Abstract
The internal predictive adaptive response (internal PAR) hypothesis predicts that individuals born in poor conditions should start to reproduce earlier if they are likely to have reduced performance in later life. However, whether this is the case remains unexplored in wild populations. Here, we use longitudinal data from a long-term study of Svalbard reindeer to examine age-related changes in adult female life-history responses to environmental conditions experienced in utero as indexed by rain-on-snow (ROSutero). We show that females experiencing high ROSutero had reduced reproductive success only from 7 years of age, independent of early reproduction. These individuals were able to maintain the same annual reproductive success between 2 and 6 years as phenotypically superior conspecifics that experienced low ROSutero. Young females born after high ROSutero engage in reproductive events at lower body mass (about 2.5 kg less) than those born after low ROSutero. The mean fitness of females that experienced poor environmental conditions in early life was comparable with that of females exposed to good environmental conditions in early life. These results are consistent with the idea of internal PAR and suggest that the life-history responses to early-life conditions can buffer the delayed effects of weather on population dynamics.
Keywords: climate change, cohort, development, predictive adaptive response, phenotypic plasticity, Svalbard reindeer
1. Introduction
There is now overwhelming evidence that ‘early life’ represents a sensitive window influencing the phenotype in various species [1,2]. Environmental conditions prevailing during this period, when the architecture of the body is being established [3], often show short-term effects, for instance by influencing juvenile survival [4]. Conditions early in life can also have long-lasting effects on adult phenotypes, from physiology and metabolism [5], to life-history traits such as body growth, reproduction and survival [6–8]. Early-life conditions can thus influence life-history trajectories of entire cohorts (set of individuals born within the same birth pulse). Such ‘delayed cohort quality effects’ (sensu [9]) can affect population dynamics [10,11]. Less well understood is how individuals respond to these long-term, environmentally induced changes. Studies have typically considered that an adverse early environment imposes severe constraints on development, resulting in stunted, poorly performing individuals (silver spoon effect) [12,13]. There is an emerging view, however, that individuals can mitigate the effects of poor early-life conditions with adaptive changes in behaviour, morphology or reproduction [14,15], but supporting data are still scarce [16,17].
The internal predictive adaptive response (internal PAR, [15]) hypothesis was recently developed to account for observed associations between poor early-life conditions and acceleration of reproductive timing in humans. Nettle and co-workers proposed that ‘early-life adversity has a lasting negative impact on the individual's somatic state, such that her health is likely to fail more rapidly as she gets older, and there is an advantage to adjusting her reproductive schedule accordingly’ [15, p. 1]. More generally, individuals born under poor conditions should start to reproduce earlier if they are likely to have reduced performance (survival and/or reproductive success) in later life or faster rates of senescence compared with individuals born in better conditions. The internal PAR differs from the external PAR, which is a form of developmental plasticity where individuals anticipate their adult environment and adjust their physiology accordingly [18]. In the internal PAR, what individuals are ‘predicting’ from their early environment is not the state of their future environment but rather the future state of their own body [15]. The internal PAR hypothesis appears more relevant than the external PAR in long-lived species living under variable environments, where the assumption that the environment in early life provides a reliable clue of the environmental conditions during adulthood is unlikely [19,20]. However, while tests of the external PAR hypothesis in long-lived species have repeatedly led to its rejection (see [21] in roe deer, [22] in baboon, [23] in preindustrial humans), direct tests of the internal PAR hypothesis are currently lacking in wild populations.
An increasing number of studies have shown that poor environmental conditions in early life can result in either accelerated senescence or reduced reproductive success and survival at old ages [24–30], but the pathways leading to these patterns are poorly understood. There are at least two mechanisms through which such relationships could occur. First, poor early-life conditions may directly affect physiological functions, such as antioxidant defences and telomere dynamics whose negative consequences on performance often do not manifest themselves until later in life [31]. Second, individuals born in poor environmental conditions may suffer from increased costs of reproduction during early adulthood [26]. The disposable soma theory predicts that increased reproductive effort during early adulthood should be accompanied by reductions in late-life performance [32,33]. However, costs of reproduction can only be evident among low-quality individuals that consistently acquire less resources than high-quality individuals [34]. Because the internal PAR assumes that reduced performance in later life associated with poor early-life conditions results from direct effects of environment rather than increased costs of early reproduction, disentangling these two mechanisms of long-lasting effects of early environment is important.
In this paper, we take advantage of a long-term study of female Svalbard reindeer (Rangifer tarandus platyrhynchus) to evaluate the support for the internal PAR hypothesis. Living at high latitudes, Svalbard reindeer experience considerable variation in winter resource availability [35], particularly due to variation in rain-on-snow (ROS) events, which create ice layers on the ground or in the snow pack [36], and limit access to vegetation in winter [37]. We focus our analysis on ROS events in utero (ROSutero) because females that experienced high ROSutero were lighter and smaller during both the juvenile and adult stages than females experiencing low ROSutero (figure 1).
Figure 1.
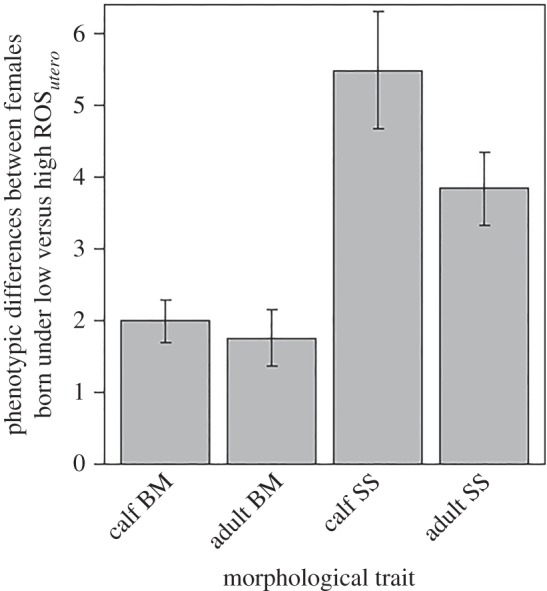
High ROSutero has a long-lasting negative influence on body development of female Svalbard reindeer. Differences (±s.e.) in body mass (BM in kilogram) and skeletal size (SS in millimetres indexed via measurement of hind foot length) between calves (1 year, n = 445) and adults (4–9 years, n = 849) that experienced low ROSutero and those that experienced high ROSutero. All differences are statically significant (p < 0.001). We used linear models to estimate values for calves with ROS in the current year as covariate in the body mass model. We used linear-mixed models to estimate values for adults with year and female identity as random factors. We also included ROScurrent and Julian date of capture as covariates in the model of adult body mass.
