Abstract
Mechanical properties of cells reflect differences in cellular subpopulations, differentiation potency, and cell behaviors. Previous study has revealed that intervertebral disc (IVD) degeneration leads to alterations in cell behavior and differentiation potency. Human nucleus pulposus-derived progenitor cells (NPPCs) are an attractive cell sources for IVD regeneration. However, the relationship between mechanical properties and differentiation potential in different NPPC subpopulations is few known. In this study, mechanical properties of different NPPC subpopulations were measured via atomic force microscopy (AFM) and correlated with differentiation potential of NPPCs. We found that elastic modulus, relaxed modulus, and instantaneous modulus were positively correlated with osteogenic potential of NPPCs. And apparent viscosity was correlated with chondrogenic potential of NPPCs. These results indicated that the mechanical properties were predictive markers for differentiation potential of NPPC subpopulations, and could be used for enrichment based on differentiation potential, which could significantly improve the outcome of IVD regeneration.
Keywords: Cellular mechanical properties, stem cell, nucleus pulposus, intervertebral disc degeneration
Introduction
Intervertebral disc (IVD) degeneration is widely known as major contributor to low back pain [1,2]. IVD degeneration is characterized by dehydration of the nucleus pulposus (NP) and a loss of disc height, which can be visualized as a low-intensity signal by MRI, together with structural deficits, inflammation and herniation of the NP. However, the pathogenesis of IVD degeneration is largely unknown and there are no effective therapies. Stem cell-based transplantation to the degenerated IVD has shown promise in decelerating or arresting the degenerative process of IVD [3].
Previously, it has been found of IVD progenitor cells and a stem cell system that participates in IVD regeneration [4-6]. Nucleus pulposus progenitor cells (NPPCs) were capable of differentiating along osteogenic, chondrogenic, adipogenic lineages in vitro, and also into oligodendrocytes, neurons and astroglial-specific precursor cells in vivo, demonstrating their multipotency is similar with bone marrow-derived MSCs (BMSCs) [7].
Disc regeneration needs stem/progenitor cell to repair and regenerate the degenerated disc. As NPPCs could be isolated from disc patients and amplifying cultured in vitro, these cells could be one of cell sources in cell therapy for IVD degeneration. Though conflicting results exist, previous findings have indicated that the regenerative potential of MSCs deteriorates with age, which suggests a possible limitation in their use [8]. However, cell aging is accompanied by changes in mechanical properties of stem cells. Thus, examining how the elastic properties of NPPCs varies with disc degeneration and how this subsequently impacts the differentiation potential is of key importance.
The goal of the study was to investigate the relationship between the mechanical properties of NPPCs and their lineage differentiation capabilities. Specifically, 30 single-cell-derived clonal populations were established using methylcellulose medium. Cellular elastic and viscoelastic properties for each clonal population were determined via atomic force microscopy (AFM) by testing individual cells. Clones were then assessed for differentiation potential along osteogenic, chondrogenic, and adipogenic lineages. Correlations were determined between individual mechanical parameters and lineage differentiation capabilities. Analyses showed that osteogenic potential was positively correlated with elastic modulus, relaxed modulus, and instantaneous modulus of undifferentiated NPPCs, and chondrogenic potential was correlated with apparent viscosity of undifferentiated NPPCs. These results indicated the cellular mechanical properties were predictive of differentiation capability of NPPC clonal subpopulations, and could be used to enrich for high potentiated progenitor cells for dramatically improving the quality of stem cell-based IVD regeneration.
Materials and methods
Isolation and expansion of NPPCs
NP tissues were obtained from 11 donors who underwent spinal fusion surgery for DDD (Table 1). NPPCs were isolated as previously described [9]. Briefly, NP tissues were enzymatically digested with 0.15% collagenase II (Sigma, USA) in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, HyClone, USA) containing 3% fetal calf serum (FCS, HyClone, USA) for 6 hours at 37°C. After digestion, the suspended cells were filtered through a 70-μm cell filter to minimize cell aggregates. NP cells (total cell number of 103 per dish) were cultured in 1 mL of MethoCult H4230 methylcellulose medium (Stem Cell Technologies) and were seeded into 35-mm diameter dishes (Stem Cell Technologies) for 2 weeks. Cell clusters (diameter greater than 50 μm) were isolated using a sterile Pasteur pipette and subcultured in 35-mm diameter dishes. All cell culture was performed under a humidified atmosphere containing 5% CO2 at 37°C. The Medical Ethics Committee of the Xinqiao Hospital of Third Military Medical University has approved this protocol, and all patients provided written informed consent.
