Abstract
European eels (Anguilla anguilla) undertake an impressive 5 000 km long migration from European fresh waters through the North Atlantic Ocean to the Sargasso Sea. Along with sexual maturation, the eel skeleton undergoes a remarkable morphological transformation during migration, where a hitherto completely obscure bone loss phenomenon occurs. To unravel mechanisms of the maturation-related decay of the skeleton, we performed a multiscale assessment of eels' bones at different life-cycle stages. Accordingly, the skeleton reflects extensive bone loss that is mediated via multinucleated bone-resorbing osteoclasts, while other resorption mechanisms such as osteocytic osteolysis or matrix demineralization were not observed. Preserving mechanical stability and releasing minerals for energy metabolism are two mutually exclusive functions of the skeleton that are orchestrated in eels through the presence of two spatially segregated hard tissues: cellular bone and acellular notochord. The cellular bone serves as a source of mineral release following osteoclastic resorption, whereas the mineralized notochord sheath, which is inaccessible for resorption processes due to an unmineralized cover layer, ensures sufficient mechanical stability as a part of the notochord sheath. Clearly, an eel's skeleton is structurally optimized to meet the metabolic challenge of fasting and simultaneous sexual development during an exhausting journey to spawning areas, while the function of the vertebral column is maintained to achieve this goal.
Keywords: European eel, bone loss, spawning migration, osteoclasts
1. Background
European eels (Anguilla anguilla) undertake a 5 000 km long spawning migration from European fresh waters through the ocean to the Sargasso Sea [1]. The complex life cycle of eels starts with larvae called leptocephali [2] that are carried back by currents to European coasts where they metamorphose into glass eels. Entering fresh water, the glass eels develop into sequential morphological stages known as ‘elvers’ and ‘yellow eels’. Eels remain in fresh water for 5–20 years until they become ‘silver eels’. These eels then enter marine waters again to migrate to spawning areas (figure 1a,b). During the long and energy-consuming journey, silver eels complete gonad maturation [2] and entirely abstain from food intake [3]. Furthermore, eels not only deplete their energy reserves, they also lose substantial amounts of bone volume during the spawning migration [4]. The specific factors driving this bone loss as well as the exact mechanisms of bone loss are not yet well understood.
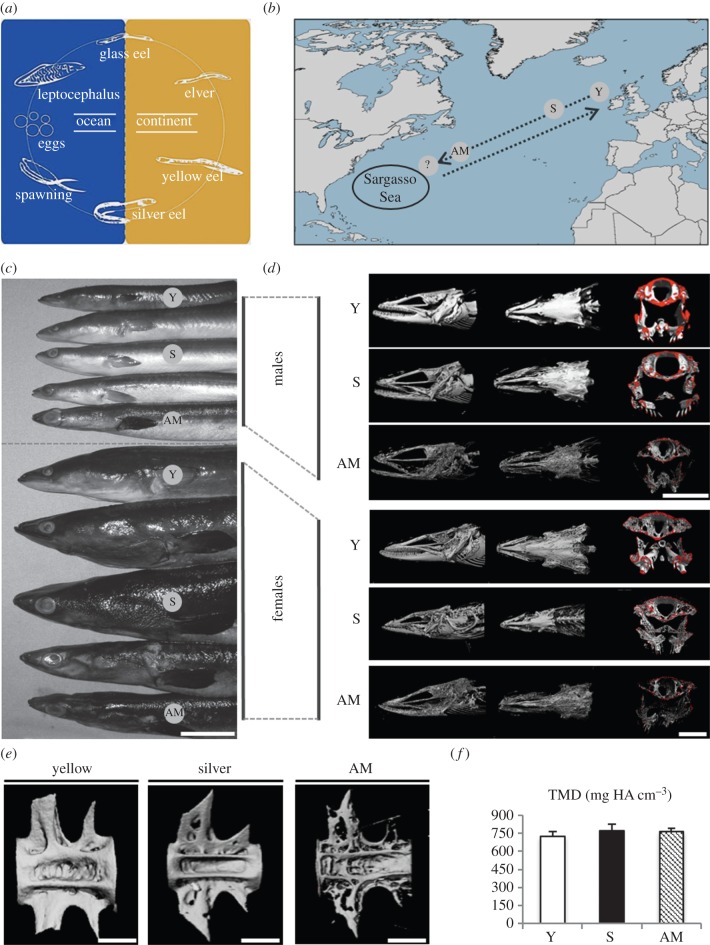
Figure 1.
Life cycle of the European eel Anguilla anguilla. (a,b) Silver eels travel to the Sargasso Sea to spawn. Larvae (leptocephalus) are transported back to European fresh water where they develop into glass eels and eventually yellow eels. (c) Photographs of the eels show the morphological change during maturation. Note the increase in eye size. Scale bar, 2 cm. (d) Images obtained by microcomputed tomography (µ-CT) point out severe bone loss in skulls during maturation among yellow (Y), silver (S), and artificially matured eels (AM) in both sexes. Red colouring indicates the cutting plane for histologic analysis of the skull base. Scale bar, 1 cm. (e) Representative three-dimensional reconstructions of µ-CT scans equally show the dramatic bone loss in vertebral bodies among the three groups. Scale bar, 1 mm. (f) Overall mineralization (tissue mineral density, TMD) obtained by µ-CT did not change significantly with maturation. (Online version in colour.)
Since eel migration takes place in the deep sea, and tag sizes in satellite tracking exclude small-sized animals like eels [5], it is not possible to trace the animals or to obtain samples directly from migrating eels. Therefore, studies investigating the effect of maturation on the animals' skeleton require experimentally induced maturation through hormone injections [2,6]. Moreover, eels are an endangered species, which is why an artificial maturation procedure has been developed as an experimental attempt of artificial fertilization and reproduction [7]. The artificial maturation method as applied here is based on previously reported protocols used to induce maturing of males and females for controlled reproduction in aquaculture [7,8].
Although much is still unknown about precise hormonal regulation of bone metabolism in many groups of teleost fish [9], it has been shown that eel bone responds to injections of 1,25-dihydroxycholecalciferol, calcitonin, and thyroid hormone [10–12]. In particular, cortisol can trigger bone resorption in eels, suggesting that cortisol is a conserved endocrine regulator of bone loss in vertebrates [13].
The vertebrate skeleton has a variety of functions. It gives protection and support for internal organs and provides stability and mobility together with the muscular system. The skeleton is also an important storage organ for minerals and it participates in regulation of plasma mineral homeostasis [4]. In tetrapods, the skeleton is mainly involved in the animal's calcium metabolism, whereas in teleost fish, the skeleton is primary involved in phosphorous metabolism [9,14,15]. Clearly, some skeletal functions mutually compete, such as using the mineralized bone to ensure mechanical stability versus using the bone as a reservoir of calcium and phosphorus. The comparative structural and compositional analyses of bone tissue in different species can help to gain insights into these two major roles of the skeleton, specifically into the different pathways in which the vertebrate skeleton responds to internal and external challenges.
