inhibition of SIRT1 and AMPK deactivated the eNOS expression. The lung W/D ratio and biomarkers in BALF were increased while mice were given inhibitors of eNOS, SIRT1 and AMPK. We concluded that over-expression of HSP70 had protective effect on LIRI and HSP70 might be involved in the protection through a SIRT1/AMPK/eNOS pathway.
Keywords: HSP70, lung ischemia/reperfusion injury, SIRT1, AMPK, Enos
Introduction
Lung ischemia/reperfusion injury (LIRI) usually occurs during in lung transplantation and extracorporeal circulation operation [1,2]. In lung transplantation or cardiac surgery with cardiopulmonary bypass, as the bronchial arterial blood flow can be decreased to approximately 10%, it may cause extensive LIRI [3,4], which is characterized by neutrophil extravasation, interstitial edema, disruption of epithelial integrity and enhanced vascular permeability [5]. LIRI may also develop into pulmonary infections, acute rejection and bronchiolitis obliterans syndrome and reduce the survival rate following lung transplantation 6. Some studies have revealed that induction of inflammatory mediators such as chemokines, cytokines, and oxygen radicals is one of the crucial underlying mechanisms, but effective treatments for its prevention are not yet available.
Heat shock proteins (Hsps) are a family of proteins that are produced by cells in response to exposure to stressful conditions, including heat, hypoxia, blood loss, ischemia-reperfusion injury, surgical and anesthetic stress. In 2000, Hiratsuka et al discovered heat pretreatment of the donor 6 hours before harvest resulted in increased synthesis of heat shock protein 70 (HSP70) and protected against subsequent ischemia-reperfusion injury in the lung isograft [7]. Later, Jayakumar et al. showed HSP70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury [8]. Moreover, several studies have confirmed that over expression of HSP70 decreases subsequent ischemia-reperfusion injury in rat lung isografts [9], neuron ischemic injury [10] and myocardial infarction [11]. However, the mechanism underlying the protection of HSP70 on LIRI was not explored yet.
Endothelial NOS (eNOS), a nitric oxide synthase that generates NO in blood vessels, plays an important role in the maintenance of endothelial barrier properties and the prevention of leukocyte infiltration [12]. Kaminski et al reported that up-regulation of eNOS inhibited pulmonary leukocyte migration following lung ischemia-reperfusion and attenuated LIRI [13]. Moreover, several studies have revealed the interaction of HSP70/eNOS in multiple disease prevention [14-16]. Specially, Li et al reported that HSP70 attenuated cardiac dysfunction, prevented cardiomyocyte apoptosis and promoted myocardial angiogenesis via an eNOS-dependent mechanism [11]. However, how HSP70 regulates NOS remains unclear. Recently, many studies show that silent information regulator 1 (SIRT1), one of the sirtuins, plays an important role in the maintenance of vascular endothelial cell homoeostasis [17-19]. Moreover, Li et al reported that SIRT1 promoted the migration and proliferation of endothelial progenitor cells through eNOS signaling pathway [20]. NO derived from eNOS can also interact with SIRT1 in the attenuation of mitochondrial dysfunction in type 2 diabetic hearts [21]. AMP-activated protein kinase (AMPK), an enzyme that plays a role in cellular energy homeostasis, is closely related to Sirt1 [22,23]. In addition, it was reported that the stimulation of NO production from eNOS and enhancement of NO bioavailability might involve both SIRT1 and AMPK [24]. Hence, in the present study, we explored the protective effect of HSP70 against LIRI and the underlying mechanism. Lung Wet/Ddy (W/D) ratio, biomarkers in the bronchoalveolar lavage fluid (BALF), lung histological changes and apoptosis markers, oxidative products and proinflammatory cytokines in the lung tissues were analyzed to evaluate the protective effects of HSP70; Next, the expression of eNOS, SIRT1 and AMPK were measured. Finally, the involvement of SIRT1/AMPK/eNOS pathway was evaluated by examining the changes of the lung W/D ratio and biomarkers in the BALF using their inhibitors.
Methods
Animals and drugs
The generation of HSP70 transgenic mice (Tg) has been previously described 25. Mice were housed in the hospital animal center. The house was maintained under standard conditions (22°C room temperature, 33% humidity) with a 12 h light/dark cycle. Mice received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). Approval for all experimental protocols was granted by the Animal Care Committee of People’s hospital, Liaocheng. L-NIO (L-N 5-[1-iminoethylornithine]), dorsomorphin and EX 527 were products of Sigma Aldrich (St. Louis, MO, USA). L-NIO was given to mice 30 min before the ischemia/reperfusion procedure (3.0 mg/kg, sc) to inhibit eNOS; Dorsomorphin was injected via the tail vein (10 mg/kg) 10 min prior to the ischemia/reperfusion procedure to inhibit SIRT1; EX-527 was dissolved in dimethyl sulfoxide (DMSO) and intraperitoneally injected (40 mg/kg) 30 min before the ischemia/reperfusion procedure to inhibit AMPK.
Experimental protocol of LIRI
Mice were anesthetized with air containing 1% isoflurane, then incubated and connected to a MiniVent mice ventilator (Hugo Sachs Elektronik, March, Germany). Heparin (30 U/kg) is given via the right external jugular vein. The chest was opened by a cut to the left fourth rib. A left thoracotomy was performed before the left lung hilum was exposed. A non-crushing clamp was placed across the left lung hilum, occluding the pulmonary artery, vein, and main stem bronchus. The left lung was rendered ischemic for 60 min before the reperfusion was established by removing the clamp for 90 min. The chest was temporarily closed during the ischemia and reperfusion procedures. Mice in the Normal+WT and Normal+Tg groups had the same procedure except that no clamp was placed across the left lung hilum.