We begin by testing the assumptions of the internal PAR hypothesis. We investigate whether females experiencing high ROSutero show steeper rates of reproductive and actuarial senescence or reduced performance in late life compared with females born under more favourable conditions to low ROSutero. We evaluate whether these differences result from differential costs of early reproduction. We also examine the effects of ROSutero on reproductive success and survival during early adulthood to determine if individuals born in poor conditions are at a permanent disadvantage, as predicted by the silver spoon hypothesis.
The internal PAR predicts an early onset of reproduction for individuals born under poor conditions. However, this does not take into account biological constraints on age at first reproduction. In large mammalian herbivores such as Svalbard reindeer, young females must reach a threshold body mass to ovulate (see [38] for a review). Information on age at first reproduction is unavailable for most female reindeer because we did not capture them every year, but there is good quality data available on pregnancy rate and mass. Therefore, we focus on how the mass-specific probability of pregnancy for young females varies with ROSutero. We test the prediction derived from the internal PAR hypothesis that young females born after high ROSutero should achieve a 50% probability of pregnancy at a lower body mass compared to females born after low ROSutero. Finally, we compare mean fitness of females born after high versus low ROSutero. The silver spoon hypothesis predicts that individuals experiencing good environmental conditions during early life should have greater fitness than individuals facing poor environmental conditions, whereas no fitness differences should be detected according to the internal PAR.
2. Material and methods
(a). Svalbard reindeer as a biological model
The Svalbard reindeer is a subspecies of Rangifer tarandus endemic to the Arctic archipelago of Svalbard. It represents one of the northernmost populations of Rangifer and is highly sedentary with no migration [39]. Maximum documented longevity is 17 years in females and 12 years in males [40]. The mating system is polygynous with the main rutting activity peaking in early October, and a subsequent highly synchronized birth period in early June [41]. Females are iteroparous and can give birth to a single calf each year from 2 years of age onwards. There are no large terrestrial predators for reindeer in Svalbard and human harvesting of reindeer is limited.
(b). Weather data
The weather data were collected at Longyearbyen airport (77°54′ N, 16°48′ E) by the Norwegian Meteorological Institute. We calculated ROS as the amount of precipitation that fell at temperatures above 1°C, between 1 November and 30 April when females were currently gestating [42]. We did not detect any evidence of temporal autocorrelation in ROS (electronic supplementary material, figure S1). In all models, we entered ROSutero as a two-level factor separating high ROSutero (more than or equal to 10 mm) from low ROSutero (less than 10 mm). The threshold of 10 mm, determined from the distribution of ROS that shows a clear grouping (electronic supplementary material, figure S2), has previously been used for investigating the effects of heavy ROS on Svalbard reindeer population growth rates [43].
(c). Study area and population
The study was carried out in Nordenskiöld Land, Spitsbergen (77°50′–78°10′ N, 15°00′–17°00′ E). The population has been monitored by capture–mark–recapture (CMR) since 1994. A small number of females were captured in August 1994 using chemical immobilization but since 1995, females have been caught using a net attached to two handheld poles between snow-scooters in the winter (mostly in April/early May) [44]. Annual population size estimates (all female adults plus calves of both sexes) ranged between 733 in 1996 and 1758 in 2014 [45]. All females included in this study were of known age, because they have been marked as calves at 10–11 months or yearlings at 22–23 months, when age can be reliably established on the basis of size and tooth eruption [46]. Approximately 25% of the females present in the population were marked [44]. Although this study is based on individual-based longitudinal data, not all females were captured every year with an estimated annual recapture probability ranging between 0.25 and 0.68 [47]. When age is referred to in this study, it corresponds to the individual age in June, one to two months after capture and around the normal timing of birth. Animals captured at the age of 1 year and 10 months are therefore referred to as 2 year olds, etc. As we are interested in long-lasting effects of environmental conditions in early life, we restricted the latest cohort in our sample to females born in 2010, yielding a total of 18 cohorts, born from 1993 to 2010. Our last year of data was 2013 and 2014 for reproduction and survival analyses, respectively.
At capture, body mass was measured to the nearest 0.5 kg and pregnancy status was determined from the progesterone concentration in blood samples and ultrasound diagnosis [48]. In addition to the winter captures, annual surveys were performed every summer between 25 June and 25 August (mostly in early August) to assess the presence or absence of calf at heel. Because pre-weaning calf mortality typically occurs in the first days after birth, these behavioural observations provide good measures of reproductive success [49].
On average, 42% of the females captured in April/May were observed in the following summer but females experiencing high ROSutero had a higher re-sighting probability than females born under more favourable conditions (49% versus 38%; 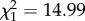 , p < 0.001). Related to this, 53% of non-pregnant females were not observed during the following summer if they were born after high ROSutero compared with 66% for non-pregnant females born after low ROSutero (
, p < 0.001). Related to this, 53% of non-pregnant females were not observed during the following summer if they were born after high ROSutero compared with 66% for non-pregnant females born after low ROSutero (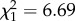 , p = 0.009). In order to avoid bias in the analyses, we relied solely on summer observations to estimate annual reproductive success.
, p = 0.009). In order to avoid bias in the analyses, we relied solely on summer observations to estimate annual reproductive success.
(d). Statistical analyses
Statistical analyses were performed using R v. 3.1.2 R (http://www.r-project.org). All response variables were individual-year observations. We used the ‘lme4’ library [50] for fitting generalized linear-mixed models (GLMMs) with binomial errors and a logit link function. All mixed models included female identity and year of sample collection as random effects to control for the non-independence of repeated measure of the same female and unmeasured sources of between-year variation. We used a backward selection procedure, testing successively the first-order interactions and, if not statistically significant, the main effects of variables. Statistical significance was assessed by likelihood ratio test and Wald statistics [51].