Within the teleost group there is an evolutionary trend towards the loss of osteocytes (acellular bone) [9,16]. By contrast, in vertebrates with cellular bone, osteocytes are the most numerous bone cells and are central regulators of bone remodelling in response to bone mineral metabolism and mechanical load [17]. Some small teleost fish such as zebrafish (Danio rerio) and medaka (Oryzias latipes), which are established models for biomedical bone research [18], are deprived of osteocytes in the early stages of life, while medaka do not feature osteocytes at all [9,19,20]. Therefore, studying teleost fish models can reveal conserved and alternative pathways for bone adaptation and metabolism [15,21–24]. Furthermore, analyses of bone metabolism in various species are essential for clarifying the evolutionary physiology of bone [15,25].
Eels belong to a basal teleost group whose skeleton contains osteocytes [26], as is the case in all tetrapods including humans. It is, therefore, essential to understand how the bone tissue of eels is organized and for which of the functions (mechanical stabilizer and mineral storage) it is optimized. In this study, the European eel was used as a model to study the mechanism of bone resorption and mineral homeostasis, as these processes seem to be part of their natural biology. Osteocytic pathways that are known to orchestrate bone remodelling in mammals are examined for their involvement in eel bone resorption, which is triggered by artificial maturation with hormones in male and female eels prior to the start of their spawning migration. This is the first study that investigates the skeleton of adult, sexually mature eels in both sexes. The skeleton of matured eels is compared with the skeleton of earlier life stages: juvenile yellow eels and sexually immature silver eels. In-depth understanding of the structural organization of eel bones as well as the mechanisms driving bone resorption in maturation contribute to the understanding of the opposing forces that maintain the mechanical stability of the skeleton and that exploit the skeleton as a mineral resource.
2. Material and methods
A detailed description of the applied procedures and methods is given in the electronic supplementary material.
(a). European eels
A total of 30 male and female eels were investigated (nine yellow, 10 silver, and 11 artificially matured-AM eels). Yellow and silver eels were caught in coastal waters, whereas the last developmental stage before spawning was accomplished by hormone injections. Here, artificial maturation was performed in males by weekly injections of human chorionic gonadotropin (hCG, Sigma Aldrich, 1 IU g−1 body weight) for 12–14 weeks following a standardized protocol [8]. In females, maturation was performed with carp pituitary extract (CPE, Catvis, 20 mg week−1) for 8–13 weeks and then, within 18 h after the last CPE injection, with an injection of 17,20β-dihydroxy-4-pregnen-3-one (DHP, Sigma Aldrich, 2 µg g−1 body weight) into the ovary to induce ovulation [8,27–29]. According to their natural behaviour in open sea, hormone-treated eels fasted from their first injection. The maturation process was performed in a water recirculation system saturated with sea salt (18°C, [30–35] Practical Salinity Unit). Eye index—a parameter for sexual maturity was calculated [30]. Blood levels of calcium, phosphorus, and cortisol were measured.
(b). Histology
von Kossa-stained bone sections were used to quantify bone volume per tissue volume (BV/TV) [31]. Toluidine blue and Mason-Goldner trichrome stained sections were used to study the morphology of bone and resorption parameters (osteoclast surface per bone surface, Oc.S/BS; osteoclast number per bone perimeter, N.Oc/B.Pm; eroded surface per bone surface, ES/BS). Collagen fibril orientation in the bone matrix was visualized via polarized light microscopy.
(c). Mineralization analysis
Quantitative backscattered-electron imaging (qBEI) was used to assess the degree of mineralization [32] based on the relationship between calcium weight percentage and qBEI signal intensities [32–34]. qBEI images were also used to quantify the osteocyte lacunar number (Tt.Lc.N/B.Ar, 1 mm−2) and area (Lc.Ar, µm2).
(d). Fourier transform infrared spectroscopy
FTIR further evaluated the bone matrix characteristics: mineral : matrix ratio (amount of mineral per organic matrix), carbonate : phosphate ratio (degree of carbonate substitution for hydroxyl and phosphate groups within hydroxyapatite), and proteoglycan content [35].
(e). High-resolution computed tomographic imaging
HR-pQCT and µ-CT were applied to assess bone microarchitecture, tissue mineral density (TMD, mgHA cm−3), and orbital cavity distance.
(f). Acid etching and analysis of canalicular connections
The osteocyte lacunocanalicular network was visualized via scanning electron microscopy on acid-etched methyl-methacrylate-embedded bone samples, according to the established protocol [36].
(g). Second harmonic generation microscopy
SHG microscopy [18,37,38] was used to further visualize the collagen orientation and its pervasion by osteocyte canalicular connections.
(h). Statistical analysis
The Kolmogorov–Smirnov test was used for verifying normal distribution of the measured parameters. Inter-group comparisons were performed using ANOVA with Bonferroni's post hoc comparisons. The analyses were performed using SPSS v. 22 (IBM Corporation) at a 0.05 significance level.
3. Results
In this study, European eels were investigated as an experimental model of severe bone loss in vertebrae and skull bones related to the sexual maturation of female and male eels prior to spawning (figures 1a–e and 2a,b).
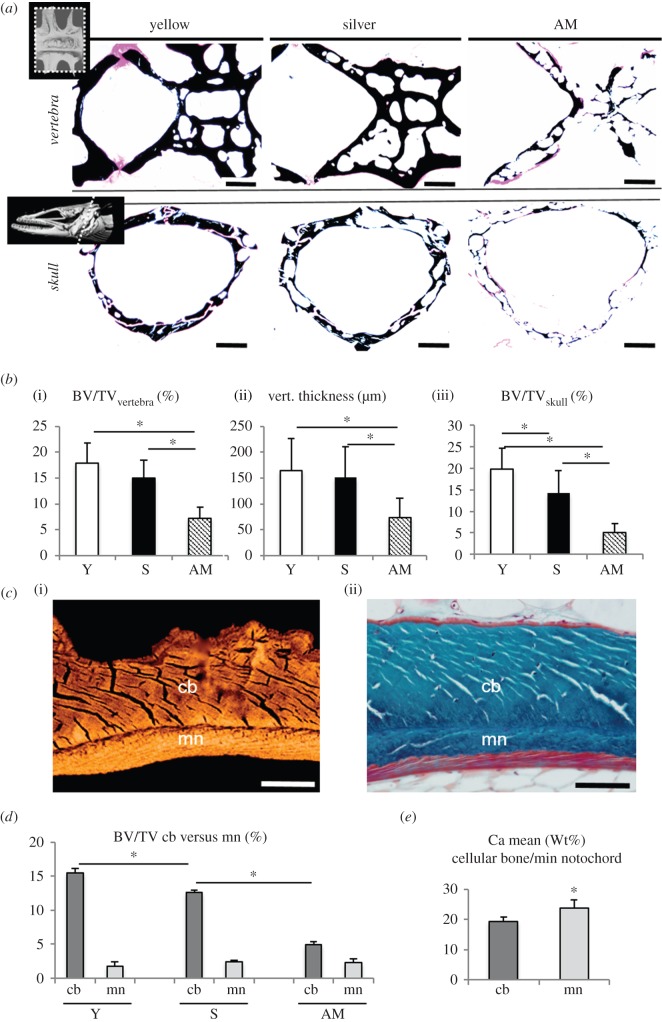
Figure 2.
