Abstract
Purpose: This study aims to investigate the expression and clinical significance of p190RhoGAP, a member of the RhoGAP family, in colorectal cancer (CRC). Methods: The expression p190RhoGAP was detected by RT-PCR, western blot (WB) and immunohistochemistry (IHC) in 14 paired CRCs and matched non-cancerous mucosal tissues. The protein content of p190RhoGAP was identified in 114 CRCs by IHC. In addition, the association of the expression of p190RhoGAP with carcinogenesis, distant metastasis and prognosis was further evaluated. Results: In 14 paired fresh tissues, the mRNA (P<0.0001) and protein (P = 0.003) expression levels of p190RhoGAP were significantly higher in primary CRCs than in paired non-cancerous mucosal tissues; and was consistent with WB results. The expression of p190RhoGAP increased from normal mucosa to adenoma, and became even greater in primary carcinoma (P = 0.001). The expression level of p190RhoGAP was highest in liver metastasis compared to primary carcinoma (P = 0.028). The incidence of p190RhoGAP expression-positive cases was 58.77% in 114 CRC tissues. Furthermore, the enhanced expression of p190RhoGAP was significantly associated with shorter disease-specific survival (P<0.001) and shorter disease-free survival (P<0.001). Cox regression analysis indicated that p190RhoGAP was an independent prognostic parameter for CRC. Conclusion: p190RhoGAP may be an independent predictive factor for the prognosis of CRC, and the abnormal expression of p190RhoGAP may play a crucial role in colorectal carcinogenesis and distant metastasis.
Keywords: p190rhogap, colorectal cancer, metastasis, poor survival
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal malignancies, and its incidence has increased rapidly in the past decades [1]. Most cancer deaths including CRC are due to metastases that are resistant to conventional therapies. However, CRC patients are often diagnosed when their cancer has already metastasized or have been considered to be advanced and metastatic [2]. Clinicopathological factors such as TNM stage could not accurately predict results in CRC patients. Therefore, there is an urgent need to identify biomarkers that can help generate personalized treatment strategies, predict responses to therapy, and investigate the mechanism of metastatic dissemination.
p190RhoGAP is a member of the Rho GTPase-activating protein (RhoGAP) family [3], and the p190RhoGAP-encoding gene is located at 19q13.3 of the human chromosome [4]. Acting as molecular switches, RhoGTPases plays a critical role in signal transduction from the extracellular environment to cellular responses such as morphology, gene expression and migration [5,6]. Depending on its GTPase-activating protein domain, the overexpression of p190RhoGAP leads to a multinucleated phenotype; while a transient decrease in endogenous p190 protein could be observed during late mitosis. These observations indicate that p190RhoGAP plays a crucial role in the cell cycle and cytokinesis. Furthermore, the overexpression of p190RhoGAP during the late mitosis of the cell cycle in breast and cervical cancer cells affects cleavage furrow plane specification and subsequent ring ingression, resulting in the multinucleated phenotype [7]. As a major family of transmembrane receptors, cadherins mediate cell-cell adhesion and contact the inhibition of cell growth [8,9]. By dynamically regulating cadherin clustering, the activation of Rac induces p190RhoGAP translocation to cadherin complexes, where it transiently interacts with p120; inducing the local inhibition of Rho and the formation of adherens junctions. Adherens junctions are usually targeted by imbalances during tumor progression in oncogenic signaling. The overexpression of p190RhoGAP decreases RhoA activity, promotes the formation of membrane protrusions, and enhances motility [10,11]. In addition, Brk promotes the formation and progression of breast tumors by inducing p190Y1105 phosphorylation and its downstream events. Meanwhile, p190RhoGAP expression in breast cancer cells can partially rescue inhibitory effects in proliferation and migration by the knockdown of Brk [12]. Lastly, through signaling pathways, the activation of the Rho GTPase family can also be induced by leptin, allowing the Rho GTPase family to aid in regulating cell motility and invasion in human colon cancer cells [13]. All of these observations suggest that the expression of p190RhoGAP can be utilized as a promising diagnostic and/or prognostic marker in CRC.
