Abstract
Transforming growth factor-β1 (TGF-β1) and inflammation play important roles in the cardiac fibrosis development associated with myocardial infarction (MI). Puerarin is wildly used for treatment of diabetes, cardiovascular disease and cerebrovascular disease in China, and recently some studies have shown its anti-cardiac fibrotic effect on myocardial hypertrophy. The purpose of our study was to determine whether puerarin has an anti-cardiac fibrotic effect after MI and find the potential mechanism. A mouse model of MI was established by standard LAD coronary artery ligation, and cardiac fibrosis was confirmed by Masson’s staining and the expression of collagen I, III and α-SMA. The expression level of F4/80 (macrophage/monocyte marker in mouse), monocyte chemoattractant protein (MCP)-1 and TGF-β1 in cardiac tissue treated with or without puerarin was evaluated by immunohistochemistry analysis, enzyme-linked immunosorbent assay (ELISA) and quantitative polymerase chain reaction (qPCR). The downstream protein phospho-Smad (small mother against decapentaplegic) 2/3 was evaluated by westernblot. The results displayed that puerarin could inhibit the recruitment and activation of monocytes/macrophages, decrease the expression of TGF-β1 in the cardiac tissues, and consequently significantly attenuated cardiac fibrosis after MI. Our results also displayed a strong positive correlation between MCP-1 and TGF-β1 expression in MI. Thus, this study revealed the mechanism by which prevented cardiac fibrosis after MI through a decrease in MCP-1 expression and an inhibition TGF-β1 pathway, and indicated puerarin could be a potential agent in attenuating MI-induced cardiac fibrosis.
Keywords: Puerarin, monocyte chemoattractant protein (MCP-1), transforming growth factor-β1 (TGF-β1), cardiac fibrosis, myocardial infarction (MI)
Introduction
Maladaptive pathological changes lead to heart failure in the cardiac remodeling process after myocardial infarction (MI), including cardiomyocyte hypertrophy, enhanced inflammation and fibrosis. Several evidences indicated that cardiac fibroblasts proliferation and excessive extracellular matrix (ECM) deposition induced myocardial fibrosis and contributed to ventricular dysfunction, ventricular dilation and heart failure [1]. Myofibroblast was transformed from fibroblasts and other cell types and played essential roles in fibrosis [2,3]. However, the exact etiology of myocardial fibrosis and available pharmacological interventions are eliminated remains poorly investigated [4-6]. Therefore, there is an urgent need to seek novel therapeutic strategies for improving functional recovery from myocardial fibrosis.
Previous intensive studies have paid attention to the TGF-β-induced ECM and fibrosis in various diseases, including cardiac fibroblast-myofibroblast transition [7,11]. TGF-β1 function was mediated by TGF-β type I and type II receptors. Activation of this receptor complex leads to Smad2/Smad3 phosphorylating, binding to Smad4 and then entering the nucleus. Nuclear Smad2/3 complex activates transcription of α-SMA and collagen I [12,13]. In addition, fibrosis typically results from simultaneous chronic inflammation, tissue remodeling during repair processes [14,15]. Therefore, therapies that target the inflammatory response or TGF-β1 signaling pathways might effectively attenuate the progression of fibrosis in cardiac remodeling.
Interestingly, various traditional Chinese medicine materials have been shown to safely suppress the pro-inflammatory and pro-fibrotic pathway and control myocardial fibrosis in several studies [16,18]. Puerarin, [7-hydroxy-3- (4-hydroxyphenyl)-1-benzopyran-4-one-8- (β-D-gluco-pyranoside)], the major bioactive ingredient derived from the pueraria lobata (ohwi), has been widely used for thousands of years in traditional Chinese medicine. With few side effects, it is used to treating cardiovascular disease, cerebrovascular disease, diabetes and diabetic complications [19]. Recently, several reporters demonstrated it had healing and anti-fibrotic effects on cardiac remodeling through its anti-inflammatory, anti-oxidant effects [20,22].