Massive bone loss during maturation. (a) Images of von Kossa-stained sections show the bone loss during maturation in the vertebra (above) and skull base (below). Scale bar, 200 µm. (b) Bar graphs indicate decreasing bone volume per tissue volume (BV/TV) in the (i) vertebra and (iii) skull, as well as decreasing (ii) thickness of the vertebral endplates. (c) Higher magnification reveals that vertebral bone is divided into two compartments. There are two types of mineralized tissue: cellular (osteocytic) bone (cb) and a mineralized notochord sheath (mn), (i) image obtained by qBEI and (ii) trichrome Goldner staining. Scale bar, 50 µm. (d) Quantification of osteocytic bone and acellular notochord bone separately showed that only osteocytic bone was resorbed, whereas the mineralized notochord sheath persists in artificially matured eels. * denotes the significant decrease of cellular bone among the groups. (e) The mineralized part of the notochord shows higher mineralization (Ca mean) than the cellular bone when measured separately. *p < 0.05.
Laboratory tests showed that plasma calcium levels were maintained in silver eels and hormone-treated eels, but plasma phosphate decreased significantly in hormone-treated male eels compared with yellow and silver eels, whereas it increased in female eels. Also, cortisol levels dropped significantly in the hormone-treated male group to a level of 12.2 ± 5.1 mg dl−1 (electronic supplementary material, table S1). Total body length and weight did not differ significantly between the male groups but increased significantly in female silver eels (electronic supplementary material, table S1).
There are various morphological differences between mature males and mature females, however, an enlargement of eye size (eye index) could be identified in both sexes (figure 1c; electronic supplementary material, table S1). Bone loss was detected in mature males and females to a similar degree (figures 1d,e and 2a,b). Specifically, the thickness of the vertebral end plates was significantly reduced in artificially matured eels (73 ± 38 µm) compared with 164 ± 63 µm in yellow eels (p < 0.001) and 151 ± 60 µm in silver eels (p < 0.001). Furthermore, the thinning of the skull's cortical bone was accompanied by a significant increase of the Euclidean distance between the orbital cavities (electronic supplementary material, table S1).
Vertebral bone volume/tissue volume (BV/TV) was significantly decreased in artificially matured eels (7.2 ± 2.1%) compared with yellow eels (17.9 ± 3.9%, p < 0.001) and silver eels (15 ± 3.5%, p < 0.001) in both sexes, however, the transition from yellow to silver eels was not accompanied by a significant decline in BV/TV (p > 0.05). In the skull base, BV/TV decreased significantly with maturation (p < 0.001), declining already at the stage of silver eels versus yellow eels (14.3 ± 5.1% versus 19.8 ± 4.9%, p = 0.023) and further to 5.1 ± 2.1% in artificially matured eels (p < 0.001, figure 2b). The decline of bone volume and vertebral thickness measured separately for both sexes is shown in the electronic supplementary material, table S2.
Microstructural analysis of skull bones confirmed the presence of osteocytes. By contrast, the vertebral bone of all three groups showed the typical composition of a vertebral body of a basal teleost fish with two types of mineralized tissue: an inner layer of the acellular (anosteocytic) mineralized notochord sheath and an outer layer of cellular (osteocytic) woven and lamellar bone (figure 2c; electronic supplementary material, figure S2). The cellular bone showed clear signs of osteoclastic resorption in terms of eroded surfaces, whereas the mineralized notochord sheath was not affected by osteoclastic resorption, maintaining its volume fraction among all three investigated groups of eels (figures 2d and 4e,f).
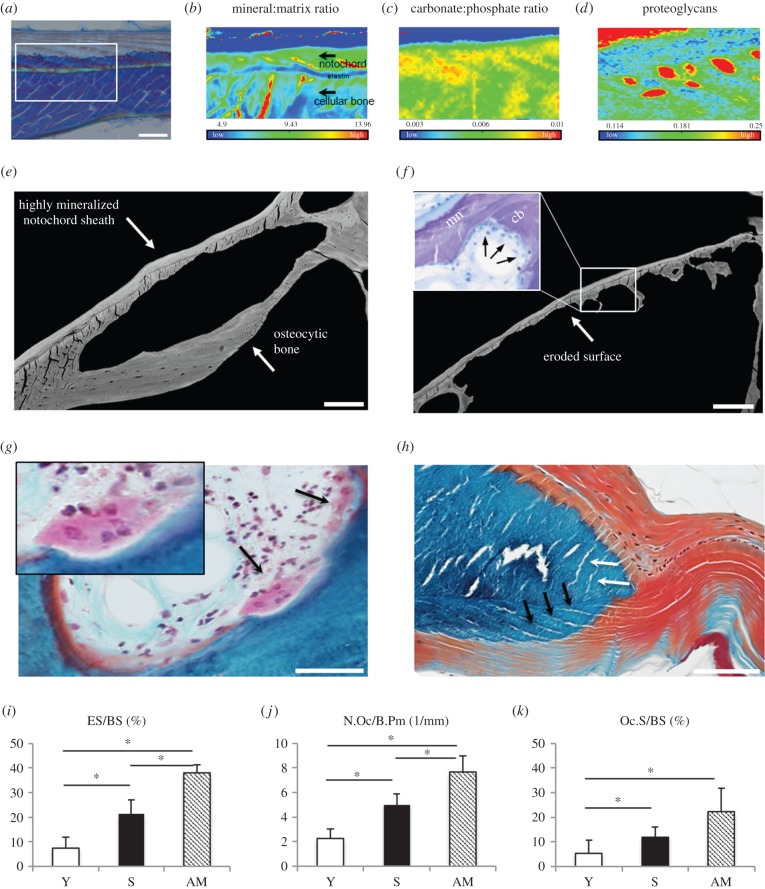
Figure 4.
