Abstract
Seizures, which result from synchronized aberrant firing of neuronal populations, can cause long-term sequelae, such as epilepsy, cognitive and behavioral issues, in which the synaptic plasticity alteration may play an important role. Long-term potentiation (LTP) is a persistent increase in synaptic strength and is essential for learning and memory. In the present study, we first examined the alteration of cognitive impairments and synaptic plasticity in mice with seizures, then explored the underlying mechanism involving pro-inflammatory factors and PI3K/Akt pathway. The results demonstrated that: (1) PTZ-induced seizure impairs learning and memory in mice, indicated by Morris water maze test; (2) PTZ-induced seizure decreased LTP; (3) the mRNA expression of IL-1β, IL-6 and TNF-α in the hippocampus were increased in mice with seizures; (4) LTP was increased by IL-1β receptor antagonist anakinra, but not inhibitors of IL-6 or TNF-α receptor; (5) Antagonist of IL-1β receptor rescues deficits in learning and memory of mice with seizures through PI3K/Akt pathway. It is concluded that the IL-1β induced by PTZ-induced seizures may impair the synaptic plasticity alteration in hippocampus as well as learning and memory ability by PI3K/Akt signaling pathway.
Keywords: Seizure, synaptic plasticity, hippocampus, IL-1β, PI3K/Akt pathway
Introduction
Seizures, which result from synchronized aberrant firing of neuronal populations, can cause long-term sequelae, such as epilepsy, cognitive and behavioral issues [1,2]. It was suggested in some clinical data that febrile seizures in some children might cause cognitive impairments [3,4] as well as in adults. Previous studies on adult rats with early life seizures found that synaptic plasticity and hippocampal dependent memory was impaired [5,6]. The behavioral phenotype of the adult rats with early life seizures includes abnormal working memory [5,7]. Recent studies in a rat model of hyperthermia-induced febrile seizures showed that seizures induced transient neuronal injury to the hippocampus without significant neuronal loss [8,9], indicating that prolonged early-life febrile seizures have long-lasting effects on the hippocampus and induce cognitive deficits. Long-term potentiation (LTP) is a persistent increase in synaptic strength. It is essential for many behavioral adaptations, such as learning and memory, visual and somatosensory system functional development [10]. Multiple studies showed that LTP could be altered by seizures, thus impacting certain behavioral adaptations. Isaeva et al showed in rats that early-life seizures caused alteration in the LTP in layer IV to layer II/III synapses of the somatosensory cortex [11]. Another study demonstrated that LTP of the hippocampal Schaffer collateral-CA1 region was impaired in postnatal animals with kainic acid (KA)-induced seizure [12]. However, whether seizure status affects the synaptic plasticity in adult animals was not well studied.
It is known that seizures can induce fast release of proinflammatory factors in the brain, such as Interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) [13,14]. It was shown that the increase of IL-1β after prolonged febrile seizures promoted adult seizure susceptibility, in which the IL-1β/IL-1R1 pathway and endocannabinoid signaling were involved [15]. A number of research groups have shown that both TNF-α and IL-1β could alter synaptic scaling and inhibited LTP in the hippocampus. Exogenous TNF-attenuated LTP induced by theta burst stimulation [16], and anti-IL-1β antibody was able to protect rats from the repeated restraint stress-induced alterations in synaptic transmission and LTP in the rat frontal cortex [17]. Moreover, a study of Vezzani et al indicated that the rapid post-translational changes in ion channels were regulated by the IL-1R/TLR signaling. It increased excitability, and transcriptional changes in genes involved in neurotransmission and synaptic plasticity, which decreased seizure thresholds chronically, suggesting that this system could be targeted to inhibit seizures in epilepsy [18]. Similarly, Serou et al found that the synaptic plasticity changes following injury might be affected by IL-1β-PAF-COX-2 signaling [19]. All these findings suggest the importance of IL-1β in synaptic plasticity.
The serine/threonine kinase Akt plays a critical role in the manipulation of diverse cellular functions, such as metabolism, proliferation, survival, transcription and protein synthesis. The Akt signaling cascade can be activated by many factors, including receptor tyrosine kinases, integrins, cytokine receptors and other stimuli that cause production of phospha-tidylinositol (3,4,5) trisphosphates (PIP3) by phosphoinositide 3-kinase (PI3K) [20]. Studies showed that the PI3K/Akt pathway is also associated with the alteration of synaptic plasticity. It was reported that magnesium sulfate treatment increased the activity of Akt at Ser473 and PI3K at Tyr458/199 and protected cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model [21]. On the other hand, IL-1β may activate the PI3K/Akt pathway under certain circumstance. For instance, the inflammatory response induced by IL-1β promotes seizures and plays an important role in the pathogenesis of MTLE via the PI3K/Akt/mTOR signaling pathway [22]. However, the interaction between IL-1β and PI3K/Akt pathway in the alteration of synaptic plasticity induced by seizures is not explored yet. Hence, in the present study, we first examined the alteration of cognitive impairments and synaptic plasticity in mice with seizures, then explored the underlying mechanism involving pro-inflammatory factors and PI3K/Akt pathway.