Lung wet/dry weight ratio
At the end of the experiments, the left lower lobe of the lung was dissected and weighed. After that, it was dried in an oven at a constant temperature of 60°C for 48 h to obtain a dehydrate consistency, then weighed again. The lung wet/dry (W/D) ratio was calculated as an indicator of edema.
Bronchoalveolar lavage fluid (BALF) collection and assays
Similarly to Enoki et al [26], after the reperfusion was finished, BALF was collected by cannulating the trachea with repeated 200 μL of sterile PBS containing heparin up to a total volume of 1.0 mL. After the BALF was collected, the left lung was removed en bloc and drained of blood, then homogenized in 2 mL PBS on ice for following studies. The BALF was centrifuged at 4,000 × g for 5 min at 4°C to separate the cells in the BALF from the liquid. The supernatant (10 μl) was used in the assay. The protein concentration was measured with a BCA protein assay reagent (Beyotime Biotechnology, Shanghai, China). LDH levels were examined using a commercial assay kit (Beyotime Biotechnology, Shanghai, China). Cells were re-suspended in PBS. Leukocytes in the BALF were counted in ten randomly chosen fields with optical microscopy, while the total cell count was determined on a fresh fluid specimen using a hemocytometer.
Histological examination by hematoxylin and eosin (H&E) staining
After the reperfusion was done, the left lung tissue were fixed in 10% formalin for 24 hours, then embedded in paraffin. Next, slices were cut and stained with routine H&E. Finally, they were observed by light microscopy for the area analysis of cell damage.
Measurement of oxidative products and proinflammatory cytokines in lung tissues
20 mg of lung tissue was homogenized in 2 mL PBS on ice. After they were centrifuged at 12,000 g for 20 min, the MDA and protein carbonyl content in the supernatant were measured using the corresponding kits (Jiancheng Bioengineering Institute, Nanjing, China) following the instructions. For 8-OHdG assay, DNA was extracted from the homogenized lung tissue with a DNA Extraction Kit (Wako Chemical; Osaka, Japan), then washed with 70% ethanol, dried, and dissolved in 200 μL of 10 mM Tris-HCl (pH 7.0) for digestion. 8-OHdG levels then were measured with the method of Qian et al [23]. The protein concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). The levels of TNF-α, IL-1β and IL-6 were measured using sandwich enzyme-linked immunosorbent assay (ELISA) commercially available from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions. These experiments were performed according to the manufacturer’s instructions.
Western blot
After the reperfusion was done, the mouse’s left lung were harvested and placed in ice-cold homogenizing buffer (20 mmol/L Tris, 100 mmol/L NaCl, and 2.7 mg/mL heparin) and then homogenized. The homogenate was centrifuged at 1500 g for 10 min at 4°C to collect the supernatant as a total protein preparation. After centrifugation, collected supernatants were transferred to a fresh tube. The protein concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). Next, equal amounts of protein were combined with 5 × sodium dodecyl sulfate, then loaded onto a sodium dodecylsulfate-polyacrylamide gel for electrophoresis and subsequently transferred to polyvinyldine diflouride membrane for immunoblot analysis. The membranes were blocked in 5% nonfat milk for 2 h at room temperature and then incubated overnight at 4°C with primary antibodies against Bcl-2, Bax, pro-caspase-3, cleaved caspase-3, p-eNOS, eNOS, SIRT1 and AMPK (Santa Cruz Biotechnology). After being washed three times with Tris-buffered saline with 0.05% Tween 20, the membranes were incubated with proper peroxidase-conjugated secondary antibody for 2 hours. The membrane was washed three times with Tris-buffered saline, and then examined with the ECL Plus Western Blotting Detection System (Amersham Life Science, UK). The results were normalized to β-actin to correct for loading.
Statistical analysis
All data are presented as Mean ± SEM. Statistical analysis was performed using one-way analysis of variance followed by the Student-Newman-Keuls post-hoc test with SPSS17.0. P value less than 0.05 was considered significant.
Results
Changes in lung W/D ratio and biomarkers in BALF with LIRI and HSP70 over-expression
Table 1 shows the changes in lung W/D ratio and biomarkers (protein level, LDH level, leukocytes and total cells) in BALF. In Sham groups, transgenic mice with HSP70 (Sham+Tg) had no changes in these indicators. In LIRI groups, WT mice had significant increases in all these indicators compared to Sham group (P<0.05). These indicators in LIRI+Tg group were dramatically decreased compared to that in the LIRI+WT group (P<0.05).
Table 1.
Changes in lung W/D ratio and biomarkers in BALF
| Sham | LIRI | |||
|---|---|---|---|---|
|
|
||||
| WT | Tg | WT | Tg | |
| Lung W/D ratio | 5.36±1.25 | 5.45±1.02 | 9.68±1.29* | 6.24±1.41& |
| Protein level in BALF (µg/ml) | 39.65±3.21 | 42.56±3.96 | 126.74±10.26* | 88.96±10.24*,& |
| LDH level in BALF (U/ml) | 10.65±2.66 | 11.63±2.94 | 46.64±5.22* | 23.31±2.67*,& |
| Leukocytes in BALF (1 × 106/ml) | 4.96 ±1.26 | 4.58±2.11 | 15.29±3.62* | 5.22±2.14& |
| Total cells in BALF (1 × 106/ml) | 5.69±1.59 | 5.27±1.18 | 19.32±2.05* | 9.69±2.37*,& |
WT: Wild type mice; Tg: transgenic mice of HSP70; LIRI: lung ischemia/reperfusion injury; W/D: Wet/dry; BALF: bronchoalveolar lavage fluid; LDH: lactate dehydrogenase. Values are expressed as Mean ± SEM.