Table 1.
Characteristic details of the patients enrolled in this study
| Case No. | Age (years) | Gender | Symptoms | Diagnosis | Disc level | Pfirrmann grading |
|---|---|---|---|---|---|---|
| Case 1 | 46 | F | BP | Lumbar discogenic pain | L4/5 | III |
| Case 2 | 44 | M | BP | Lumbar disc herniation | L5/S1 | IV |
| Case 3 | 64 | M | BP-RP | Lumbar discogenic pain | L5/S1 | IV |
| Case 4 | 57 | M | BP-RP | Lumbar disc herniation | L5/S1 | III |
| Case 5 | 41 | F | BP-RP | Spondylolisthesis | L5/S1 | III |
| Case 6 | 45 | M | BP | Spondylolisthesis | L4/5 | IV |
| Case 7 | 52 | F | BP | Lumbar disc herniation | L5/S1 | III |
| Case 8 | 48 | F | BP-RP | Spondylolisthesis | L4/5 | III |
| Case 9 | 56 | M | BP-RP | Lumbar disc herniation | L5/S1 | III |
| Case 10 | 51 | F | BP | Lumbar disc herniation | L4/5 | IV |
| Case 11 | 49 | F | BP-RP | Lumbar discogenic pain | L4/5 | III |
BP: back pain; RP: radicular pain.
AFM single-cell mechanical measurements
The mechanical properties of individual NPPCs were measured using an atomic force microscope (Nanowizard II, JPK Instruments, Germany) using previously established techniques [10,11]. Briefly, spherically tipped cantilevers (5 μm diameter, k ~0.03 N/m, Novascan Technologies, USA) were used for indentation and stress relaxation experiments. Individual cells were mechanically tested using a single indentation/stress relaxation test over the perinuclear region of the cell. An approach velocity of 15 μm/s was used, followed by a 30 s relaxation period. During testing, indentation depths were maximized for 0.5 μm, but never exceeded 10% strain. The elastic modulus was extracted from force vs. indentation data using a modified Hertz model [12], where R is the relative radius of the tip, and v is the Poisson’s ratio, assumed to be ~0.5 for an incompressible material. However, testing procedures were identical for all cells, which allows for valid comparisons among clones in this study.
Osteogenic differentiation
The cells were seeded on 24-well culture plates and incubated for 24 h; the normal culture media were then replaced with osteogenic induction media (Osteogenic Differentiation Kit, GIBCO, USA). The induction process lasted for 21 days and the media were changed every 3 days. The control cells were maintained in DEME/F12 with 10% FCS. Finally, the induction media were removed, and the cells were rinsed with PBS, followed by fixation with a 4% paraformaldehyde solution at room temperature for 1 h for alizarin red staining. Alizarin red staining was used to assess calcified matrix deposition in osteogenic and control samples. After digital images were taken, alizarin red staining dyes were eluted from each sample, and optical densities were measured at 540 nm.
Chondrogenic differentiation
A pellet culture was employed for chondrogenic induction as previously described [13]. The cells were centrifuged at 500 g for 10 min in a polypropylene tube to form a pellet. Without being disturbed, the cell pellets were incubated for 24 h with normal medium; the normal medium was subsequently replaced by chondrogenic induction medium (Chondrogenic Differentiation Kit, GIBCO, USA). Thereafter, the induction medium was refreshed every 3 days. Blank controls were cultured with DMEM/F12 with 10% FCS. On day 14, the cell aggregates were weighed to determine the wet weights and fixed in 4% paraformaldehyde solution for 15 min at room temperature; the cells were then dehydrated in serial ethanol solutions and embedded in paraffin blocks. The paraffin sections were stained with alcian blue dye for histological analysis. The papain-digested pellets were used to quantify sGAG content via the dimethylmethylene blue assay (Sigma, USA). The PicoGreen assay (Invitrogen, USA) was used to quantify DNA amounts (480 nm excitation, 520 nm emission). For sGAG quantification, optical densities were measured at 595 nm. A standard curve was used to calculate total sGAG amounts in each pellet, which were then normalized on a per-DNA basis.
