Abstract
Many vertebrates are known to show behavioural lateralization, whereby they differentially use one side of their body or either of their bilateral organs or limbs. Behavioural lateralization often manifests in a turning bias in fishes, with some individuals showing a left bias and others a right bias. Such biases could be the source of considerable conflict in fish schools given that there may be considerable social pressure to conform to the group to maintain effective group evasion. Here, we show that predation pressure is a major determinant of the degree of lateralization, both in a relative and absolute sense, in yellow-and-blueback fusiliers (Caesio teres), a schooling fish common on coral reefs. Wild-caught fish showed a bias for right turning. When predation pressure was experimentally elevated or relaxed, the strength of lateralization changed. Higher predation pressure resulted in an increase in the strength of lateralization. Individuals that exhibited the same turning bias as the majority of individuals in their group had improved escape performance compared with individuals that were at odds with the group. Moreover, individuals that were right-biased had improved escape performance, compared with left-biased ones. Plasticity in lateralization might be an important evolutionary consequence of the way gregarious species respond to predators owing to the probable costs associated with this behaviour.
Keywords: group living, schooling, predation, alarm cues, behavioural lateralization, coral reef fish
1. Introduction
Group living reflects a fine balance of costs and benefits. Any given individual within a group may find food faster, benefit from group evasion and reduce the cost of finding mates, but at the same time may experience increased competition for food and mates and have a greater level of exposure to disease and parasites [1,2]. Given that not all individuals are equal, the costs and benefits of being in a group vary from individual to individual.
For vertebrates, we know that many individuals differentially use one side of their body or either of the bilateral organs or limbs [3], a phenomenon known as behavioural lateralization [4–6]. Differences in lateralization between any given individual and the majority of its groupmates may represent a major source of conflict and hence may be the source of differential costs and benefits for members of the group. Lateralization has been documented in a variety of contexts. For example, toads, lizards and birds show more aggression towards conspecifics on their left side than their right [7–9]. Feeding responses, on the other hand, are often, but not always, biased towards the right [10–14]. Individuals within a species often show considerable variation in their degree of lateralization. Sovrano et al. [15] documented that highly lateralized fish perform better in spatial tasks than non-lateralized fish. Highly lateralized fish also show enhanced learning [16] and highly lateralized parrots have enhanced problem-solving abilities [17]. Moreover, lateralized birds are better than non-lateralized birds at multitasking [18].
In the context of predation, prey often show a bias in their response to threatening stimuli. Several experiments have documented that prey have greater escape performance when predators are detected in their left visual field [19,20]. Prey from high-risk populations often preferentially use one eye over the other when observing predators [21]. Moreover, prey often move closer to predators when a companion is on their left side [22]. During an escape response, different taxa show differential turning biases with some favouring a right-turning bias, whereas others have a left-turning bias [23–25]. Understanding lateralization in the context of predation is challenging. Given that predators are equally likely to attack on both sides of the prey, there should be negative selection on individuals that preferentially attend to predator-related information on one side or the other. When an entire population shows the same average turning bias, then the situation is even more confusing, because having cognitive functions that are specialized in the right or in the left hemisphere is, theoretically, equivalent [3]. Vallortigara & Rogers [3] argue that alignment of behavioural asymmetries at the population level arise as an evolutionary arms race from social pressure to coordinate behaviour. Ghirlanda et al. [26,27] formalized this hypothesis showing that populations consisting of left- and right-type individuals in unequal numbers can be evolutionarily stable if being lateralized in one or the other direction has frequency-dependent costs and benefits. In prey–predator interactions, prey that can coordinate their escape response with others in a group should have a considerable advantage. This supposition is supported by the work of Bisazza et al. [28], who investigated turning responses in 16 species of fishes, 10 of which showed a consistent lateral bias to turn preferentially to the right or to the left. All of the gregarious species that relied heavily on schooling as a defence showed population lateralization, whereas only 40% of the non-gregarious species did so. If social pressure is the driver of behavioural lateralization at the population level, then we should see variation in the extent of relative lateralization that individuals exhibit based on their perceived cost of not doing so. Four recent experiments that manipulated predation pressure suggest that prey exhibit flexibility in their lateralization responses. Embryonic pre-exposure to predator odours resulted in a strong left bias in cuttlefish [29] and guppies raised for five weeks under high risk exhibited an increase in behavioural lateralization [30]. In the most recent studies, varying levels of risk over as little as 4 days caused considerable changes in lateralization in juvenile coral reef damselfish [31,32].
This research examines how predation pressure affects lateralization and how lateralization conflict influences escape performance in the yellow-and-blueback fusilier (Caesio teres), a schooling fish common on coral reefs throughout the Indo-west and central Pacific. We started by collecting individuals from four distinct schools and assessing their relative lateralization (left- or right-turning preference) to determine whether there was an overall population bias. Examining how individual schools compared with the overall population allowed us to assess whether individuals in different schools were coordinated with each other. Even in the absence of an overall population turning bias, individuals within schools could be coordinated, i.e. biased left or right. If lateralization is indeed highly flexible with short-term changes in risk, as recent evidence suggests, then we may also be able to observe different levels of lateralization in wild-caught schools of fish if we assume that fish schools experience different levels of threat over relatively short temporal and spatial scales. Differences in the strength of lateralization irrespective of turning direction (i.e. absolute lateralization) could occur. A high degree of absolute lateralization has been linked to higher escape reactivity in fishes [33]. If the benefit of exhibiting lateralization is reduced when the risk of predation is lowered, then fish should quickly reduce lateralization if placed into a common low-risk environment, while maintaining the ability to quickly increase lateralization, should predation pressure again increase. Here, we documented changes in lateralization among schools of fish held in the laboratory without risk and conducted a manipulative experiment to test whether subsequent variation in predation pressure would alter the strength of lateralization. Moreover, we examined the consequences of lateralization conflict that occurred within schools. Given that any given individual could be lateralized in either direction, it may find that its turning bias conforms or conflicts with others in the group. Therefore, we examined whether this conformity, or lack thereof, influences escape performance.