In light of above points, we supposed that puerarin could abolish cardiac fibrosis by inhibition of TGF-β1 signaling pathways and inflammatory responds. For this purpose, we developed a mouse model to investigate whether puerarin is a potential therapeutic compound for cardiac fibrosis induced by MI.
Materials and methods
Drugs
Injections, made of the extract of puerarin (98% purity as determined by high-performance liquid chromatography analysis) were from Shanghai Winherb Medical S&T Development Co. Ltd. (Shanghai, China).
Animal groups
C57BL/6J male mice, weight from 18 to 22 g were bought from the animal center of Nanjing Medical University Animal Centre. All protocols of animal experiments were approved by the Animal Care and Use Committee of Nanjing Medical University. Mice myocardial infarction (MI) model was generated by surgical ligation of LAD coronary artery according to previous description [23,24]. Briefly, after initial anesthesia with pentobarbital sodium (50 mg/kg), mice were incubated with a tracheal cannula. Then the chest cavity was opened to expose the heart, and the 8-0 silk suture was used for permanent ligation of LAD coronary artery. The suture was passed approximately 2-3 mm below the tip of the left auricle. The thorax was closed layer by layer with 6-0 silk suture. MI was confirmed by the pale color of the anterior wall of the left ventricle (LV). Fifty survived MI mice and 10 sham mice (thoracotomy without ligation) were randomly divided into six groups: Sham group (sham mice injected intraperitoneally with equal volume of saline for 28 days, n=10); Sham + puerarin group (injected intraperitoneally with 200 mg/kg/day puerarin for 28 days, n=10); Myocardial infarction group (MI mice injected intraperitoneally with equal volume of saline for 28 days, n=10); MI+P50 group (MI mice injected intraperitoneally with 50 mg/kg/day for 28 days, n=10); MI+P100 group (MI mice injected intraperitoneally with 100 mg/kg/day for 28 days, n=10); MI+P200 group (MI mice injected intraperitoneally with 200 mg/kg/day for 28 days, n=10). The treatment was started at 48 h from MI operation. After 4 weeks of MI, the animals of each group were sacrificed by carbon dioxide. The hearts were taken for further examination. For histology study, the hearts were perfused with 20 ml of 0.01 M PBS and followed by 30 ml of 4% paraformaldehyde after anesthetized. The myocardial samples were then dipped into 4% paraformaldehyde for another 24 h at 4°C and embedded in paraffin.
Histology
The 5 μm-thick sections of paraffin embedded myocardial samples were dewaxed and rehydrated in xylene and ethyl alcohol and washed in tap water. The quantification for cardiac fibrosis was used by routine Masson’s Trichrome Staining.
Immunohistochemistry
For immunohistochemical staining, paraffin embedded myocardial samples were dewaxed and rehydrated in xylene and ethyl alcohol followed by incubation in 0.3% methanol/H2O2 to block endogenous peroxidases. Antigen retrieval was performed by boiling slides in a 10 mM citrate pH 6.0 solution for 20 min using a microwave oven. Next, sections were incubated overnight at 4ºC with the primary antibodies. Two-step technique (SuperPictureTM3rd Gen IHC Detection kit; Invitrogen, CA, USA) was used for visualization, with 3,3’-diaminobenzidine (DAB, 0.1 mg/ml, 0.02% H2O2) (Vector Laboratories, Burlingame, USA) as a chromogen and sections were counterstained with haematoxylin. Primary antibodies for immunohistochemistry were as follows: MCP-1, F4/80 (Macrophages/Monocytes markers in mouse) (Millipore Corporation, MA, USA), α-SMA, Collagen I (Col 1), Collagen III (Col 3) (Abcam, MA, USA) and TGF-β1 (Santa Cruz, CA, USA).
