Abstract
Acetaminophen (APAP), commonly used in clinical prescription, has time- and dose-dependent side effects. Thus, further animal study warrants to be investigated to assess possible adverse effect of APAP application. Here, we conducted pre-clinical research to elucidate the molecular mechanism regarding APAP-mediated toxicological action. Our data showed that serous/urinary and hepatic/renal APAP concentrations were significantly increased when compared with normal control, which the liver tissue showed the highest level. As an acute liver damage model induced by APAP, absolute liver weight, serum enzyme (ALT), urine protein content were notably elevated. Representatively, APAP-damaged liver resulted in increased pro-apoptotic Bax and compensatory Ki-67 positive cells, while the number of anti-apoptotic Bcl2 positive cells was reduced. In addition, the immunoactivity markers for NF-κB, TRL4, TNF-α in the kidney were increased, respectively. Furthermore, intracellular TRL4 and TNF-α mRNAs in the liver and kidney showed significant up-regulation. In summary, our current findings demonstrate that APAP-mediated the specific cytotoxicity is linked to the molecular mechanisms of facilitating apoptosis and inflammatory stress in the liver and kidney.
Keywords: Acetaminophen, liver, kidney, cytotoxicity
Introduction
In therapeutic practice, acetaminophen (APAP) is widely applied in managing pain and fever, even cancer pain or post-surgery [1]. Once being abused, unregulated dose can lead to hepatic failure and other complications. APAP can induce serious liver injury if more than recommended dosage is taken, as reported worldwide [2]. Specifically, some unwanted symptoms, such as asthma, hearing loss and renal toxicity, are found in many clinical cases, in which kidney dysfunction occurs in around 2% of patients taking overdose of APAP [3]. APAP is metabolized mainly by hepatic cytochrome P450, however, the excessive production of quinone metabolite is toxic to liver cells in turn. As one of intermediate products, N-acetyl-p-benzoquinonimine (NAPQI) is toxic byproduct and it is primarily responsible for the cytotoxicity mediated by APAP [4]. Acute liver damage represents a pathological condition characterized by pronounced inflammatory response. On the other hand, increasing evidences have found that dosed use of APAP can induce kidney dysfunction, including acute and chronic renal failure [5]. However, the molecular mechanism of APAP-induced hepatotoxicity and nephrotoxicity has not completely understood. On the basis of our preliminary observations, the current study was designed to highlight the adverse effects of APAP-associated structural dysfunctions of the liver and kidney, as well as to discuss the underlying mechanisms.
Materials and methods
Chemicals
Commercially available acetaminophen (APAP) was purchased from Smith Kline & French Laboratorles Ltd. (Tianjin, China). The other reagents used were listed in following experimental necessity, respectively.
Laboratory mice
Adult male Kunming (KM) mice (8-week-old, approximately 25±2 g), were purchased from the Experimental Animal Centre of Guangxi Medical University (Nanning, China). Laboratory processes were implemented following by the protocols of Institutional Ethical Committee of Guangxi Medical University.
As reported previously, APAP-induced acute liver damage in mice were prepared [6]. All the animals were acclimatized for one week prior to further experiments. The mice in APAP-damaged group were treated by oral gavage with single dose of freshly-prepared APAP solution in physiological saline (300 mg/kg, w/w), while the control mice were administered with the same volume of saline. After 16 h fasting, the urine was harvested, and then all mice were killed via cervical dislocation and collected serum. After recording the liver weight, respective liver and kidney specimens were isolated and stored until further assays.
Liver index (%) = Liver weight/Body weight × 100%
Instrumental analysis of HPLC
Blood, urine and tissue samples were processed with acid-reagents for deproteinization. In addition, dosed concentrations of internal standard of APAP (Yuanye Biology Co. Ltd. Shanghai, China) were prepared. The supernatants were harvested by centrifuging on 1,4500 rmp for 10 min at room temperature. Aliquot vial samples were injected into the HPLC system (Shimadzu, Japan) for the determination of APAP. Chromatography was conducted by Shimadzu LC-20A System, equipped with a SIL-20AC detector and an automatic sample injector. The separation equipment was achieved by using a ASTON RG C18 column (4.6 mm × 50 mm i.d., 5 μm; ANAX, China). The column condition was maintained at 45°C. A mixture of a ratio with acetonitrile:isopropanol:purified water = 23:8:69 in triethylamine solution (pH = 6.9) was used as the mobile phase at a flow rate of 1.1 mL/min. The optical signal detector of HPLC was set on 230 nm wavelengths. Peak of APAP was identified by comparison of retention time of internal standard and tested samples. The contents of APAP from each sample were quantified, and final data were expressed as ug/ml per protein.