2. Material and methods
(a). Test species
Juvenile yellow-and-blueback fusiliers form large schools in the midwater above shallow coral reefs where they forage on zooplankton brought by currents. The fish are vulnerable to a variety of resident reef predators, including groupers and transient predators, including trevally, snappers and barracuda. In early March 2015, we located four distinct schools (school name referring to the location found—Loomis, Entrance Lagoon, Entrance Bommie and Horseshoe) of juvenile fish on the reefs fringing Lizard Island (14°40′ S, 145°28′ E), northern Great Barrier Reef, Australia. Schools were separated by a minimum distance of 300 m and a maximum distance of 2.5 km. Given that the fish are site attached and the schools could be located in the same location for the duration of our study, there is little possibility that the fish mixed between schools. The mean (±s.e.) standard length of the fish at testing was 35.9 (±0.32) mm.
We captured the groups of fish from each of the four schools on several different occasions, using a barrier net and hand nets while on SCUBA. The fish were transported back to the Lizard Island Research Station where they were held in 30 l flow-through tanks, and fed brine shrimp three times per day.
(b). Experiment 1: differences in lateralization among schools and the effect of captivity on behavioural lateralization
In this experiment, we wanted to characterize the lateralization scores, both in a relative and absolute sense, of newly caught fish from the four different schools. Relative lateralization refers to the left/right-turning bias of the fish, whereas absolute lateralization refers to the strength of the bias (see below for calculation method). Characterizing relative lateralization allowed us to test whether the overall population had a turning bias and second whether each of the schools exhibited the same lateralization bias. We were also interested in absolute behavioural lateralization, particularly, because fish with high absolute lateralization have higher escape reactivity and greater escape distance [33].
Additionally, we wanted to test whether being in a low-risk environment altered their pattern of lateralization over a 4 day period. We tested fish from each school on the day of capture (after being in the laboratory for a minimum of 4 h) and from the remaining laboratory stock tested new fish each day for 3 days giving us four tests for each of the four schools. Each fish was tested only once. We captured fish from each of the schools in the field several times over the course of the experiment, testing between 49 and 139 fish from each school over the four test periods. Our sample size for some of the test periods was reduced, because we were forced to stop collecting fish for safety reasons associated with Cyclone Nathan. However, even if the reduced sample size limits the power of inference for particular schools, then it still provides important information about population-level lateralization.
We used a detour test to examine behavioural lateralization following the methodology of Ferrari et al. [31]. The apparatus consisted of an opaque PVC tank (60 × 30 × 15.4 cm), with a runway in the middle (25 × 3 × 12 cm) and at both ends of the runway (3 cm ahead of the runway) an opaque barrier (12 cm long × 12 cm height) was positioned perpendicular to the orientation of the runway. The tank was filled to a depth of 6 cm. Each trial started with a single fish being introduced into the middle of the runway and left for 2 min. The fish was then gently manoeuvred such that it swam along the runway until it faced the barrier. Fish then had the choice to turn left or right around the barrier. To account for any possible asymmetry in the set-up, tests were carried out alternately on the two ends of the runway. To avoid fish taking ‘a familiar route’, the fish entered the runway from a different side from which it exited. The water was changed after five trials to avoid changes in water temperature and dissolved oxygen, both of which are known to affect neurological function. Water temperature in the lateralization chamber was 27–28°C.
To determine the relative lateralization (LR) of each fish, we computed the number of right turns made by the fish out of 10 times it travelled down the runway. We calculated an absolute lateralization index (LA) according to the following formula: absolute value of [(number of right turn − number of left turn)/(total number of trials, i.e. 10) × 100]. The LA index ranges from 0 (an individual that turned in equal proportion to the right and to the left—no bias) to 100 (an individual that turned in the same direction in all 10 trials). LA allowed us to compare the strength of the lateralization (irrespective of its direction) among groups.
(c). Experiment 2: the effect of background risk on behavioural lateralization and escape performance
The goal of this experiment was to specifically test whether elevated levels of background risk would influence behavioural lateralization and escape performance in C. teres. We captured approximately 220 fish from the Loomis school (the largest of all the schools) and kept them in the laboratory for 5 days in the absence of risk prior to starting the experiment. Based on the results of experiment 1, a reduced level of lateralization was expected, but we wanted to know how much the extended period without risk would reduce the scores. Consequently, we tested a sample of 37 fish to establish a baseline level of lateralization. The remaining fish were then split into eight groups of 20 fish and placed in clear plastic 15 l flow-through tanks. Following a well-established procedure [34,35], fish in half of the tanks were exposed to elevated risk, whereas the remainder were held in the absence of risk (low-risk control). The high- or low-risk conditions were simulated by introducing a solution of alarm cues (high risk) or a seawater control (low risk) into the tanks three times per day over the course of 5 days. The alarm cue solution was prepared just prior to being used, by making vertical cuts on each side of two freshly euthanized donor conspecific fish and then rinsing the fish in 60 ml of seawater. The fish were euthanized using cold shock followed by decapitation. We injected 10 ml of this standard alarm cues solution into the conditioning tanks, giving us a concentration of two cuts per litre once injected. This concentration has been shown to elicit strong antipredator responses in coral reef fishes [34]. The timing of the three injections occurred randomly between 08.00 and 18.00, with a minimum of 1.5 h between consecutive injections.
