Abstract
Understanding the processes that promote signal reliability may provide important insights into the evolution of diverse signalling strategies among species. The signals that animals use to communicate must comprise mechanisms that prohibit or punish dishonesty, and social costs of dishonesty have been demonstrated for several fixed morphological signals (e.g. colour badges of birds and wasps). The costs maintaining the honesty of dynamic signals, which are more flexible and potentially cheatable, are unknown. Using an experimental manipulation of the dynamic visual signals used by male veiled chameleons (Chamaeleo calyptratus) during aggressive interactions, we tested the idea that the honesty of rapid colour change signals is maintained by social costs. Our results reveal that social costs are an important mechanism maintaining the honesty of these dynamic colour signals—‘dishonest’ chameleons whose experimentally manipulated coloration was incongruent with their contest behaviour received more physical aggression than ‘honest’ individuals. This is the first demonstration, to the best our knowledge, that the honesty of a dynamic signal of motivation—physiological colour change—can be maintained by the social costliness of dishonesty. Behavioural responses of signal receivers, irrespective of any specific detection mechanisms, therefore prevent chameleon cheaters from prospering.
Keywords: signalling, communication, colour, conventional signals, chameleons, physiological colour change
1. Introduction
Animal signals are wildly diverse, yet all signals must contain reliable information to remain evolutionarily stable [1–6]. When the interests of signallers and receivers are aligned, as in the case of related individuals, minimal enforcement mechanisms are required to ensure signal honesty [7]. However, when animals with different interests rely on signals to mediate social interactions the costs and constraints of signal production, maintenance, or display are required to preserve signal reliability [2,5,8]. Although the specific costs vary with different classes of signals, costs should generally prevent low-quality individuals from dishonestly signalling high-quality or ‘bluffing’ [2,9].
Conventional signals are commonly used by animals to minimize the costs associated with competition over limited resources. Unlike performance or indicator signals [10,11], conventional signals usually have low production constraints/costs and are arbitrarily linked to the signalled quality [12–14]. The absence of a direct cost limiting the production of conventional signals would leave them open to invasion by dishonest ‘cheaters’, unless there was some other means of ensuring signal honesty [15,16]. A key hypothesis regarding the function, evolution and maintenance of conventional signals is that the costs that keep these signals honest are receiver-dependent, whereby conspecifics impose significant costs on dishonest individuals [17]. Although several lines of evidence suggest that differential aggression directed towards dishonest individuals can provide the means by which signals with low production costs can be kept honest [17–22], social costs of dishonesty have not been demonstrated for any dynamic signal. Interestingly, one study investigating receiver responses to dishonest songs found that territorial banded wrens responded more quickly to ‘dishonest’ playback vocalizations [19], but this study did not quantify differential costs to honest versus dishonestly signalling animals.
When signal production is well-understood, concrete predictions can be made regarding the processes maintaining signal honesty [4–6,14]. Some signals, however, are sufficiently complex that identifying the costs maintaining their honesty a priori is not possible. Dynamic colour changes represent one such class of signals, where the flexibility of rapid colour change allows individuals to display different colour signals under different conditions [23–25]. On the one hand, this plasticity suggests that colour change signals have low production costs (sensu behavioural displays [26–29]), whereas the pigmentary and structural basis of display colours [30–32] suggests concrete physiological production costs [33,34]. This dual nature of physiological colour change makes it difficult to predict whether the honesty and reliability of these signals are maintained by production or social costs.
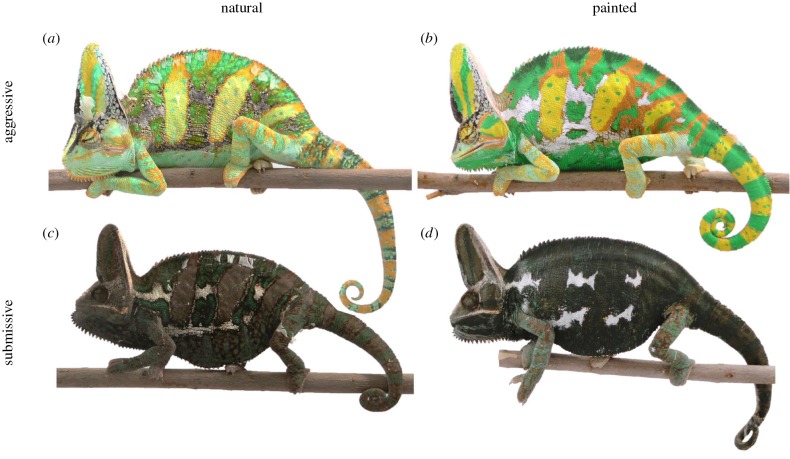
Here, we test the hypothesis that punishment costs are an important mechanism maintaining the honesty of the dynamic colour change signals used by chameleons to mediate social interactions. Widely known for cryptic colour changes [35], many species of chameleon (Squamata: Chameleonidae) exhibit dramatic chromatic shifts during conspecific displays [36], and comparative evidence suggests that selection for conspicuous signals has driven the evolution of display coloration in some chameleons [37,38]. In this study, we experimentally uncoupled chameleon colour expression from the typically associated behavioural displays by painting individuals to mimic the two ends of the aggression–submission colour spectrum used by veiled chameleons during agonistic displays (figure 1). In this species, bright coloration serves as a signal of aggression and fighting ability [39,40], whereas dark coloration serves as a signal of submission [41]. Because individuals who under-report or exaggerate (‘Trojan-chameleons’ [15] and ‘bluffers’ [42], respectively) their ability are expected to experience greater social costs during competitive interactions than honestly signalling individuals [1,17], we predicted that ‘dishonest’ (bright–submissive and dark–aggressive) chameleons would receive more aggression than ‘honest’ (bright–aggressive and dark–submissive) chameleons.