Western blot analysis
Cardiac tissues were homogenized in RIPA buffer and the protein concentrations were determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA). Total 30 μg proteins was separated on a 10% SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.). After blocking with 5% non-fat dry milk, the membranes were incubated with primary antibodies, then with corresponding secondary anti-mouse or anti-rabbit IgG peroxidase (1:5000, Santa Cruz Biotechnology, Texas, USA) at room temperature for 50 min. The primary antibodies were used as followed: p-Smad 2/3, Smad2/3, GAPDH (1:1000, Cell Signaling Technology, Boston USA), α-SMA (1:1000, Abcam, MA, USA), Collagen I, Collagen III, (1:500, Santa Cruz Biotechnology, Texas, USA). The bands were visualized by an ECL kit (Millipore). Images were quantified by densitometry. The results were normalized to GAPDH. All experiments were repeated for 3 times.
RNA preparation and quantitative real-time PCR
Total RNA in cardiac tissues was isolated with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Ottawa, Canada) refer to the manufacturer’s instructions. The random RT primer 5’- (dN) 9-3’ was used for MCP-1, TGF-β and GAPDH reverse transcription. The polymerase chain reaction (PCR) was performed using an SABI SYBR Green Master Mix (Invitrogen) according to the manufacturer’s protocol. Primers were designed as follows: TGF-β: Sense: 5’-TTCCTGGCGTTACCTTGGT-3’; Anti-sense: 5’-TACGCCTGAGTGGCTGTCTTTTGA-3’; MCP-1: Sense: 5’-CGCCTCCAGCATGAAAGTCT-3’; Antisense: 5’-GGGAATGAAGGTGGCTGCTA-3’; GAPDH: Sense: 5’-TGCACCACCAACTGCTTAGC-3’; Antisense: 5’-GGCATGGACTGTGGTCATGAG-3’. All mRNA quantification data were normalized to GAPDH as an endogenous control for the mRNA detection. The data were processed using 2-ΔΔCt methods.
Concentration of inflammatory cytokines
Cardiac tissues were homogenized in Iscove’s protein medium using a polytron and centrifuged twice at 10,000 × g at 4°C for 15 min. The supernatants were removed and protein concentrations were determined by BCA method. The cytokine levels were measured using mouse MCP-1 and TGF-β1 ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions and all samples were run in duplicate and expressed as a pg per 100 μg of total protein.
Statistical analysis
Data were expressed as mean ± SD. Differences between groups were analyzed by Student’s t test or by one-way analysis of variance (One-way ANOVA). P < 0.05 was considered statistically significant. Data were analyzed using the GraphPad Prism 5.0.
Results
Effect of puerarin on cardiac fibrosis in MI mice
According to the data from the Masson’s trichrome staining analysis, fibrosis was dramatically increased in both infarcted and border zone in MI mice compared to the sham and puerarin alone animals. The MI mice treated with the middle and high doses of puerarin exhibited significantly ameliorated fibrosis in both infarcted and border zone compared to MI mice (Figure 1). However, puerarin treatment alone did not affect the morphology and fibrosis of the cardiac tissues. Immunohistochemical staining and westernblot revealed deposition of extracellular matrices, including type I and III collagen and activated fibroblast (increased α-SMA-positive staining and expression) was extensive in MI mice and decreased in MI mice treated with a high dose and middle dose of puerarin (Figure 2). These results indicated that treatment with puerarin both in middle and high doses of puerarin could reduce the cardiac fibrosis in vivo. Based on these results, we chose the high dose of puerarin for subsequent experiments to determine the underlying mechanisms of these effects.
Figure 1.