In order to elucidate the association of p190RhoGAP with the clinicopathological characteristics and survival status of CRCs after surgery, we detected the expression of p190RhoGAP in different colorectal tissues such as normal mucosa, adenoma, primary carcinoma, lymphnodal metastasis and liver metastasis.
Materials and methods
Tissues and patients
Paired samples of non-cancerous adjacent and carcinoma tissues were collected from 14 patients undergoing resection performed by one surgeon between November 2011 and January 2012. All cases were confirmed as colorectal carcinoma by pathological examination. Carcinoma samples were taken from areas of the tumors, and necrotic tissues were avoided. The resection of matched non-cancerous mucosa included tissues located at least 5 cm from the edge of the tumor, in which the dissection depth reached the mucosa and submucosa. Quantitative RT-PCR and immunohistochemistry (IHC) was conducted on these samples to assess p190RhoGAP transcript and expression. Additionally, six paired samples were randomly selected to confirm IHC results by western blot (WB) analysis.
The 164 formalin-fixed paraffin-embedded tissue samples used for IHC analysis were collected from 114 CRC patients who underwent surgery in 2005. These samples were grouped as follows: normal mucosa (n = 14), adenoma (n = 14), primary carcinoma (n = 114), lymphnode metastasis (n = 14) and liver metastasis tissue (n = 8). Matching normal tissues and adenoma tissues were available in 14 of the primary carcinoma tissues. In addition, all normal mucosa and adenoma tissues were collected from the 114 CRC patients. Similarly, there were matching lymph node metastasis tissues for the 14 primary carcinomas and matching liver metastasis tissues (from 13 patients with liver metastases that underwent hepatectomy in one stage or by stages) for eight primary carcinomas. Primary carcinomas were assessed according to the 7th Edition American Joint Committee on Cancer (AJCC) staging system. Patients were followed up from the time of primary surgery until death or January 2011. Median follow-up time for survivors was 52.23 months (range, 2.87-70.57 months).
All data and tissues of CRC patients were collected from the Affiliated Tumor Hospital of Harbin Medical University. No patients received preoperative radiotherapy or chemotherapy. This study was approved by the Ethics Committee of the Affiliated Tumor Hospital of Harbin Medical University, Harbin, China. All donors or the next of kin provided written informed consent for the use of tissue samples for this study.
Quantitative RT-PCR
Total RNA from tissue specimens preserved in liquid nitrogen was extracted with RNA-dsoI (Takara, Japan). First-strand cDNA was synthesized from total RNA using a Prime Script RT reagent Kit (Takara, Japan). In order to quantify mRNA expression, RT-PCR was performed with the ABI7500 using the SYBR Premix Ex Taq II (Takara, Japan), according to manufacturer’s protocol. Primer sequences for each gene were as follows: p190RhoGAP, forward: 5’-CCAAAGGAAAACCACGAAGT-3’, reverse: 5’-GGGCACTGCTGGATAAAGAG-3’; GAPDH, forward 5’-GAAGGTGAAGGTCGGAGTC-3’, reverse 5’-GAAGATGGTGATGGGATTTC-3’. Human GAPDH gene was used as an endogenous control. Relative mRNA levels were calculated on the basis of Ct values corrected by GAPDH mRNA expression, according to the equation: 2-ΔCt [ΔCt = Ct (p190RhoGAP) - Ct (GAPDH)].