P<0.05 compared to Sham+WT;
P<0.05 compared to LIRI+WT.
N = 10 per group.
Histological changes and apoptotic proteins expression
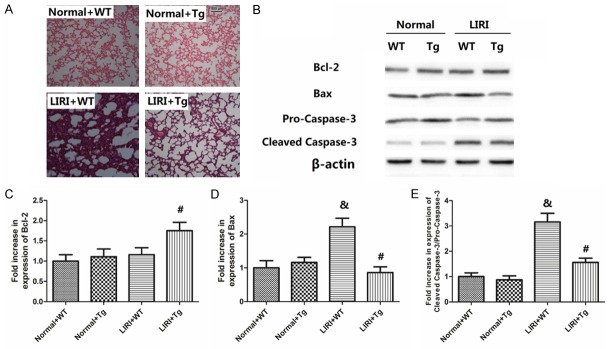
Figure 1 shows the histological changes and apoptotic proteins expression caused by LIRI or HSP70. As shown in Figure 1A, LIRI caused apparent hemorrhage, alveolar septal thickening as well as fibrin deposition. In mice in the LIRI+Tg group, histological damages was also caused, but to a lesser degree. As shown in Figure 1B-E, the levels of Bcl-2 was not altered by LIRI, but greatly enhanced by over-expression of HSP70 (P<0.05); both Bax and cleaved caspase-3/pro caspase-3 ratio was increased by LIRI (P<0.05), but significantly suppressed by HSP70 (P<0.05).
Figure 1.
Histopathological changes and apoptotic protein expression. Microscopic examinations of the lung tissues were stained with hematoxylin and eosin was shown in (A. The magnification used in these photos was 200 ×. LIRI: lung ischemia/reperfusion injury; Tg: transgenic mice of HSP70; Vehicle: mice treated with saline. Apoptotic protein expression (Bcl-2, Bax, pro-caspase-3 and cleaved caspase-3) is shown in (B-E). Values are expressed as Mean ± SEM. &P<0.05 compared to Sham+WT; #P<0.05 compared to LIRI+WT. N = 8 per group.
Changes in oxidative products in lung tissues
Changes in oxidative products (MDA, protein carbonyl and 8-OHdG) were shown in Figure 2A-C. The over-expression of HSP70 only had no effects on the levels of MDA, protein carbonyl and 8-OHdG. LIRI caused significant oxidative stress in lung, as shown in the dramatic increase in MDA, protein carbonyl and 8-OHdG levels (P<0.05). However, HSP70 over-expression significantly decreased these parameters compared with those in LIRI+WT group (P<0.05).
Figure 2.
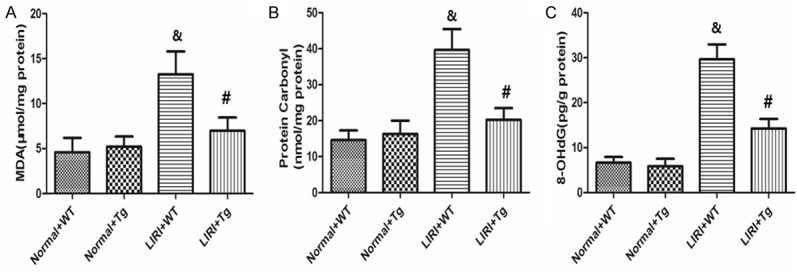
Changes in oxidative products (MDA, protein carbonyl and 8-OHdG) in the lung tissues. It shows the levels of MDA, protein carbonyl and 8-OHdG in the lung tissues. Values are expressed as Mean ± SEM. &P<0.05 compared to the Sham+WT; #P<0.05 compared to LIRI+WT. N = 10 per group.
Changes in TNF-α, MPO and IL-6 levels in lung tissues
As demonstrated in Figure 3A-C, the over-expression of HSP70 only had no effects on the levels of TNF-α, MPO and IL-6 levels, but their levels in lung tissue were remarkably increased by LIRI compared with Sham+WT group (P<0.05). Over-expression of HSP70, however, significantly reduced the alteration of TNF-α, MPO and IL-6 levels in lung tissues (P<0.05).
Figure 3.
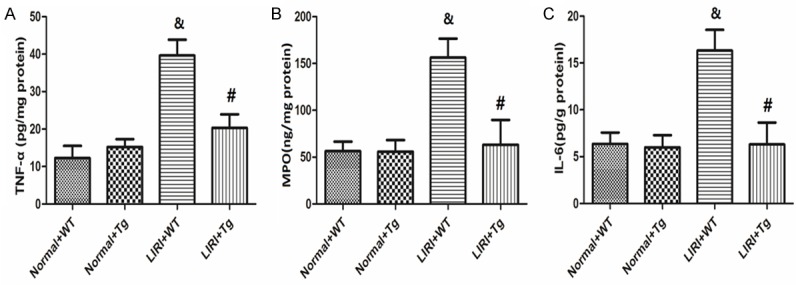
Changes in lung inflammation markers (TNF-α, MPO and IL-6). The levels of TNF-α, MPO and IL-6 levels in the lung tissues are shown in (A-C), respectively. Values are expressed as Mean ± SEM. &P<0.05 compared to the Sham+WT; #P<0.05 compared to the LIRI+WT. N = 10 per group.