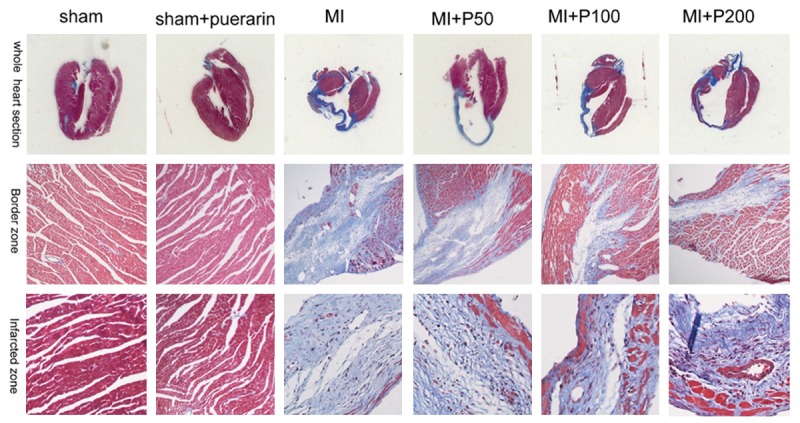
Micrograph of Masson’s Trichrome Staining in mouse myocardium. The fibrotic area in MI model increased dramatically compared with that in the sham and puerarin treated alone groups both in infracted zone and border zone. The level of fibrosis was reduced in those treated with puerarin in middle and high dose groups.
Figure 2.
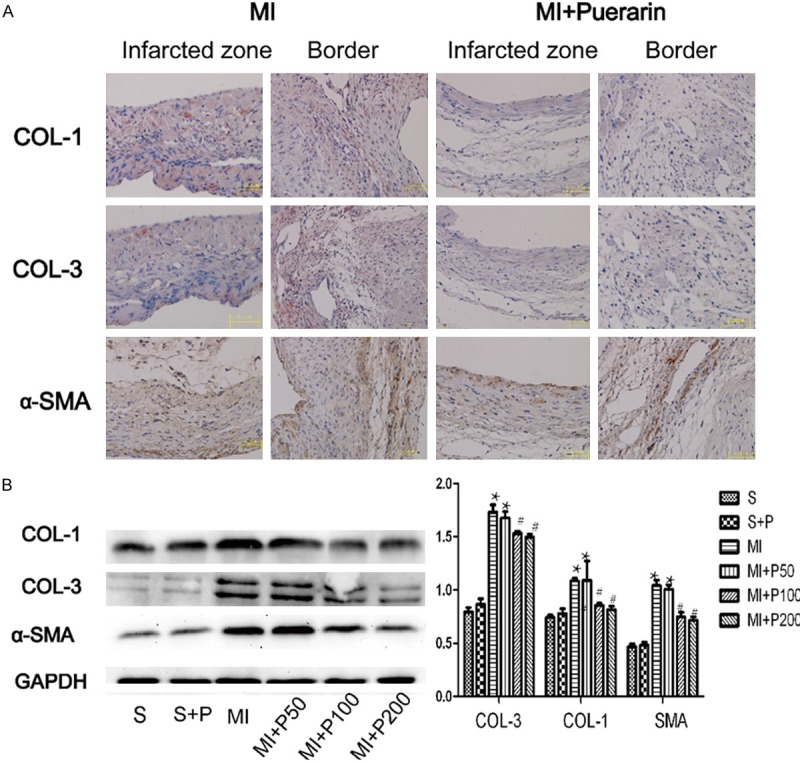
The deposition of extracellular matrices in the MI myocardium treated with or without puerarin. A. Micrograph of Collagen I, III and α-SMA immunohistochemical staining in MI (left panel) and MI plus high dose puerarin treatment (right panel). B. Representative micrograph of expression of Collagen I, III and α-SMA in westernblot analysis and quantitative results. #P < 0.05 VS MI, *P < 0.05 VS sham group, n=5.