Adipogenic differentiation
The cells were seeded in 24-well culture plates and incubated for 24 h; the normal medium was subsequently replaced by adipogenic induction medium (Adipogenic Differentiation Kit, GIBCO, USA). The negative controls were maintained in normal medium. On day 21, the cells layers were fixed with 4% paraformaldehyde solution for 15 min at room temperature and stained with oil red O for an additional 15 min to confirm the newly formed lipid droplets. Oil red O staining was used to assess lipid accumulation in adipogenic and control samples. After digital images were taken, oil red O dyes were eluted from each sample, and optical densities were measured at 500 nm.
Statistical analysis
All statistical tests were performed using SPSS 13.0 software (IBM). Data collected from clonal populations (n=32) were subjected to a Shapiro-Wilk normality test. Non-normally distributed mechanical properties were log-transformed before statistical analyses. P-values between differentiated and undifferentiated controls for each clone were calculated using two-tailed, unpaired student’s t-tests (α=0.05). To investigate correlations between mechanical properties and differentiation potential, Pearson correlation coefficients (r) were calculated from log-transformed data. Correlation coefficients are expressed as r ± 95% confidence intervals. Statistical significance was achieved if P < 0.05. All values are reported as means ± SD.
Results
Characterization of NPPCs from human degenerated nucleus pulposus
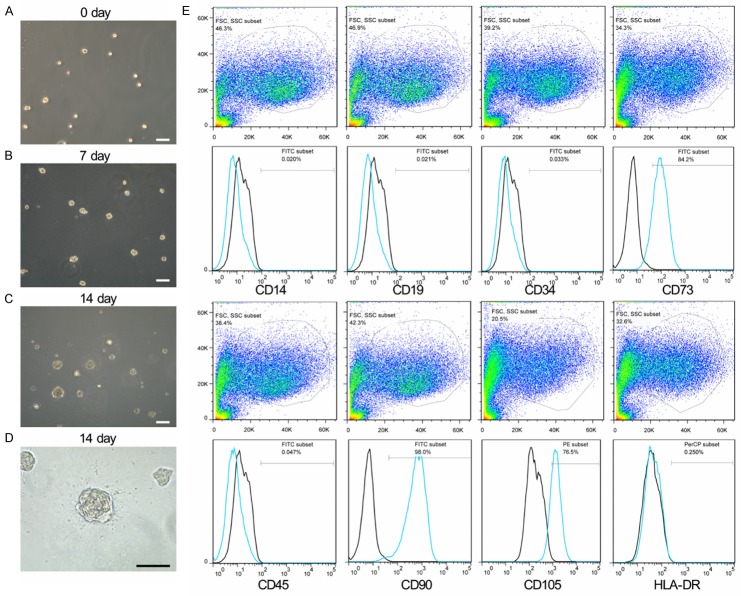
To identify and isolate NP stem/progenitor cells, we began with a colony-forming assay (CFA) using methylcellulose semi-solid medium, which was established to evaluate endothelial stem/progenitor cells [14] and has been used to identify tissue-specific stem/progenitor cells from various organs [15,16]. During the culture of primary NP cells, 1×103 NP cells were collected and subjected to the methylcellulose (Figure 1A). After 7 days, adhesive spheroid colonies were observed (Figure 1B). After being cultured in methylcellulose for 2 weeks, some of the seeded cells formed cell clusters with diameters greater than 50 μm and each colony was subcultured in multi-well plates for expansion (Figure 1C, 1D). The antigenic phenotype of NPPCs was checked by flow cytometric analysis (Figure 1E). NPPCs were positive for many stem cell markers common to cartilage endplate-derived stem cell (CESCs) [6], including, CD73, CD90, and CD105. Similar with CESCs, the NPPCs were negative for the pan-monocytic antigen CD14, the hematopoietic stem cell marker CD34, the pan-B-cell marker CD19, the pan-hematopoietic marker CD45, and the class 2 HLA antigen HLA-DR. It suggested that NPPCs and CESCs might derive from the same stem cell niches in degenerated IVD.