After the fish were held in the different risk treatments for 5 days, we calculated the lateralization score of each fish as per experiment 1. We tested a total of 64 fish in the low-risk treatment and 63 fish in the high-risk treatment (15–16 fish tank−1). Time constraints precluded us from testing all 80 fish in each treatment. For each individual, we also determined the ‘conformity’ of the individual to its school within each treatment tank. A fish that has a particular turning bias (either left or right) may find itself in a tank with a majority of individuals that have the same turning bias or with individuals that have a different turning bias. When a fish had a turning bias that matched the majority of the individuals in its group, we considered that the fish conformed to the group. If the individual had a turning bias that was different from the majority of fish in the group, then we considered the fish to be in conflict.
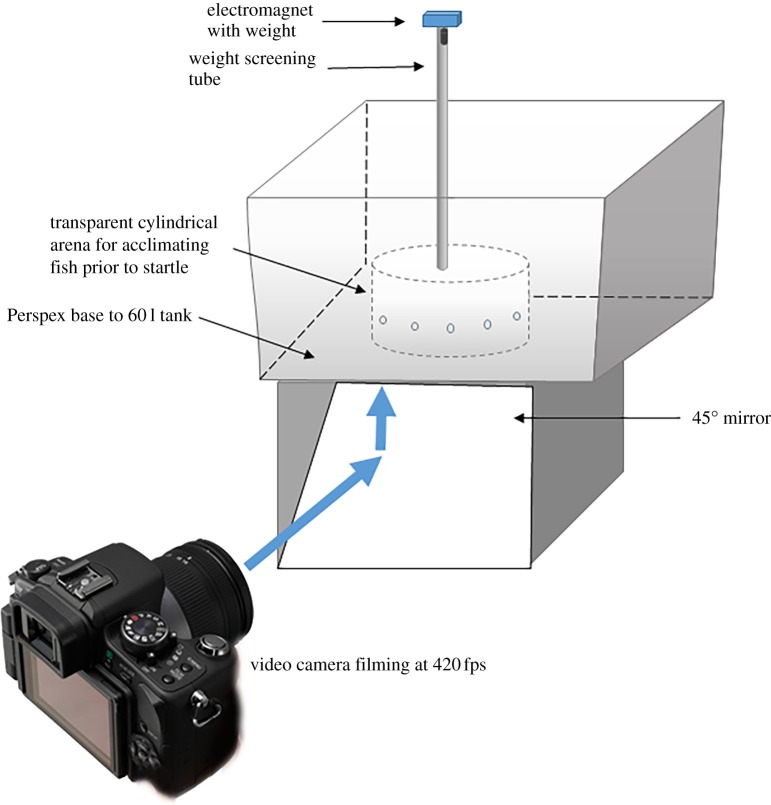
Immediately after completing the lateralization experiment, we assayed 39 high-risk and 40 low-risk fish for characteristics of their escape response. Individuals were placed into the testing arena (figure 1), which consisted of a transparent circular acrylic arena (diameter 200 mm) within a large plastic tank (585 × 420 × 330 mm; 60 l). Shallow water depth (100 mm) was used in the experimental arena in order to minimize displacement in the vertical dimension. Water temperature in the experimental arena was 27–28°C. The arena was illuminated by LED strip lighting (750 lumens) placed above the water surface on the outside of the tank. Five minutes after being released into the testing arena, an escape response was elicited by the release of a tapered metal weight from above the water surface. This was accomplished by turning off an electromagnet to which the metal weight was attached. The metal weight was controlled by a piece of fishing line that was long enough such that the tapered tip only just touched the surface of the water. In order to provide a sudden stimulation and allow calculation of the escape latency, the stimulus was released through a white PVC tube (diameter 40 mm, length 550 mm) suspended above the experimental arena, with the bottom edge at a distance of 10 mm above the water level. Fish were startled only when they moved to the middle portion of the tank, allowing an individual to move an equal distance in any direction and standardizing for fish position relative to the stimulus. Escape responses were recorded at 480 frames per second (Casio EX-ZR1000) as a silhouette from below obtained through pointing the camera at a mirror angled at 45°. From the videos, we quantified latency to initiate an escape, maximum escape speed, maximum acceleration and escape distance. A 1 cm line was drawn in the centre of the inner arena to enable calibration for video analysis. Trials were conducted between 08.00 and 16.00.
Figure 1.
Schematic of test arena used to test measure fish burst performance. (Online version in colour.)
Kinematic variables associated with the fast-start response were analysed using the image-analysis software ImageJ, with a manual tracking plug-in. The centre of mass of each fish was tracked for the duration of the response. The following kinematic variables were measured
1. Latency to respond (seconds) was measured as the time interval between the stimulus onset and the first detectable movement leading to the escape of the animal.
2. Maximum escape speed (m s−1) was measured as the maximum speed achieved at any time during the first two axial bends (i.e. stage 1 and stage 2, as defined by Domenici & Blake [36]).
3. Maximum acceleration (m s−2) was measured as the maximum acceleration within a fixed time (first 30 ms after initial response).
4. Escape distance (m) is a measure of the total distance covered by the fish during stages 1 and 2 [36], which are the periods considered crucial for avoiding ambush predator attacks [37].
(d). Statistical analysis
(i). Experiment 1
To determine whether the population as a whole showed a non-random turning bias, we compared the relative lateralization score of all of the fish to zero. We completed the same analysis for each of the four schools separately. To compare absolute lateralization, we tested the effect of school origin (random factor) and time held in the laboratory (fixed factor) in a two-way ANOVA.
(ii). Experiment 2
We compared both the LR and LA scores of high-risk and low-risk treatment fish using a two-way nested ANOVA, whereby fish were nested within tanks, and tanks within risk group. This ensured that tank, not fish, was used as our replicative unit, because fish coming from the same tank cannot be considered independent (the degrees of freedom for risk reflects that tank was used as the level of replication). To compare those scores to that of fish tested prior to the start of the experiment, we conducted a one-way ANOVA (pre-treatment versus low risk versus high risk), followed by post hoc Tukey tests.