In most iteroparous species, fitness components initially increase with age and then decrease after a certain age threshold (the onset of senescence). This onset of senescence is 7 years in both reproductive success and survival of female Svalbard reindeer (see Results). Thus, to investigate late-life performance, our models only included the probabilities of reproductive success and survival at 7 years and over. For reproductive success, we constructed a GLMM containing ROSutero and age (fitted as a linear or quadratic function) as the fixed effects of interest. We included an interaction between ROSutero and both age and its square to determine whether reproductive senescence differed in relation to early-life environment. Age at last observation was also included as fixed effect term to control to for selective disappearance (the non-random departure from the dataset of individuals as age increase) so that age effect reflected an unbiased estimate of within-individual change [52]. Age at last observation was retained in the model independent of its statistical significance. To further characterize the breeding environment, we included ROS in the previous winter (ROScurrent) as this is strongly negatively associated with reproductive success [42]. ROScurrent was fitted as a continuous variable after a log + 1 transformation to reduce nonlinearity [42]. We tested whether ROSutero affected late-life reproductive success through differences in costs of early reproduction by re-running our previously selected model of reproductive success with the three-way interaction of the proportion of years in which a female produced a calf between age at first reproduction and 6 years old, ROSutero, and age. The full model of ‘late-life’ survival included ROSutero, age, ROScurrent and the interaction between ROSutero and age. We analysed survival by using CMR models [53] with E-surge [54] because of the imperfect detection of individuals. Survival data included capture histories of 407 females. Following previous CMR analyses in this population [47,49], capture probability was allowed to vary between years. Capture probability did not vary with ROSutero (ΔAIC = 2.80 compared with a time dependent model). Owing to small sample sizes at high ages, we pooled data from females more than or equal to 14 years of age in analyses of reproductive success, and data from females more than or equal to 12 years of age in analyses of survival.
We tested whether there was any significant relationship between ROSutero and reproductive success during early adulthood by considering females aged between 2 and 6 years. Our full GLMM included the effects of ROSutero, age (fitted as a quadratic function), ROScurrent and age at last observation within this age class. Based on survival changes with age (see Results), we also tested the effect of ROSutero on survival of yearling females (1 year olds) and survival between 2 and 6 years after accounting for the influence of ROScurrent.
We examined whether the relationship between pregnancy rate and body mass of young females (between 2 and 6 years) varied according to environmental conditions experienced in utero by assessing the evidence supporting an interaction between body mass adjusted to 12 April (the mean Julian date of the whole capture period) and ROSutero in a GLMM. We also repeated the analysis for each age between 2 and 6 separately using generalized linear models. To obtain a measure of effect size, we compared predicted body mass at 50% probability of pregnancy between females that experienced high versus low ROSutero.
In order to quantify the fitness consequences of ROSutero, we built two Leslie matrix models [55], one for each modality of ROSutero. We entered the age-specific values of reproductive success and survival, with their uncertainty, into the matrix models (electronic supplementary material, figure S3) to obtain the asymptotic growth rate (λ, the mean fitness sensu [56]) and its 95% confidence interval (CI) of each of these groups with bootstrap methods (10 000 simulations). Estimates of λ were approximately normally distributed and were compared using the percentile method.
3. Results
(a). Age-specific variation in reproductive success and survival
Reproductive success increased with female age until about 5 years and then remained relatively stable until 7 years before declining (figure 2a). A linear function of female age adequately explained changes in reproductive success between 7 and 14 years (table 1). Survival between 1 and 2 years was around 0.80 (figure 2b). Survival slightly increased with age between 2 and 6 years (from about 0.90 to 0.95) and declined strongly after age 7 (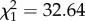 , p < 0.001, slope on a logit scale: −0.30 ± 0.05; figure 2b).
, p < 0.001, slope on a logit scale: −0.30 ± 0.05; figure 2b).
Figure 2.
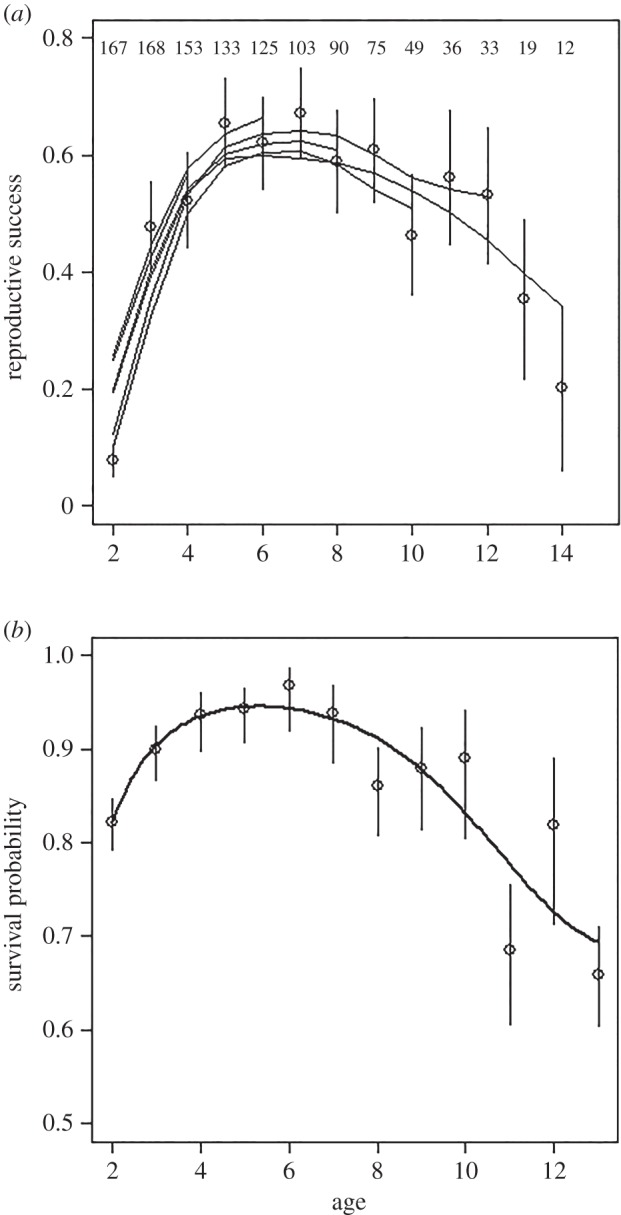
Age-specific probability (±s.e.) of reproductive success and survival in female Svalbard reindeer. (a) Lines are predicted age curves from a generalized additive mixed model for females with different ages at last observation, as indicated by the age where the lines end. Sample size for each age is indicated at the top of the figure. (b) The line represents the predicted values obtained from a generalized linear model with a cubic effect of age.