Immunohistochemistry
The formalin-fixed, paraffin-embedded sections (4 µm) were deparaffinized, rehydrated and quenched with 3% H2O2 for 10 minutes. Antigen retrieval was performed by autoclave in 10 mM of sodium citrate buffer (pH 6.0) for three minutes at 121°C. After washing with PBS, the sections were incubated with p190RhoGAP antibody (Abnova, diluted at 1:150) overnight at 4°C. Then, the sections were incubated with secondary antibody for 20 minutes, and the reaction products were visualized with diaminobenzidine and counterstained with hematoxylin. The primary antibody was omitted for negative controls. All samples were reviewed by two independent pathologists, who are experienced in conducting IHC analyses and blinded to the clinical outcome of these patients. The expression of p190RhoGAP was semi-quantitatively determined by assessing the percentage of positively stained immunoreactive cells and staining intensity. The percentage of immunoreactive cells was rated as follows: 0 points, <10%; 1 point, 10-50%; 2 points, >50%. Staining intensity was rated as follows: 0 (no staining or weak staining = light yellow), 1 (moderate staining = yellow brown), and 2 (strong staining = brown). The overall score for p190RhoGAP expression was the sum of points determined for the percentage of positively stained immunoreactive cells and their expression, and an overall score ranging from 0 to 4 was assigned. For statistical analysis, patients were divided into two groups: negative (low expression) group and positive (high expression) group. An overall score between 0 and 2 was defined as negative, and an overall score between 3 and 4 was defined as positive. Independent scores assigned by two pathologists were combined into a final score, which was reported in this study. Any differences in the scores were resolved by discussion between these two pathologists.
Western blot
All tissues were washed with PBS and resuspended in RIPA lysis buffer. Lysates were centrifuged for 15 minutes at 4°C, and protein content of the supernatant was determined using the DC protein assay reagents package (Bio-Rad Laboratories, CA, USA). Aliquots of these proteins were separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes purchased from Bio-Rad Laboratories (CA, USA). For WBs, membranes were incubated with antibodies. Antibodies against p190RhoGAP was purchased from BD Biosciences (CA, USA), and β-actin was obtained from Cell Signaling Technology (Danvers, MA). Bands were visualized using the ECL™ Western Blotting Detection Reagent (GE Healthcare, UK).
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 for Windows. Chi-square test, Fisher’s exact test and Student’s t-test were used for comparison between groups. If variances in groups were not homogeneous, the Mann-Whitney U-test was used. Kaplan-Meier curves were plotted to assess the association of p190RhoGAP expression with disease-specific survival (DSS) and disease-free survival (DFS) rates. Survival curves were compared using log-rank test. Cox regression analysis was performed to evaluate the statistical power of individual covariates as predictor of unfavorable prognosis. P<0.05 was considered statistically significant.
Results
Enhanced expression of p190RhoGAP in CRCs compared with matched non-cancerous mucosa
In order to evaluate the mRNA expression of p190RhoGAP, quantitative RT-PCR was performed in 14 primary CRC tissues and 14 matched non-cancerous tissues. Results revealed that mRNA levels of p190RhoGAP significantly increased in primary CRCs compared to non-cancerous mucosa (P<0.0001, Figure 1A). Furthermore, IHC revealed that the protein content of p190RhoGAP in primary CRCs was also considerably higher than in matched non-cancerous tissues (P = 0.003, Figure 1B). In addition, six of the above paired tissues were also analyzed for p190RhoGAP protein expression by WB. Increased expression levels of p190RhoGAP were detected in all cancers, except for one sample, which remained consistent with IHC results. Both primary CRC tissues and matched non-cancerous tissues did not indicate any expression of p190RhoGAP, compared with corresponding adjacent non-cancerous mucosal tissues (Figure 1D).
Figure 1.