The kinematics variables (latency to respond, max speed, max acceleration and escape distance) were reduced into two variables using a PCA (correlation matrix), which explained 77% of the variance. The first eigenvector, which explained 53% of the variance, loaded heavily on the first three variables, and we referred to this synthetic variable as the ‘temporal response’. The second vector, which explained 24% of the variance, loaded heavily on escape distance, and was referred to as the ‘spatial response’. These two orthogonal variables were first used to investigate the possible relationship between kinematic performance and lateralization using correlations. We also used them as response variables in a three-way nested ANOVA, testing the effect of conformity and risk. We introduced ‘tank’ as a nested factor, to reflect the lack of independence of fish coming from the same tank. Conformity was determined as the concordance between the bias of the fish (right or left) compared with the bias of its school (majority right or left-biased fish). Fish turning right between zero and four times were considered left lateralized (indicative of a right eye preference), whereas fish turning right six to 10 times were considered right lateralized (indicative of a left eye preference). Fish turning right five times (non-lateralized) were excluded from the analyses, as were two schools were there were equal numbers of right- and left-biased fish. This left us with 42 fish in six schools. For all analyses, data met the assumptions of normality and heteroscedasticity.
3. Results
(a). Experiment 1
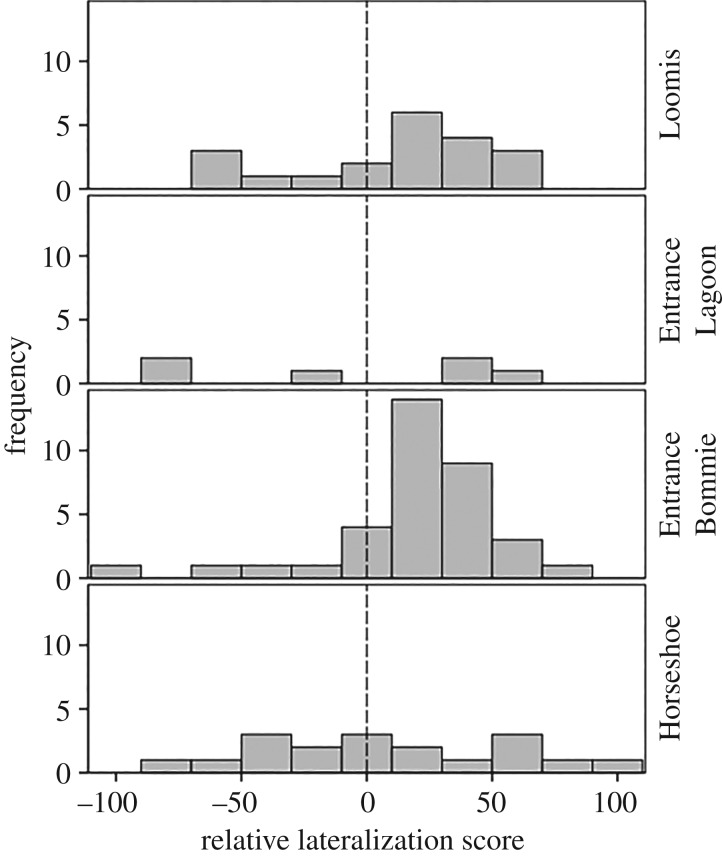
We determined the lateralization score of 79 fish the day they were brought to the laboratory. This includes 35, 20, 18 and six fish from the Entrance Bommie, Loomis, Horseshoe and Entrance Lagoon schools, respectively. Considering all of the fish together, we found a significant right bias in the population (one-sample t-test, t78 = 2.7, p = 0.009). Indeed, of the fish tested, nine showed no bias, 19 showed a left bias and 51 showed a right bias. However, this overall population bias might be driven by one school only (entrance Bommie school, one-sample t-test: t34 = 0.002—p > 0.05 for other schools; figure 2). With sample sizes of 20, 18 and 6, in the Loomis, Horseshoe and Entrance Lagoon schools, respectively, our power to detect a difference was minimal as we would need 75%, 78% and 100% right turns to show a significant turning bias. For the entrance Bommie school, a significant turning bias would need only 69% (24/35) of the fish to have a right bias.
Figure 2.
Distribution of fish of varying relative lateralization scores (LR) upon arrival in the laboratory for each of the four schools (n = 20, 6, 35 and 18, respectively). Positive values indicate right turns and negative values indicate left turns.
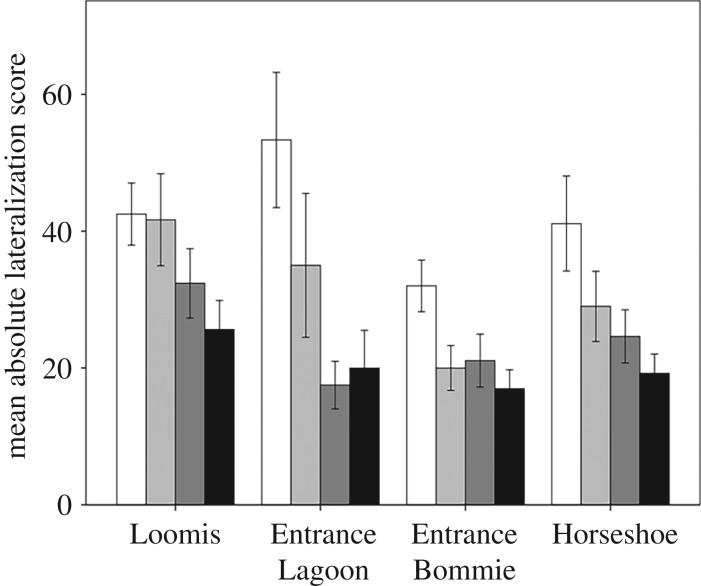
The two-way ANOVA examining absolute lateralization revealed a significant effect of time (F3,11.7 = 11.5, p = 0.001) and school identity (F3,10.3 = 5.7, p = 0.015, figure 3) on the lateralization scores. There was no interaction between these two factors (F9,337 = 1.0, p = 0.43), indicating that all schools were affected by time in the same way; namely they all decreased their absolute lateralization score through time.