Materials and methods
Animals
C57BL/6 mice were purchased from the Shanghai animal center. Animals were housed in the animal center of the Tongji university school of medicine. The room temperature was kept at 24 ± 1°C and humidity at 50%-60% under a 12:12 light-dark cycle. The animals were allowed access to water and food ad libitum. All of the experiments were approved by the local animal care and use committee of Tongji university school of medicine and performed under the guidelines in the “Principles of Laboratory Animal Care” and the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, revised 1996).
Experimental TLE induction
The induction of experimental TLE was similar to that used in the study by Reddy et al [23]. Briefly, animals received a s.c. injection of pentylenetetrazol (PTZ, 85 mg/kg) and were then observed for a 30-min period for clonic seizures. Animals that showed clonic seizures lasting longer than 5 s were scored positive for seizure occurrence.
Morris water maze
The Morris water maze test was performed as described by Morris [24]. Briefly, mice were taken 4 trials per day. A different starting position was used on each trial. The duration of a trial was 90 seconds. Escape latencies (time spent swimming from start point to the target) and path length (the distance from start point to the platform) before reaching the platform were recorded for 5 consecutive days. The escape latency of the mice at the first training day was normalized to 1.0. The relative escape latencies in the following training day to that of the first day were analyzed (escape latency in the following day/escape latency in the first day) and labeled as learning trend. For probe trials, the platform was removed after the last trial of the acquisition period. The mice were tested 24 hours later to assess memory consolidation. The time spent in the target quadrant within 60 seconds was recorded. The latency to the first target site was measured, and the numbers of platform-site crossovers were recorded.
Novel object recognition
The mice were exposed to 2 identical objects for 10 minutes placed in 2 opposite corners of the apparatus 8.5 cm from the sidewall. Ninety minutes after the training session, the animal explored the open field for 10 minutes in the presence of 1 familiar and 1 novel objects. Location preference = time exploring one of the identical objects/time exploring the identical object pairs × 100%. Recognition index (RI) = time exploring novel object/(time exploring novel object + time exploring familiar object) × 100%.
Extracellular field recording
The extracellular field recording was done with method of Clark et al [25]. Long-term synaptic plasticity was induced using a multiple train stimulus induction protocol previously shown to elicit a compound potentiation consisting of both NMDAR and non-NMDAR components of LTP [26]. The non-NMDAR component of LTP was measured utilizing the selective NMDAR antagonist d, l-AP5 to block the NMDAR component of LTP. Briefly, hippocampal slices were prepared from mice 10-17 days after maze testing. Mice were sacrificed with quick decapitation, then the brain was removed and submerged in ice-cold, oxygenated dissection artificial cerebrospinal fluid (ACSF) and sectioned into 400 μm thick slices. The hippocampus was dissected free from slices obtained between the levels of Bregma -4.0 mm to Bregma -2.4 mm. Slices were placed in a recording chamber and perfused with oxygenated standard ACSF for 45 min at room temperature and 45 min at 30°C. A bipolar stimulating electrode (Kopf Instruments, Tujunga, CA, USA) was placed within the stratum radiatum of CA1 and an extracellular recording microelectrode (World Precision Instruments, Sarasota, FL, USA) was positioned in the same layer of CA1 to record the field xxcitatory post-synaptic potentials (fEPSPs). Synaptic responses for LTP experiments were normalized by dividing all fEPSP slope values by the average of the five responses recorded during the 5 min immediately before high frequency stimulation (HFS). To measure the non-NMDAR component of LTP, the NMDAR antagonist d,l-AP5 (50 μM, Tocris Bioscience, Minneapolis, MN, USA) was applied before LTP measurement. LTP values for the 1 h time point were determined by averaging 5 min of normalized slope values at 55-60 min post-HFS.
Measurement of mRNA expression by polymerase chain reaction (PCR)
The IL-1β, IL-10, IL-6, IFNγ and TNF-α mRNA expression were evaluated by reverse transcription-polymerase chain reaction (RT-PCR) using the kit GoTaq DNA Polymerase with the methods of Sitges et al [27]. Briefly, 1.5 µl of cDNA (250 ng/µl) were amplified in a mixture containing 2 µl of 5X green buffer, 0.8 µl of MgCl2 (25 mM), 0.25 µl of PCR nucleotide mix (10 mM), 0.5 µl of the sense primer (10 pM), 0.5 µl of the antisense primer (10 pM), 0.05 µl of DNA Polymerase (5 u/µl) and 4.4 µl of sterile Milli-Q water. The temperature cycling conditions were: initial denaturation at 95°C for 5 min, followed by 34 cycles, including denaturation at 94°C for 30 s, primer annealing for 45 s at 60°C, and primer extension at 72°C for 1 min. A final primer extension was performed at 72°C for 10 min after which the samples were immediately cooled at 4°C. PCR products were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide and the resulting bands were quantified by densitometry using a MiniBIS Pro Gel Documentation System (Bio-America, Miami FL, USA) and ImageJ version 1.42 software (National Institute of Health, USA). Results are expressed as relative mRNA level expression.