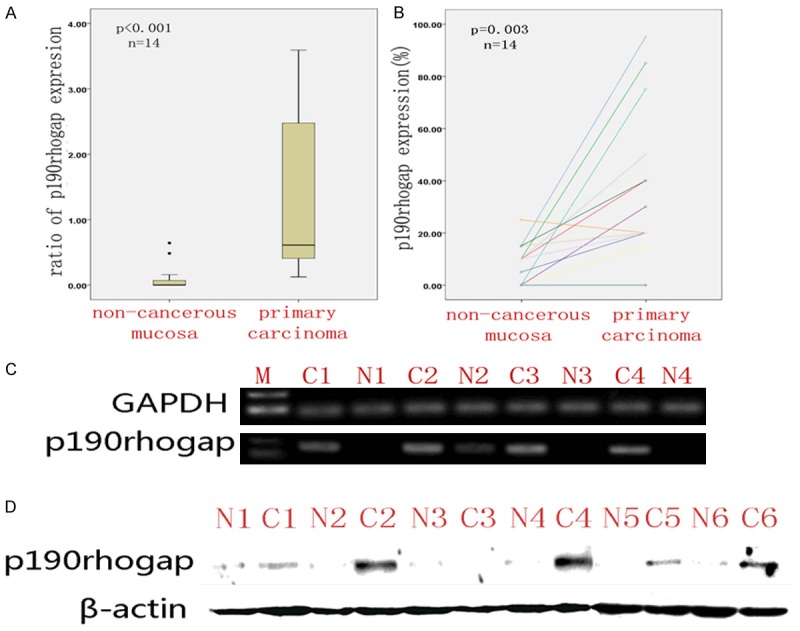
Increased p190rhogap expression in primary carcinoma (C) compared with non-cancerous mucosa (N). A: Quantitative RT-PCR analysis of p190rhogap mRNA expression. B: IHC analysis of p90rhogap protein expression in paired tissues. C: Electropherogram analysis of p190rhogap mRNA (selected from quantitative RT-PCR randomly) expression. D: Western blotting analysis of p190rhogap protein expression. β-Actin is the loading control.
p190RhoGAP expression increased during tumorigenesis
In order to validate whether p190RhoGAP activation is involved in CRC tumorigenesis, the expression of p190RhoGAP was examined by immunostaining the paired adenomas and normal mucosa tissues. It was found that p190RhoGAP staining was localized within the cytoplasm of colorectal epithelial cells (Figure 2A-C). The expression of p190RhoGAP significantly increased in adenomas compared to normal mucosa (P = 0.031), which became even higher in carcinomas (P = 0.001); although there was no significant difference between these expression in adenomas and carcinomas (P = 0.150, Figure 2D).
Figure 2.
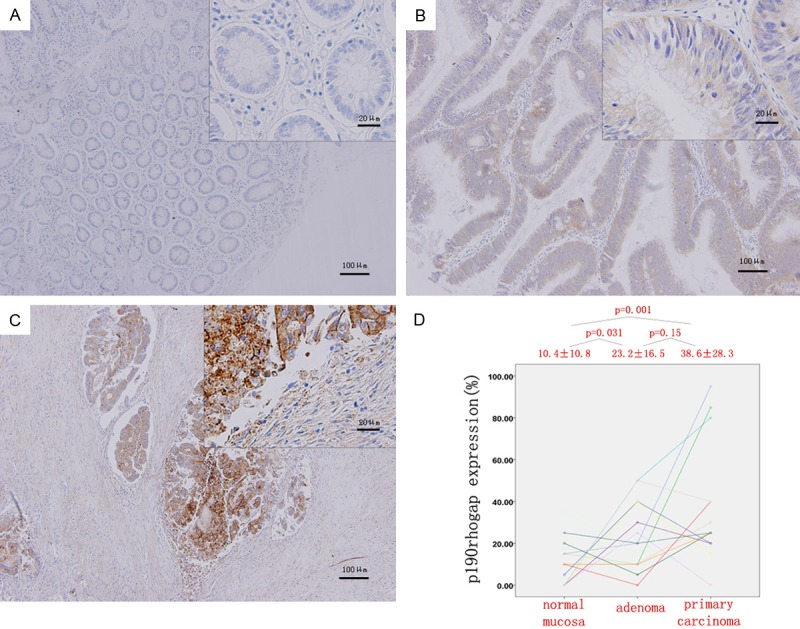
Quantitative analysis of the IHC staining in CRC. (A-C) p190rhogap staining was localized within the cytoplasm of colorectal epithelial cells. (D) The p190rhogap expression increased significantly from normal mucosa (A) to adenomas (B) and from normal mucosa (A) to carcinomas (C) though no significantly up-regulation from adenomatous (B) to cancerous (C) tissue. Magnification, 100× and 400×.