Table 1.
Generalized linear-mixed model of the probability of reproductive success based on 417 observations of 157 female Svalbard reindeer aged 7 years and over. (Parameter values are given for the final model, excluding non-significant terms except age at last observation. Interactions are denoted by ×. We reported standardized regression coefficients for the final model by first centring and then dividing all continuous variables by 2 s.d [57].)
| analysis of deviance | χ2 | d.f. | p-value |
|---|---|---|---|
| ROSutero × age2 | 1.14 | 1 | 0.29 |
| age2 | 0.13 | 1 | 0.72 |
| ROSutero × age | 0.30 | 1 | 0.58 |
| ROSutero | 5.05 | 1 | 0.02 |
| log (ROScurrent + 1) | 4.50 | 1 | 0.03 |
| age | 3.95 | 1 | 0.04 |
| final model | estimate | s.e. | p-value |
| intercepta | −0.312 | 0.330 | 0.34 |
| age at last observation | −0.183 | 0.305 | 0.54 |
| log (ROScurrent + 1) | −1.255 | 0.533 | 0.02 |
| age | −0.608 | 0.308 | 0.04 |
| ROSutero | 0.612 | 0.275 | 0.02 |
aFemales born under high ROSutero were considered as the reference.
(b). Senescence
Females aged more than or equal to 7 years born after high ROSutero had 1.84 times lower chances of rearing a calf than those born after low ROSutero (table 1 and figure 3). There was no evidence of an interaction between age and ROSutero on reproductive success of these females (table 1). Thus, females that experienced poor conditions in early life did not suffer steeper rates of reproductive senescence. We found no evidence for a significant interaction between average reproductive success between 2 and 6 years and ROSutero or age on late-life reproductive success (early reproduction × ROSutero: 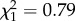 , p = 0.37; early reproduction × age:
, p = 0.37; early reproduction × age: 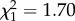 , p = 0.19; early reproduction × ROSutero × age:
, p = 0.19; early reproduction × ROSutero × age: 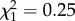 , p = 0.62). The correlation between early and late reproductive success is positive rather than negative (slope = 0.66 ± 0.25, p = 0.007). The effect of ROSutero on reproductive success of older females was independent of the positive influence of reproduction in early adulthood (electronic supplementary material, table S1). Annual survival after age 6 was negatively influenced by ROScurrent (
, p = 0.62). The correlation between early and late reproductive success is positive rather than negative (slope = 0.66 ± 0.25, p = 0.007). The effect of ROSutero on reproductive success of older females was independent of the positive influence of reproduction in early adulthood (electronic supplementary material, table S1). Annual survival after age 6 was negatively influenced by ROScurrent (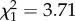 , p = 0.05, slope on a logit scale: −0.36 ± 0.18). However, ROSutero did not have a significant effect fitted alone or in interaction with age on survival (ROSutero × age:
, p = 0.05, slope on a logit scale: −0.36 ± 0.18). However, ROSutero did not have a significant effect fitted alone or in interaction with age on survival (ROSutero × age: 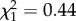 , p = 0.51; ROSutero:
, p = 0.51; ROSutero: 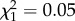 , p = 0.81).
, p = 0.81).
Figure 3.
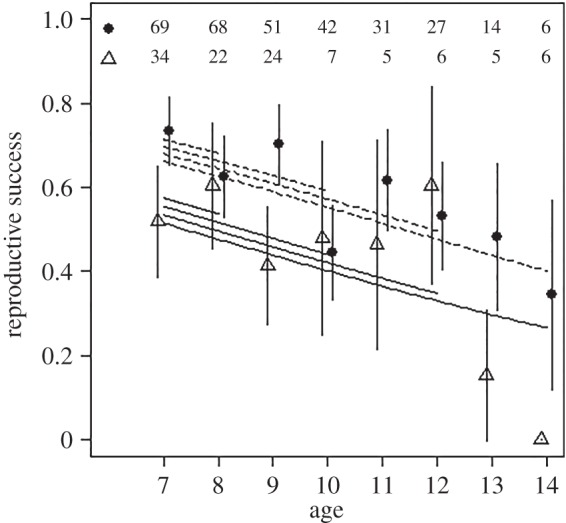
Relationship between reproductive success and age for female reindeer aged 7 years and over that experienced high (triangles and solid line) or low (filled circles and dashed line) ROSutero. The lines represent model predictions for females with different ages at last observation, as indicated by the age where the lines end. Age-specific estimates ±s.e. (points with errors bars) are obtained by fitting age as a factor. The number of individuals of each group for each age is indicated at top of the figure.
(c). Performance during early adulthood
After accounting for a quadratic age effect and negative influence of ROScurrent, we found no effect of ROSutero on annual reproductive success of females aged between 2 and 6 years (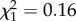 , p = 0.68; figure 4; electronic supplementary material, table S2). There was no relationship between age at last observation and reproductive success of young females (electronic supplementary material, table S2), regardless of ROSutero (
, p = 0.68; figure 4; electronic supplementary material, table S2). There was no relationship between age at last observation and reproductive success of young females (electronic supplementary material, table S2), regardless of ROSutero (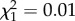 , p = 0.93). Neither ROScurrent nor ROSutero influenced the survival of young females (all p > 0.20). The probability of survival between 1 and 2 years was estimated to be 0.79 (95% CI = (0.70, 0.86)) for females born after high ROSutero and 0.81 (95% CI = (0.75, 0.87)) for females born after low ROSutero. Annual survival rate between 2 and 6 years was 0.94 for both groups.
, p = 0.93). Neither ROScurrent nor ROSutero influenced the survival of young females (all p > 0.20). The probability of survival between 1 and 2 years was estimated to be 0.79 (95% CI = (0.70, 0.86)) for females born after high ROSutero and 0.81 (95% CI = (0.75, 0.87)) for females born after low ROSutero. Annual survival rate between 2 and 6 years was 0.94 for both groups.
Figure 4.