Effect of puerarin on MCP-1 expression and macrophage recruitment in MI mice
Compared to the MI mice, the number of MCP-1 positive cells was significantly decreased in the border zone of the cardiac tissue from MI mice treated with a high dose of puerarin (Figure 3A). These results were confirmed by qPCR (Figure 3B) and ELISA (Table 1). As the result of MCP-1 production, the increased number of anti-F4/80 positive cells (macrophage recruitment) in the MI mice was also reduced in the MI mice treated with a high dose of puerarin (Figure 3A). These results indicated that puerarin effectively reduced MCP-1 expression and macrophage recruitment in the MI cardiac tissues.
Figure 3.
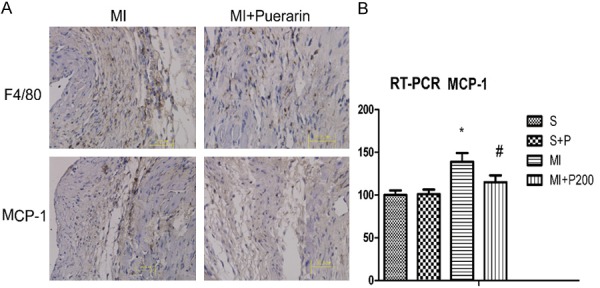
A. Micrograph of MCP-1 and F4/80 immunohistochemical staining in the MI (left panel) and MI plus high dose puerarin treatment (right panel). B. Real-time qPCR quantification of MCP-1. All data are shown as means ± SD and experiments were repeated three times. #P < 0.05 VS MI, *P< 0.05 VS sham group, n=5.
Table 1.
Concentration of inflammatory cytokines in the myocardial infarction mice
| Cytokines (pg per 100 μg Protein n=10) | Normal Control | Puerarin | Myocardial infarction | Myocardial infarction + Puerarin |
|---|---|---|---|---|
| MCP-1 | 8.7 ± 0.4 | 8.9 ± 0.1 | 21.5 ± 2.7* | 9.1 ± 1.0# |
| TGF-β | 24.3 ± 2.8 | 26.0 ± 1.9 | 59 ± 1.7* | 29.3 ± 2.2# |
Mean ± SEM:
P < 0.05 compared to the normal controls;
P < 0.05 compared to myocardial infarction.
Effect of puerarin on TGF-β1 expression in MI mice
The expression of TGF-β1 was determined by qPCR, ELISA and immunohistochemical staining. The downstream of TGF-β1, phosphorylated Smad2/3 was also detected by westernblot. Compared to the level in MI mice, TGF-β1 expression in the cardiac tissue was reduced following treatment with a high dose of puerarin (Figure 4A, 4B and Table 1). The results of phospho-Smad2/3, which is downstream of TGF-β1, confirmed the effect of puerarin (Figure 4C, 4D) and demonstrated its inhibitory effect on the TGF-β/Smad pathway.
Figure 4.
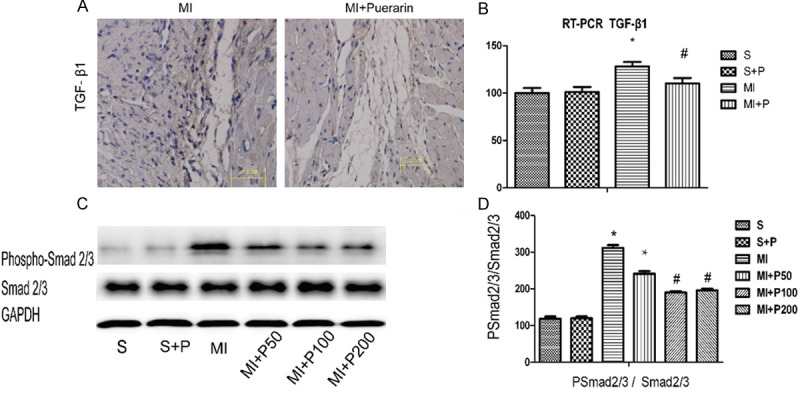
The expression of TGF-β1 in the MI myocardium treated with or without puerarin. A. Micrograph of TGF-β1 immunohistochemical staining in MI (left panel) and MI plus high dose puerarin treatment (right panel). B. Real-time qPCR quantification of TGF-β1. All data are shown as means ± SD and experiments were repeated three times. #P < 0.05 VS MI, *P < 0.05 VS sham group, n=5. C. Representative micrograph of expression of phospho-Smad2/3 in westernblot analysis which is downstream of TGF-β1. D. Quantitative results. #P < 0.05 VS MI, *P < 0.05 VS sham group, n=5.