The protein expression of eNOS, p-eNOS, SIRT1 and AMPK
As shown in Figure 4A and 4D, there was no significant difference between Sham+WT, Sham+Tg and LIRI+WT groups, but in the LIRI+Tg group, the eNOS expression was greatly increased (P<0.05 compared to LIRI+WT). Figure 4B, 4E and 4F show that both the SIRT1 and AMPK expression were increased by LIRI (P<0.05 compared to Sham+WT), and further enhanced in the LIRI+Tg group (P<0.05 compared to LIRI+WT). To investigate the effects of SIRT1 and AMPK on the eNOS activation, we used the inhibitors of eNOS, SIRT1 and AMPK (L-NIO, dorsomorphin and EX 527), and then observed the expression of eNOS and p-eNOS. As shown in Figure 4C and 4G, the p-eNOS/eNOS was greatly decreased while mice were treated with L-NIO, dorsomorphin or EX 527 (P<0.05 compared to LIRI+Tg).
Figure 4.
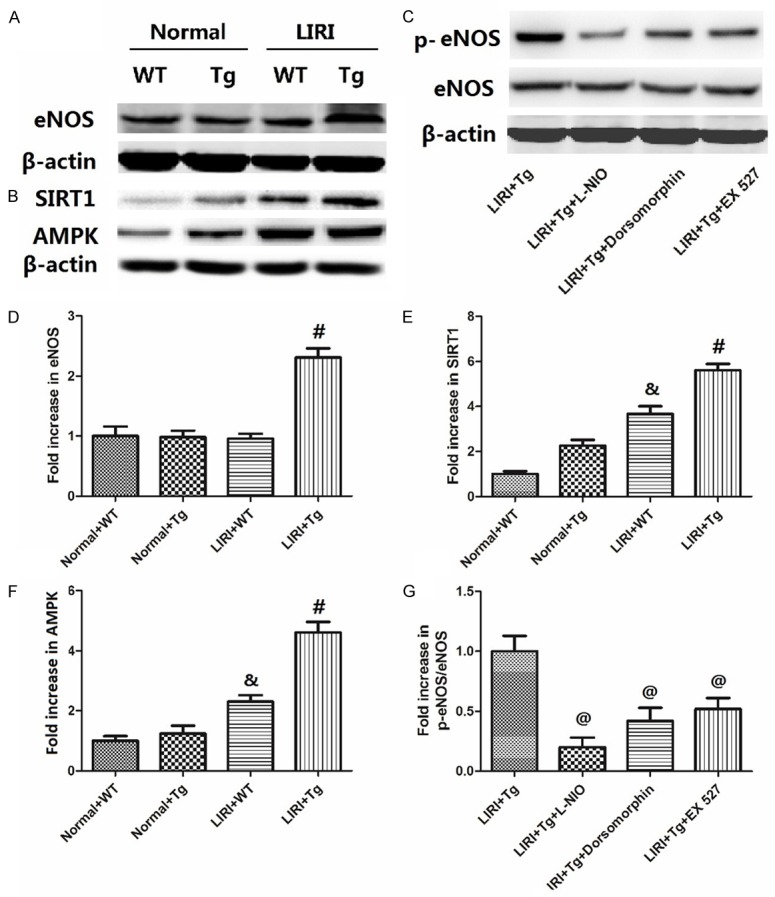
Changes in the expression of eNOS, SIRT1, AMPK and p-eNOS in lung tissues. The blots of eNOS, SIRT1, AMPK and eNOS phosphorylation are shown in (A-C); the value changes are shown in (D-G). The values are expressed as Mean ± SEM. &P<0.05 compared to the Sham+WT; #P<0.05 compared to the LIRI+WT, @P<0.05 compared to the LIRI+Tg. N = 8 per group.
Changes in lung W/D ratio and biomarkers in BALF with inhibitors of eNOS, SIRT1 and AMPK
Table 2 shows the changes in lung W/D ratio and biomarkers in BALF with the treatment of inhibitors of eNOS, SIRT1 and AMPK. Compared to the LIRI+Tg group, all these indicators were increased while mice were given L-NIO, dorsomorphin or EX 527 (P<0.05 compared to LIRI+Tg), demonstrating the roles of eNOS, SIRT1 and AMPK in the protective effects of HSP70.
Table 2.
Changes in lung W/D ratio and biomarkers in BALF with inhibitors of eNOS, SIRT1 and AMPK
| LIRI | ||||
|---|---|---|---|---|
|
|
||||
| Vehicle | L-NIO | Dorsomorphin | EX 527 | |
| Lung W/D ratio | 6.38±0.86 | 10.26±1.03* | 9.08±1.31* | 9.47±1.21* |
| Protein level in BALF (µg/ml) | 80.23±9.67 | 121.23±11.01* | 132.25±9.66* | 121.28±10.34* |
| LDH level in BALF (U/ml) | 26.31±2.37 | 49.67±5.31* | 52.33±4.21* | 42.33±4.05* |
| Leukocytes in BALF (1 × 106/ml) | 5.67±1.56 | 16.33±3.24* | 14.20±3.27* | 13.29±3.15* |
| Total cells in BALF (1 × 106/ml) | 10.22±2.07 | 18.66±2.31* | 21.03±2.69* | 18.21±2.15* |
LIRI: lung ischemia/reperfusion injury; Tg: transgenic mice of HSP70; Vehicle: mice treated with saline; W/D: Wet/dry; BALF: bronchoalveolar lavage fluid; LDH: lactate dehydrogenase. Values are expressed as Mean ± SEM.