Increased expression of p190RhoGAP in metastatic tissues compared to CRC lesions
IHC assay was also performed in metastatic tissues in lymph nodes and livers. Compared with primary CRC lesions, lymph node and liver metastatic tissues broadly and intensively expressed abundant p190RhoGAP protein (Figure 3A). The mean value of p190RhoGAP expression in liver metastatic tissues, but not in lymph node tissues, was significantly higher than in paired primary CRC lesions (P = 0.028, Figure 3B).
Figure 3.
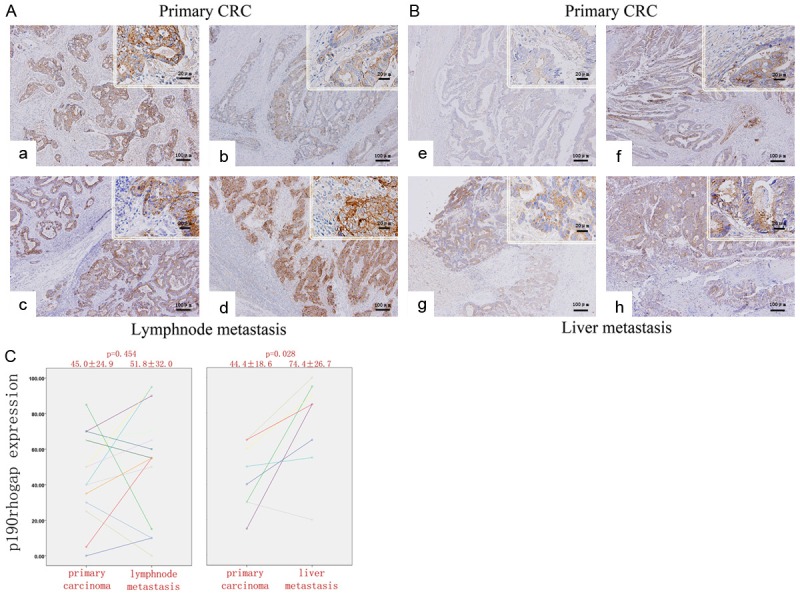
Increase of p190rhogap expression during metastasis. (A) Immunostaining of metastatic lesions and paired primary CRC: (a, b) primary CRC and (c, d) lymph node metastasis. (B) (e, f) primary CRC and (g, h) liver metastasis. (C) Quantitive analysis of the p190rhogap expression in paired primary CRC and lymphoid (left panel) and liver (right panel) metastatic tissues. The p190rhogap expression significantly increased in liver metastatic tissues. Magnification, 100× and 400×.
Abnormal expression of p190RhoGAP and clinicopathologic characteristics in CRC
The correlation between p190RhoGAP expression and the clinicopathologic characteristics of CRC patients were analyzed (Table 1). The expression of p190RhoGAP was highly correlated with grade (includes: low-grade, well and moderately differentiated; high-grade, poorly differentiated and undifferentiated) (X 2 = 4.429, P = 0.035), pT (X 2 = 8.480, P = 0.027), pN (X 2 = 7.053, P = 0.008), AJCC stage (X 2 = 10.931, P = 0.011), and Ki-67 expression (X 2 = 7.384, P = 0.007). However, there was no significant association between the expression of p190RhoGAP with age, gender, tumor location, tumor size, pM or adjuvant therapy after operation.
Table 1.