Figure 1.
Characterization of NPPCs from human degenerated nucleus pulposus. Single NP cells were inoculated into methylcellulose medium in 96-well culture plates, and were observed on day 0 (A), day 7 (B), and day 14 (C). The colonies derived from human primary NP cells in methylcellulose medium at 14 days (D). Immunophenotypic profile of nucleus pulposus-derived progenitor cells (NPPCs) from colony-forming assay (CFA) analyzed by FACS (E). The blue lines represent the fluorescence intensity of cells stained with the indicated antibodies and the black lines represent the negative control cells, which were stained with a non-immunoreactive isotype control antibody.
Cell stiffness varies in different clonal population
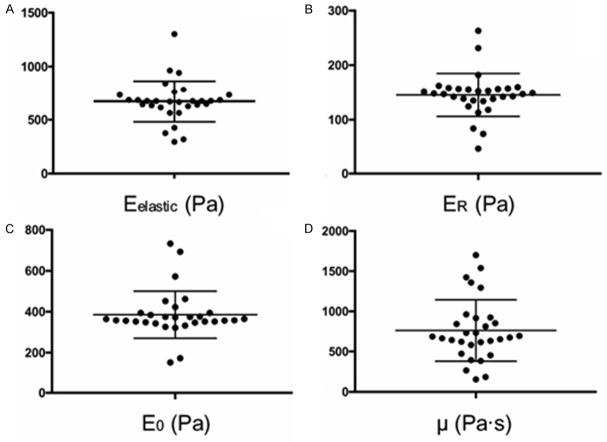
Single-cell mechanical properties were measured by AFM for 30 NPPCs clonal populations. All cells exhibited stress-relaxation behavior consistent with a viscoelastic solid material, reaching an equilibrium reaction force in approximately 30 seconds. There was highly agreement between the theoretical model fits and the experimental data for both elastic (R2=0.8736 ± 0.052) and viscoelastic (R2=0.943 ± 0.027) models were obtained. Each clone of NPPCs exhibited substantial heterogeneity in their elastic and viscoelastic properties (Figure 2), including elastic modulus, relaxed modulus, instantaneous modulus, and apparent viscosity. These results suggested that the existence of different subpopulations in NPPCs in human degenerated NP tissues, which may have different biophysical characteristics.
Figure 2.
Cell stiffness varies in different clonal population. The mechanical properties of NPPCs were heterogeneous, which suggested a possible means to identify lineage-specific subpopulations. Elastic and viscoelastic properties of 30 NPPCs clones were measured via AFM indentation and stress relaxation tests, respectively. Within each clonal population, an average of 25 cells was tested. The following cellular mechanical properties were measured: elastic modulus (A), relaxed modulus (B), instantaneous modulus (C), and apparent viscosity (D). Elastic and viscoelastic data fit well to Hertzian mathematical models (R2=0.8736 ± 0.052 and R2=0.943 ± 0.027, respectively). Data are presented as scatter dot plot overlaid with the individual geometric means of each clone.
Cell stiffness is correlated with the differentiation potential of NPPCs
Each NPPC clonal population was assessed for multipotentiality by differentiation along the osteogenic, chondrogenic, and adipogenic lineages. Osteogenic differentiation was confirmed by the deposition of an alizarin red positive mineralized matrix (Figure 3D). For in vitro chondrogenesis and adipogenesis, the alcian blue staining (Figure 3E) and oil red O staining (Figure 3F) were used to confirm the chondrogenic and adipogenic differentiation, respectively. NPPCs treated with growth culture medium alone showed negative staining (Figure 3A-C). Of all clones tested, 72% exhibited osteogenic differentiation potential, 83% exhibited positive chondrogenic differentiation and positive adipogenic differentiation was observed for 58% of all clones. Overall, 36% of clones were tripotent, 38% were bipotent, and 29% were unipotent. No clones showed a total lack of differentiation capability (Figure 3G).