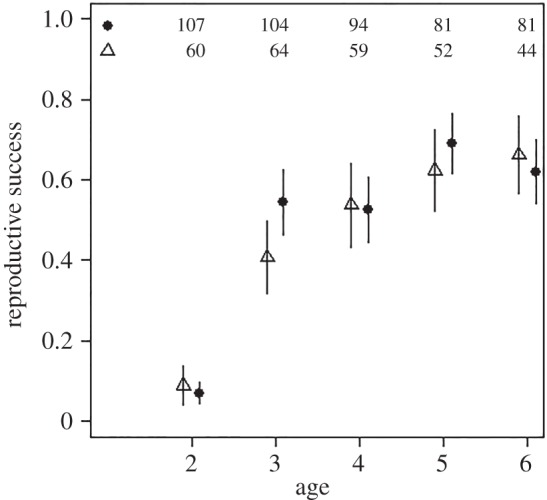
Relationship between reproductive success and age for female reindeer aged 2–6 years that experienced high (triangles) or low (filled circles) ROSutero. The number of individuals of each group for each age is indicated at top of the figure.
(d). Pregnancy rate for a given body mass
The proportion of pregnant females between 2 and 6 years did not depend on ROSutero (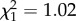 , p = 0.31, see also electronic supplementary material, figure S4). However, the relationship between annual pregnancy rate and body mass varied according to ROSutero (
, p = 0.31, see also electronic supplementary material, figure S4). However, the relationship between annual pregnancy rate and body mass varied according to ROSutero (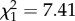 , p = 0.006; figure 5a). Most young females more than 48 kg were pregnant. Below this mass, however, females experiencing high ROSutero achieved higher pregnancy rate for a given body mass than females born under more favourable conditions (figure 5a). To have a 50% chance of pregnancy, young females born after low or high ROSutero weighed 43.7 ± 0.3 kg and 41.2 ± 0.6 kg, respectively. This corresponded to an average difference of 2.5 kg. When we repeated the analysis for each age between 2 and 6, we found that females born after high ROSutero consistently had a lower mass at 50% pregnancy probability than females born after low ROSutero except at 2 years of age when only 11% of the females were pregnant (figure 5b; electronic supplementary material, figure S4).
, p = 0.006; figure 5a). Most young females more than 48 kg were pregnant. Below this mass, however, females experiencing high ROSutero achieved higher pregnancy rate for a given body mass than females born under more favourable conditions (figure 5a). To have a 50% chance of pregnancy, young females born after low or high ROSutero weighed 43.7 ± 0.3 kg and 41.2 ± 0.6 kg, respectively. This corresponded to an average difference of 2.5 kg. When we repeated the analysis for each age between 2 and 6, we found that females born after high ROSutero consistently had a lower mass at 50% pregnancy probability than females born after low ROSutero except at 2 years of age when only 11% of the females were pregnant (figure 5b; electronic supplementary material, figure S4).
Figure 5.
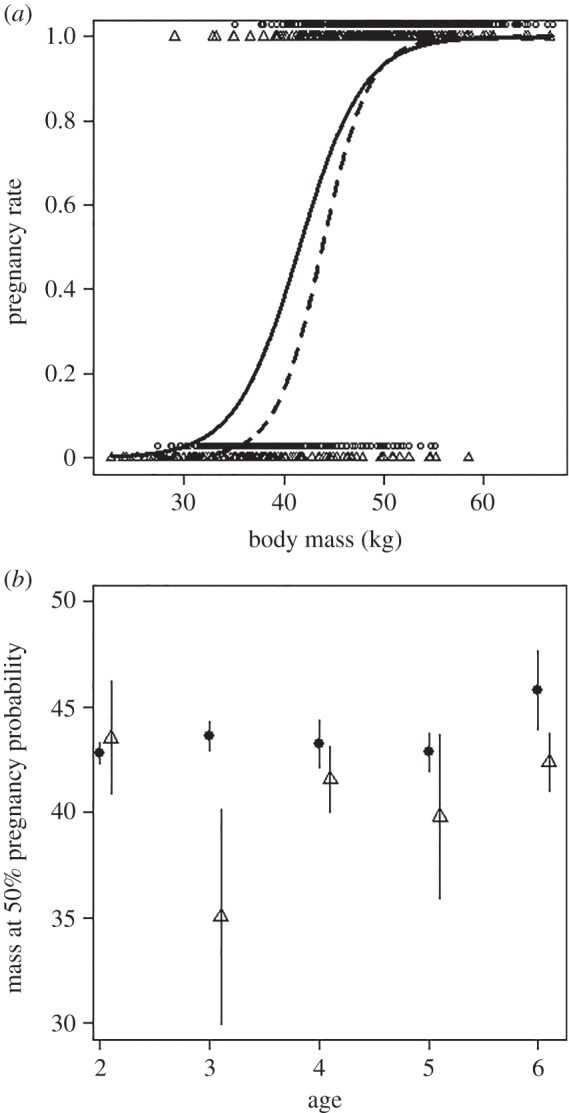
(a) Pregnancy rate as a function of body mass and the quality of early environment (high ROSutero: triangles and solid line, low ROSutero: circles and dashed line) in young female (2–6 years of age) Svalbard reindeer. Lines show the predicted values and points indicate the raw data (n = 1050). (b) Age-specific mass ±s.e. at 50% pregnancy probability for female reindeer that experienced high (open triangles) or low (filled circles) ROSutero.
(e). Fitness consequences of ROSutero
Leslie matrix models indicate that the asymptotic growth rate (λ) of cohorts born under poor conditions was only marginally lower that of cohorts born under favourable conditions, with a near complete overlap in confidence limits (high ROSutero: λ = 1.04, 95% CI = (0.96, 1.09); low ROSutero: λ = 1.07, 95% CI = (1.03, 1.10)).
4. Discussion
We found that early-life environmental conditions influence reproductive success of female Svalbard reindeer but in a highly age-dependent manner. The negative effects of ROSutero on annual reproductive success occurred only among females aged 7 years and older. Females experiencing high ROSutero were able to maintain the same annual reproductive success between 2 and 6 years as phenotypically superior conspecifics born after low ROSutero. Young females born after high ROSutero engaged in reproductive events at a lower body mass than those born after low ROSutero, which is consistent with the internal predictive adaptive response (internal PAR) hypothesis. Furthermore, mean fitness of females that experienced poor environmental conditions in early life was comparable to that of females exposed to more favourable conditions.