Figure 1.
Experimental manipulation used to mimic natural colour signals. Individual-specific application of custom paints allowed us to manipulate the chromatic signals of focal chameleons during agonistic interactions replicating (a) naturally bright, aggressive coloration (cf. b) and (c) naturally dark, submissive coloration (cf. d). (Online version in colour.)
2. Methods
(a). Study species and husbandry
Veiled chameleons are territorial, arboreal lizards native to southwestern Arabia [36]. Veiled chameleons use rapid colour changes to communicate during intraspecific interactions [36,39,41,43], and males typically display aggressive behaviours towards one another when they come into contact. In addition to behavioural and morphological changes, aggression is conveyed by rapid brightening [39] and submission is conveyed by rapid darkening during male–male contests [41].
Our chameleons, obtained from feral populations and a private breeder in Florida, USA, were housed individually in opaque-walled cages containing a variety of perches and climbing substrates. All cages were located in a temperature-controlled vivarium at Arizona State University, and each cage was equipped with a UV light source and heat lamp. Additional housing and husbandry details are detailed elsewhere [44].
(b). Aggression trials
To test the hypothesis that social costs are involved in maintaining the honesty of dynamic colour signals used by veiled chameleons, we staged a series of aggressive, dyadic encounters, using 36 adult male veiled chameleons. Every dyadic interaction was unique—chameleons had never previously interacted with opponents. In each trial, one chameleon was painted (see section Chameleon colour manipulation) to appear either (i) brightly coloured (aggressive) or (ii) darkly coloured (submissive) and one chameleon was unmanipulated. Unmanipulated chameleons were not painted to increase the likelihood that painted chameleons would exhibit natural behavioural responses to their opponent. Each painted chameleon participated in two encounters as the experimentally manipulated participant, one in which he was painted bright to appear aggressive and one in which he was painted dark to appear submissive. Trials in which a given chameleon participated as the painted individual were separated by 2–8 days (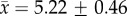 ; mean ± s.e.m.). The order of paint treatment was balanced such that half of the painted chameleons were painted bright first and half of the chameleons painted dark first. Each of the two fights in which a painted chameleon participated was against a novel, relatively size-matched, unpainted opponent. The average mass difference between painted and unpainted opponents, expressed as a percentage of painted chameleon body mass, was −3.78% (±3.54%). Average absolute difference in body mass between painted and unpainted chameleons was 19.11% (±2.42%).
; mean ± s.e.m.). The order of paint treatment was balanced such that half of the painted chameleons were painted bright first and half of the chameleons painted dark first. Each of the two fights in which a painted chameleon participated was against a novel, relatively size-matched, unpainted opponent. The average mass difference between painted and unpainted opponents, expressed as a percentage of painted chameleon body mass, was −3.78% (±3.54%). Average absolute difference in body mass between painted and unpainted chameleons was 19.11% (±2.42%).
Overall, we conducted 54 aggression trials using 27 painted chameleons. The 54 total contests were conducted in two rounds that were 2.5 months apart to allow time for chameleons painted in the first round to complete ecdysis and serve, if necessary, as unmanipulated chameleons in the second round. The first round consisted of 36 contests, in which 18 chameleons served as painted chameleons, and the second round consisted of 18 trials, in which nine previously unpainted chameleons served as painted chameleons (i.e. painted chameleons experienced both treatments within a single round).
Agonistic trials were conducted similarly to those previously conducted [39,41]. Briefly, we measured the body mass of each chameleon before placing them on opposite, visually isolated sides of the trial arena, where they were allowed to acclimate for 5 min before we removed a central divider and began the trial. Trials were recorded using Panasonic HDC-TM 700 video cameras (Osaka, Japan), which we also used to take still photographs of each chameleon throughout the trials (concurrent with video recording). Trials were conducted for 10 min or until the losing chameleon retreated from his opponent twice. Additionally, we stopped one trial, because chameleon combatants were in a precarious position that, if left unattended, may have increased the likelihood of injury.