Western blot
After the hippocampus in mice was collected, the tissues were washed in ice-cold saline, then homogenized in 4°C RIPA lysis buffer (with 1 mM PMSF) and centrifuged at 3000 g at 4°C for 15 min. The supernatants were collected and the protein concentration was determined with a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). 40 μg protein sample was loaded per lane and separated on SDS-PAGE. The target protein (p-Akt/Akt, p-JNK/JNK, p-ERK/ERK) were then electrophoretically transferred to nitrocellulose membranes. The protein blots were blocked overnight at 4°C, followed by incubations with a primary antibodies against p-Akt, Akt, p-JNK, JNK, p-ERK or ERK (Santa Cruz, Dallas, USA). Each sample was also probed with ß-actin antibody (Sigma-Aldrich Corp) as a loading control. Finally, blots were washed with PBST and then examined with the ECL plus western blotting detection system (Amersham Life Science, UK).
Data analysis
Values were presented as mean ± SEM. Statistical analysis was performed using SPSS 17.0 with one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. P<0.05 was considered significant.
Results
PTZ-induced seizure impairs learning and memory in mice
The effects of PTZ-induced seizure on spatial memory were examined using Morris water maze test. As shown in Figure 1A, mice with PTZ-induced seizures showed a significant deficit in locating the submerged escape platform. Figure 1B demonstrated the escape latencies in both groups. Mice with PTZ-induced seizures showed increased escape latencies compared with control mice. Figure 1C quantified the escape latencies to that in the first trail day to get the relative escape latencies and compares the difference. The results revealed that the relative escape latencies in consecutive trials were also higher in the Seizure group. Consistently, the mice with PTZ-induced seizures had longer swimming length compared to that of control mice, as shown in Figure 1D. In the probe trial experiment, mice with PTZ-induced seizures exhibited impaired memory retention as indicated that they swam to cross over the target site less times than control mice (Figure 1E). As shown in Figure 1F, no significant difference was observed in location preference during the training phase, indicating that the location of the objects does not affect the exploratory behavior of mice (Figure 1F). In the testing phase, mice with PTZ-induced seizures displayed a shorter time period with RI than control mice (Figure 1G), indicating a deficit in their memory for the familiar object.
Figure 1.
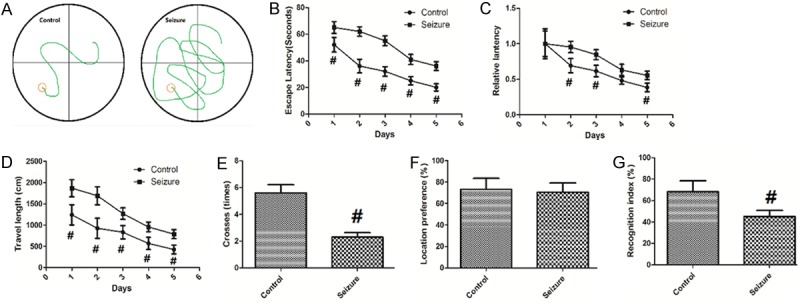
PTZ-induced seizure impairs learning and memory in mice. Values are expressed as mean ± SEM. #: P<0.05 compared to control.
Changes of LTP induced by seizure
The total LTP and non-NMDAR component of LTP in control mice and mice with PTZ-induced seizures were shown in Figure 2A and 2B and the values were summarized in Figure 2C. There was significant difference in total LTP between control mice and mice with PTZ-induced seizures (P<0.05). In the presence of d,l-AP5 (50 μM), the potentiated fEPSP slope value was significantly reduced in control mice and mice with PTZ-induced seizures as compared with their respective total LTP values (P<0.05, Figure 2C). In addition, the non-NMDAR component of LTP was significantly lower in mice with PTZ-induced seizures than in the control mice (P<0.05, Figure 2C).
Figure 2.
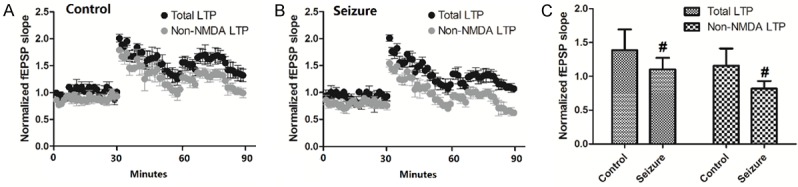
Changes of LTP induced by seizure. Values are expressed as mean ± SEM. #: P<0.05 compared to control.