P<0.05 compared to LIRI+Tg+Vehicle.
N = 10 per group.
Discussion
The present study aims to study the protective effects of HSP70 against the LIRI and to explore the underlying mechanism. Mice exposed to LIRI procedure had significant increases in lung W/D ratio and biomarkers (protein level, LDH level, leukocytes and total cells) in BALF. LIRI also caused histological injury, demonstrated by hemorrhage, alveolar septal thickening and fibrin deposition. Apoptosis, oxidative products and proinflammatory cytokines in lung tissue were also induced by LIRI. The over-expression of HSP70, however, antagonized the impacts of LIRI by attenuating these parameters. Further investigation revealed that HSP70 significantly increased the expression of eNOS, SIRT1 and AMPK, while the inhibition of SIRT1 and AMPK deactivated the eNOS expression. Finally, lung W/D ratio and biomarkers in BALF were increased while mice were given inhibitors of eNOS, SIRT1 and AMPK, demonstrating their roles in the protective effects of HSP70.
Previous studies and ours have shown that LIRI is characterized by increased microvascular permeability and the pulmonary sequestration of leukocytes [27]. ROS and proinflammatory cytokines are essential in the pathology of LIRI. The burst of ROS caused by lung reperfusion overwhelms the anti-oxidative capacity in body and causes damage to the cellular membrane, alveolar epithelial, capillary endothelial cells and mitochondria [28], as well as stimulate the production of several inflammatory cytokines [4], resulting in cellular necrosis and apoptosis, which is shown by the increase of Bax and cleaved caspase-3. Consistent with previous studies, the present study confirms that the oxidative products and proinflammatory cytokines in lung tissue were induced by LIRI. MDA (a naturally occurring product of lipid peroxidation), carbonyl protein (an indicator of oxidative injury in proteins) and 8-OHdG (a marker of oxidative damage to DNA) were all increased by LIRI. TNF-α is a proinflammatory cytokine secreted from macrophages in response to a variety of pathologic processes and has been identified as one of the pivotal factors accelerating LIRI [29]. The activity of MPO was used as a marker of neutrophil infiltration [30]. Several previous studies have shown the active role of MPO in the lung tissue in inflammatory response after LIRI [31,32]. IL-6 acts as a mediator of acute lung injury and the induction of edema [33]. It is suggested that these proinflammatory cytokines might amplify lung injury and the systemic inflammatory response [34]. The increase of TNF-α, MPO and IL-6 in the lung tissues after LIRI demonstrates their involvement in the pathology of LIRI.
HSP70, the largest and most conserved family of HSP, usually acts as a signaling molecule and regulator in cellular processes and protects cells from various noxious conditions, including apoptosis, oxidative stress, ischemic and/or reperfusion injury [35-40]. Some studies suggested that HSP70 could regulate cellular apoptosis, oxidative stress and inflammation, three important factors in the pathogenesis of LIRI, in the process of ischemia and/or reperfusion. Li et al showed that HSP70 protected heart from myocardial infarction by prevention of cardiomyocyte apoptosis [11], while Ma and Kang et al. found that HSP70 prevented expression of active caspase-3 and neural apoptosis and improved neurological functions after a cerebral ischemia/reperfusion injury [9,41]. Consistent with these findings, Cui et al discovered HSP70 expression after LPS-induced inflammation in the brain [42]. Our results showed that HSP70 attenuated the increase of lung W/D ratio and biomarkers in BALF (protein level, LDH level, leukocytes and total cells) as well as the histological injury, demonstrating its protective effect against LIRI. In the HSP70 over-expressed mice, we found that the levels of Bcl-2 was greatly enhanced and both Bax and cleaved caspase-3/pro caspase-3 ratio was suppressed, indicating the attenuation of cellular apoptosis. For oxidative stress, it was confirmed that HSP70 was capable of restore the oxidative/anti-oxidative balance caused by LIRI, as shown by the decreases of oxidative products (8-OHdG, MDA and protein carbonyl). Moreover, over-expression of HSP70 greatly mitigated the inflammatory response, demonstrated by the decrease in the inflammation markers (TNF-α, MPO and IL-6), which are all activated by the LIRI. Together with previous studies, these results confirm the protective effects of HSP70 against cellular injury, inflammation and oxidative stress.
To further explore the involvement of eNOS in the protective effects of HSP70, we investigated their expression in the HSP70 over expressed mice. ENOS is a kind of enzyme catalyzing the production of NO from L-arginine, which may protect cell from various injuries, such as mediating the vascular response to the oxidative stress and inhibiting neutrophil adhesion to the vascular endothelium [43-45]. Many investigators have explored the regulation between HSP70 and eNOS [14,46,47]. It was shown that HSP70 and the PI3/Akt/eNOS pathway might be involved in the cardioprotection of periodic acceleration preconditioning [14], while another study indicated HSP72 could increase angiotensin-(1-7) signaling through the Mas/eNOS/SIRT1 pathway [46,48]. Moreover, it was directly demonstrated that HSP70 prevented cardiomyocyte apoptosis by an eNOS-dependent mechanism [11]. Here, we found that HSP70 significantly increased the expression of eNOS and lung W/D ratio and biomarkers in BALF were increased while mice were given inhibitors of eNOS. The similarity between previous studies and our results is that the expression of eNOS can be regulated by HSP70, which plays an important role in the in the protection of LIRI.