(c). Chameleon colour manipulation
We used six colours of non-toxic acrylic paint (Golden Artist Colors Inc., New Berlin, NY) to mimic natural chameleon display coloration (electronic supplementary material, figure S1). Five of the colours we used were custom mixtures of paint designed to match naturally occurring colours, and one colour (white) was unmixed (i.e. straight from the bottle). Each paint mixture was measured with a UV–vis reflectance spectrometer (Ocean Optics, Dunedin, FL) and compared with a series of representative spectra taken from displaying chameleons, using chameleon-specific visual models. To compare the chromatic match between real and artificial colours, the spectral sensitivity of four classes of chameleon photoreceptors ([45]; electronic supplementary material, figure S2) was incorporated into receptor-noise-limited visual models [46] and discriminability was calculated in units of just notable differences (JNDs), taking into account lighting conditions of the trial arenas (electronic supplementary material, figure S3). Additionally, we calculated achromatic discriminability following Siddiqi et al. [47]. Based on the range of calculated perceptual distances, our custom paints were similar to chameleon coloration (JNDs between custom paints and chameleon colours ranged from 0.80 to 5.57 JNDs depending on the cone ratios modelled; electronic supplementary material, table S1). Achromatic contrasts between paint and chameleon colours ranged from 1.03 to 4.01 (electronic supplementary material, table S1).
To facilitate detailed paint application to the intricate stripes and patches of chameleon body colour, each to-be-painted chameleon was temporarily anaesthetized using inhaled isoflurane. Each paint was applied to the relevant body regions of a given chameleon in an attempt to manipulate only the coloration displayed while leaving individual-specific body patterning unchanged (figure 1). We applied enough paint to anaesthetized chameleons that the painted surfaces were opaque and not, therefore, influenced by changes in underlying skin colour. Additionally, we did not paint the legs (based on a subjective decision to maximize colour manipulation area and accuracy while minimizing the time each chameleon was anaesthetized) or the areas around the mouth, nostrils and eyes (to avoid mucous membranes). The entire painting process took 30–45 min per chameleon, and chameleons were returned to their visually isolated home cages following the procedure to prevent any social feedback based on their appearance prior to behavioural trials. Painted chameleons always had at least 24 h to recover from painting prior to participation in a contest.
(d). Behavioural quantification
Two trained observers, blind to experimental treatment (see electronic supplementary material), used a customized version of the open-source behaviour logging software CowLog [48] to record chameleon behaviours during aggressive interactions. Observers quantified numerous agonistic behaviours (electronic supplementary material, table S2) from each recording which allowed us to calculate interobserver repeatability [49] for 15 quantified behavioural metrics of competitiveness (electronic supplementary material, table S2). Repeatability of scored behaviours was high (mean = 0.85, median = 0.92), so we used averaged behaviour scores in all subsequent analyses.
For each trial, we calculated the total number of physical and non-physical aggressive behaviours (electronic supplementary material, table S2) the painted chameleon received (i.e. those exhibited by the unpainted chameleon). Non-physical aggressive behaviours were those behaviours shown by chameleons in aggressive contexts but which did not have the potential to inflict any direct costs. Physical aggressive behaviours were those with the direct potential to inflict a cost upon an opponent. We also used the behaviours exhibited by trial participants to qualify the ‘winners’ and ‘losers’ of each trial. Losing chameleons were those that retreated (exhibiting directed movement away from their opponent) at some point during the trial. In exactly half of the trials, we were able to assign a winner and loser, and all subsequent analyses were conducted on this subset of definitive trials (n = 27).
(e). Statistical analyses
To analyse how paint treatment, the aggressive behaviour of painted chameleons and their interaction influenced unpainted chameleon behaviour, we coded paint treatment as ‘bright’ or ‘dark’, and the behaviour of painted chameleons as ‘aggressive’ or ‘not aggressive’, based on whether or not the painted chameleon approached his opponent during the contest. The approach behaviour of chameleons provided a strong measure of his overall aggression (electronic supplementary material, table S3) providing support for the aggressive/non-aggressive categorization. This approach also differentiates between chameleons that initiated aggression as opposed to those simply involved in aggression as a consequence of opponent behaviour. We used generalized linear-mixed models (using the glmer command from the lme4 package [50] in R) to analyse the number of aggressive behaviours received (physical aggression and non-physical aggression analysed separately), including painted chameleon identity as a random effect. Following the detection of significant effects, we used the glht function in the lsmeans package [51] to test for differences in aggression received among different categories of painted chameleons, and we used the ‘r.squaredGLMM’ function in the MuMIn package [52] to estimate both marginal and conditional R2-values [53]. Marginal R2 (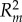 ) values represent variance explained by fixed factors, whereas conditional R2 (
) values represent variance explained by fixed factors, whereas conditional R2 (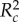 ) values provide information about the variance explained by the complete model (both fixed and random factors; [53]). All analyses were conducted in the R computing environment [54].