Changes of mRNA expression of proinflammatory factors
Changes of mRNA expression of IL-1β, IL-10, IL-6, IFNγ and TNF-αwere measured in the hippocampus and cortex in mice. As shown in Figure 3A, the mRNA expression of IL-1β, IL-6 and TNF-α in the hippocampus were increased in mice with seizures (P<0.05). No difference was observed in the mRNA expression ofIL-10 or IFNγ was seen in the hippocampus. As shown in Figure 3B, No difference was observed in the mRNA expression of IL-1β, IL-10, IL-6, IFNγ or TNF-α was seen in the cortex.
Figure 3.
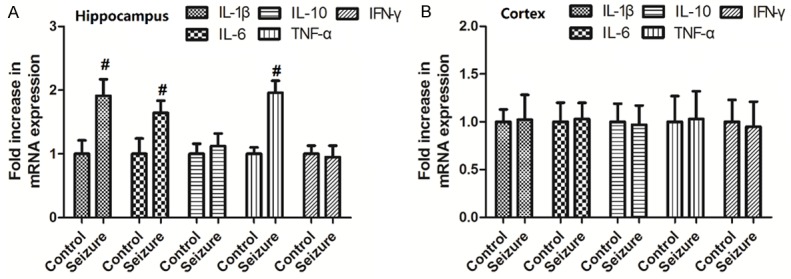
Changes of mRNA expression of pro-inflammatory factors. Values are expressed as mean ± SEM. #: P<0.05 compared to control.
Changes of LTP induced by antagonists of receptors of IL-1β, IL-6 and TNF-α
To examine the roles of IL-1β, IL-6 and TNF-α in the changes of LTP caused by PTZ-induced seizures, we treated mice with antagonists of receptors of IL-1β, IL-6 and TNF-α (anakinra, tocilizumab and R-7050, respectively), then examined the changes of LTP. As shown in Figure 4, both the total LTP and non-NMDAR component of LTP in Seizure + anakinra mice were increased compared with mice with PTZ-induced seizures, while in the Seizure + tocilizumab or Seizure + R-7050 group, there is no significant change in total LTP or non-NMDAR component of LTP.
Figure 4.
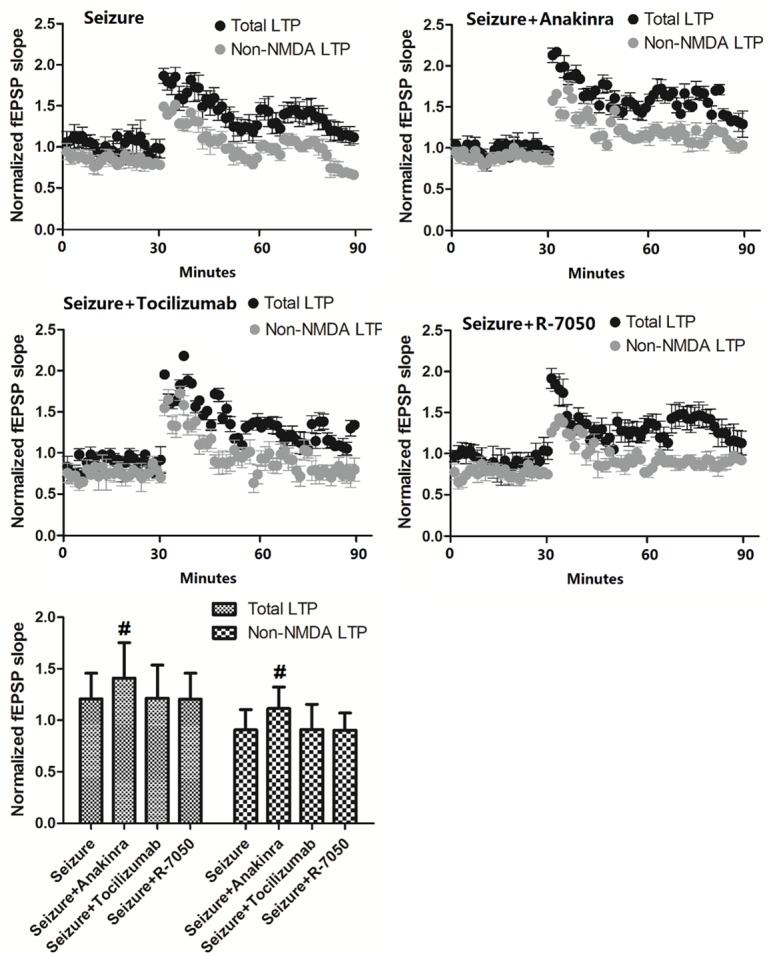
Changes of LTP induced by antagonists of receptors of IL-1β, IL-6 and TNF-α. Values are expressed as mean ± SEM. #: P<0.05 compared to control.