Many studies have shown that individuals born in poor environmental conditions are at permanent reproductive disadvantage regardless of their adult environment [21,22,25,58], the so-called reverse silver spoon effect [12,13]. However, such effects are not universal in food-limited environments. For instance, captive female guppies compensated for experimental food restrictions during the juvenile stage by accelerating growth rates in the adult stage and achieved the same reproductive success than those that experienced high food levels as juveniles [17]. Here, we show that young female Svalbard reindeer mitigated the negative long-lasting effects of ROSutero through a change in reproductive tactic. Thus, females that experienced poor conditions in utero were able to maintain the same reproductive success through their first 6 years of life as phenotypically superior females who experienced good conditions in utero. The negative impact of ROSutero on reproductive success appeared only from 7 years of age. This is an important point with respect to fitness consequences of early-life conditions because in a growing population, as is the case here [45], offspring produced early in life contribute more to fitness than do offspring produced late in life [59]. Hence, by using Leslie matrix models, we found that asymptotic growth rate, a measure of mean fitness, was comparable between individuals exposed to contrasting ROSutero. A different conclusion would probably have been reached with a measure of lifetime reproductive success, which neglects timing of reproduction within the life cycle. As a cautionary note, however, we entered the same estimate of calf winter survival for both groups in matrix models, whereas we do not know whether this parameter varied with ROSutero. Unfortunately, information on calf winter survival is not available because calves are marked for the first time at ca 10 months of age.
How might the delayed effects of ROSutero on reproductive success that we report arise? First, this seems to correspond to a direct effect of ROSutero rather than differential costs of early reproduction between individuals born under contrasting ecological conditions. Indeed, we report a lack of interaction between early-life reproduction and ROSutero on late-life reproductive success. Instead, high early-life reproductive output was positively associated with subsequent age-specific reproductive success. In red deer, the effects of early-life reproduction and population density in year of birth on reproductive senescence rates were also independent of one another [24]. However, contrary to Svalbard reindeer, female red deer that produced more offspring during early adulthood showed higher rates of reproductive senescence [24,60] as predicted by the disposable soma theory ([32], see [33] for a review). Tests for trade-offs between allocation to early reproduction and late-life performance can yield positive rather than negative correlations (e.g. [61,62]), because some individuals consistently acquire a large amount of resources such that they are able to allocate much energy to several functions without suffering from any costs across their lifespan [63]. An explanation for direct effects of ROSutero on late-life reproductive success is that under-nutrition during gestation affects gene expression associated with changes in the physiology and metabolism of the offspring. The effects of epigenetic modifications in utero on performance may not manifest until later in life [64]. We cannot, however, exclude the possibility that the physiological changes occur during post-natal development as females in poor condition can allocate less energy to offspring through lactation after experiencing high ROS the preceding winter. Irrespective of the precise mechanism, our study provides evidence of intergenerational climate impacts on reproduction in a wild population.
There are a few studies conducted in the laboratory, which support the internal PAR hypothesis [15]. For instance in the rat, maternal caloric restriction during pregnancy led to early pubertal onset of offspring [65]. We cannot measure the influence of ROSutero on age at first reproduction of female reindeer because this life-history trait is unknown for most of them. Rather, we investigated how the mass-specific probability of pregnancy for young females varies with environmental conditions in early life. Thus, we take into account biological constraints imposed by body mass on pregnancy rate. The relationship between maturity and size is often considered for studying the potential evolutionary consequences of fishing. For example, before Canadian populations of Atlantic cod (Gadus morhua) collapsed in the 1990s, young females showed a decline over time in size at which the probability of maturing was 50%, supporting the idea that fishing may select for and cause evolution of maturity at smaller size [66]. In red deer, the threshold mass required for females to conceive was higher in high-density populations than in low-density populations, suggesting a conservative strategy that minimizes mortality risks [67]. Our results show that substantial differences can exist among individuals in a population. Young females (2–6 years old) born after high ROSutero achieve a 50% probability of pregnancy at about 2.5 kg lower body mass than females born after low ROSutero. Young females that experienced poor conditions in early life engage in reproduction at lower body mass possibly in anticipation of their reduced reproductive performance in later life.
Climate change is particularly pronounced in the high Arctic and ROS events are predicted to become increasingly frequent [36,68]. These extreme weather events have major ecosystem wide implications as they synchronize population fluctuations across the entire community of terrestrial species on Svalbard, including Svalbard reindeer [69]. ROS events have immediate negative effects on body mass and reproductive success of adult female reindeer [42,45], as well as on survival of older females (this study). However, asymptotic growth rate of cohorts born after high ROSutero was comparable to that of cohorts born after low ROSutero, suggesting that the long-term delayed effects of ROSutero on reproductive success had no major demographic consequences. The reproductive tactics of females born under poor environmental conditions have the potential to limit some of the negative effects of climate change. To date, most studies of climate change impacts have focused on plasticity in adulthood such as the date of egg laying [70] or hibernation emergence [71]. Our findings highlight the need to consider the role of developmental plasticity in the ability of wild populations to track, buffer and adapt to environmental change.
Supplementary Material
Acknowledgements
We thank the Governor of Svalbard for permission to undertake the research, R. Langvatn and O. Halvorsen who helped set up the project, the many field assistants involved and the UNIS Logistics Department. We thank J.-M. Gaillard, S. Dobson and three anonymous reviewers for constructive comments on the manuscript.
Ethics
All capture and live animal handling procedures were performed under licences from the Norwegian Food Inspection Authority and its predecessor the Norwegian National Research Authority.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fp505 [72].
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the Norwegian Research Council (project number 216051), UK Natural Environment Research Council (GR3/1083) and the Macaulay Development Trust. M.D. was supported by PhD scholarships from the French Ministry of Higher Education and Research and enjoyed a postdoctoral fellowship at the University of Sherbrooke during the revision stage.