) values provide information about the variance explained by the complete model (both fixed and random factors; [53]). All analyses were conducted in the R computing environment [54].
3. Results
The physical aggression received by painted chameleons in our experiment indicates that there are significant social costs associated with dishonest signals of motivation/strategy in veiled chameleons. Specifically, the number of physically aggressive acts that painted chameleons received from opponents was strongly influenced by the interaction between their painted appearance and aggression level (table 1). When evaluating painted chameleons within discrete aggression (aggressive versus not aggressive) categories, we found that bright, non-aggressive chameleons (‘bluffers’) received more aggression than dark, non-aggressive chameleons (Tukey's post hoc comparison, p = 0.003) and that dark, aggressive chameleons (‘Trojans’) received more aggression than bright, aggressive chameleons (Tukey's post hoc comparison, p = 0.031; figure 2). Furthermore, as the mismatch between colour signal and contest behaviour increased, so too did the amount of physical aggression received (figure 3 and table 2). In contrast to the pattern observed for physical aggression, the number of non-physical aggressive acts (i.e. displays) received by painted chameleons was not influenced by the interaction between aggression category and experimentally manipulated colour (electronic supplementary material, table S4). Rather, chameleons that were not aggressive to their opponents received more non-physical aggressive displays, as did those painted to appear bright (electronic supplementary material, figure S4).
Table 1.
Physical aggression received by painted chameleons is greater when colour signals are dishonest. Physical aggression received by painted chameleons depends on the interaction between their external, experimentally manipulated colour and whether they were aggressive during the trial. Parameters calculated using GLMM with painted chameleon identity included as random effect and Poisson distribution. Estimate, slope estimate; s.e., standard error. Italicized values indicate significant effects.
| model | fixed effect | estimate | s.e. | z | p |
|---|---|---|---|---|---|
physical aggression received 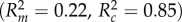
|
|||||
| intercept | 0.72 | 0.45 | 1.58 | 0.114 | |
| treatment (painted bright/dark) | 1.29 | 0.38 | 3.42 | <0.001 | |
| aggressive (yes/no) | 0.23 | 0.76 | −0.31 | 0.760 | |
| treatment × aggression | −2.18 | 0.51 | −4.31 | <0.0001 | |
Figure 2.
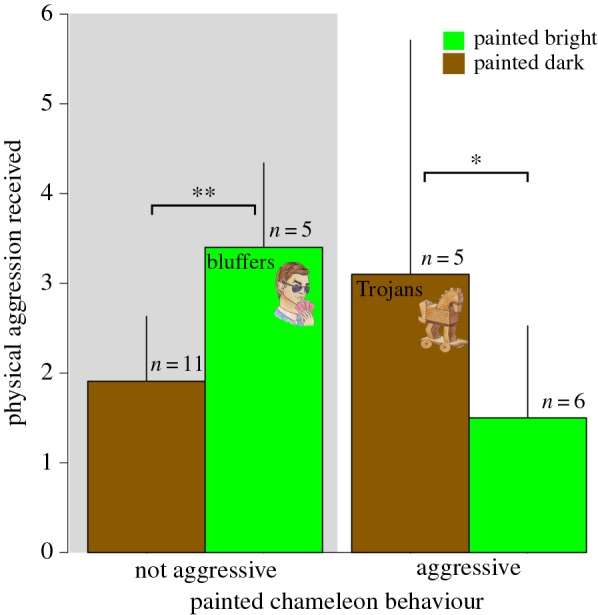
Chameleons exhibiting ‘dishonest’ colour signals (bright and non-aggressive = bluffer, dark and aggressive = Trojan) received more physical aggression than their honestly signalling counterparts (means ± s.e.m.). Asterisks indicate significant differences between groups identified with Tukey post hoc tests. (Online version in colour.)
Figure 3.