Serological and urinary assay
Serum aminotransferase (ALT) level and proteinuria content were calculated by using commercially available kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Routine and immunocytochemical stains
As referenced in our pervious study [6], the liver and kidney samples were pre-fixed with 10% neutral formalin before being cut into 5 μm sections. Then, some of slices were subjected to hematoxylin and eosin staining. In addition, other sections were dewaxed by xylene and rehydrated via different levels of ethanol. When being blocking with 10% BSA for 1 h at room temperature, liver samples were incubated with rabbit anti-Bcl2, BAX, Ki67 antibodies, and kidney sections were exposed to rabbit anti-NF-κB, TLR4, TNF-α (1:400; Boster, Wuhan, China), followed by horseradish peroxidase (HRP) conjugated anti-rabbit secondary antibody (1:1000; Boster, Wuhan, China) for 1 h at room temperature. Diaminobenzidine (DAB) was used for colour development on antigen-antibody complex before counterstaining with hematoxylin. Subsequently, the sections were mounted, and imaged.
PCR analysis
Total RNAs of liver and kidney were isolated by using Trizol kit (Aidlab Biotechnologies Co., Ltd., Beijing, China). The purity of extracted RNAs concentrations was validated by using a spectrophotometer (Bio-Rad, USA) during the readings of 1.8-2.0 in A260/A280 ratio. 0.5 mg of RNA from each sample was adversely converted to cDNA through Revert Aid First Strand cDNA Synthesis kit (Thermo Scientific, USA). The designed PCR primers (TRL4 and TNF-α mRNAs) were commercially provided by Takara Biotechnology Co., Ltd (Dalian, China). The detailed sequences and PCR reactions were described as our previous report [6].
Statistical analysis
Statistical data was conducted by using statistical product and service solutions (SPSS) 19.0 software. Differences between two compared groups were assessed by a one-way analysis of variance (ANOVA) followed by Tukey’s test using in HPLC analysis or Student’s t-test using in biochemical and PCR assays. Result was expressed as mean ± SD. A P less than 0.05 was considered as statistically significant.
Results
APAP induced toxicokinetics and hepatic weight change
As shown in Figure 1A, instrumental analyzed data showed that APAP levels were detected in all the collected samples from APAP-treated mice, which the contents were greater than those in control (P<0.05). In addition, the urine APAP content was higher than that in serum (P<0.05), and the intra-liver APAP concentration in kidney was greater than that in liver (P<0.05), which the results showed statistical significance. Visibly, APAP-induced hepatomegaly (liver enlargement) was significant when compared to the control, with the result of increased liver weight and liver index (P<0.05) (Figure 1B).
Figure 1.
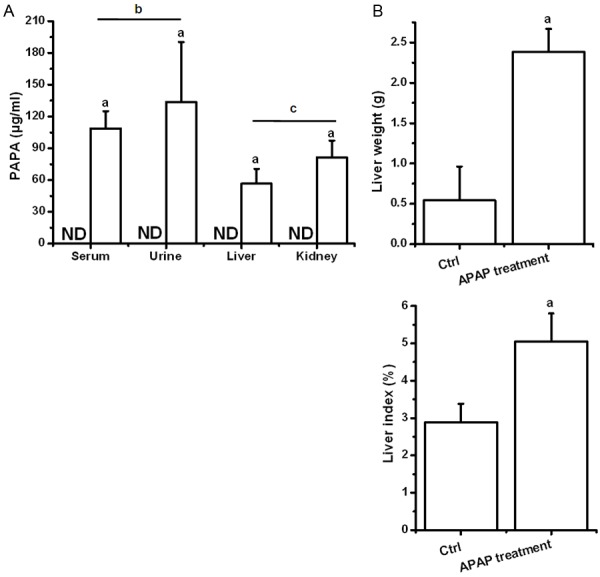
APAP mediated toxicokinetics and liver weight change. Statistical data was analyzed by using SPSS 19.0 software. Differences between two-compared groups were assessed by a one-way analysis of variance (ANOVA) followed by Tukey’s test. Result was expressed as mean ± SD. Notes: vs. Control (ctrl), a P<0.05; Serum vs Urine. b P<0.05; Liver vs kidney. c P<0.05 in APAP treatment. ND = undetected condition.