References
- 1.Lucas A. 1991. Programming by early nutrition in man. In The childhood environment and adult disease (eds Bock GR, Whelan J), pp. 38–55. Chichester, UK: Wiley. [Google Scholar]
- 2.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12 (Suppl 1), 1–14. ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later. Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 4.Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C. 2000. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 31, 367–393. ( 10.1146/annurev.ecolsys.31.1.367) [DOI] [Google Scholar]
- 5.McMillen IC, Robinson JS. 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633. ( 10.1152/physrev.00053.2003) [DOI] [PubMed] [Google Scholar]
- 6.Albon SD, Clutton-Brock TH, Guinness FE. 1987. Early development and population dynamics in red deer. II. Density-independent effects and cohort variation. J. Anim. Ecol. 56, 69–81. ( 10.2307/4800) [DOI] [Google Scholar]
- 7.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 8.Lummaa V, Clutton-Brock TH. 2002. Early development, survival and reproduction in humans. Trends Ecol. Evol. 17, 141–147. ( 10.1016/S0169-5347(01)02414-4) [DOI] [Google Scholar]
- 9.Gaillard J-M, Loison A, Toïgo C, Delorme D, Van Laere G. 2003. Cohort effects and deer population dynamics. Ecoscience 10, 412–420. [Google Scholar]
- 10.Albon SD, Clutton-Brock TH, Langvatn R. 1992. Cohort variation in reproduction and survival: implications for population demography. In The biology of deer (ed. Brown RD.), pp. 15–21. New-York, NY: Springer. [Google Scholar]
- 11.Lindström J, Kokko H. 2002. Cohort effects and population dynamics. Ecol. Lett. 5, 338–344. ( 10.1046/j.1461-0248.2002.00317.x) [DOI] [Google Scholar]
- 12.Grafen A. 1988. On the uses of data on lifetime reproductive success. In Reproductive success: studies of individual variation in contrasting breeding systems, (ed. Clutton-Brock TH.), pp. 454–471. Chicago, IL: University of Chicago Press. [Google Scholar]
- 13.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bateson P, et al. 2004. Developmental plasticity and human health. Nature 430, 419–421. ( 10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 15.Nettle D, Frankenhuis WE, Rickard IJ. 2013. The evolution of predictive adaptive responses in human life history. Proc. R. Soc. B 280, 20131343 ( 10.1098/rspb.2013.1343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217. ( 10.1126/science.1235765) [DOI] [PubMed] [Google Scholar]
- 17.Auer SK. 2010. Phenotypic plasticity in adult life-history strategies compensates for a poor start in life in Trinidadian guppies (Poecilia reticulata). Am. Nat. 176, 818–829. ( 10.1086/657061) [DOI] [PubMed] [Google Scholar]
- 18.Gluckman PD, Hanson MA, Spencer H. 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533. ( 10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 19.Wells JCK. 2006. Is early development in humans a predictive adaptive response anticipating the adult environment? Trends Ecol. Evol. 21, 424–425. ( 10.1016/j.tree.2006.05.006) [DOI] [PubMed] [Google Scholar]
- 20.Kuzawa CW. 2005. Fetal origins of developmental plasticity: are foetal cues reliable predictors of future nutritional environments? Am. J. Hum. Biol. 17, 5–21. ( 10.1002/ajhb.20091) [DOI] [PubMed] [Google Scholar]
- 21.Douhard M, Plard F, Gaillard J-M, Capron G, Delorme D, Klein F, Duncan P, Loe LE, Bonenfant C. 2014. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. R. Soc. B 281, 20140276 ( 10.1098/rspb.2014.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lea AJ, Altmann J, Alberts SC, Tung J. 2015. Developmental constraints in a wild primate. Am. Nat. 185, 809–821. ( 10.1086/681016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward AD, Rickard IJ, Lummaa V. 2013. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc. Natl Acad. Sci. USA 110, 13 886–13 891. ( 10.1073/pnas.1301817110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussey DH, Kruuk LE, Morris A, Clutton-Brock TH. 2007. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 17, R1000–R1001. ( 10.1016/j.cub.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 25.Descamps S, Bountin S, Berteaux D, McAdam AG, Gaillard J-M. 2008. Cohort effects in red squirrels: the influence of density, food abundance and temperature on future survival and reproductive success. J. Anim. Ecol. 77, 305–314. ( 10.1111/j.1365-2656.2007.01340.x) [DOI] [PubMed] [Google Scholar]
- 26.Reed TE, Kruuk LEB, Wanless S, Frederiksen M, Cunningham EJA, Harris MP. 2008. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance. Am. Nat. 171, E89–E101. ( 10.1086/524957) [DOI] [PubMed] [Google Scholar]
- 27.Millon A, Petty SJ, Little B, Lambin X. 2011. Natal conditions alter age-specific reproduction but not survival or senescence in a long-lived bird of prey. J. Anim. Ecol. 80, 968–975. ( 10.1111/j.1365-2656.2011.01842.x) [DOI] [PubMed] [Google Scholar]
- 28.Cartwright SJ, Nicoll MAC, Jones CG, Tatayah V, Norris K. 2014. Anthropogenic natal environmental effects on life histories in a wild bird population. Curr. Biol. 24, 536–540. ( 10.1016/j.cub.2014.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balbontín J, Møller AP. 2015. Environmental conditions during early life accelerate the rate of senescence in a short-lived passerine bird. Ecology 96, 948–959. ( 10.1890/14-1274.1) [DOI] [PubMed] [Google Scholar]
- 30.Mumby HS, Mar KU, Hayward AD, Htut W, Htut-Aung Y, Lummaa V. 2015. Elephants born in the high stress season have faster reproductive ageing. Sci. Rep. 5, 13946 ( 10.1038/srep13946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monaghan P, Charmantier A, Nussey DH, Ricklefs RE. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. ( 10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 32.Kirkwood TB, Rose MR. 1991. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. Lond. B 332, 15–24. ( 10.1098/rstb.1991.0028) [DOI] [PubMed] [Google Scholar]
- 33.Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard J-M. 2015. Early-late trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamel S, Côté SD, Gaillard J-M, Festa-Bianchet M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151. ( 10.1111/j.1365-2656.2008.01459.x) [DOI] [PubMed] [Google Scholar]
- 35.Solberg EJ, Jordhøy P, Strand O, Aanes R, Loison A, Sæther BE, Linnell JDC. 2001. Effects of density-dependence and climate on the dynamics of a Svalbard reindeer population. Ecography 24, 441–451. ( 10.1034/j.1600-0587.2001.d01-200.x) [DOI] [Google Scholar]