(a–d) Fourier transform infrared (FTIR) spectroscopy in the eel's vertebral endplates. (a) Toluidine blue staining signifies the scanning area for FTIR. Scale bar, 50 µm. (b) The mineral : matrix ratio was higher in the mineralized notochord sheath. Note the prominent non-mineralized elastin layer in between the cellular bone and the notochord. (c) The increased carbonate : phosphate ratio is either due to higher carbonate or lower phosphate contents in the mineralized notochord compared with the cellular bone. (d) The proteoglycan content was substantially lower in the mineralized notochord sheath, which reveals that its structural composition is different from the cellular bone matrix (red patches are due to cutting artefacts). (e–k) Mechanism of osteoclastic resorption. (e) The qBEI of a silver eel in higher magnification shows mineralized notochord and cellular bone where only cellular bone can be resorbed. Scale bar, 100 µm. (f) Hormone-treated eels present large eroded surfaces. The cellular bone was almost completely resorbed. Scale bar, 100 µm. (g) Histology of a trichrome Goldner stained specimen shows multinucleated osteoclasts in Howship's lacuna. Scale bar, 50 µm. (h) Fibres of the enlarged notochord sheath in the intervertebral space continue in the mineralized part of the sheath (black arrows). In the outer bone layer there are equivalent structures that have been identified as Sharpey's fibres (white arrows). Scale bar, 100 µm. (i–k) Resorption parameters such as eroded surface per bone surface (ES/BS), number of osteoclasts per bone perimeter (N.Oc./B.Pm), and osteoclast surface per bone surface (Oc.S/BS) increased significantly among the groups. *p < 0.05.
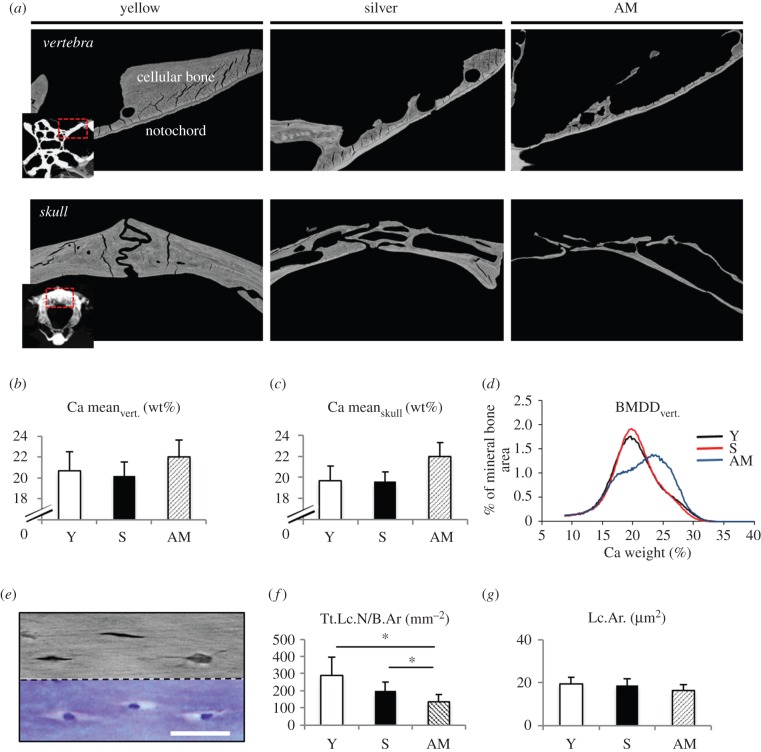
The resorption process was not accompanied by a decreasing calcium content in the remaining mineralized bone (i.e. demineralization) (figures 1e,f and 3a–c). In fact, qBEI analysis revealed no changes in overall mineralization of the remaining matrix in vertebral bodies and skull bones of artificially matured eels. However, in hormone-treated eels, qBEI showed a peak shift towards higher matrix mineralization (figure 3d) similar to the mineral content of the acellular notochord sheath when measured separately (figure 2e).
Figure 3.
Bone mineralization and osteocyte parameters during maturation. (a) qBEI images confirm the massive bone loss in vertebra and skull. Scale bar, 100 µm. (b,c) Ca mean values did not differ significantly in any of the three groups although there was a trend towards higher values in AM. (d) Ca weight analysis shows that this is due to a higher proportion of high-mineralized tissue in AM. The second peak represents the remaining high-mineralized notochord and conforms to the results when analysing mineralized notochord alone. (BMDD = bone mineral density distribution.) (e) Observing corresponding areas within the vertebra, osteocyte lacunae identified by qBEI clearly show a cell nucleus in corresponding histology (toluidine blue staining). Scale bar, 20 µm. (f) The number of osteocyte lacunae was measured per cellular bone area (Tt.Lc.N./B.Ar.). There is a decreased number of osteocyte lacunae between artificially matured and yellow eels and matured and silver eels (*p < 0.05). (g) Lacunar area did not change significantly among the three groups. (Online version in colour.)
Measurements of the osteocyte number per bone area showed a declining number of osteocyte lacunae in cellular bone (N.Ot.Lc/B.Ar = 287.8 ± 109.5 versus 201.3 ± 50.3 versus 133.7 ± 46.3 mm−2) but no significant enlargement of osteocyte lacunae during artificial maturation (figure 3e–g). qBEI and acid-etching techniques, as well as SHG microscopy, revealed no clear signs of existing osteocyte canaliculi in eel bone, apart from scarce extensions at some lacunar walls. The line-like connections that are seen in the mineralized notochord sheath are direct extensions of the fibrous notochord sheath that connects the vertebral bodies (figure 4h, black arrows). Furthermore, there are abundant collagen fibre bundles anchored into osteocytic bone that forms around the notochord sheath (figure 4h, white arrows; electronic supplementary material, figure S1, white arrows). These are most likely Sharpey's fibres.
FTIR spectroscopy indicated a higher mineral-to-matrix ratio in the mineralized part of the notochord sheath (8.18 ± 1.78) than in cellular bone (6.58 ± 1.37), which supports the qBEI data (figure 4b). Between these two layers there was a thin layer that showed a very low mineral : matrix ratio (5.54 ± 0.47) indicating a low mineral content, which was also seen in toluidine blue staining, where this layer was stained light blue (figure 4a). Furthermore, the carbonate : phosphate ratio was lower in the osteocytic bone (0.0061 ± 0.0011 versus 0.00727 ± 0.00089) exposed to resorption (figure 4c). The proteoglycan content of the non-mineralized inner layer of the notochord sheath was very high, whereas the mineralized part of the notochord sheath showed lower proteoglycan content than the adjacent osteocytic bone (0.142 ± 0.019 versus 0.154 ± 0.015; figure 4d).