p190rhogap staining in tumor cells and associations with clinicopathologic characteristics
| Variable | No. | P190RhoGAP | χ2 | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Negative | Positive | ||||
| Age (years) | 1.081 | 0.298 | |||
| <60 | 60 | 28 | 32 | ||
| ≥60 | 54 | 20 | 34 | ||
| Gender | 0.726 | 0.394 | |||
| Male | 67 | 26 | 41 | ||
| Female | 47 | 22 | 25 | ||
| Location | 1.215 | 0.27 | |||
| Colon | 62 | 29 | 33 | ||
| Rectum | 52 | 19 | 33 | ||
| Tumor size (cm) | 0.521 | 0.471 | |||
| <5 | 52 | 20 | 32 | ||
| ≥5 | 62 | 28 | 34 | ||
| Grade | 4.429 | 0.035* | |||
| Low | 70 | 38 | 32 | ||
| High | 44 | 15 | 29 | ||
| pT classification | 8.48 | 0.027* | |||
| T1 | 3 | 3 | 0 | ||
| T2 | 17 | 11 | 6 | ||
| T3 | 29 | 10 | 19 | ||
| T4 | 65 | 24 | 41 | ||
| pN classification | 7.053 | 0.008* | |||
| N0 | 57 | 31 | 26 | ||
| N1-N2 | 57 | 17 | 40 | ||
| pM classification | 1.387 | 0.232 | |||
| M0 | 101 | 45 | 56 | ||
| M1 | 13 | 3 | 10 | ||
| AJCC stage | 10.931 | 0.011* | |||
| I | 16 | 12 | 4 | ||
| II | 41 | 19 | 22 | ||
| III | 44 | 14 | 30 | ||
| IV | 13 | 3 | 10 | ||
| Ki-67 expression | 7.384 | 0.007* | |||
| Negative | 59 | 32 | 27 | ||
| Positive | 55 | 16 | 39 | ||
| Adjuvant therapy after operation | 1.888 | 0.169 | |||
| NO | 70 | 33 | 37 | ||
| YES | 44 | 15 | 29 | ||
p<0.05.
p190RhoGAP expression correlated with unfavorable survival outcome
Furthermore, the prognostic value of p190RhoGAP expression on DSS in all patients (n = 114) and DFS in patients with curative resection (n = 101, except for cases that underwent palliative surgery) were evaluated. Log-rank test revealed that both DSS and DFS time were significantly different between the p190RhoGAP-negative and p190RhoGAP-positive groups (62.68 ± 2.28 vs. 46.67 ± 2.88, P<0.001; 64.20 ± 1.95 vs. 45.18 ± 3.23, P<0.001; respectively). As shown in Figure 4, the cumulative 5-year DSS and DFS rates of p190RhoGAP-negative patients were 43.4% and 42.4%, while rates in the positive groups were 79.8% and 84.2%, respectively. In addition, based on Cox regression analysis results, p190RhoGAP expression was regarded as an independent prognostic indicator of survival (Table 2). Therefore, our findings demonstrated that the expression of p190RhoGAP in CRC tumors might be associated with increased metastatic potential and poor survival outcome.
Figure 4.
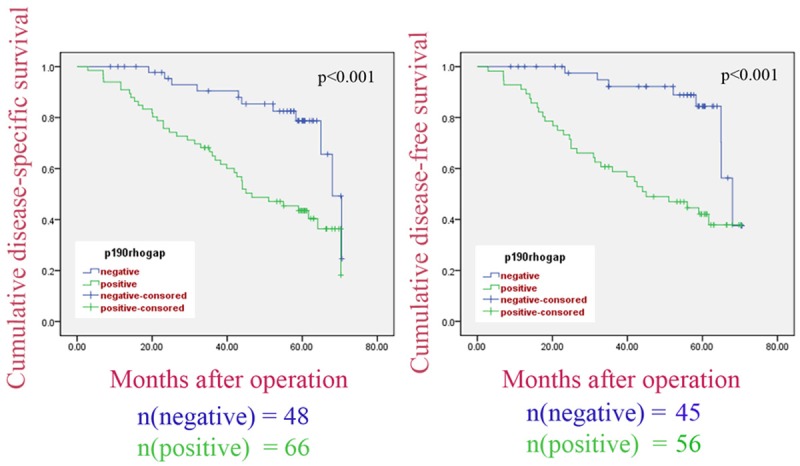
DSS and DFS periods in CRC patients. The p190rhogap-positive group showed significantly shorter DSS and DFS compared with the p190rhogap-negative group.
Table 2.