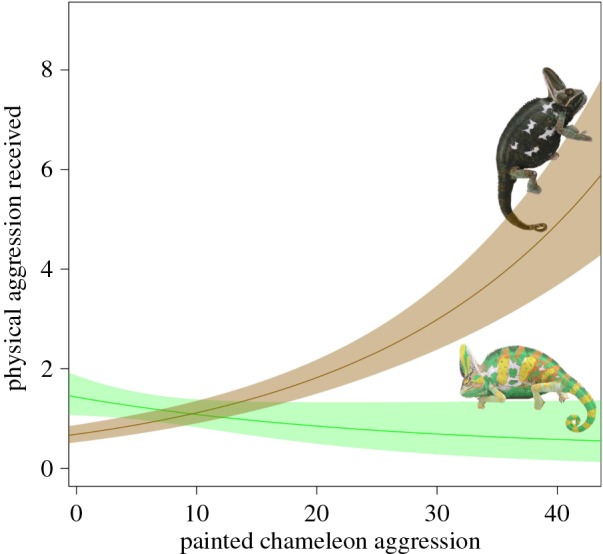
Costs of displaying dishonest colour signals of motivation increased with degree of dishonesty. Chameleons painted with bright, aggressive coloration (green line) that did not exhibit aggressive behaviours receive higher levels of physical aggression than those who displayed aggressively. Conversely, those chameleons painted with dark, submissive colours (brown line) that did not exhibit aggressive behaviours received low levels of aggression from their opponents but those who displayed aggressive behaviours while bearing these dark submissive colours received high levels of physical aggression. Solid lines represent maximum-likelihood predictions and shaded regions represent confidence intervals based on 100 000 random samples from the multivariate normal distribution of model coefficients.
Table 2.
Physical aggression received by painted chameleons increases with colour signal/behaviour mismatch. Physical aggression received by painted chameleons depends on the interaction between their external, experimentally manipulated colour and their overall level of aggression. Parameters calculated using GLMM with painted chameleon identity included as random effect and Poisson distribution. Estimate, slope estimate; s.e., standard error. Italicized values indicate significant effects.
| model | fixed effect | estimate | s.e. | z | p |
|---|---|---|---|---|---|
physical aggression received 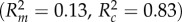
|
|||||
| intercept | 0.52 | 0.41 | 1.29 | 0.198 | |
| treatment (painted bright/dark) | 1.15 | 0.36 | 3.25 | 0.001 | |
| number of aggressive behaviours | 0.00 | 0.02 | 0.14 | 0.887 | |
| treatment × number of aggressive behaviours | −0.10 | 0.04 | −2.78 | 0.005 | |
The categorization of painted chameleons as ‘honest’ or ‘dishonest’ was possible only as a consequence of the interaction between their paint treatment and behaviour in a given trial. Consequently, it was necessary to determine if any individual differences in behaviour underlay the differentiation of chameleons into ‘honest’ or ‘dishonest’ categories, because such differences might also underlie the increased physical aggression directed towards dishonest chameleons. Because latency to aggression can be a good indicator of a chameleon's motivation and contest strategy [40] and is a behavioural output that is unlikely to be confounded by the downstream (i.e. later) contest dynamics, we investigated potential differences in latency to aggression among painted chameleons. Specifically, using a generalized least-squares model (which outperformed a mixed model including chameleon ID), we found that ‘honest’ and ‘dishonest’ painted chameleons did not differ in latency to aggression (t = −0.71, p = 0.49). Additionally, we examined the behaviour of a subset of chameleons that were each involved in two definitive trials (one as a bright painted individual, one as a dark painted individual). All seven chameleons were behaviourally consistent, exhibiting the same behavioural response (either aggressive or not aggressive) to both opponents. This pattern resulted in a perfect pairwise comparison, where we could also compare the aggression individual chameleons received when their behaviour matched their appearance (honest signals) and when their behaviours did not match their appearance (dishonest signals). In all seven cases, chameleons received more physical aggression when their colour did not match their behaviour (paired t-test, t6 = 3.27, p = 0.017). Collectively, these analyses provide further support for the idea that high levels of physical aggression were directed towards dishonest individuals as a consequence of the mismatch between signal and behaviour.
4. Discussion
Experimentally manipulated ‘dishonest’ chameleons received more physical aggression than their ‘honest’ counterparts, providing the first evidence that social costs ensure signal honesty of a dynamic colour signal of motivation. Interestingly, the non-physical aggression received by experimentally manipulated ‘dishonest’ chameleons was not significantly different from that received by ‘honest’ chameleons. This pattern suggests that the experimentally induced mismatch between colour and behaviour primarily influences the physical stages of aggressive interactions between adult male chameleons. Non-aggressive chameleons experience social costs associated with dishonesty, perhaps because they are treated as greater threats when exhibiting bright coloration, consequently receiving more aggression from motivated opponents. Aggressive chameleons, on the other hand, may experience increased social costs of dishonesty when their dark coloration prevents them from sufficiently intimidating rivals—these chameleons must therefore show (and receive) higher overall escalation and aggression to win agonistic encounters. The increased physical aggression received by dishonest chameleons, therefore, does not appear to require any recognition of dishonesty by signal receivers, instead arising as a consequence of the mismatch between aggressive behaviour and display colour.