Antagonists of IL-1β receptor rescues deficits in learning and memory of mice with seizures
To examine the role of IL-1β receptor in the effects of PTZ-induced seizures in learning and memory deficits, we measured the learning and memory ability of mice in the presence of antagonists of IL-1β receptor anakinra using Morris water maze test. As shown in Figure 5A, when treated with anakinra, mice with PTZ-induced seizures showed decreased escape latencies (P<0.05). Figure 5B showed that when mice in the Seizure + anakinra group swam to cross over the target site more times than Seizure mice (P<0.05). As demonstrated in Figure 5C, mice with PTZ-induced seizures had longer swimming length compared to that of Seizure + anakinra group (P<0.05). In the testing phase, mice in the Seizure + anakinra group displayed a longer time period with RI than mice with PTZ-induced seizures (Figure 5D), indicating a rescue in their memory for the familiar object.
Figure 5.
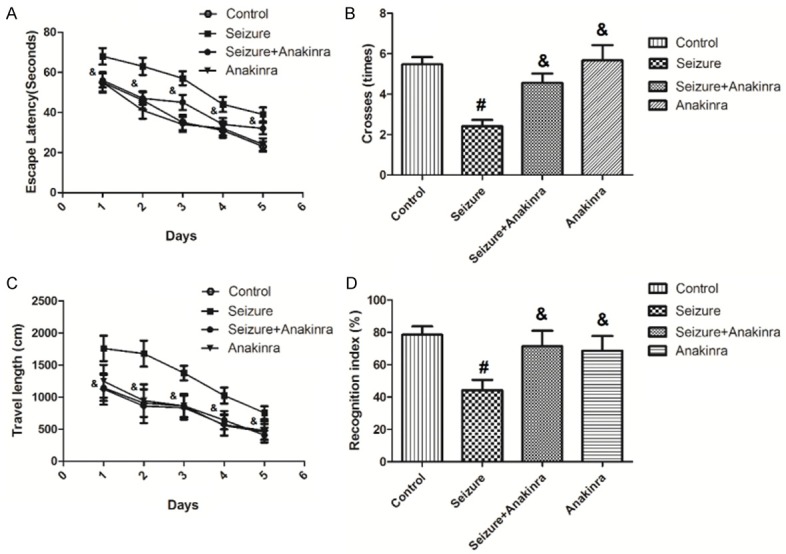
Antagonists of IL-1β receptor rescues deficits in learning and memory of mice with seizures. Values are expressed as mean ± SEM. #: P<0.05 compared to control; &: P<0.05 compared to Seizure.
Changes in Akt activation and recognition index by inhibitors of IL-1β receptor or PI3K/Akt pathway
Figure 6 shows the changes of p-Akt/Akt, p-JNK/JNK and p-ERK/ERK induced by antagonist of receptors of IL-1β anakinra and recognition index by inhibitors of PI3K/Akt pathway (LY294002 and triciribine). Figure 6A-F show that only the ratio of p-Akt/Akt, but not p-JNK/JNK or p-ERK/ERK, was enhanced by seizure (P<0.05), and then decreased by treatment of anakinra (P<0.05). Figure 6G shows that the decrease in the recognition index by seizure was greatly inhibited by LY294002 and triciribine (P<0.05).
Figure 6.
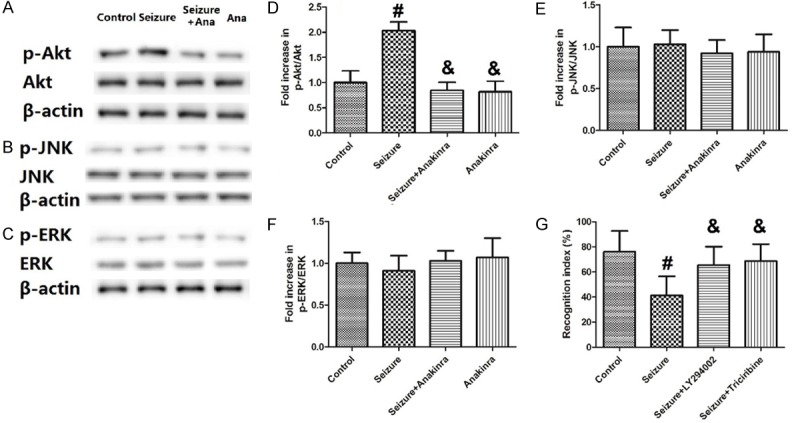
Changes in Akt activation and recognition index by inhibitors of IL-1β receptor or PI3K/Akt pathway. Values are expressed as mean ± SEM. #: P<0.05 compared to control; &: P<0.05 compared to Seizure.