Multivariate Analyses of Factors Associated With DSS and DFS
| Survival | Category | HR (95% CI) | p-value |
|---|---|---|---|
| Disease-specific survival | |||
| p190rhogap | negative vs. positive | 2.472 (1.202-5.084) | 0.014 |
| AJCC stage | I-II vs. III-IV | 3.295 (1.684-6.448) | 0.0005 |
| ki-67 | negative vs. positive | 3.787 (1.776-6.893) | 0.0003 |
| Disease-free survival | |||
| p190rhogap | negative vs. positive | 3.142 (1.404-7.031) | 0.005 |
| AJCC stage | I-II vs. III-IV | 3.395 (1.671-6.897) | 0.001 |
| ki-67 | negative vs. positive | 4.454 (1.988-7.657) | <0.0001 |
Discussion
By regulating the activity of the Rho family, p190RhoGAP plays an important role in various cellular processes including cell adhesion, proliferation, migration, transformation and cytokinesis; which varies tremendously in different cancer cells [7,14]. However, not all results of p190RhoGAP expression in different cases are consistent. For example, the tyrosine phosphorylation of p190RhoGAP induced by endogenous Brk, which is overexpressed in cancer cells, play indispensable roles in both migration and proliferation. Indeed, the disruption of p190RhoGAP phosphorylation by the p190/p120 complex in breast cancer cells not only results in a defect of the Brk-induced regulation of RhoA and Ras, but also eliminates the stimulatory effects of Brk on cell proliferation, invasion, transformation, migration and tumorigenesis [12]. On the other hand, p190RhoGAP can inhibit PDGF-induced glioma by downregulating Rho activity [3]. In order to identify whether p190RhoGAP plays an oncogenic or inhibitory role in the progression and metastasis of CRC, RT-PCR, IHC and WB were used to detect the expression of p190RhoGAP in different colorectal tissues.
In order to evaluate the regulatory activity of p190RhoGAP in cell growth, p190RhoGAP mRNA and protein expression levels were detected in 14 paired fresh CRC tissues via quantitative RT-PCR, IHC and WB. We found that the expression of p190RhoGAP in CRC tissues was higher than in matched non-cancerous tissues at both mRNA and protein levels. These data suggests that the upregulation of p190RhoGAP occurred not only at the transcriptional level, but also at the post-transcriptional stage. Thus, these results indicate that abnormal p190RhoGAP activation may play an oncogenic role in the development of CRC.
Furthermore, in order to investigate whether alterations of p190RhoGAP expression occurred in malignant transformation and metastasis compared to normal tissues, we collected different tissues from CRCs patients including normal mucosa, adenoma, carcinoma, lymph node metastasis and liver metastasis. The expression of p190RhoGAP significantly increased in adenoma and carcinomas tissues compared to normal mucosa, although no significant difference was observed between adenoma and carcinoma tissues. This observation suggests that p190RhoGAP activation increased during colorectal carcinogenesis, which initially occurs during the transformation from normal tissue into adenoma. Meanwhile, metastatic lesions of the liver, and not of the lymph node, broadly expressed abundant p190RhoGAP expression, compared with paired primary CRC tissues. Our results suggest that the activation of p190RhoGAP may act as a promoter in tumor progression and metastatic dissemination to the liver. These results may be explained as follows. (1) p190RhoGAP positive colorectal cancer cells have a specific adhesion effect and affinity to vascular endothelial cells and metastatic tissues. (2) The expression of p190RhoGAP in metastatic foci might be correlated to its metastatic distance. Most of the lymph nodes that have been removed are the first and second station lymph nodes, which are relatively closer to the original focus compared to hepatic metastasis. (3) p190RhoGAP positive cell proliferation and metastasis are affected by differences in growth factors in the entrails microcirculation. (4) Differences in quantity and immunity of immune cells in different tissues may also influence cell growth and apoptosis. However, all these hypotheses require more research and experiments to be verified.
In order to further confirm our hypothesis, we analyzed the correlation between p190RhoGAP expression and the clinical characteristics of CRC. Our results demonstrate that the expression of p190RhoGAP significantly correlated with grade, pT, AJCC stage and Ki-67 expression; while there was no considerable difference in p190RhoGAP expression in CRC patients categorized according to age, gender, tumor location, tumor size, pM or adjuvant therapy after operation. These data suggest that p190RhoGAP can be used as a molecular marker to identify subsets of CRC patients with more aggressive diseases. Additionally, Kaplan-Meier analysis and Cox regression analysis results confirm that p190RhoGAP expression correlates with the risk of cancer-related deaths after surgery. Therefore, p190RhoGAP expression may be used as an independent prognostic indicator of survival in CRC patients.