APAP increased the levels of aminotransferase and urine protein
To validate APAP-related hepatotoxicity and nephrotoxicity, biochemical analysis was tested. As a result, APAP-treated mice exhibited elevated concentrations of liver functional enzyme (ALT) and urine protein when compared to these in untreated mice (P<0.05) (Figure 2).
Figure 2.
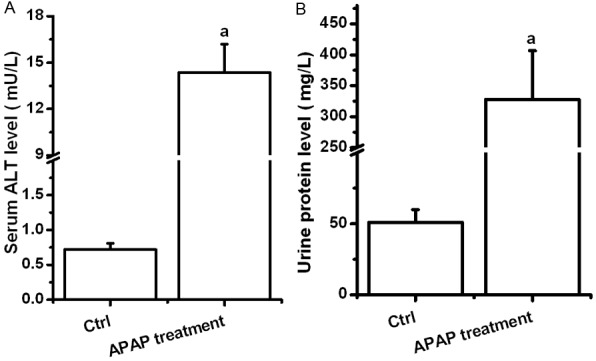
APAP elevated concentrations of aminotransferase and urine protein. Statistical data was analyzed by using SPSS 19.0 software. Differences between two-compared groups were assessed by a one-way analysis of variance (ANOVA) followed by Student’s t-test. Result was expressed as mean ± SD. Notes: vs. Control (ctrl), a P<0.05.
APAP affected inflammation-related mRNAs in the liver and kidney
In PCR readings, intrahepatic mRNAs of TLR4, TNF-α in APAP-treated mice was increased significantly, when compared to those in control (P<0.05), whereas the notably up-regulated levels of TLR4, TNF-α mRNAs were observed in APAP-treated kidney (P<0.05). However, these expressed mRNAs were no significant difference between the liver and kidney samples (Figure 3).
Figure 3.
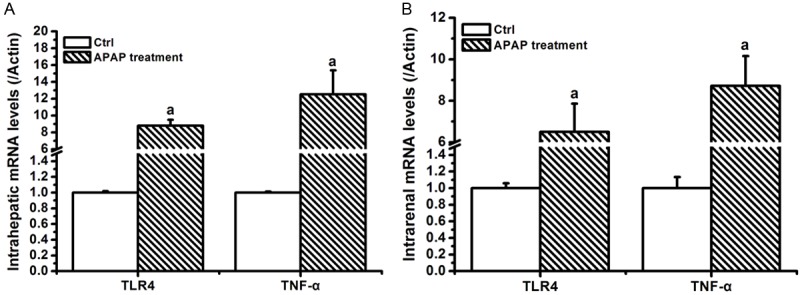
APAP affected inflammation-related mRNAs in liver and kidney samples. Statistical data was analyzed by using SPSS 19.0 software. Differences between two-compared groups were assessed by a one-way analysis of variance (ANOVA) followed by Student’s t-test. Result was expressed as mean ± SD. Notes: vs. Control (ctrl), a P<0.05.
APAP altered expression of regulator proteins in the liver and kidney
To characterize the APAP-induced cytotoxicity, signaling-associated proteins were validated by immunostaining. Compared to control, APAP-damaged mice resulted in increased pro-apoptotic Bax and compensatory Ki-67 immunoreactive cells in liver cells, while the amount of anti-apoptotic Bcl2 positive cells was reduced (P<0.05). In addition, the immunoactivity markers for NF-κB, TRL4, TNF-α in the kidney were visibly increased, respectively, when compared to those in the untreated kidney (P<0.05) (Figure 4).
Figure 4.