Figure 3.
Mean (±s.e.) absolute lateralization scores of fish from each of the four schools (x-axis), tested 1 (white bars), 2 (light grey bars), 3 (dark grey bars) or 4 (black bars) days after collection. Each fish was only tested once (n = 75–108 day−1, and n = 50–140 school−1).
(b). Experiment 2
The two-way nested ANOVA on absolute lateralization revealed that high-risk fish were significantly more lateralized than low-risk ones (F1,6 = 152, p < 0.001) without any effect of tank (F6,119 = 0.6, p = 0.72). The one-way ANOVA comparing the high- and low-risk fish to the pre-treatment fish indicated that there was an overall difference (F2,161 = 41.9, p < 0.001) in absolute lateralization scores. Pre-treatment fish and low-risk fish did not differ in their lateralization scores (Tukey's HSD: p = 0.23), whereas the high-risk fish had a significantly higher lateralization score compared with both the low-risk and pre-treatment fish (both p < 0.001).
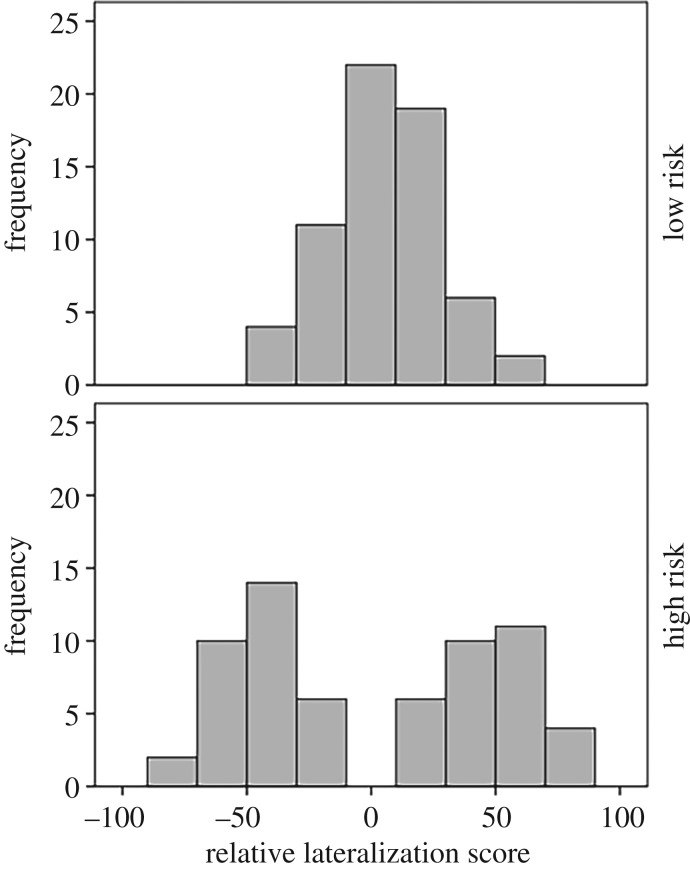
Upon inspection of the relative lateralization of high- and low-risk fish, we found that while most low-risk fish were not lateralized, we failed to have a single non-lateralized fish in the high-risk group (figure 4).
Figure 4.
Distribution of relative lateralization scores (LR) of fish exposed for 4 days under low- or high-risk conditions.
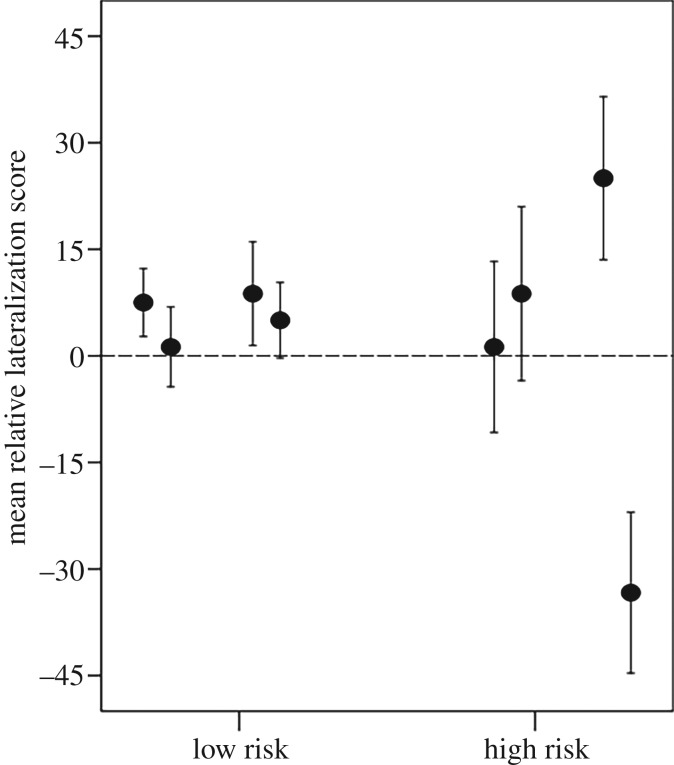
The two-way nested ANOVA on relative lateralization revealed no overall effect of risk (F1,6 = 0.12, p = 0.7, figures 4 and 5), but a significant effect of tank (F6,119 = 3.4, p = 0.004). This tank effect was absent when looking at the low-risk fish (F3,60 = 0.3, p = 0.8), but present for the high-risk fish (F3,59 = 4.1, p = 0.01). None of the four low-risk tanks differed from zero (all p > 0.25). The relative lateralization of two high-risk tanks did not differ from zero (one-sample t-test: both p > 0.5). However, two other high-risk tanks both showed high consistency in the directionality of lateralization of the fish (one-sample t-test: p = 0.046 and 0.011, respectively), with fish from one tank being mostly right-lateralized, whereas those from another tank being mostly left-lateralized.