Figure 3.
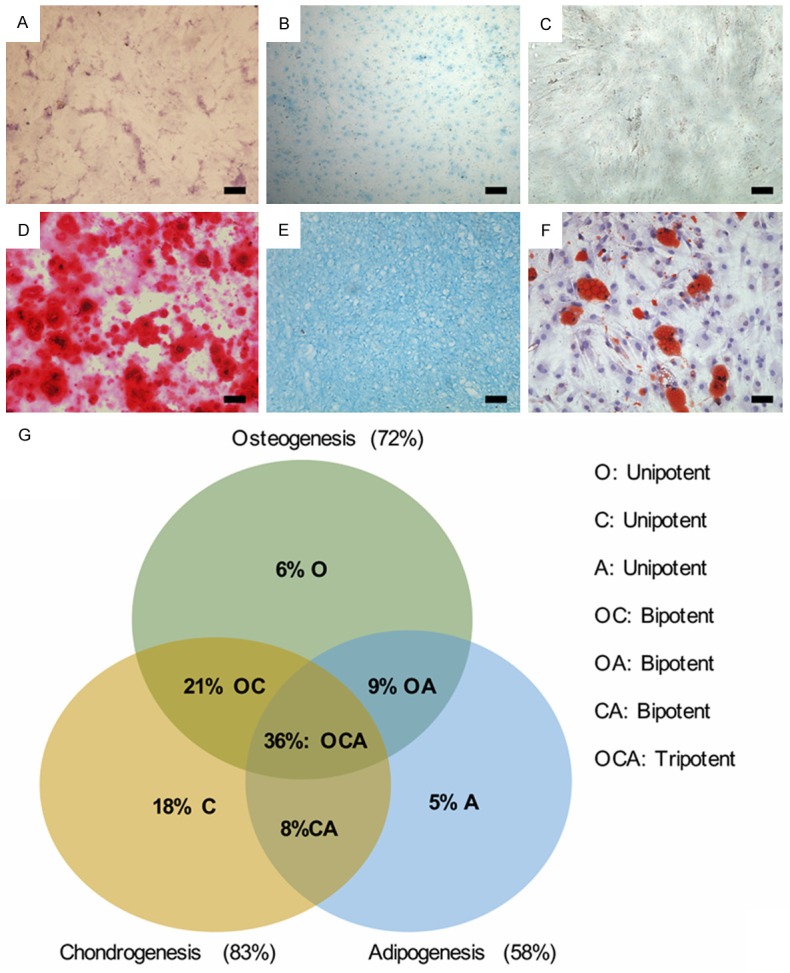
Cell stiffness is correlated with the differentiation potential of NPPCs. NPPCs treated with growth culture medium alone showed negative alizarin red staining (A), negative alcian blue staining (B), negative oil red O staining (C). NPPCs treated with osteogenic medium for 14 days showed mineral deposition around the cells to form nodular aggregates (D). NPPCs treated with chondrogenic medium for 21 days showed positive alcian blue staining (E). NPPCs treated with adipogenic medium for 4 days showed negative oil red O staining (F). Bar=100 μm. Each differentiation lineage showed extensive variability in differentiation potential, suggesting heterogeneity among the clonal populations. Results were used to determine the multipotentiality of clones investigated in this study (G).
To confirm whether mechanical properties reflect the differentiation potential of NPPCs, we determined the associations between NPPC mechanical properties and differentiation ability. The mechanical biomarkers for these clones showed distinct differences from the other populations. Significant correlations existed for cellular mechanical properties and the three lineages examined above (Table 2). Analyses showed that osteogenesis was positively correlated with Eelastic, ER, and R0. Highly osteogenic clones exhibited significantly higher Eelastic, ER, and E0 values (P < 0.05). Chondrogenesis was positively correlated with μ. Highly chondrogenic clones showed significantly higher μ values (P < 0.05). Adipogenesis was not significantly correlated with Eelastic, ER, and E0. This finding suggested that when clonal populations typically were stiffer, it was increased in highly osteogenic clones, and when clonal populations were more viscous, it was increased in highly chondrogenic clones.