Based on these results, we can generate some hypothesis as follows: p190RhoGAP expression plays a positive role in the progression and metastasis of CRC. However, it remains unclear whether the overexpression of p190RhoGAP could represent a genetically, biologically and clinically distinct type of CRC with high malignancy and metastasis.
Most cancer-related deaths are due to metastasis, a process with a long series of sequential and interrelated steps. Meanwhile, metastasis is usually resistant to conventional therapies [15]. The invasion of cancer cells, which is one of the main steps in the formation of metastasis, requires adhesion, proteolysis and migration; and is regulated by various signaling molecules [16]. These molecules include the p190RhoGAP-regulated Rho family [11]. A number of environmental factors regulate the motility of cancer cells by stimulating intracellular responses, which include the polarization and reorganization of the actin-cytoskeleton [17]. The expression and activity of focal adhesion kinase (FAK) plays an important role in regulating fibronectin-stimulated Src-PTK activation and cell motility [18,19].
FAK is also a key mediator of p190RhoGAP localization and tyrosine phosphorylation, which links p190RhoGAP in regulating the polarity of cells in mouse embryonic fibroblasts, endothelial cells and colon carcinoma cells. It was reported that increased levels of p120RasGAP is important for migration, and the formation of the p120RasGAP-p190RhoGAP complex is related to actin-cytoskeleton structural changes and directed cell movement. Additionally, the SH2-SH3-SH2 region of p120RasGAP also plays a role to link FAK to p190RhoGAP [20,21]; and this may lead to the elevation of Ras activity by reducing p120RasGAP. Importantly, it has been reported that Brk-induced p190Y1105 phosphorylation promotes the binding of p190RhoGAP to p120RasGAP, and result in the activation of Ras by sequestrating p120RasGAP. Ras is an oncogene that has been studied to a great extent for the past years [12]. Therefore, p190RhoGAP may contribute to tumorigenesis by regulating Ras activity.
In summary, the present study suggests that the abnormal expression of p190RhoGAP may play an important role in promoting the progression and metastasis of CRC. Furthermore, p190RhoGAP may be applied as an independent diagnostic and/or prognostic marker in CRC patients. However, further studies need to be performed to better understand the relationship between p190RhoGAP expression and liver metastasis in CRC, and to determine whether the inactivation of Rho, the activation of Ras, or some other functional change(s) regulated by p190RhoGAP play a dominant role in tumor progression and metastasis in CRC.
Acknowledgements
This research was supported by grants from Educational Commission of Heilongjiang Province, China (No. 1153H13).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Wolf RM, Draghi N, Liang X, Dai C, Uhrbom L, Eklö C, Westermark B, Holland EC, Resh MD. P190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human Chromosome 19q13.3. Genes Dev. 2003;17:476–487. doi: 10.1101/gad.1040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 5.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Sastry SK, Burridge K. Focal adhesions: a nexus forintracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 7.Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 10.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-Catenin and p190RhoGAP Regulate Cell-Cell Adhesion by Coordinating Antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Arthur WT, Burridge K. RhoA Inactivation by p190RhoGAP Regulates Cell Spreading and Migration by Promoting Membrane Protrusion and Polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen CH, Chen HY. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer. 2008;123:2543–2556. doi: 10.1002/ijc.23821. [DOI] [PubMed] [Google Scholar]
- 14.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 16.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Bernard-Trifilo JA, Lim Y, Lim ST, Mitra SK, Uryu S. Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene. 2008;27:1439–1448. doi: 10.1038/sj.onc.1210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP -p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni SV, Gish G, van der Geer P, Henkemeyer M, Pawson T. Role of P120 Ras-Gap in Directed Cell Movement. JCB. 2000;149:457–470. doi: 10.1083/jcb.149.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]