Discussion
The present study aimed to determine the alteration of cognitive impairments and synaptic plasticity in mice with seizures and the underlying mechanism involving proinflammatory factors and PI3K/Akt pathway. The results demonstrated that: (1) PTZ-induced seizure impairs learning and memory in mice, indicated by Morris water maze test; (2) PTZ-induced seizure decreased LTP; (3) The mRNA expression of IL-1β, IL-6 and TNF-α in the hippocampus were increased in mice with seizures; (4) LTP was increased by IL-1β receptor antagonist anakinra, but not inhibitors of IL-6 or TNF-α receptor; (5) Antagonist of IL-1β receptor rescues deficits in learning and memory of mice with seizures through PI3K/Akt pathway.
PTZ is a classical drug to induce experimental seizures. In the first part of our study, we injected mice with PTZ to produce an experimental TLE model and observe its impacts on learning and memory ability using Morris water maze and novel object recognition method. The results revealed that mice with PTZ-induced seizures had: (1) a significant deficit in locating the submerged escape platform; (2) increased escape latencies; (3) longer swimming length; (4) less times to cross over the target site; (5) a shorter time period with RI. All these results indicated a deficit in their learning and memory. This result is consistent with a number of previous studies. Cognitive impairment, such as memory deficiency, is a common comorbidity of epilepsy and is even persistent once seizures are under control with antiepileptic medication [28]. Kiasalari et al found that kainate (KA) injection caused a higher severity and rate of seizures and deteriorated learning and memory performance in passive avoidance paradigm and spontaneous alternation as an index of spatial recognition memory in Y-maze task [29]. Mice with an intrahippocampal injection of KA show deficits of spatial learning and short- and long-term memories in a hippocampus-dependent large diameter pool Morris water maze task [30]. Learning and memory were also impaired in pilocarpine epileptic mice [31].
However, regarding the exact mechanism of how seizure induces learning and memory loss, there is no conclusion yet. Some studies suggested that the impairment of cognitive processes in epileptic rats is attributed to the neurodegenerative processes in brain structures including hippocampal areas [30], astrocytic hypertrophy [32] and sprouting of new connections [33]. Some studies found that oxidative stress played an important role in the cognitive impairment [29]. Recent studies suggested that the alteration of synaptic plasticity in hippocampus might be another mechanism. Gangarossa et al reported that repeated systemic administration of SKF81297 induces kindled seizures in mice, which parallelled the hyperactivation of the mTOR signaling in the hippocampus and disrupted LTP in the dentate gyrus (DG) and altered recognition memories [34]. Isaeva et al showed in rats that early-life seizures result in long-term alteration in the maintenance phase of LTP in layer IV to layer II/III synapses of the somatosensory cortex without alteration of basal synaptic transmission, the induction phase of LTP and short-term depression [11]. Another study demonstrated that LTP of the Schaffer collateral-CA1 region was impaired in postnatal animals with kainic acid (KA)-induced seizure [12]. Hence, in the present study, we focused on the alteration of synaptic plasticity. Our results showed that PTZ-induced seizures significantly changed both the total LTP and non-NMDAR component of LTP, indicating the alteration of synaptic plasticity in the hippocampus.
To further explore the mechanism of synaptic plasticity alteration, we examined the mRNA expression of pro-inflammatory factors (IL-1β, IL-10, IL-6, IFNγ and TNF-α) in the hippocampus and cortex. The mRNA expression of IL-1β, IL-6 and TNF-α in the hippocampus were increased by seizures, while no change of them was seen in the cortex. Next, we treated mice with antagonists of receptors of IL-1β, IL-6 and TNF-α (anakinra, tocilizumab and R-7050, respectively), then observed the changes of LTP. The results showed that both the total LTP and non-NMDAR component of LTP was changed by anakinra, but not by tocilizumab and R-7050. These findings suggest that the increase of proinflammatory factors IL-1β in the hippocampus is responsible for the synaptic plasticity alteration. Furthermore, we treated mice with anakinra, then measured the learning and memory ability of mice. It turned out that mice treated with anakinra had: (1) decreased escape latencies; (2) shorter swimming length; (3) more times to cross over the target site; (4) a longer time period with RI. Both TNF-α and IL-1β have been shown to alter synaptic scaling and to inhibit LTP in the hippocampus when induced by specific high-frequency stimulation. Exogenous TNF-α attenuated LTP induced by theta burst stimulation [16], and anti-interleukin-1β antibody prevents the occurrence of repeated restraint stress-induced alterations in synaptic transmission and LTP in the rat frontal cortex [17]. But in the present study, it shows only IL-1β participates in the LTP alteration. IL-1β is an inflammatory cytokine whose expression is elevated in brain during seizures. It has been shown that the antagonists of IL-1β receptor could inhibit the impairment of learning and memory by seizures, indicating the role of IL-1β in the procedure. Transient increase of IL-1β after prolonged febrile seizures promoted adult seizure susceptibility, in which the postnatal IL-1β/IL-1R1 pathway was involved [15]. Moreover, a study of Vezzani et al indicated that the IL-1R/TLR signaling mediated the changes in voltage- and ligand-gated ion channels and synaptic plasticity [18]. Similarly, Serou’s study suggested that following injury, synaptic plasticity changes may be affected by IL-1β-PAF-COX-2 neuronal signaling [19]. All these results revealed the important role of IL-1β in the LTP alteration by seizures.