Because signals need only be honest ‘on average’ to remain evolutionarily stable [55], bluffing can exist within signalling populations—either by a mixture of exclusively honest and exclusively deceptive individuals, or with individuals adopting different signalling strategies over time and context [56–58]. Across four different studies involving 41 chameleons and 100 definitive dyadic interactions, we found that chameleons exhibited ‘dishonest’ strategies in 53 of 200 instances (26.5%). Although every instance of a colour/behaviour mismatch is not necessarily indicative of a dishonest strategy (e.g. aggressively brightening chameleons might face opponents that submit without a need for further escalation), most chameleons exhibited such a mismatch at some point (28 of 41 chameleons = 68%). However, only five chameleons were predominantly ‘dishonest’ (two who employed the ‘Trojan-chameleon’ strategy in more than 50% of the trials in which they participated, three who ‘bluffed’ in more than 50% of their interactions). Hence, colour change signals for veiled chameleons appear to be honest ‘on average’, because most chameleons are honest most of the time, but the flexibility of signal appears to enable occasional dishonesty by a large portion of individuals in our experimental population. Investigating additional flexible signals (e.g. songs, behavioural postures and dynamic colour changes) in natural populations across time and context will provide important new insights into the conditions associated with when, why and how dishonest signalling naturally occurs (sensu [58,59]), as well as the costs and benefits of over- and under-signalling [60].
A number of investigations have explored the idea that social costs provide the mechanism ensuring signal honesty for conventional or low-cost signals (as first proposed by Rohwer [17]), and this phenomenon has perhaps been most convincingly demonstrated in Polistes dominulus paper wasps. Specifically, experimentally manipulated ‘dishonest’ individuals experience high social costs [20,21]. Interestingly, context-specific signal evaluation by receivers appears to facilitate the occasional ‘testing’ of the quality signals required to maintain signal honesty [61]. Importantly, however, signalling phenotypes do not vary contextually in this species (facial pattern is fixed at adulthood) and the added complexity that context-specific signalling strategies such as colour change, combined with context-specific receiver strategies, can have on contest dynamics is an important consideration that has recently been modelled [60]. A key finding of this work [60] is that the relative abundance of different communication strategies will have important consequences on the relative costs and benefits of each strategy. Furthermore, the error in a given signal influences the likely maintenance of alternative signalling strategies, in some cases favouring the existence of ‘bluffers’ and ‘modest’ signallers (the Trojan strategy). Experimental manipulation of individual quality and motivation will allow explicit tests of Botero et al.'s predictions and may provide key insights into the factors promoting the evolution of dynamic signaller and receiver strategies. Additionally, new investigations of the interaction between strategic signals of motivation (e.g. brightening versus not) and variable signal expression within brightening individuals (i.e. graded signals) may shed additional light on the patterns and processes regulating communicative complexity and further illuminate the costs and benefits of dynamic versus static signals used to mediate competitive interactions.
Supplementary Material
Acknowledgements
We thank Kristen McCartney, Sarah Bruemmer, Megan Best, Brianna Bero-Buell, Andrea Carpenter and Tierney Coates for assistance with chameleon husbandry, data collection and photographic analyses. We thank Dale DeNardo, Ron Rutowski, Rick Simpson, Brett Seymoure, Pierce Hutton and two anonymous reviewers for detailed comments and suggestions on earlier versions of this manuscript. We thank Ellis Loew and Jim Bowmaker for providing the raw data necessary to complete our visual models and Matthew Toomey for providing assistance in designing and implementing the chameleon visual models. We thank Sarah Bruemmer for the illustrations of ‘bluffing’ and ‘Trojan’ strategies and Conor Taff for providing plotting assistance for figure 3. We also thank David and Sandy Ligon for early financial support, and Veronica Ligon for all-around life support.
Ethics
This work was approved by the Arizona State University Institutional Animal Care and Use Committee, protocol no. 13-1308R.
Data accessibility
The datasets supporting this article have been uploaded as part of the supplementary material.
Authors' contributions
R.A.L. and K.J.M. conceived of the study. R.A.L. designed, coordinated, performed and analysed the experiments. R.A.L. wrote the paper with input from K.J.M.
Competing interests
We declare we have no competing interests.
Funding
We thank the American Society of Ichthyologists and Herpetologists, the Animal Behavior Society, the American Society of Naturalists and the Arizona State University Graduate and Professional Students Association for funding. R.A.L. was supported by the ASU Graduate College Completion Fellowship and NSF grants nos. 1401236 and 1523895 during the creation of this manuscript.