SIRT1, a homologue of silent information regulator (Sir2) protein, is the closest human homologue of yeast Sir2 [19]. It is classified as a NAD-dependent deacetylase and a nuclear sirtuin, although it is not restricted to the nucleus and has important non-nuclear functions 49 Many studies have revealed that SIRT1 is a key component in several stress-responsive pathways involved in apoptotic cell death, cellular senescence and vascular growth (e.g., upon ischemia/reperfusion) [49]. How SIRT1 protects vascular from stress-induced endothelial dysfunction remains to be determined, but some studies suggested that eNOS, which plays a key role in maintaining vascular homeostasis, might be involved. Resveratrol, a polyphenolic activator of SIRT1, has been shown to increase the expression of eNOS 50; Xia et al reported that both SIRT1 and AMPK were involved in the stimulation of NO production from eNOS and enhancement of NO bioavailability [24]. As eNOS-derived NO is essential for endothelial-dependent vasorelaxation as well as endothelial cell survival, this pathway might be an important mechanism for the protective effect of SIRT1 in the LIRI. It is reported that AMPK modulates many signaling cascades, and then protects endothelial cells from injury and dysfunction, such as endothelial oxidative injuries [51]. SIRT1/AMPK pathway is involved in the protection induced by many factors, such as methylene blue, aspirin, alpha-lipoic acid and resveratrol [52-55]. Enlightened by these findings, we examined the role of SIRT1/AMPK pathway to explore how SIRT1 regulates the eNOS expression. Interestingly, SIRT1 and AMPK expression were both increased by HSP70 over-expression. Moreover, the p-eNOS/eNOS was greatly decreased while mice were treated with dorsomorphin or EX 527 (inhibitors of SIRT1 or AMPK), indicating the role of SIRT1 and AMPK in the activation of eNOS. Further studies demonstrated that the changes in lung W/D ratio and biomarkers in BALF were increased while mice were given L-NIO, dorsomorphin or EX 527, demonstrating the roles of eNOS, SIRT1 and AMPK in the protective effects of HSP70. These evidence support our hypothesis that over-expression of HSP70 protects mice against lung ischemia/reperfusion injury through SIRT1/AMPK/eNOS pathway.
Taken together, the present study demonstrates that over-expression of HSP70 had protective effect on LIRI, demonstrated by attenuation of histological injury, cellular apoptosis, oxidative products and proinflammatory cytokines. The SIRT1/AMPK/eNOS pathway is involved in the protective effects of HSP70. Although the precise mechanism needs further investigation, the present study suggests that HSP70 may serve as a novel target in the treatment of LIRI.
Disclosure of conflict of interest
None.
References
- 1.Mukherjee S, Kim S, Gibbons LE, Nho K, Risacher SL, Glymour MM, Habeck C, Lee GJ, Mormino E, Ertekin-Taner N, Montine TJ, Decarli C, Saykin AJ, Crane PK Alzheimer’s Disease Neuroimaging Initiative. Genetic architecture of resilience of executive functioning. Brain Imaging Behav. 2012;6:621–633. doi: 10.1007/s11682-012-9184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Z, Shen L, Sun X, Tong G, Sun D, Han T, Yang G, Zhang J, Cao F, Yao L, Wang H. Variation of NDRG2 and c-Myc expression in rat heart during the acute stage of ischemia/reperfusion injury. Histochem Cell Biol. 2011;135:27–35. doi: 10.1007/s00418-010-0776-9. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 5.Allison RC, Kyle J, Adkins WK, Prasad VR, McCord JM, Taylor AE. Effect of ischemia reperfusion or hypoxia reoxygenation on lung vascular permeability and resistance. J Appl Physiol. 1990;69:597–603. doi: 10.1152/jappl.1990.69.2.597. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6:39–46. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 7.Hiratsuka M, Yano M, Mora BN, Nagahiro I, Cooper JD, Patterson GA. Heat shock pretreatment protects pulmonary isografts from subsequent ischemia-reperfusion injury. J Heart Lung Transplant. 1998;17:1238–1246. [PubMed] [Google Scholar]
- 8.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. HSP70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104:I303–I307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- 9.Hiratsuka M, Mora BN, Yano M, Mohanakumar T, Patterson GA. Gene transfer of HSP70 protects lung grafts from ischemia-reperfusion injury. Ann Thorac Surg. 1999;67:1421–1427. doi: 10.1016/s0003-4975(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 10.Chi W, Meng F, Li Y, Wang Q, Wang G, Han S, Wang P, Li J. Downregulation of miRNA-134 protects neural cells against ischemic injury in N2A cells and mouse brain with ischemic stroke by targeting HSP70. Neuroscience. 2014;277:111–122. doi: 10.1016/j.neuroscience.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhang Y, Li C, Xie J, Liu Y, Zhu W, Zhang X, Jiang S, Liu L, Ding Z. HSP70 attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism. Cardiovasc Res. 2013;99:674–684. doi: 10.1093/cvr/cvt139. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski A, Kasch C, Zhang L, Kumar S, Sponholz C, Choi YH, Ma N, Liebold A, Ladilov Y, Steinhoff G, Stamm C. Endothelial nitric oxide synthase mediates protective effects of hypoxic preconditioning in lungs. Respir Physiol Neurobiol. 2007;155:280–285. doi: 10.1016/j.resp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski A, Pohl CB, Sponholz C, Ma N, Stamm C, Vollmar B, Steinhoff G. Up-regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia-reperfusion in mice. Am J Pathol. 2004;164:2241–2249. doi: 10.1016/S0002-9440(10)63780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia IM, Mazzei L, Benardon ME, Oliveros L, Cuello-Carrión FD, Gil Lorenzo A, Manucha W, Vallés PG. Caveolin-1-eNOS/Hsp70 interactions mediate rosuvastatin antifibrotic effects in neonatal obstructive nephropathy. Nitric Oxide. 2012;27:95–105. doi: 10.1016/j.niox.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Manucha W, Kurban F, Mazzei L, Benardón ME, Bocanegra V, Tosi MR, Vallés P. eNOS/Hsp70 interaction on rosuvastatin cytoprotective effect in neonatal obstructive nephropathy. Eur J Pharmacol. 2011;650:487–495. doi: 10.1016/j.ejphar.2010.09.059. [DOI] [PubMed] [Google Scholar]
- 16.Hwang J, Lee HI, Chang YS, Lee SJ, Kim KP, Park SI. 15-deoxy-Delta12,14-prostaglandin J2-induced down-regulation of endothelial nitric oxide synthase in association with HSP70 induction. Biochem Biophys Res Commun. 2007;357:206–211. doi: 10.1016/j.bbrc.2007.03.127. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breitenstein A, Wyss CA, Spescha RD, Franzeck FC, Hof D, Riwanto M, Hasun M, Akhmedov A, von Eckardstein A, Maier W, Landmesser U, Lüscher TF, Camici GG. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLoS One. 2013;8:e53106. doi: 10.1371/journal.pone.0053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potente M, Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle. 2008;7:2117–2122. doi: 10.4161/cc.7.14.6267. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Du D, Wang H, Liu Y, Lai X, Jiang F, Chen D, Zhang Y, Zong J, Li Y. Silent information regulator 1 (SIRT1) promotes the migration and proliferation of endothelial progenitor cells through the PI3K/Akt/eNOS signaling pathway. Int J Clin Exp Pathol. 2015;8:2274–2287. [PMC free article] [PubMed] [Google Scholar]
- 21.Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol. 2014;306:H1558–H1568. doi: 10.1152/ajpheart.00865.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyton L, Hott M, Acuna F, Caroca J, Nuñez M, Martin C, Zambrano A, Concha MI, Otth C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015;205:63–72. doi: 10.1016/j.virusres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Han JY, Lee S, Yang JH, Kim S, Sim J, Kim MG, Jeong TC, Ku SK, Cho IJ, Ki SH. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015;39:105–115. doi: 10.1016/j.jgr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia N, Forstermann U, Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L, Díaz-Martín J, Dillmann WH, López-Barneo J. Heat shock protein 70 kDa over-expression and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal degeneration in mice. Neuroscience. 2011;193:323–329. doi: 10.1016/j.neuroscience.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Enoki Y, Ishima Y, Tanaka R, Sato K, Kimachi K, Shirai T, Watanabe H, Chuang VT, Fujiwara Y, Takeya M, Otagiri M, Maruyama T. Pleiotropic Effects of Levofloxacin, Fluoroquinolone Antibiotics, against Influenza Virus-Induced Lung Injury. PLoS One. 2015;10:e0130248. doi: 10.1371/journal.pone.0130248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reignier J, Sellak H, Lemoine R, Lubineau A, Mazmanian GM, Detruit H, Chapelier A, Hervé P. Prevention of ischemia-reperfusion lung injury by sulfated Lewis(a) pentasaccharide. The Paris-Sud University Lung Transplantation Group. J Appl Physiol. 1997;82:1058–1063. doi: 10.1152/jappl.1997.82.4.1058. [DOI] [PubMed] [Google Scholar]
- 28.Fisher AB, Dodia C, Tan ZT, Ayene I, Eckenhoff RG. Oxygen-dependent lipid peroxidation during lung ischemia. J Clin Invest. 1991;88:674–679. doi: 10.1172/JCI115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxey TS, Enelow RI, Gaston B, Kron IL, Laubach VE, Doctor A. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:541–547. doi: 10.1016/j.jtcvs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Sener G, Sehirli O, Ercan F, Sirvanci S, Gedik N, Kacmaz A. Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg Today. 2005;35:575–580. doi: 10.1007/s00595-004-2985-0. [DOI] [PubMed] [Google Scholar]
- 31.Ai S, Fan X, Fan L, Sun Q, Liu Y, Tao X, Dai K. Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydr Polym. 2013;94:731–736. doi: 10.1016/j.carbpol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu KX, Wu WK, He W, Liu CL. Ginkgo biloba extract (EGb 761) attenuates lung injury induced by intestinal ischemia/reperfusion in rats: roles of oxidative stress and nitric oxide. World J Gastroenterol. 2007;13:299–305. doi: 10.3748/wjg.v13.i2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marqui CE, Silva HC, Ferez D, Cavassani SS, Moraes JB, Silva DA, Simões RS, Lopes CA, Taha MO, Oliveira-Júnior IS. Pretreatment with pentoxifylline attenuates lung injury induced by intestinal ischemia/reperfusion in rats. Acta Cir Bras. 2011;26:438–444. doi: 10.1590/s0102-86502011000600006. [DOI] [PubMed] [Google Scholar]