The induction of sexual maturation caused an increase in resorption-related structural changes (figure 4e,f). Identified osteoclasts were multinucleated and were located in typical resorption lacunae at the surface of the osteocyte-containing bone (figure 4g). Namely, in mature eels, an increased eroded surface per bone surface (ES/BS) in comparison with yellow and silver eels was evident (p < 0.001, p < 0.001). The number of multinucleated osteoclasts per bone perimeter (N.Oc/B.Pm) was notably increased in comparison with yellow eels (p < 0.001) and silver eels (p = 0.012). The osteoclast surface per bone surface (Oc.S/BS) in mature eels was substantially higher than in yellow eels (p = 0.011) and tended to be higher than in silver eels (figure 4i–k).
4. Discussion
In general, three mechanisms of bone resorption have been observed in teleost fish in response to metabolic demands or for adaptation to mechanical load: osteoclastic resorption, osteocyte-mediated resorption (osteocytic osteolysis), and halastatic demineralization [9]. The latter describes the removal of mineral substance with no degradation of the organic matrix of the bone [39]. Previous studies suggest that these processes may also occur in eels [26], but it is still unknown what mechanism triggers bone resorption in eels in connection with sexual maturation.
In mammals, bone is an important reservoir of calcium ions that can be deposited or released depending on the current needs of tightly regulated plasma calcium levels [40]. Teleost fish maintain similar tightly regulated plasma calcium levels but they can take in calcium from the water and release excess calcium via the gills [9,16]. Thus, teleosts need not involve bone in their calcium metabolism. By contrast, phosphorus cannot be obtained from the water through the gills and, therefore, teleost fish rely on dietary phosphorous intake [9,41]. As phosphorus is an essential factor for eel metabolism, sexual maturation, and primary yolk synthesis [4], eels that stop feeding prior to spawning have to find an alternative source of phosphorus. Hence, it was suggested that they might mobilize phosphorus from the endoskeleton through bone resorption [4].
Moreover, it has not been clarified whether calcium or phosphate deficiency may be an initiating stimulus for bone resorption. Our findings revealed that the plasma calcium concentration remains unchanged during artificial sexual maturation. Furthermore, in contrast with previous suggestions [13] we showed that the mechanism of increased bone resorption in sexually mature eels was obviously cortisol independent, given that measurements of blood cortisol levels showed considerably low values in mature eels. In addition, excessive bone resorption liberates a large amount of organic material (mainly collagen), which under certain circumstances may mainly contribute as an additional source for energy production, given that protein oxidation was suggested to be an equally important process during starvation in eels [42,43]. Thus, bone loss in fasting eels may have a partly similar function to the bone resorption occurring during hibernation in some animals, another condition involving prolonged fasting [44,45]. Nevertheless, spawning migration in eels relies heavily on extensive fat reserves that are mostly found in muscles, skin, and liver [46,47].
We observed abundant multinucleated osteoclasts in both female and male eels. Similar observations have been made in mature and starving male Atlantic salmon where the extension of the lower jaw (kype) is resorbed after spawning by giant multinucleated osteoclasts [48]. As has been done for Atlantic salmon it can be speculated that multinuclear osteoclasts are needed for fast extensive resorption [49], a mode of resorption that is characterized by the presence of deep Howship lacunae and extended areas of eroded bone surface. The fact that osteoclasts in sexually mature eels are multinucleated and are located at the surface of the osteocyte-containing bone lends support to the general notion that there is a possible link between the presence of osteocytes and multinucleated osteoclasts as a primary osteoclast type [9]. However, revealing the potential role of osteocytes in triggering fast and extensive bone resorption requires further investigation.
In this study, enlarged osteocyte lacunae associated with osteocytic osteolysis were not observed. While resorption-related enlargement of osteocyte lacunae by approximately 20–40% was previously found in Japanese eels [12,13], our experiments provide no evidence that European eels' osteocytes are potent contributors of mineral release at this stage of life. Osteocyte lacunar quantification revealed that, with a density of approximately 200 mm−2 and an area approximately 20 µm2, they are less densely packed and much smaller in eels than in mammals, such as humans [50] or mice [51], and also than in other teleost fish (approx. 400 mm−2, 40 µm2) [16]. Osteocyte canaliculi were found to be scarce or absent (figure 3e; electronic supplementary material, figure S1), as described for several teleost species. Given that other members of the order Anguilliformes such as the spotted moray (Gymnothorax moringa) have acellular bone [52], the reduction of canaliculi in A. anguilla could perhaps represent a bone type that is intermediate between cellular and acellular bone.
Bone resorption was indeed evident at the osteocyte-containing bony areas; yet, there are several examples of anosteocytic tissues, including teleost bone, being remodelled and resorbed by (mostly mononucleated) osteoclasts [53–57]. Our experiments question if/how the small and not densely packed osteocytes are involved in signalling bone resorption in eels, especially considering that they show almost no canalicular connections. Furthermore, osteocyte lacunar numbers further decreased during maturation, which is an effect similar to ageing human bone or osteoporosis [50]. However, the reduction of osteocytes also has anatomical reasons unrelated to ageing. Namely, the first layer of bone around the notochord is woven bone with low numbers of osteocytes and abundant fibres. The built second layer is lamellar bone with higher numbers of osteocytes (electronic supplementary material, figure S2). When the first layer of lamellar bone is resorbed by osteoclasts, woven bone with lower osteocyte numbers remains. Further research is needed to clarify the osteocytes' role in signalling bone resorption in eels.
A shift in the histogram to higher calcium values in the bone matrix of hormone-treated eels was evident because of the resorbed lower mineralized cellular bone portions, while the highly mineralized notochord sheath remained (figures 2d,e and 4d). FTIR spectroscopy confirmed the findings of higher mineralization and lower proteoglycan content in the mineralized notochord in comparison with the cellular bone matrix. A lower carbonate : phosphate ratio, as present in osteocytic bone, highlights younger tissue age [35,58,59] and reflects a low carbonate and/or a high phosphate content. Low proteoglycan content in the acellular mineralized notochord sheath reflects that its basic structure is related to a different embryologic tissue origin than the adjacent osteocytic bone.
Bone development in teleost fish and in mammals requires bone resorption and remodelling at an early stage [20]. By contrast, teleost vertebral bodies develop within and grow around the notochord sheath, in a process that initially does not involve bone resorption or bone remodelling [9]. The notochord sheath of teleost fish is resorbed and penetrated by osteoclasts under special conditions, such as after upregulation of RANKL (receptor activator of nuclear factor kappa-B ligand) in transgenic medaka models and is connected to skeletal malformations in non-genetically manipulated animals [22,23,60–62]. However, the mineralized notochord sheath was not resorbed in mature eels, most likely due to specific anatomical organization that features a non-mineralized layer between the mineralized notochord and the osteocytic bone (figure 4a,b; electronic supplementary material, figure S1a). This non-mineralized area has been found to be composed of elastin [62] and, in contrast with some other species where it becomes fragmented allowing invasion of the notochord [63], the elastin layer was apparently intact in mature eels and served as an unmineralized barrier to osteoclastic attachment. Namely, osteoclasts need a mineralized substrate and cannot attach to non-mineralized surfaces [59,64,65]. Similarly, in salmon with severe phosphorus deficiency and an enormous amount of non-mineralized bone, bone and the notochord were not resorbed as they were protected from resorption by the non-mineralized bone layer [41].