References
- 1.Enquist M. 1985. Communication during aggressive interactions with particular reference to variation in choice of behaviour. Anim. Behav. 33, 1152–1161. ( 10.1016/S0003-3472(85)80175-5) [DOI] [Google Scholar]
- 2.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RA. 1997. The evolution of animal signals. In Behavioural ecology (eds Krebs JR, Davies NB), pp. 155–178. Oxford, UK: Blackwell Science. [Google Scholar]
- 4.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, MA: Sinauer. [Google Scholar]
- 5.Maynard Smith J, Harper D. 2003. Animal signals. New York, NY: Oxford University Press. [Google Scholar]
- 6.Searcy WA, Nowicki S. 2005. The evolution of animal communication. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Searcy WA, Nowicki S. 2005. Signaling when interest overlap. In The evolution of animal communication, pp. 24–77. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 9.Maynard Smith J, Harper DG. 1988. The evolution of aggression: can selection generate variability? Phil. Trans. R. Soc. Lond. B 319, 557–570. ( 10.1098/rstb.1988.0065) [DOI] [PubMed] [Google Scholar]
- 10.Kodric-Brown A, Brown JH. 1984. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 124, 309–323. ( 10.1086/284275) [DOI] [Google Scholar]
- 11.Biernaskie JM, Grafen A, Perry JC. 2014. The evolution of index signals to avoid the cost of dishonesty. Proc. R. Soc. B 281, 20140876 ( 10.1098/rspb.2014.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilford T, Dawkins MS. 1995. What are conventional signals? Anim. Behav. 49, 1689–1695. ( 10.1016/0003-3472(95)90090-X) [DOI] [Google Scholar]
- 13.Senar JC. 1999. Plumage coloration as a signal of social status. Proc. Int. Ornithol. Congr. 22, 1669–1686. [Google Scholar]
- 14.Hurd PL, Enquist M. 2005. A strategic taxonomy of biological communication. Anim. Behav. 70, 1155–1170. ( 10.1016/j.anbehav.2005.02.014) [DOI] [Google Scholar]
- 15.Owens IPF, Hartley IR. 1991. ‘Trojan sparrows’: evolutionary consequences of dishonest invasion for the badges-of-status model. Am. Nat. 138, 1187–1205. ( 10.1086/285277) [DOI] [Google Scholar]
- 16.Johnstone RA, Norris K. 1993. Badges of status and the cost of aggression. Behav. Ecol. Sociobiol. 32, 127–134. ( 10.1007/BF00164045) [DOI] [Google Scholar]
- 17.Rohwer S. 1977. Status signaling in Harris sparrows: some experiments in deception. Behaviour 61, 107–129. ( 10.1163/156853977X00504) [DOI] [Google Scholar]
- 18.Caryl PG. 1982. Telling the truth about intentions. J. Theor. Biol. 97, 679–689. ( 10.1016/0022-5193(82)90366-6) [DOI] [Google Scholar]
- 19.Molles LE, Vehrencamp SL. 2001. Songbird cheaters pay a retaliation cost: evidence for auditory conventional signals. Proc. R. Soc. Lond. B 268, 2013–2019. ( 10.1098/rspb.2001.1757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218–222. ( 10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 21.Tibbetts EA, Izzo A. 2010. Social punishment of dishonest signalers caused by mismatch between signal and behavior. Curr. Biol. 20, 1637–1640. ( 10.1016/j.cub.2010.07.042) [DOI] [PubMed] [Google Scholar]
- 22.Dey CJ, Dale J, Quinn JS. 2014. Manipulating the appearance of a badge of status causes changes in true badge expression. Proc. R. Soc. B 281, 20132680 ( 10.1098/rspb.2013.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muske LE, Fernald RD. 1987. Control of a teleost social signal. I. Neural basis for differential expression of a color pattern. J. Comp. Physiol. A 160, 89–97. ( 10.1007/BF00613444) [DOI] [PubMed] [Google Scholar]
- 24.Summers CH, Greenberg N. 1994. Somatic correlates of adrenergic activity during aggression in the lizard, Anolis carolinensis. Horm. Behav. 28, 29–40. ( 10.1006/hbeh.1994.1003) [DOI] [PubMed] [Google Scholar]
- 25.O'Connor KI, Metcalfe NB, Taylor AC. 1999. Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar? Anim. Behav. 58, 1269–1276. ( 10.1006/anbe.1999.1260) [DOI] [PubMed] [Google Scholar]
- 26.Oberweger K, Goller F. 2001. The metabolic cost of birdsong production. J. Exp. Biol. 204, 3379–3388. [DOI] [PubMed] [Google Scholar]
- 27.Ward S, Lampe HM, Slater PJ. 2004. Singing is not energetically demanding for pied flycatchers, Ficedula hypoleuca. Behav. Ecol. 15, 477–484. ( 10.1093/beheco/arh038) [DOI] [Google Scholar]
- 28.Weiner SA, Woods WA, Starks PT. 2009. The energetic costs of stereotyped behavior in the paper wasp, Polistes dominulus. Naturwissenschaften 96, 297–302. ( 10.1007/s00114-008-0464-y) [DOI] [PubMed] [Google Scholar]