To further explore the underlying mechanism of IL-1β’s action, we tried to measure the involvement of PI3K/Akt pathway. We measured the expression of p-Akt/Akt, p-JNK/JNK and p-ERK/ERK in the presence of antagonist of IL-1β receptor anakinra. It was demonstrated that only the ratio of p-Akt/Akt, but not p-JNK/JNK or p-ERK/ERK, was enhanced by seizure, and then decreased by treatment of anakinra. Finally, we examined the changes in recognition index when mice were treated with inhibitors of PI3K/Akt pathway (LY294002 and triciribine). The results revealed a significant increase in the recognition index by LY294002 and triciribine, indicating the involvement of PI3K/Akt pathway in the alteration of synaptic plasticity and learning and memory ability impairment. It has been reported that the activation of PI3K/Akt pathway altered cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model [21]. Another study showed that lixisenatide, can prevent Aβ-related impairments in synaptic plasticity and spatial memory of rats by affecting the PI3K-Akt-GSK3β pathway [35]. GSK3β, a multifunctional serine/threonine (ser/thr) kinase that is originally identified as a regulator of glycogen metabolism, is highly enriched in the brain, where it has been implicated in several CNS dysfunctions, including synaptic plasticity and spatial memory [36-38]. The result that the ratio of p-Akt/Akt was enhanced by seizure and then decreased by treatment of IL-1β receptor antagonist anakinra indicates the manipulation of IL-1β on PI3K/Akt pathway. Previous studies have shown that IL-1β may activate the PI3K/Akt pathway under certain circumstance. For instance, the inflammatory response induced by IL-1β promotes seizures and mediated the pathogenesis of temporal lobe epilepsy via the PI3K/Akt/mTOR signaling pathway [22]. Yang et al reported that IL-1β activated phosphorylation of Akt and upregulated human endothelial progenitor cells (EPCs)’ functions, indicating the role of PI3K/Akt signaling pathway in the regulation of EPCs functions induced by IL-1β [39]. IL-1β could also induce PI3K and nuclear factor κB signaling pathways in human tenocytes, which can be inhibited by resveratrol, an activator of histone deacetylase Sirt-1 [40]. Taken together, these results indicate that it is very likely the IL-1β induced by PTZ-induced seizures may impair the synaptic plasticity alteration in hippocampus as well as learning and memory ability by PI3K/Akt signaling pathway. Further studies should be done to explore the detailed mechanism and the strategy to prevent seizure-induced learning and memory ability loss.
Disclosure of conflict of interest
None.
References
- 1.Holmes GL. Effects of early seizures on later behavior and epileptogenicity. Ment Retard Dev Disabil Res Rev. 2004;10:101–105. doi: 10.1002/mrdd.20019. [DOI] [PubMed] [Google Scholar]
- 2.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 3.Notenboom RG, Ramakers GM, Kamal A, Spruijt BM, de Graan PN. Long-lasting modulation of synaptic plasticity in rat hippocampus after early-life complex febrile seizures. Eur J Neurosci. 2010;32:749–758. doi: 10.1111/j.1460-9568.2010.07321.x. [DOI] [PubMed] [Google Scholar]
- 4.Holmes GL, Ben-Ari Y. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:379–81. doi: 10.1002/ana.21136. [DOI] [PubMed] [Google Scholar]
- 5.Bernard PB, Castano AM, O’Leary H, Simpson K, Browning MD, Benke TA. Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis. 2013;59:1–17. doi: 10.1016/j.nbd.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 7.Castelhano AS, Cassane Gdos S, Scorza FA, Cysneiros RM. Altered anxiety-related and abnormal social behaviors in rats exposed to early life seizures. Front Behav Neurosci. 2013;7:36. doi: 10.3389/fnbeh.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender RA, Dube C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube C, Yu H, Nalcioglu O, Baram TZ. Serial MRI after experimental febrile seizures: altered T2 signal without neuronal death. Ann Neurol. 2004;56:709–714. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirila AM, Brown TE, Bishop RA, Bellono NW, Pucci FG, Kauer JA. Long-term potentiation of glycinergic synapses triggered by interleukin 1beta. Proc Natl Acad Sci U S A. 2014;111:8263–8268. doi: 10.1073/pnas.1401013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaeva E, Isaev D, Holmes GL. Alteration of synaptic plasticity by neonatal seizures in rat somatosensory cortex. Epilepsy Res. 2013;106:280–283. doi: 10.1016/j.eplepsyres.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YH, Kuo TT, Chu MT, Ma HI, Chiang YH, Huang EY. Postnatal systemic inflammation exacerbates impairment of hippocampal synaptic plasticity in an animal seizure model. Neuroimmunomodulation. 2013;20:223–232. doi: 10.1159/000348440. [DOI] [PubMed] [Google Scholar]