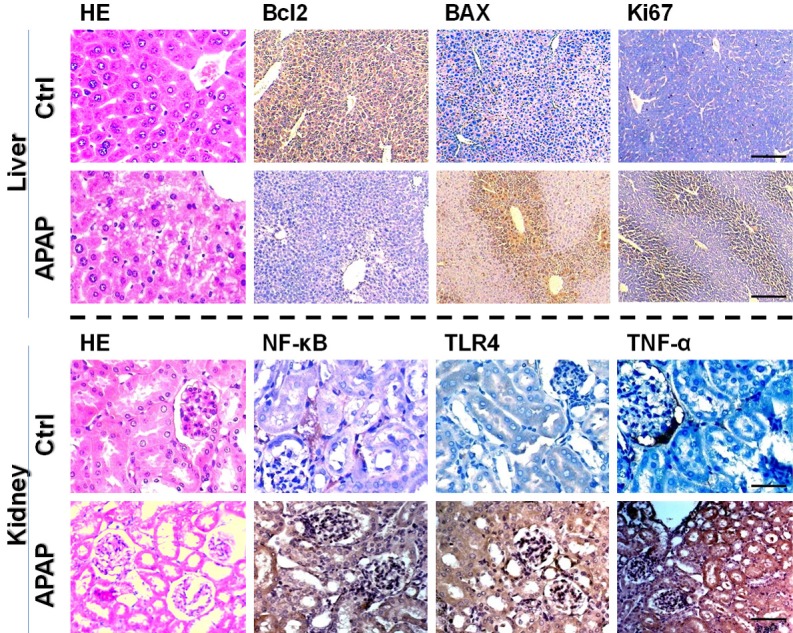
APAP altered expression of regulator proteins in the liver and kidney (Immunohistochemistry stains, scale bar = 200 μm). As a result, APAP exposure showed up-regulated expression of apoptosis-regulated protein in liver cells, and elevated level of inflammation-regulated protein in kidney cells.
Discussion
Acute liver injury induced by acetaminophen (APAP) may occur when overdose intake [7]. Increasing evidences have found APAP-induced liver cell necrosis [8], however, the molecular mechanism behind this action has not yet completely elucidated. In addition to liver toxicity, APAP-induced abnormalities in other key organs warrant to be further investigated. In the present study, APAP resulted in significantly increased liver weight and liver index, which these visible observations showed APAP-induced hepatotoxicity. In addition, APAP accumulation in blood, urine, liver and kidney was significant when compared to those in untreated mice. Notably, the APAP contents in kidney and urine were greater than these in the liver as shown in HPLC assay, implying that APAP-medicated immediate damage might be occurred in the kidney cells. Therefore, the possible adverse effects induced by APAP could be identified on the liver and kidney tissues.
To validate the adverse effects of APAP-induced cytotoxicity on target organs, some representative parameters were determined. Alanine transaminase (ALT) with abnormally elevated serum level can be used in clinical measurement for liver damage [8]. Albuminuria (urine protein) refers to the pathological condition that excessive albumin is abnormally accumulated in the urine, resulting in kidney damage over time [9]. As a result, APAP-treated mice had higher levels of serum ALT and urine protein, suggesting immediate impairments of APAP-induced toxicity to liver and kidney cells. However, the possible underlying mechanism needed to be explored.
Bcl-2 (B-cell lymphoma 2) represents a specific functional protein that regulates cell fate [10], especially inhibition of apoptosis. Instead, apoptosis regulator BAX is a protein that functions as pro-apoptotic regulator involved in cell death [11]. Ki-67 is a nuclear protein that is commonly used as a cell biomarker, and it is necessary for cellular proliferation [12]. Therefore, our findings exhibited that abnormally altered expressions of Bcl-2, BAX and BAX proteins in liver cells by APAP treatment, indicating that apoptosis-dependent pathway was occurred in APAP-damaged liver. Hepatic Ki-67 positive cell was consistent with the consequence of APAP-induced apoptosis.