Table 2.
Correlations between mechanical properties of NPPCs and their differentiation potential
| Mechanical properties | Lineage | Pearson’s r (± 95% Cl) | P value |
|---|---|---|---|
| Eelastic | Osteogenic | 0.38 ± 0.17 | 0.004 |
| Chondrogenic | 0.52 ± 0.18 | 0.003 | |
| Adipogenic | -0.58 ± 0.31 | 0.008 | |
| Osteogenic | 0.47 ± 0.28 | 0.002 | |
| E0 | Chondrogenic | 0.63 ± 0.25 | 0.005 |
| Adipogenic | -0.32 ± 0.18 | 0.003 | |
| Osteogenic | 0.51 ± 0.31 | 0.006 | |
| ER | Chondrogenic | 0.42 ± 0.27 | 0.008 |
| Adipogenic | -0.52 ± 0.31 | 0.004 | |
| Osteogenic | 0.18 ± 0.21 | 0.32 | |
| μ | Chondrogenic | 0.09 ± 0.32 | 0.72 |
| Adipogenic | 0.03 ± 0.18 | 0.53 |
Cellular mechanical properties are indicated by the following abbreviations: Eelastic (elastic modulus), E0 (instantaneous modulus), ER (relaxed modulus), and μ (apparent viscosity). Error values for Pearson’s correlation coefficient r represent 95% confidence intervals (95% CI). Osteogenic, chondrogenic, and adipogenic characteristic metabolites are intracellular lipids, extracellular matrix-bound calcium, and sulfated GAGs, respectively. Correlations were calculated using log-transformed geometric means. To allow for comparisons among lineages, metabolite data were normalized to their respective, lineage-specific arithmetic mean and then fit with a linear regression.
Discussion
In this study, we demonstrated that the cellular mechanical properties of clonal populations of NPPCs were different, and the cellular mechanical properties are predictive of NPPC differentiation potential. Significant correlations existed between mechanical properties and lineage-specific differentiation ability of NPPCs. These findings represent an advantageous means to characterize the differentiation potential of stem cells. Mechanical properties act as an indicator of the biochemical and structural characteristics of a cell and hold promise as a composite biomarker capable of identifying beneficial NPPCs subpopulations.
The observed heterogeneity in mechanical properties among the different clones can be attributed to variations in intracellular composition and organization. Previous study has shown that differentiated cells and stem/progenitor cells differ in the elastic and viscoelastic properties [10]. When stem cells differentiate towards specific lineages, their cytoskeleton rearranges before differentiation is achieved [17,18]. During the process of differentiation, rearrangement is concurrent with a modulation of the cellular mechanical properties and any accumulation of intracellular metabolites [19]. With the process of osteogenic differentiation, cells become stiffer, whereas when adipogenic differentiation, cells become more compliant. Chondrogenesis induces intracellular changes that result in higher instantaneous and relaxed moduli as well as higher apparent viscosity [20]. These mechanical changes can occur in varying degrees before irreversible commitment to a single lineage. However, it is hypothesized that each NPPCs exhibits a mechanical phenotype associated with its preferred lineage, possibly because it has already begun the differentiation process [18]. the purpose of tissue-specific enrichment is to collect all cells capable of expressing the proper phenotype whether they are initially multipotent, unipotent, or fully differentiated.
As has been reported previously, NP tissue contains subpopulations of progenitor cells with distinct cell surface markers [9]. However, surface antigen expression produces low cell yields and requires antibody conjugation, which may affect cellular function. The mechanical properties are distinct from those measured for stem cells and require no modification of or invasion into individual NPPCs [21].
In the current study, we use a small number (n=30) of clones that are assumed to be representative of NPPC populations. Further intensive experiments have been needed for the underlying mechanisms in the relationship between mechanical properties and lineage-specific differentiation, which are critical for stem cell-based tissue regeneration. The current findings are consistent with previous reports of mechanical differences between undifferentiated stem cells and fully differentiated osteoblasts, adipocytes, and chondrocytes [10,22]. Furthermore, it remains to be known whether this mechanical biomarker-lineage relationship can be used for improving functional tissue growth compared to using unsorted populations. In summary, this study supports the hypothesis that NPPC mechanical properties are indicative of differentiation potential. Future studies can build on these findings by targeting mechanically similar subpopulations that are well suited for IVD regeneration.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472076, 81271982, 81301944 and 81401801).