In summary, although eels present two types of mineralized tissue (cellular bone and the mineralized notochord sheath), only the osteocytic bone was subject to osteoclastic resorption to release mineral (and organic components) for metabolic functions needed during sexual maturation and spawning migration. By contrast, the elastin layer protects the notochord from resorption. This reveals structural optimization of the eel skeleton to fulfil two potentially conflicting functions of mineralized tissue (preserving mechanical strength and releasing minerals when necessary). Specifically, these functions are spatially segregated to two different hard tissue compartments: cellular bone that may function as a reservoir of minerals, especially phosphorus, and the acellular mineralized notochord sheath that is unaffected by osteoclastic actions and, therefore, ensures enough stability even under phosphorus-deficient conditions. The notochord alone, without surrounding bone from vertebral bodies, is a main contributor to a stable axial skeleton even in very large bony fish such as sturgeons, coelacanths, and lungfish [41]. The notochord as a hydroskeleton is under high pressure [66], such that fenestration of the notochord sheath by osteoclasts would be dangerous.
5. Conclusion
During the spawning migration to the Sargasso Sea, European eels abstain from food and lose substantial amounts of bone. Here, we show not only the degree of skeletal decay, but also the mechanisms responsible for bone degradation in eels. While eels clearly show two types of mineralized tissues, exclusively cellular bone is subject to osteoclastic resorption and required mineral release. By contrast, the mineralized notochord sheath remains structurally intact ensuring sufficient mechanical stability of the skeleton during the exhausting migration. Studying marine vertebrates highlights conserved and alternative pathways for bone adaptation to mechanical and metabolic challenges in an evolutionary context. In-depth characterization of bone homeostatic mechanisms in teleosts may open avenues for the prevention and treatment of human bone loss syndromes.
Supplementary Material
Acknowledgements
We thank Marion Dietzmann and Mona Neven for support in preparing histological specimens, Jozef Zustin for help with histologic evaluation of cellular entities and Bülent Peker for support with Multiphoton microscopy.
Ethics
Animal care and experimental procedures were performed with approval from the environmental authority of Schleswig-Holstein (91-6/12).
Data accessibility
Detailed data are available in the electronic supplementary material.
Authors' contributions
T.R., F.N., M.A., and B.B. designed the study. T.R., F.N., S.W., R.P.M., A.J., F.N.S., M.H., and B.B. performed the experiments. T.R., F.N., P.M., R.P.M., P.E.W., M.A., and B.B. analysed the data. T.R., P.M., and B.B. drafted the manuscript. T.R., P.M., P.E.W., and B.B. revised the manuscript content. All authors approved the final version of manuscript. T.R. and B.B. take responsibility for the integrity of the data analysis.
Competing interests
The authors have no competing interests.
Funding
B.B. is supported by PIER (Partnership for Innovation, Education and Research) under grant no. PIF-2014-28 and is a fellow of the DFG (Deutsche Forschungsgemeinschaft—German Research Foundation; BU 2562/2-1/3-1). P.M. is a fellow of Alexander von Humboldt Foundation. F.N.S. was supported by the Joachim Herz Stiftung in cooperation with PIER.
References
- 1.Tesch FW. 2003. The Eel, 408 pp. Oxford, UK: Blackwell Science. [Google Scholar]
- 2.van Ginneken VJT, Maes GE. 2005. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev. Fish Biol. Fish. 15, 367–398. ( 10.1007/s11160-006-0005-8) [DOI] [Google Scholar]
- 3.van Ginneken V, Antonissen E, Muller UK, Booms R, Eding E, Verreth J, van den Thillart G. 2005. Eel migration to the Sargasso: remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 208, 1329–1335. ( 10.1242/jeb.01524) [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y, Okamura A, Tanaka S. 2002. The roles of bone and muscle as phosphorus reservoirs during the sexual maturation of female Japanese eels, Anguilla japonica Temminck and Schlegel (Anguilliformes). Fish Physiol. Biochem. 24, 327–334. ( 10.1023/A:1015059524947) [DOI] [Google Scholar]
- 5.Aarestrup K. 2009. Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325, 1660 ( 10.1126/science.1178120) [DOI] [PubMed] [Google Scholar]
- 6.van Ginneken V, et al. 2007. Does a 5500-km swim trial stimulate early sexual maturation in the European eel (Anguilla anguilla L.)? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 1095–1103. ( 10.1016/j.cbpa.2007.03.021) [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Yamauchi K. 1974. Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature 251, 220–222. ( 10.1038/251220a0) [DOI] [PubMed] [Google Scholar]
- 8.Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N, Hirose K. 1997. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol. Biochem. 17, 163–169. ( 10.1023/A:1007720600588) [DOI] [Google Scholar]
- 9.Witten PE, Huysseune A. 2009. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol. Rev. Camb. Philos. Soc. 84, 315–346. ( 10.1111/j.1469-185X.2009.00077.x). [DOI] [PubMed] [Google Scholar]
- 10.Lopez E, Mac Intyre I, Martelly E, Lallier F, Vidal B. 1980. Paradoxical effect of 1,25 dihydroxycholecalciferol on osteoblastic and osteoclastic activity in the skeleton of the eel Anguilla anguilla L. Calcif. Tissue Int. 32, 83–87. ( 10.1007/BF02408525) [DOI] [PubMed] [Google Scholar]
- 11.Lopez E, Peignoux-Deville J, Lallier F, Martelly E, Milet C. 1976. Effects of calcitonin and ultimobranchialectomy (UBX) on calcium and bone metabolism in the eel, Anguilla anguilla L. Calcif. Tissue Res. 20, 173–186. ( 10.1007/BF02546406) [DOI] [PubMed] [Google Scholar]
- 12.Sbaihi M, Kacem A, Aroua S, Baloche S, Rousseau K, Lopez E, Meunier F, Dufour S. 2007. Thyroid hormone-induced demineralisation of the vertebral skeleton of the eel, Anguilla anguilla. Gen. Comp. Endocrinol. 151, 98–107. ( 10.1016/j.ygcen.2006.12.009) [DOI] [PubMed] [Google Scholar]