Tumor necrosis factor (TNF-α) is a regulator cytokine that is responsible for pro-inflammatory stress and promoting the acute damage [13]. Further, NF-κB is a nucleoprotein that controls gene and cell activities, such as transcription of DNA, production of cytokine and cell survival. Incorrect regulation of NF-κB has been associated with inflammatory disorder [14]. Biologically, toll-like receptor 4 (TLR4) has found to be a vital controller in promotion of the immune and inflammation activities in the body. The post-effect of TLR4 is achieved via NF-κB signaling, leading to activation of downstream effectors [15], such as TNF-α. Therefore, suppression of TLR4/NF-κB signaling pathway can be attributed to reduction of organ toxicity. In this study, our current representative data showed that intrarenal inflammation-associated effectors, including TNF-α, TLR4 and NF-κB positive cells, were increased in the kidney, respectively. Meanwhile, the mRNA levels of TNF-α, TLR4 had been validated in APAP-treated liver and kidney, respectively. Thus, these adverse effects showed that APAP-mediated organ toxicity was involved in activating intrarenal and intrahepatic TLR4/NF-κB signaling pathway, resulting in induction of cytotocixity and inflammatory stress.
Conclusions
Taken together, our current findings indicate that APAP induces cytotoxicity in key metabolic organs of the liver and kidney, as validated in instrumental and biochemical analyses. Representatively, APAP-induced organ damage is linked to activating intracellular TLR4/NF-κB/TNF-α signaling, thereby inducing apoptosis and inflammatory stress in the liver and kidney.
Acknowledgements
This research is supported by Talents Highland of Emergency and Medical Rescue of Guangxi Province in China (No. GXJZ201510), Science and Technology Research Projects of Guangxi Universities (YB2014275 and KY2015LX283), and National Nature Science Foundation of China (No. 81560134 and No. 81660091).
Disclosure of conflict of interest
None.
References
- 1.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12:133–141. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 2.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 3.Bass S, Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose. Am J Health Syst Pharm. 2013;70:1496–1501. doi: 10.2146/ajhp120645. [DOI] [PubMed] [Google Scholar]
- 4.Kalinec GM, Thein P, Parsa A, Yorgason J, Luxford W, Urrutia R, Kalinec F. Acetaminophen and NAPQI are toxic to auditory cells via oxidative and endoplasmic reticulum stress-dependent pathways. Hear Res. 2014;313:26–37. doi: 10.1016/j.heares.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoine DJ, Sabbisetti VS, Francis B, Jorgensen AL, Craig DG, Simpson KJ, Bonventre JV, Park BK, Dear JW. Circulating Kidney Injury Molecule 1 Predicts Prognosis and Poor Outcome in Patients With Acetaminophen-Induced Liver Injury. Hepatology. 2015;62:591–599. doi: 10.1002/hep.27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu K, Guo C, Wu X, Su M. Ameliorative effectiveness of allicin on acetaminophen-induced acute liver damage in mice. Journal of Functional Foods. 2015;8:665–672. [Google Scholar]
- 7.Bass S, Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose. Am J Health Syst Pharm. 2013;70:1496–1501. doi: 10.2146/ajhp120645. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H, Xie Y, McGill MR. Acetaminophen-induced Liver Injury: from Animal Models to Humans. J Clin Transl Hepatol. 2014;2:153–161. doi: 10.14218/JCTH.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz A, Schütten-Faber S, van Es N, Unland J, Schulte L, Kossmehl P, de Heer E, Kreutz R. Induction of albuminuria and kidney damage in SHR by transfer of chromosome 8 from Munich Wistar Frömter rats. Physiol Genomics. 2012;44:110–116. doi: 10.1152/physiolgenomics.00123.2011. [DOI] [PubMed] [Google Scholar]
- 10.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct Activation of Bax Protein for Cancer Therapy. Med Res Rev. 2016;36:313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodzki A, Łopuszyński W, Brodzki P, Tatara MR. Diagnostic and prognostic value of cellular proliferation assessment with Ki-67 protein in dogs suffering from benign and malignant perianal tumors. Folia Biol (Krakow) 2014;62:235–241. doi: 10.3409/fb62_3.235. [DOI] [PubMed] [Google Scholar]
- 13.Vigerust NF, Bjørndal B, Bohov P, Brattelid T, Svardal A, Berge RK. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. Eur J Nutr. 2013;52:1315–1325. doi: 10.1007/s00394-012-0441-2. [DOI] [PubMed] [Google Scholar]
- 14.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289:2457–2468. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]