Discussion
In this study, we provided evidence that puerarin inhibited MI-induced cardiac fibrosis through attenuating MCP-1 and TGF-β1 expression. Some studies have demonstrated the healing and scarring processes and the subsequent fibrosis were always the results of myocardial infarction. At the 4th week after MI established, a series of pathological alterations could be observed in the MI mice, including extensive fibrosis in the heart along the infarcted and the border zone, the increased expression of collagen I and III, and enhanced myofibroblast recruitment, as previously reports [3,25,26]. In our study, it has shown that puerarin effect on cardiac fibrosis was dose-dependent. For example, puerarin in the middle and high dose groups significantly attenuated the pathological changes in the MI, such as the myofibroblast recruitment, collagen synthesis and fibrosis formation in the cardiac tissues, while the low dose puerarin did not show the similar effect. These results indicated that puerarin had an anti-fibrotic effect on cardiac fibrosis, which was in consistent with results displayed in other tissues [27,29].
Activated fibroblasts transformation into myofibroblasts expressing α-SMA plays essential roles in the healing and scarring processes following myocardial infarction. However, chronic inflammation and prolonged repair process could trigger myofibroblasts transformation leading to exceed synthesis of new collagen and fibrosis [30,31]. Monocytes/macrophages are widely distributed and potent inflammation regulators. Under conditions of injury or inflammation, resident cells at sites of injury or inflammation secrete chemokines to recruit monocytes from the bloodstream, which are involved in various inflammatory respond by switching into macrophages phenotype. One of the most effective chemokine is monocyte chemoattractant protein (MCP-1), which plays a critical role in the process of various fibrotic and inflammatory diseases progression, such as renal fibrosis [32], diabetic nephropathy [33], and peritoneal dialysis [34]. Compelling evidence indicates that MCP-1 regulates several functions involved in cardiac fibrosis, which is associated with the recruitment and activation of monocytes/macrophages [35,36]. In this study, puerarin has been experimentally validated as the novel inhibitor of MCP-1. Firstly, we confirmed by immunohistochemistry assay that puerarin significantly decreased the sum of MCP-1 positive cells in the border zone from MI mice. Secondly, elevated MCP-1 expression in the MI cardiac tissues was significantly abolished by puerarin (Table 1). Thirdly, puerarin repressed the augmented macrophage recruitment (increased F4/80 positive cells), in which associated with the MCP-1 production. These results demonstrated that puerarin could effectively downregulate the expression of MCP-1 and inhibit macrophage recruitment in the MI mice.
The TGF-β/Smad pathway is closely associated with the cardiac fibrosis development and Macrophage-derived TGF-β1 is observed to promote fibrosis, which is attributed to the EMT of resident mesenchymal and epithelial cells [37]. In this study, the decreased expression of MCP-1 and macrophage recruitment was accompanied by a decrease of TGF-β1 expression both in mRNA and protein level, indicating the essential roles of TGF-β1 in cardiac fibrosis. On the other hand, compared to the MI mice, puerarin in a high dose could decrease the products of the downstream of TGF-β1 phospho-Smad2/3. The results confirmed the effect of puerarin and indicated inhibition of TGF-β/Smad pathway was involved in puerarin anti-fibrotic effect. At last, our study demonstrated that puerarin could attenuate cardiac fibrosis after MI, and its mechanisms may be involved in mediating the expression of TGF-β/Smad pathway and MCP-1. It could be used as a potential therapeutic drug to retard the process of cardiac fibrosis after MI.