- 13.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 15.Feng B, Tang Y, Chen B, Xu C, Wang Y, Dai Y, Wu D, Zhu J, Wang S, Zhou Y, Shi L, Hu W, Zhang X, Chen Z. Transient increase of interleukin-1beta after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci Rep. 2016;6:21931. doi: 10.1038/srep21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall AM, Mukandala G, Greig NH, O’Connor JJ. Tumor necrosis factor-alpha potentiates long-term potentiation in the rat dentate gyrus after acute hypoxia. J Neurosci Res. 2015;93:815–829. doi: 10.1002/jnr.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobula B, Sowa J, Hess G. Anti-interleukin-1beta antibody prevents the occurrence of repeated restraint stress-induced alterations in synaptic transmission and long-term potentiation in the rat frontal cortex. Pharmacol Rep. 2015;67:123–128. doi: 10.1016/j.pharep.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Serou MJ, DeCoster MA, Bazan NG. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. J Neurosci Res. 1999;58:593–598. [PubMed] [Google Scholar]
- 20.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Xu ZP, Li L, Bao J, Wang ZH, Zeng J, Liu EJ, Li XG, Huang RX, Gao D, Li MZ, Zhang Y, Liu GP, Wang JZ. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS One. 2014;9:e108645. doi: 10.1371/journal.pone.0108645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z, Peng J, Yang L, Kong H, Yin F. Interleukin-1beta plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol. 2015;282:110–117. doi: 10.1016/j.jneuroim.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 24.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Clark JK, Furgerson M, Crystal JD, Fechheimer M, Furukawa R, Wagner JJ. Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol Learn Mem. 2015;125:152–162. doi: 10.1016/j.nlm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 27.Sitges M, Gomez CD, Aldana BI. Sertraline reduces IL-1beta and TNF-alpha mRNA expression and overcomes their rise induced by seizures in the rat hippocampus. PLoS One. 2014;9:e111665. doi: 10.1371/journal.pone.0111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titiz AS, Mahoney JM, Testorf ME, Holmes GL, Scott RC. Cognitive impairment in temporal lobe epilepsy: role of online and offline processing of single cell information. Hippocampus. 2014;24:1129–1145. doi: 10.1002/hipo.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiasalari Z, Khalili M, Shafiee S, Roghani M. The effect of Vitamin E on learning and memory deficits in intrahippocampal kainate-induced temporal lobe epilepsy in rats. Indian J Pharmacol. 2016;48:11–14. doi: 10.4103/0253-7613.174394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miltiadous P, Stamatakis A, Koutsoudaki PN, Tiniakos DG, Stylianopoulou F. IGF-I ameliorates hippocampal neurodegeneration and protects against cognitive deficits in an animal model of temporal lobe epilepsy. Exp Neurol. 2011;231:223–235. doi: 10.1016/j.expneurol.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Groticke I, Hoffmann K, Loscher W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol. 2007;207:329–349. doi: 10.1016/j.expneurol.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;2:33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- 33.Mansouri Z, Sabetkasaei M, Moradi F, Masoudnia F, Ataie A. Curcumin has neuroprotection effect on homocysteine rat model of Parkinson. J Mol Neurosci. 2012;47:234–242. doi: 10.1007/s12031-012-9727-3. [DOI] [PubMed] [Google Scholar]
- 34.Gangarossa G, Ceolin L, Paucard A, Lerner-Natoli M, Perroy J, Fagni L, Valjent E. Repeated stimulation of dopamine D1-like receptor and hyperactivation of mTOR signaling lead to generalized seizures, altered dentate gyrus plasticity, and memory deficits. Hippocampus. 2014;24:1466–1481. doi: 10.1002/hipo.22327. [DOI] [PubMed] [Google Scholar]
- 35.Cai HY, Holscher C, Yue XH, Zhang SX, Wang XH, Qiao F, Yang W, Qi JS. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein-induced impairments in rats. Neuroscience. 2014;277:6–13. doi: 10.1016/j.neuroscience.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 37.Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Guo XG, Du CQ, Yang JX, Jiang DM, Li B, Zhou WJ, Zhang FR. Interleukin-1 beta increases activity of human endothelial progenitor cells: involvement of PI3K-Akt signaling pathway. Inflammation. 2012;35:1242–1250. doi: 10.1007/s10753-012-9434-9. [DOI] [PubMed] [Google Scholar]
- 40.Busch F, Mobasheri A, Shayan P, Lueders C, Stahlmann R, Shakibaei M. Resveratrol modulates interleukin-1beta-induced phosphatidylinositol 3-kinase and nuclear factor kappaB signaling pathways in human tenocytes. J Biol Chem. 2012;287:38050–38063. doi: 10.1074/jbc.M112.377028. [DOI] [PMC free article] [PubMed] [Google Scholar]