- 13.Sbaihi M, Rousseau K, Baloche S, Meunier F, Fouchereau-Peron M, Dufour S. 2009. Cortisol mobilizes mineral stores from vertebral skeleton in the European eel: an ancestral origin for glucocorticoid-induced osteoporosis? J. Endocrinol. 201, 241–252. ( 10.1677/JOE-08-0492) [DOI] [PubMed] [Google Scholar]
- 14.Favus MJ, Goltzman D. 2013. Regulation of calcium and magnesium. In Primer on the metabolic bone diseases and disorders of mineral metabolism, pp. 171–179. New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- 15.Doherty AH, Ghalambor CK, Donahue SW. 2015. Evolutionary physiology of bone: bone metabolism in changing environments. Physiology (Bethesda) 30, 17–29. ( 10.1152/physiol.00022.2014) [DOI] [PubMed] [Google Scholar]
- 16.Cohen L, Dean M, Shipov A, Atkins A, Monsonego-Ornan E, Shahar R. 2012. Comparison of structural, architectural and mechanical aspects of cellular and acellular bone in two teleost fish. J. Exp. Biol. 215, 1983–1993. ( 10.1242/jeb.064790). [DOI] [PubMed] [Google Scholar]
- 17.Bonewald LF. 2013. Osteocytes. In Primer on the metabolic bone diseases and disorders of mineral metabolism, pp. 34–41. New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- 18.Bruneel B, Witten PE. 2015. Power and challenges of using zebrafish as a model for skeletal tissue imaging. Connect. Tissue Res. 56, 161–173. ( 10.3109/03008207.2015.1013193) [DOI] [PubMed] [Google Scholar]
- 19.Ekanayake S, Hall BK. 1987. The development of acellularity of the vertebral bone of the Japanese medaka, Oryzias latipes (Teleostei; Cyprinidontidae). J. Morphol. 193, 253–261. ( 10.1002/jmor.1051930304) [DOI] [PubMed] [Google Scholar]
- 20.Witten PE, Hansen A, Hall BK. 2001. Features of mono- and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J. Morphol. 250, 197–207. ( 10.1002/jmor.1065) [DOI] [PubMed] [Google Scholar]
- 21.Apschner A, Schulte-Merker S, Witten PE. 2011. Not all bones are created equal—using zebrafish and other teleost species in osteogenesis research. Methods Cell Biol. 105, 239–255. ( 10.1016/B978-0-12-381320-6.00010-2) [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Witten PE, Huysseune A, Buettner A, To TT, Winkler C. 2016. Live imaging of osteoclast inhibition by bisphosphonates in a medaka osteoporosis model. Dis. Model. Mech. 9, 155–163. ( 10.1242/dmm.019091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To TT, Witten PE, Renn J, Bhattacharya D, Huysseune A, Winkler C. 2012. Rankl-induced osteoclastogenesis leads to loss of mineralization in a medaka osteoporosis model. Development 139, 141–150. ( 10.1242/dev.071035) [DOI] [PubMed] [Google Scholar]
- 24.Shahar R, Dean MN. 2013. The enigmas of bone without osteocytes. Bonekey Rep. 2, 343 ( 10.1038/bonekey.2013.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall BK, Witten PE. 2007. Plasticity of and transitions between skeletal tissues in vertebrate evolution and development. In Major transitions in vertebrate evolution (eds Anderson JS, Sues H-D), 432 Bloomington, IN: Indiana University Press. [Google Scholar]
- 26.Lopez E. 1970. Demonstration of several forms of decalcification in bone of the teleost fish, Anguilla anguilla L. Calcif. Tissue Res. 4(Suppl), 83 ( 10.1007/BF02152363) [DOI] [PubMed] [Google Scholar]
- 27.Palstra AP, Cohen EGH, Niemantsverdriet PRW, van Ginneken VJT, van den Thillart GEEJM. 2005. Artificial maturation and reproduction of European silver eel: development of oocytes during final maturation. Aquaculture 249, 533–547. ( 10.1016/j.aquaculture.2005.04.031) [DOI] [Google Scholar]
- 28.Palstra A, Thillart G. 2009. Artificial maturation and reproduction of the European eel. In Spawning migration of the european eel: reproduction index, a useful tool for conservation management (eds Thillart G, Dufour S, Rankin JC), pp. 309–331. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 29.Pérez L, Peñaranda DS, Dufour S, Baloche S, Palstra AP, Van Den Thillart GEEJM, Asturiano JF. 2011. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen. Comp. Endocrinol. 174, 51–59. ( 10.1016/j.ygcen.2011.08.009) [DOI] [PubMed] [Google Scholar]
- 30.Pankhurst NW. 1982. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 21, 127–140. ( 10.1111/j.1095-8649.1982.tb03994.x) [DOI] [Google Scholar]
- 31.Dempster DW, et al. 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17. ( 10.1002/jbmr.1805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koehne T, Vettorazzi E, Kusters N, Luneburg R, Kahl-Nieke B, Puschel K, Amling M, Busse B. 2014. Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone 66, 31–38. ( 10.1016/j.bone.2014.05.010). [DOI] [PubMed] [Google Scholar]
- 33.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. 2008. Bone mineralization density distribution in health and disease. Bone 42, 456–466. ( 10.1016/j.bone.2007.10.021) [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann EA, et al. 2015. Modifications to nano- and microstructural quality and the effects on mechanical integrity in Paget's disease of bone. J. Bone Miner. Res. 30, 264–273. ( 10.1002/jbmr.2340) [DOI] [PubMed] [Google Scholar]
- 35.Boskey A, Pleshko Camacho N. 2007. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 28, 2465–2478. ( 10.1016/j.biomaterials.2006.11.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Puschel K, Djuric M, Amling M, Busse B. 2013. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 7, 7542–7551. ( 10.1021/nn401360u) [DOI] [PubMed] [Google Scholar]
- 37.Ambekar R, Chittenden M, Jasiuk I, Toussaint KC Jr. 2012. Quantitative second-harmonic generation microscopy for imaging porcine cortical bone: comparison to SEM and its potential to investigate age-related changes. Bone 50, 643–650. ( 10.1016/j.bone.2011.11.013) [DOI] [PubMed] [Google Scholar]
- 38.Stoller P, Reiser KM, Celliers PM, Rubenchik AM. 2002. Polarization-modulated second harmonic generation in collagen. Biophys. J. 82, 3330–3342. ( 10.1016/S0006-3495(02)75673-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kacem A, Meunier FJ. 2003. Halastatic demineralization in the vertebrae of Atlantic salmon, during their spawning migration. J. Fish Biol. 63, 1122–1130. ( 10.1046/j.1095-8649.2003.00229.x) [DOI] [Google Scholar]