Figure 5.
Mean (±s.e.) relative lateralization scores of fish maintained for 4 days in low or high risk. Each circle represents a tank (n = 20 tank−1).
There was no correlation between absolute lateralization scores and kinematic performance of the fish (temporal response: p = 0.33, n = 74; spatial response: p = 0.98, n = 74). While there was no relationship between relative lateralization and temporal response (p = 0.13, n = 74), we found a relationship between relative lateralization on spatial kinematics (r = 0.27, p = 0.023, n = 74), with increased performance associated with positive values of LR (more right-biased).
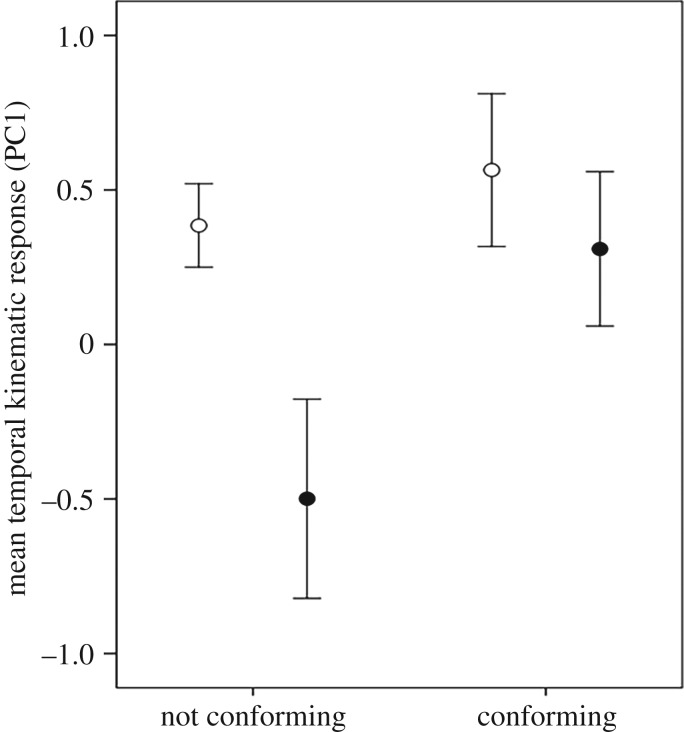
The three-way nested ANOVA on the temporal fast-start response indicated a significant effect of conformity (F1,38 = 5.1, p = 0.029), but a lack of effect of risk (F1,3.5 = 2.1, p = 0.2) and a lack of interaction between the two factors (F1,38 = 1.1, p = 0.3). Namely, the fish that conformed to the overall side preference of their group showed improved escape responses compared with the fish that did not conform (figure 6). Tank did not have an effect on the temporal response variable (F4,35 = 1.2, p = 0.3), nor for the factors on the variable ‘spatial response’ (all p > 0.5).
Figure 6.
Mean (±s.e.) synthetic temporal kinematic performance response of fish exposed, in groups of 20, to 4 days of low- (open circle) or high- (filled circle) risk conditions. Fish were determined to conform or not conform to the majority of fish in their schools, based on the bias of the fish compared with the bias of the school (fish and school without bias were excluded from the analysis). Kinematic performance scores were derived from the first axis of a PCA (53% variance explained) encompassing four kinematic variables (n = 5–15 treatment−1).
4. Discussion
Our results provide clear evidence that short-term changes in predation risk can dramatically alter the expression of lateralization in a schooling species. In our initial experiment, we documented a right-turning bias at the population level with 65% of the fish being right-biased, 24% left-biased and 11% showing no bias. There was only one school that showed a statistically significant turning bias, suggesting a large variation among schools.
Vallortigara & Rogers [3] and Ghirlanda [26] have argued that population level turning bias may represent an evolutionary arms race from social pressure to coordinate behaviour. If this is the case, then the proportion of individuals exhibiting the same turning bias should increase when the cost of not conforming increases, such as would occur with increasing predation pressure. Our manipulative experiment demonstrated that our four low-risk tanks were not strongly lateralized after experiencing an absence of risk for 5 days. Indeed, all four of the tanks had a significant proportion of fish that were non-lateralized. The percentage of fish in the tanks that had the equal numbers of right and left turns was 37.5%, 37.5%, 37.5%, 25%, and most of the remaining fish were only weakly lateralized. In contrast, there were no non-lateralized fish in high-risk tanks. While all four high-risk tanks dramatically increased their strength of lateralization, the direction and within-tank consistency of the bias differed. One tank showed a strong right bias, another a strong left bias. Fish in two tanks were strongly lateralized but had a relatively equal number of right- and left-turning fish. This raises the question of how particular groups become highly left or right lateralized. Are there dominant or bold individuals that the rest of the group follows? Why would all of the tanks not show deviation from 50/50? Perhaps we started each tank with a different mix of bold and shy fish that drove the differences observed. Future work should examine the proximate mechanisms that govern the directionality of lateralization, as well as the conditions that drive individual schools to become left lateralized even when the population is right lateralized.
Our work highlights that escape performance is closely linked to being at odds with the group. Individuals that matched the lateralization bias of the majority of their schoolmates had a stronger escape response than those that were in conflict with the majority of the school. Specifically, we observed a greater temporal response, which was an aggregate response of latency to respond, maximum speed and maximum acceleration. Future work should consider what the true causal factor behind this relationship is. If it is generally the case that escape performance is reduced for those individuals that turn against their preference, then it would be fascinating to determine whether those individuals suffer higher predation rates. The conflict between an individual's turning preference and the average preference of the group deserves attention in other systems. Indeed, many animals that are highly lateralized, including some birds and mammals, show coordinated behaviour that requires animals to turn the same direction.