Acknowledgements
This work was granted from the Priority Academic Program Development of Jiangsu Higher Education Institutions (BL2012011), the Chinese Medical Association of the Sunlight Foundation (SCRFCMDA201217), Collaborative innovation center of Nanjing Medical University, and the Fourth Period Project “333” of Jiangsu Province (BRA2012207), China.
Disclosure of conflict of interest
None.
References
- 1.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Halade GV, Lindsey ML. Extracellular matrix and fibroblast communication following myocardial infarction. J Cardiovasc Transl Res. 2012;5:848–857. doi: 10.1007/s12265-012-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouri F, Hanoun N, Mediani O, Saurini F, Hamon M, Vanhoutte PM, Lechat P. Blockade of beta 1- and desensitization of beta 2-adrenoceptors reduce isoprenaline-induced cardiac fibrosis. Eur J Pharmacol. 2004;485:227–234. doi: 10.1016/j.ejphar.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 5.Zhang RY, Wang LF, Zhang L, Meng XN, Li SJ, Wang WR. Effects of angiotensin converting enzyme inhibitor, angiotensin II type I receptor blocker and their combination on postinfarcted ventricular remodeling in rats. Chin Med J (Engl) 2006;119:649–655. [PubMed] [Google Scholar]
- 6.Galderisi M, de Divitiis O. Risk factor-induced cardiovascular remodeling and the effects of angiotensin-converting enzyme inhibitors. J Cardiovasc Pharmacol. 2008;51:523–531. doi: 10.1097/FJC.0b013e31817751a7. [DOI] [PubMed] [Google Scholar]
- 7.Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, Zecchi-Orlandini S, Bani D, Formigli L. Relaxin prevents cardiac fibroblast-myofibroblast transition via Notch-1-mediated inhibition of TGF-b/Smad3 signaling. PLoS One. 2013;8:e63896. doi: 10.1371/journal.pone.0063896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao J, Ju H, Zhao S, Junaid A. Scammell-La Fleur T and Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31:667–678. doi: 10.1006/jmcc.1998.0902. [DOI] [PubMed] [Google Scholar]
- 9.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY, Heuchel R, Yan BP, Yu CM, Lan HY. Deficiency of Smad7 enhances cardiac remodeling induced by angiotensin II infusion in a mouse model of hypertension. PLoS One. 2013;8:e70195. doi: 10.1371/journal.pone.0070195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim H, Zhu YZ. Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci. 2006;63:2584–2596. doi: 10.1007/s00018-006-6085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Finnerty CC, He J, Herndon DN. Smad ubiquitination regulatory factor 2 expression is enhanced in hypertrophic scar fibroblasts from burned children. Burns. 2012;38:236–246. doi: 10.1016/j.burns.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Qian Y, Jin R, Wo Y, Chen J, Wang C, Wang D. Effects of TRAP-1-like protein (TLP) gene on collagen synthesis induced by TGF-β/Smad signaling in human dermal fibroblasts. PLoS One. 2013;8:e55899. doi: 10.1371/journal.pone.0055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang CB, Yang Y, Zou Y, Ge J, Chen R, Chen H. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol. 2011;658:168–174. doi: 10.1016/j.ejphar.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Y, Zong J, Zhou H, Bian ZY, Deng W, Dai J, Gan HW, Yang Z, Li H, Tang QZ. Puerarin attenuates pressure overload-induced cardiac hypertrophy. J Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Xue J, Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-beta1 expression. J Nutr Biochem. 2012;23:1080–1085. doi: 10.1016/j.jnutbio.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Lu Z, Wang L. Restrictive effect of puerarin on myocardial infarct area in dogs and its possible mechanism. J Tongji Med Univ. 2000;20:43–45. doi: 10.1007/BF02887673. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Zong J, Zhou H, Bian ZY, Deng W, Dai J, Gan HW, Yang Z, Li H, Tang QZ. Puerarin attenuates pressure overload-induced cardiac hypertrophy. J Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Xue J, Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-beta1 expression. J Nutr Biochem. 2012;23:1080–1085. doi: 10.1016/j.jnutbio.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Cheng W, Wu P, Du Y, Wang Y, Zhou N, Ge Y, Yang Z. Puerarin improves cardiac function through regulation of energy metabolism in Streptozotocin-Nicotinamide induced diabetic mice after myocardial infarction. Biochem Biophys Res Commun. 2015;463:1108–1114. doi: 10.1016/j.bbrc.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 24.Zhou N, Fu Y, Wang Y, Chen P, Meng H, Guo S, Zhang M, Yang Z, Ge Y. p27 kip1 haplo-insufficiency improves cardiac function in early-stages of myocardial infarction by protecting myocardium and increasing angiogenesis by promoting IKK activation. Sci Rep. 2014;4:5978. doi: 10.1038/srep05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol. 2016;91:114–122. doi: 10.1016/j.yjmcc.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lighthouse JK, Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52–60. doi: 10.1016/j.yjmcc.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu MW, Liu R, Wu HY, Li YY, Su MX, Dong MN, Zhang W, Qian CY. Radix puerariae extracts ameliorate paraquat-induced pulmonary fibrosis by attenuating follistatin-like 1 and nuclear factor erythroid 2p45-related factor-2 signalling pathways through downregulation of miRNA-21 expression. BMC Complement Altern Med. 2016;16:11. doi: 10.1186/s12906-016-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang OH, Kim SB, Mun SH, Seo YS, Hwang HC, Lee YM, Lee HS, Kang DG, Kwon DY. Puerarin ameliorates hepatic steatosis by activating the PPARα and AMPK signaling pathways in hepatocytes. Int J Mol Med. 2015;35:803–809. doi: 10.3892/ijmm.2015.2074. [DOI] [PubMed] [Google Scholar]
- 29.Guo C, Xu L, He Q, Liang T, Duan X, Li R. Anti-fibrotic effects of puerarin on CCl4-induced hepatic fibrosis in rats possibly through the regulation of PPAR-γ expression and inhibition of PI3K/Akt pathway. Food Chem Toxicol. 2013;56:436–442. doi: 10.1016/j.fct.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Sziksz E, Pap D, Lippai R, Béres NJ, Fekete A, Szabó AJ, Vannay Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015;2015:764641. doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, Tomosugi N, Matsushima K, Egashira K, Yokoyama H. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15:940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- 33.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Kang HY, Kim KS, Nam BY, Paeng J, Kim S, Li JJ, Park JT, Kim DK, Han SH, Yoo TH, Kang SW. The monocyte chemoattractant protein-1 (MCP-1)/CCR2 system is involved in peritoneal dialysis-related epithelial-mesenchymal transition of peritoneal mesothelial cells. Lab Invest. 2012;92:1698–1711. doi: 10.1038/labinvest.2012.132. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y. CCR2 mediates the uptake of bone marrowderived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301:H538–47. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mewhort HE, Lipon BD, Svystonyuk D, Teng G, Guzzardi DG, Silva C, Yong VW, Fedak PW. Monocytes Increase Human Cardiac Myofibroblast-Mediated Extracellular Matrix Remodeling Through TGF-β1. Am J Physiol Heart Circ Physiol. 2016;310:H716–24. doi: 10.1152/ajpheart.00309.2015. [DOI] [PubMed] [Google Scholar]
- 37.Locatelli L, Cadamuro M, Spirlì C, Fiorotto R, Lecchi S, Morell CM, Popov Y, Scirpo R, De Matteis M, Amenduni M, Pietrobattista A, Torre G, Schuppan D, Fabris L, Strazzabosco M. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology. 2016;63:965–982. doi: 10.1002/hep.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]