- 36.Rennert KJ, Roe G, Putkonen J, Bitz CM. 2009. Soil thermal and ecological impacts of rain on snow events in the circumpolar arctic. J. Clim. 22, 2303–2315. ( 10.1175/2008JCLI2117.1) [DOI] [Google Scholar]
- 37.Hansen BB, Aanes R, Sæther B-E. 2010. Feeding-crater selection by high-Arctic reindeer facing ice-blocked pastures. Can. J. Zool. 88, 170–177. ( 10.1139/Z09-130) [DOI] [Google Scholar]
- 38.Bonenfant C, et al. 2009. Empirical evidences of density-dependence in populations of large herbivores. Adv. Ecol. Res. 41, 313–357. ( 10.1016/S0065-2504(09)00405-X) [DOI] [Google Scholar]
- 39.Tyler NJC, Øritsland NA. 1989. Why don't Svalbard reindeer migrate? Holarctic Ecology 12, 369–376. ( 10.1111/j.1600-0587.1989.tb00911.x) [DOI] [Google Scholar]
- 40.Reimers E. 1983. Mortality in Svalbard reindeer. Ecography 6, 141–149. ( 10.1111/j.1600-0587.1983.tb01075.x) [DOI] [Google Scholar]
- 41.Skogland T. 1989. Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv. Ethol. 29, 1–74. [Google Scholar]
- 42.Stien A, et al. 2012. Congruent responses to weather variability in high Arctic herbivores. Biol. Lett. 8, 1002–1005. ( 10.1098/rsbl.2012.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen BB, Aanes R, Herfindal I, Kohler J, Sæther BE. 2011. Climate, icing, and wild arctic reindeer: past relationships and future prospects. Ecology 92, 1917–1923. ( 10.1890/11-0095.1) [DOI] [PubMed] [Google Scholar]
- 44.Milner JM, Stien A, Irvine RJ, Albon SD, Langvatn R, Ropstad E. 2003. Body condition in Svalbard reindeer and the use of blood parameters as indicators of condition and fitness. Can. J. Zool. 81, 1566–1578. ( 10.1139/z03-152) [DOI] [Google Scholar]
- 45.Albon SD, et al. In press. Contrasting effects of summer and winter warming on body mass explain population dynamics in a food-limited Arctic herbivore. Glob. Change Biol. ( 10.1111/gcb.13435) [DOI] [PubMed] [Google Scholar]
- 46.Reimers E, Norby Ø. 1968. Relationship between age and tooth cementum in Norwegian reindeer. J. Wild. Manage. 32, 957–961. ( 10.2307/3799574) [DOI] [Google Scholar]
- 47.Lee AM, et al. 2015. An integrated population model for a long-lived ungulate: more efficient data use with Bayesian methods. Oikos 124, 806–816. ( 10.1111/oik.01924) [DOI] [Google Scholar]
- 48.Ropstad E, Johansen O, King C, Dahl E, Albon SD, Langvatn RL, Irvine RJ, Halvorsen O, Sasser G. 1999. Comparison of plasma progesterone, transrectal ultrasound and pregnancy specific proteins (PSPB) used for pregnancy diagnosis in reindeer. Acta Vet. Scand. 40, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albon SD, Stien A, Irvine RJ, Langvatn R, Ropstad E, Halvorsen O. 2002. The role of parasites in the dynamics of a reindeer population. Proc. R. Soc. Lond. B 269, 1625–1632. ( 10.1098/rspb.2002.2064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 51.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 52.Van De Pol M, Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 53.Lebreton JD, Burnham KP, Clobert J, Anderson DR. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118. ( 10.2307/2937171) [DOI] [Google Scholar]
- 54.Choquet R, Rouan L, Pradel R. 2009. Program ESURGE: a software application for fitting multievent models. In Modeling demographic processes in marked populations (eds Thomson DL, Cooch EG, Conroy MJ), pp. 845–865. New York, NY: Springer. [Google Scholar]
- 55.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 56.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 57.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865–2873. ( 10.1002/sim.3107) [DOI] [PubMed] [Google Scholar]
- 58.Van de Pol M, Bruinzeel LW, Heg D, Van der Jeugd HP, Verhulst S. 2006. A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). J. Anim. Ecol. 75, 616–626. ( 10.1111/j.1365-2656.2006.01079.x) [DOI] [PubMed] [Google Scholar]
- 59.Brommer JE, Merilä J, Kokko H. 2002. Reproductive timing and individual fitness. Ecol. Lett. 5, 802–810. ( 10.1046/j.1461-0248.2002.00369.x) [DOI] [Google Scholar]
- 60.Nussey DH, Kruuk LE, Donald A, Fowlie M, Clutton-Brock TH. 2006. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 9, 1342–1350. ( 10.1111/j.1461-0248.2006.00989.x) [DOI] [PubMed] [Google Scholar]
- 61.Bérubé CH, Festa-Bianchet M, Jorgenson JT. 1999. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecology 80, 2555–2565. ( 10.2307/177240) [DOI] [Google Scholar]
- 62.Hayward AD, Mar KU, Lahdenperä M, Lummaa V. 2014. Early reproductive investment, senescence and lifetime reproductive success in female Asian elephants. J. Evol. Biol. 27, 772–783. ( 10.1111/jeb.12350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reznick D, Nunney L, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 64.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2009. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73. ( 10.1056/NEJMra0708473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. 2009. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS ONE 4, e6744 ( 10.1371/journal.pone.0006744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. 2004. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935. ( 10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- 67.Albon SD, Mitchell B, Staines BW. 1983. Fertility and body weight in female red deer: a density-dependent relationship. J. Anim. Ecol. 52, 969–980. ( 10.2307/4467) [DOI] [Google Scholar]
- 68.Hansen BB, Isaksen K, Benestad RE, Kohler J, Pedersen ÅØ, Loe LE, Coulson SJ, Larsen JO, Varpe Ø. 2014. Warmer and wetter winters: characteristics and implications of an extreme weather event in the high Arctic. Environ. Res. Lett. 9, 114021 ( 10.1088/1748-9326/9/11/114021) [DOI] [Google Scholar]
- 69.Hansen BB, Grøtan V, Aanes R, Sæther BE, Stien A, Fuglei E, Ims RA, Yoccoz NG, Pedersen ÅØ. 2013. Climate events synchronize the dynamics of a resident vertebrate community in the high Arctic. Science 339, 313–315. ( 10.1126/science.1226766) [DOI] [PubMed] [Google Scholar]
- 70.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 71.Lane JE, Kruuk LEB, Charmantier A, Murie JO, Dobson FS. 2012. Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489, 554–557. ( 10.1038/nature11335) [DOI] [PubMed] [Google Scholar]
- 72.Douhard M, Loe LE, Stien A, Bonenfant C, Irvine RJ, Veiberg V, Ropstad E, Albon S. 2016. Data from: The influence of weather conditions during gestation on life histories in a wild Arctic ungulate. Dryad Digital Repository. ( 10.5061/dryad.fp505) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fp505 [72].