Disclosure of conflict of interest
None.
References
- 1.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Takatalo J, Karppinen J, Niinimaki J, Taimela S, Nayha S, Mutanen P, Sequeiros RB, Kyllonen E, Tervonen O. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine (Phila Pa 1976) 2011;36:2180–2189. doi: 10.1097/BRS.0b013e3182077122. [DOI] [PubMed] [Google Scholar]
- 3.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–256. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 4.Blanco JF, Graciani IF, Sanchez-Guijo FM, Muntion S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado MV, Cruz G, Gutierrez-Cosio S, Herrero C, San Miguel JF, Brinon JG, del Canizo MC. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 5.Feng G, Yang X, Shang H, Marks IW, Shen FH, Katz A, Arlet V, Laurencin CT, Li X. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LT, Huang B, Li CQ, Zhuang Y, Wang J, Zhou Y. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One. 2011;6:e26285. doi: 10.1371/journal.pone.0026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwin WM, Islam D, Eftekarpour E, Inman RD, Karim MZ, Fehlings MG. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural repair. Spine (Phila Pa 1976) 2013;38:211–216. doi: 10.1097/BRS.0b013e318266a80d. [DOI] [PubMed] [Google Scholar]
- 8.Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014;9:e115963. doi: 10.1371/journal.pone.0115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J Biomech. 2008;41:454–464. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage. 2006;14:571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J. 2007;92:1784–1791. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 14.Benndorf RA, Gehling UM, Appel D, Maas R, Schwedhelm E, Schlagner K, Silberhorn E, Hossfeld DK, Rogiers X, Boger R. Mobilization of putative high-proliferative-potential endothelial colony-forming cells during antihypertensive treatment in patients with essential hypertension. Stem Cells Dev. 2007;16:329–338. doi: 10.1089/scd.2006.0074. [DOI] [PubMed] [Google Scholar]
- 15.Kawase Y, Yanagi Y, Takato T, Fujimoto M, Okochi H. Characterization of multipotent adult stem cells from the skin: transforming growth factor-beta (TGF-beta) facilitates cell growth. Exp Cell Res. 2004;295:194–203. doi: 10.1016/j.yexcr.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Nakano T, Nakahata T, Asai H, Yagi Y, Tsuji K, Komiyama A, Akabane T, Kojima S, Kitamura Y. Formation of mast cell colonies in methylcellulose by mouse peritoneal cells and differentiation of these cloned cells in both the skin and the gastric mucosa of W/Wv mice: evidence that a common precursor can give rise to both “connective tissue-type” and “mucosal” mast cells. J Immunol. 1986;136:1378–1384. [PubMed] [Google Scholar]
- 17.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007;53:219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Tay CY, Leong WS, Tan SC, Liao K, Tan LP. Mechanical behavior of human mesenchymal stem cells during adipogenic and osteogenic differentiation. Biochem Biophys Res Commun. 2010;393:150–155. doi: 10.1016/j.bbrc.2010.01.107. [DOI] [PubMed] [Google Scholar]
- 19.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofek G, Willard VP, Koay EJ, Hu JC, Lin P, Athanasiou KA. Mechanical characterization of differentiated human embryonic stem cells. J Biomech Eng. 2009;131:061011. doi: 10.1115/1.3127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuznetsova TG, Starodubtseva MN, Yegorenkov NI, Chizhik SA, Zhdanov RI. Atomic force microscopy probing of cell elasticity. Micron. 2007;38:824–833. doi: 10.1016/j.micron.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Tan SC, Pan WX, Ma G, Cai N, Leong KW, Liao K. Viscoelastic behaviour of human mesenchymal stem cells. BMC Cell Biol. 2008;9:40. doi: 10.1186/1471-2121-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]