If the costs and benefits of being lateralized vary, then we should see that variation in lateralization should follow predictable patterns with changes in environmental conditions. Dadda et al. [33] have shown that the strength of lateralization is correlated with escape performance. Following from this, we predicted that changes in predation pressure should alter the strength of lateralization. In our first experiment, we found that holding the fish in the laboratory for several days in the absence of predation risk resulted in a very pronounced reduction in absolute behavioural lateralization. This was observed again in our empirical work (experiment 2). An increase in risk resulted in an increase in absolute lateralization. We did not find a simple relationship between escape performance and absolute lateralization, but we did find that individuals that were right-biased performed a better escape response than those that were left-biased. To the best of our knowledge, this is the first demonstration of such a bias. Non-conformers from high-risk environments show particularly poor escape performance. Why non-conformers from low-risk environments do not show poor escape performance is unclear. These results highlight the complexity of studying lateralization and escape responses in fishes.
If individuals that conform to the group have improved escape performance over those that do not, then it is not unreasonable to expect that the entire population should move towards having the same lateralization bias. Every individual in the population should be either left- or right-biased. Such complete lateralization is not observed in the wild. In fact, typically 10–35% of the population do not conform to the pattern of the majority of individuals [3]. The reason for the lack of conformity must mean that there are other advantages of being at odds with the group. Perhaps individuals at odds with the group have competitive advantages or they are advertising their quality by being able to survive despite their lateralization handicap. Of course, we cannot underestimate the role of predators in ensuring that there is not complete population-level behavioural lateralization. If every individual always turned the same direction, then it would be rather easy for predators to predict the behaviour of prey [3,26]. Future work needs to grapple with questions of costs and benefits. Are there species differences in flexibility of lateralization that reflects differential costs and benefits? If lateralization does indeed provide prey with a considerable advantage, why do they so quickly revert to minimal lateralization with the relaxation of predation pressure?
Acknowledgements
We thank the staff at the Lizard Island Research Station for assistance with this project.
Ethics
All work carried herein was in accordance with the James Cook University Animal Ethics Committee (approval numbers: A2080 and A2005).
Authors' contributions
D.C., M.I.M., B.A., M.D.M. and M.F. designed the experiments; M.I.M., B.A., M.D.M. and E.G. collected the fish; D.C., B.A., M.D.M. and M.F. conducted the experiments; M.F., B.A. and R.B. analysed the data, D.C. and M.F. wrote the first draft of the paper, all authors contributed to the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
Natural Sciences and Engineering Council of Canada to M.F. and D.C., the Australian Research Council to M.F., D.C. and M.I.M., ARC Centre of Excellence for Coral Reef Studies to M.I.M.
References
- 1.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: OUP. [Google Scholar]
- 2.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 3.Vallortigara G, Rogers LJ. 2005. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–588. ( 10.1017/S0140525X05000105) [DOI] [PubMed] [Google Scholar]
- 4.Rogers LJ, Vallortigara G, Andrew RJ. 2013. Divided brains: the biology and behaviour of brain asymmetries. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Vallortigara G, Chiandetti C, Sovrano VA. 2011. Brain asymmetry (animal). Wiley Interdiscip. Rev. Cogn. Sci. 2, 146–157. ( 10.1002/wcs.100) [DOI] [PubMed] [Google Scholar]
- 6.MacNeilage PF, Rogers LJ, Vallortigara G. 2009. Origins of the left & right brain. Sci. Am. 301, 60–67. ( 10.1038/scientificamerican0709-60) [DOI] [PubMed] [Google Scholar]
- 7.Robins A, Lippolis G, Bisazza A, Vallortigara G, Rogers LJ. 1998. Lateralized agonistic responses and hindlimb use in toads. Anim. Behav. 56, 875–881. ( 10.1006/anbe.1998.0877) [DOI] [PubMed] [Google Scholar]
- 8.Deckel AW. 1995. Laterality of aggressive responses in Anolis. J. Exp. Zool. 272, 194–200. ( 10.1002/jez.1402720304) [DOI] [Google Scholar]
- 9.Rogers L. 1991. Development of lateralization. In Neural and behavioural plasticity: the use of the domestic chick as a model (ed. RJ Andrew), pp. 507–535. Oxford: Oxford University Press. [Google Scholar]
- 10.Mench J, Andrew R. 1986. Lateralization of a food search task in the domestic chick. Behav. Neural Biol. 46, 107–114. ( 10.1016/S0163-1047(86)90570-4) [DOI] [PubMed] [Google Scholar]
- 11.Clayton NS, Krebs JR. 1994. Memory for spatial and object-specific cues in food-storing and non-storing birds. J. Comp. Physiol. A 174, 371–379. ( 10.1007/bf00240218) [DOI] [Google Scholar]
- 12.Diekamp B, Regolin L, Güntürkün O, Vallortigara G. 2005. A left-sided visuospatial bias in birds. Curr. Biol. 15, R372–R373. ( 10.1016/j.cub.2005.05.017) [DOI] [PubMed] [Google Scholar]