- 34.Hamacher J, Stammberger U, Weber E, Lucas R, Wendel A. Ebselen improves ischemia-reperfusion injury after rat lung transplantation. Lung. 2009;187:98–103. doi: 10.1007/s00408-009-9134-x. [DOI] [PubMed] [Google Scholar]
- 35.Beere HM, Green DR. Stress management-heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 36.Yao X, Bai Q, Yan D, Li G, Lu C, Xu H. Solanesol protects human hepatic L02 cells from ethanol-induced oxidative injury via upregulation of HO-1 and Hsp70. Toxicol In Vitro. 2015;29:600–608. doi: 10.1016/j.tiv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 38.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao W, Li M, Li J, Li C, Xu X, Gu W. Geranylgeranylacetone ameliorates lung ischemia/reperfusion injury by HSP70 and thioredoxin redox system: NF-κB pathway involved. Pulm Pharmacol Ther. 2015;32:109–115. doi: 10.1016/j.pupt.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Sun L, Fan H, Yang L, Shi L, Liu Y. Tyrosol prevents ischemia/reperfusion-induced cardiac injury in H9c2 cells: involvement of ROS, Hsp70, JNK and ERK, and apoptosis. Molecules. 2015;20:3758–3775. doi: 10.3390/molecules20033758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang L, Zhang G, Yan Y, Ke K, Wu X, Gao Y, Li J, Zhu L, Wu Q, Zhou Z. The role of HSP70 in regulating neuronal apoptosis. Neurochem Res. 2013;38:311–320. doi: 10.1007/s11064-012-0922-y. [DOI] [PubMed] [Google Scholar]
- 42.Cui Z, Wang P, Sun L, Liu H, Yang J, Li X, Kang L, Huang Y, Shen A, Cheng C. Lipopolysaccharide-evoked HSP70 expression by activation of MAPK cascade in microglial cells of the spinal cord. J Neurol Sci. 2010;294:29–37. doi: 10.1016/j.jns.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Lu W, Cai WW, Wang PJ, Zhang N, Yu CP, Wang DL, Liu BC, Sun W. Telmisartan attenuates monocrotaline-induced pulmonary artery endothelial dysfunction through a PPAR gamma-dependent PI3K/Akt/eNOS pathway. Pulm Pharmacol Ther. 2014;28:17–24. doi: 10.1016/j.pupt.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Qian GQ, Ding J, Zhang X, Yin X, Gao Y, Zhao GP. Preconditioning with glycyrrhizic, ferulic, paeoniflorin, cinnamic prevents rat hearts from ischemia/reperfusion injury via endothelial nitric oxide pathway. Pharmacogn Mag. 2015;11:292–296. doi: 10.4103/0973-1296.153081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinmura K, Tamaki K, Ito K, Yan X, Yamamoto T, Katsumata Y, Matsuhashi T, Sano M, Fukuda K, Suematsu M, Ishii I. Indispensable role of endothelial nitric oxide synthase in caloric restriction-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308:H894–H903. doi: 10.1152/ajpheart.00333.2014. [DOI] [PubMed] [Google Scholar]
- 46.Karpe PA, Tikoo K. Heat shock prevents insulin resistance-induced vascular complications by augmenting angiotensin-(1-7) signaling. Diabetes. 2014;63:1124–1139. doi: 10.2337/db13-1267. [DOI] [PubMed] [Google Scholar]
- 47.Uryash A, Wu H, Bassuk J, Kurlansky P, Adams JA. Preconditioning with periodic acceleration (pGz) provides second window of cardioprotection. Life Sci. 2012;91:178–185. doi: 10.1016/j.lfs.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Aramburo A, Rafikov R, Sun X, Kumar S, Oishi PE, Datar SA, Raff G, Xoinis K, Kalkan G, Fratz S, Fineman JR, Black SM. L-carnitine preserves endothelial function in a lamb model of increased pulmonary blood flow. Pediatr Res. 2013;74:39–47. doi: 10.1038/pr.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallerath T, Li H, Godtel-Ambrust U, Schwarz PM, Forstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. 2005;12:97–104. doi: 10.1016/j.niox.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Tsai KL, Chen LH, Chiou SH, Chiou GY, Chen YC, Chou HY, Chen LK, Chen HY, Chiu TH, Tsai CS, Ou HC, Kao CL. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol Nutr Food Res. 2011;55(Suppl 2):S227–S240. doi: 10.1002/mnfr.201100147. [DOI] [PubMed] [Google Scholar]
- 52.Shin SY, Kim TH, Wu H, Choi YH, Kim SG. SIRT1 activation by methylene blue, a repurposed drug, leads to AMPK-mediated inhibition of steatosis and steatohepatitis. Eur J Pharmacol. 2014;727:115–124. doi: 10.1016/j.ejphar.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 53.Tsai KL, Huang PH, Kao CL, Leu HB, Cheng YH, Liao YW, Yang YP, Chien Y, Wang CY, Hsiao CY, Chiou SH, Chen JW, Lin SJ. Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochem Pharmacol. 2014;88:189–200. doi: 10.1016/j.bcp.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q, Li J, Zhang Q, Gao Y, Gao L, Zhao J. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J Nutr Biochem. 2014;25:1207–1217. doi: 10.1016/j.jnutbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Tamaki N, Cristina Orihuela-Campos R, Inagaki Y, Fukui M, Nagata T, Ito HO. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic Biol Med. 2014;75:222–229. doi: 10.1016/j.freeradbiomed.2014.07.034. [DOI] [PubMed] [Google Scholar]