- 40.Mundy GR, Guise TA. 1999. Hormonal control of calcium homeostasis. Clin. Chem. 45, 1347–1352. [PubMed] [Google Scholar]
- 41.Witten PE, Owen MA, Fontanillas R, Soenens M, McGurk C, Obach A. 2016. A primary phosphorus-deficient skeletal phenotype in juvenile Atlantic salmon Salmo salar: the uncoupling of bone formation and mineralization. J. Fish Biol. 88, 690–708. ( 10.1111/jfb.12870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boetius I, Boetius J. 1985. Lipid and protein content in Anguilla anguilla during growth and starvation. Dana 4, 1–17. ( 10.1089/dna.1985.4.1) [DOI] [Google Scholar]
- 43.Palstra AP, van den Thillart GE. 2010. Swimming physiology of European silver eels (Anguilla anguilla L.): energetic costs and effects on sexual maturation and reproduction. Fish Physiol. Biochem. 36, 297–322. ( 10.1007/s10695-010-9397-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seger RL, et al. 2011. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus). Bone 49, 1205–1212. ( 10.1016/j.bone.2011.08.017) [DOI] [PubMed] [Google Scholar]
- 45.Steinberg B, Singh IJ, Mitchell OG. 1981. The effects of cold-stress. Hibernation, and prolonged inactivity on bone dynamics in the golden hamster, Mesocricetus auratus. J. Morphol. 167, 43–51. ( 10.1002/jmor.1051670105) [DOI] [PubMed] [Google Scholar]
- 46.Pierron F, Baudrimont M, Bossy A, Bourdineaud JP, Brethes D, Elie P, Massabuau JC. 2007. Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat. Toxicol. 81, 304–311. ( 10.1016/j.aquatox.2006.12.014) [DOI] [PubMed] [Google Scholar]
- 47.Abraham S, Hansen HJ, Hansen FN. 1984. The effect of prolonged fasting on total lipid synthesis and enzyme activities in the liver of the European eel (Anguilla anguilla). Comp. Biochem. Physiol. B 79, 285–289. ( 10.1016/0305-0491(84)90027-0) [DOI] [PubMed] [Google Scholar]
- 48.Witten PE, Hall BK. 2003. Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. J. Anat. 203, 435–450. ( 10.1046/j.1469-7580.2003.00239.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meunier F. 1989. The acellularisation process in osteichthyan bone. Trends in vertebrate morphology. In Proc. of the 2nd Int. Symp. on Vertebrate Morphology, Vienna, Austria, 1986 (ed. HHH Splechtna), pp. 443–446. Stuttgart, Germany: Fischer.
- 50.Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, Djuric M, Amling M. 2010. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9, 1065–1075. ( 10.1111/j.1474-9726.2010.00633.x) [DOI] [PubMed] [Google Scholar]
- 51.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. 2012. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 27, 1018–1029. ( 10.1002/jbmr.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moss ML. 1961. Studies of the acellular bone of teleost fish. 1. Morphological and systematic variations. Acta Anat. 46, 343–462. ( 10.1159/000141794) [DOI] [PubMed] [Google Scholar]
- 53.Witten PE. 1997. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae). Cell Tissue Res. 287, 591–599. ( 10.1007/s004410050782) [DOI] [PubMed] [Google Scholar]
- 54.Atkins A, et al. 2014. Remodeling in bone without osteocytes: billfish challenge bone structure-function paradigms. Proc. Natl Acad. Sci. USA 111, 16 047–16 052. ( 10.1073/pnas.1412372111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horton JM, Summers AP. 2009. The material properties of acellular bone in a teleost fish. J. Exp. Biol. 212, 1413–1420. ( 10.1242/jeb.020636) [DOI] [PubMed] [Google Scholar]
- 56.Weiss RE, Watabe N. 1979. Studies on the biology of fish bone. III. Ultrastructure of osteogenesis and resorption in osteocytic (cellular) and anosteocytic (acellular) bones. Calcif. Tissue Int. 28, 43–56. ( 10.1007/BF02441217) [DOI] [PubMed] [Google Scholar]
- 57.Atkins A, Milgram J, Weiner S, Shahar R. 2015. The response of anosteocytic bone to controlled loading. J. Exp. Biol. 218, 3559–3569. ( 10.1242/jeb.124073) [DOI] [PubMed] [Google Scholar]
- 58.Milovanovic P, et al. 2015. Multi-level characterization of human femoral cortices and their underlying osteocyte network reveal trends in quality of young, aged, osteoporotic and antiresorptive-treated bone. Biomaterials 45, 46–55. ( 10.1016/j.biomaterials.2014.12.024) [DOI] [PubMed] [Google Scholar]
- 59.Busse B, et al. 2013. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci. Transl. Med. 5, 193ra188. ( 10.1126/scitranslmed.3006286) [DOI] [PubMed] [Google Scholar]
- 60.To TT, Witten PE, Huysseune A, Winkler C. 2015. An adult osteopetrosis model in medaka reveals the importance of osteoclast function for bone remodeling in teleost fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 178, 68–75. ( 10.1016/j.cbpc.2015.08.007) [DOI] [PubMed] [Google Scholar]
- 61.Loizides M, Georgiou AN, Somarakis S, Witten PE, Koumoundouros G. 2014. A new type of lordosis and vertebral body compression in Gilthead sea bream, Sparus aurata L.: aetiology, anatomy and consequences for survival. J. Fish Dis. 37, 949–957. ( 10.1111/jfd.12189) [DOI] [PubMed] [Google Scholar]
- 62.Bensimon-Brito A, Cardeira J, Cancela ML, Huysseune A, Witten PE. 2012. Distinct patterns of notochord mineralization in zebrafish coincide with the localization of Osteocalcin isoform 1 during early vertebral centra formation. BMC Dev. Biol. 12, 28 ( 10.1186/1471-213X-12-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodrich ES. 1930. Studies on the structure & development of vertebrates. London, UK: Macmillan. [Google Scholar]
- 64.Manolagas SC. 2000. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137. ( 10.1210/er.21.2.115) [DOI] [PubMed] [Google Scholar]
- 65.Uchida M, Shima M, Chikazu D, Fujieda A, Obara K, Suzuki H, Nagai Y, Yamato H, Kawaguchi H. 2001. Transcriptional induction of matrix metalloproteinase-13 (collagenase-3) by 1α,25-dihydroxyvitamin D3 in mouse osteoblastic MC3T3-E1 cells. J. Bone Miner. Res. 16, 221–230. ( 10.1359/jbmr.2001.16.2.221) [DOI] [PubMed] [Google Scholar]
- 66.Stemple DL. 2005. Structure and function of the notochord: an essential organ for chordate development. Development 132, 2503–2512. ( 10.1242/dev.01812) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed data are available in the electronic supplementary material.