- 13.Wilzeck C, Kelly DM. 2013. Avian visual pseudoneglect: the effect of age and sex on visuospatial side biases. In Behavioral lateralization in vertebrates (eds D Csermely, L Regolin), pp. 55–70. Berlin, Germany: Springer. [Google Scholar]
- 14.Ventolini N, Ferrero EA, Sponza S, Della Chiesa A, Zucca P, Vallortigara G. 2005. Laterality in the wild: preferential hemifield use during predatory and sexual behaviour in the black-winged stilt. Anim. Behav. 69, 1077–1084. ( 10.1016/j.anbehav.2004.09.003) [DOI] [Google Scholar]
- 15.Sovrano VA, Dadda M, Bisazza A. 2005. Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav. Brain Res. 163, 122–127. ( 10.1016/j.bbr.2005.04.012) [DOI] [PubMed] [Google Scholar]
- 16.Bibost A-L, Brown C. 2014. Laterality influences cognitive performance in rainbowfish Melanotaenia duboulayi. Anim. Cogn. 17, 1045–1051. ( 10.1007/s10071-014-0734-3) [DOI] [PubMed] [Google Scholar]
- 17.Magat M, Brown C. 2009. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B 276, 4155–4162. ( 10.1098/rspb.2009.1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers LJ, Zucca P, Vallortigara G. 2004. Advantages of having a lateralized brain. Pro. R. Soc. Lond. B 271, S420–S422. ( 10.1098/rsbl.2004.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin N, Rogers L. 2007. Asymmetry of flight and escape turning responses in horses. Laterality 12, 464–474. ( 10.1080/13576500701495307) [DOI] [PubMed] [Google Scholar]
- 20.Lippolis G, Westman W, McAllan B, Rogers L. 2005. Lateralisation of escape responses in the stripe-faced dunnart, Sminthopsis macroura (Dasyuridae: Marsupialia). Laterality: Asymmet. Body, Brain Cogn. 10, 457–470. ( 10.1080/13576500442000210) [DOI] [PubMed] [Google Scholar]
- 21.Brown C, Gardner C, Braithwaite V. 2004. Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. Lond. B 271, S455–S457. ( 10.1098/rsbl.2004.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisazza A, De Santi A, Vallortigara G. 1999. Laterality and cooperation: mosquitofish move closer to a predator when the companion is on their left side. Anim. Behav. 57, 1145–1149. ( 10.1006/anbe.1998.1075) [DOI] [PubMed] [Google Scholar]
- 23.Cantalupo C, Bisazza A, Vallortigara G. 1995. Lateralization of predator-evasion response in a teleost fish (Girardinus falcatus). Neuropsychologia 33, 1637–1646. ( 10.1016/0028-3932(95)00043-7) [DOI] [PubMed] [Google Scholar]
- 24.Bonati B, Csermely D, López P, Martín J. 2010. Lateralization in the escape behaviour of the common wall lizard (Podarcis muralis). Behav. Brain Res. 207, 1–6. ( 10.1016/j.bbr.2009.09.002) [DOI] [PubMed] [Google Scholar]
- 25.Lippolis G, Joss JMP, Rogers LJ. 2009. Australian Lungfish Neoceratodus forsteri: a missing link in the evolution of complementary side biases for predator avoidance and prey capture. Brain, Behav. Evol. 73, 295–303. ( 10.1159/000230674) [DOI] [PubMed] [Google Scholar]
- 26.Ghirlanda S, Vallortigara G. 2004. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. R. Soc. Lond. B 271, 853–858. ( 10.1098/rspb.2003.2669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghirlanda S, Frasnelli E, Vallortigara G. 2009. Intraspecific competition and coordination in the evolution of lateralization. Phil. Trans. R. Soc. B 364, 861–866. ( 10.1098/rstb.2008.0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisazza A, Cantalupo C, Capocchiano M, Vallortigara G. 2000. Population lateralisation and social behaviour: a study with 16 species of fish. Laterality: Asymmet. Body, Brain Cogn. 5, 269–284. ( 10.1080/713754381) [DOI] [PubMed] [Google Scholar]
- 29.Jozet-Alves C, Hébert M. 2013. Embryonic exposure to predator odour modulates visual lateralization in cuttlefish. Proc. R. Soc. B 280, 20122575 ( 10.1098/rspb.2012.2575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broder ED, Angeloni LM. 2014. Predator-induced phenotypic plasticity of laterality. Anim. Behav. 98, 125–130. ( 10.1016/j.anbehav.2014.09.033) [DOI] [Google Scholar]
- 31.Ferrari MCO, McCormick MI, Allan BJM, Choi R, Ramasamy RA, Johansen JL, Mitchell MD, Chivers DP. 2015. Living in a risky world: the onset and ontogeny of an integrated antipredator phenotype in a coral reef fish. Sci. Rep. 5, 15537 ( 10.1038/srep15537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrari MC, McCormick MI, Allan BJ, Choi RB, Ramasamy RA, Chivers DP. 2015. The effects of background risk on behavioural lateralization in a coral reef fish. Funct. Ecol. 29, 1553–1559. ( 10.1111/1365-2435.12483) [DOI] [Google Scholar]
- 33.Dadda M, Koolhaas WH, Domenici P. 2010. Behavioural asymmetry affects escape performance in a teleost fish. Biol. Lett. 6, 414–417. ( 10.1098/rsbl.2009.0904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chivers DP, McCormick MI, Mitchell MD, Ramasamy RA, Ferrari MCO. 2014. Background level of risk determines how prey categorize predators and non-predators. Proc. R. Soc. B 281, 20140355 ( 10.1098/rspb.2014.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrari MCO, McCormick MI, Meekan MG, Chivers DP. 2015. Background level of risk and the survival of predator-naive prey: can neophobia compensate for predator naivety in juvenile coral reef fishes? Proc. R. Soc. B 282, 20142197 ( 10.1098/rspb.2014.2197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domenici P, Blake R. 1997. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178. [DOI] [PubMed] [Google Scholar]
- 37.Webb P. 1976. The effect of size on the fast-start performance of rainbow trout Salmo gairdneri, and a consideration of piscivorous predator–prey interactions. J. Exp. Biol. 65, 157–177. [DOI] [PubMed] [Google Scholar]