- 29.Matsumasa M, Murai M, Christy JH. 2013. A low-cost sexual ornament reliably signals male condition in the fiddler crab Uca beebei. Anim. Behav. 85, 1335–1341. ( 10.1016/j.anbehav.2013.03.024) [DOI] [Google Scholar]
- 30.Cooper WE, Greenberg N. 1992. Reptilian coloration and behavior. In Biology of the reptilia, vol. 18: hormones, brain, and behavior (ed. Gans C.), pp. 298–422. Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Teyssier J, Saenko SV, van der Marel D, Milinkovitch MC. 2015. Photonic crystals cause active colour change in chameleons. Nat. Commun. 6, 6368 ( 10.1038/ncomms7368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ligon RA, McCartney KL. 2016. Biochemical regulation of pigment motility in vertebrate chromatophores: a review of physiological color change mechanisms. Curr. Zool. 62, 237–252. ( 10.1093/cz/zow051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw KJ. 2006. Mechanics of carotenoid-based coloration. In Bird coloration: volume I, mechanisms and measurements (eds Hill GE, McGraw KJ), pp. 177–242. Cambridge, MA: Harvard University Press. [Google Scholar]
- 34.Kemp DJ. 2008. Resource-mediated condition dependence in sexually dichromatic butterfly wing coloration. Evolution 62, 2346–2358. ( 10.1111/j.1558-5646.2008.00461.x) [DOI] [PubMed] [Google Scholar]
- 35.Stuart-fox D, Whiting MJ, Moussalli A. 2006. Camouflage and colour change: antipredator responses to bird and snake predators across multiple populations in a dwarf chameleon. Biol. J. Linn. Soc. 88, 437–446. ( 10.1111/j.1095-8312.2006.00631.x) [DOI] [Google Scholar]
- 36.Nečas P. 1999. Chameleons: nature’s hidden jewels Frankfurt, Germany: Chimaira. [Google Scholar]
- 37.Stuart-Fox D, Moussalli A, Whiting MJ. 2007. Natural selection on social signals: signal efficacy and the evolution of chameleon display coloration. Am. Nat. 170, 916–930. ( 10.1086/522835) [DOI] [PubMed] [Google Scholar]
- 38.Stuart-Fox D, Moussalli A. 2008. Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 6, e25 ( 10.1371/journal.pbio.0060025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ligon RA, McGraw KJ. 2013. Chameleons communicate with complex colour changes during contests: different body regions convey different information. Biol. Lett. 9, 20130892 ( 10.1098/rsbl.2013.0892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ligon RA. 2015. Chameleon color change communicates conquest and capitulation. PhD thesis, Arizona State University. See http://repository.asu.edu/items/34900.
- 41.Ligon RA. 2014. Defeated chameleons darken dynamically during dyadic disputes to decrease danger from dominants. Behav. Ecol. Sociobiol. 68, 1007–1017. ( 10.1007/s00265-014-1713-z) [DOI] [Google Scholar]
- 42.Gardner R, Morris MR. 1989. The evolution of bluffing in animal contests: an ESS approach. J. Theor. Biol. 137, 235–243. ( 10.1016/S0022-5193(89)80209-7) [DOI] [Google Scholar]
- 43.Kelso EC, Verrell PA. 2002. Do male veiled chameleons, Chamaeleo calyptratus, adjust their courtship displays in response to female reproductive status? Ethology 512, 495–512. ( 10.1046/j.1439-0310.2002.00789.x) [DOI] [Google Scholar]
- 44.McCartney KL, Ligon RA, Butler MW, Denardo DF, McGraw KJ. 2014. The effect of carotenoid supplementation on immune system development in juvenile male veiled chameleons (Chamaeleo calyptratus). Front. Zool. 11, 26 ( 10.1186/1742-9994-11-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowmaker JK, Loew ER, Ott M. 2005. The cone photoreceptors and visual pigments of chameleons. J. Comp. Physiol. A 191, 925–932. ( 10.1007/s00359-005-0014-4) [DOI] [PubMed] [Google Scholar]
- 46.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485. ( 10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 48.Hänninen L, Pastell M. 2009. CowLog: open source software for coding behaviors from digital video. Behav. Res. Methods 41, 472–476. ( 10.3758/BRM.41.2.472) [DOI] [PubMed] [Google Scholar]
- 49.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121. ( 10.2307/4087240) [DOI] [Google Scholar]
- 50.Bates D, Machler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 51.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 52.Barton K.2013. MuMIn: multi-model inference. R Packag, version 1.9.13. See http://CRAN.R–project.org/package=MuMIn .
- 53.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 54.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R–project.org/. [Google Scholar]
- 55.Johnstone RA, Grafen A. 1993. Dishonesty and the handicap principle. Anim. Behav. 46, 759–764. ( 10.1006/anbe.1993.1253) [DOI] [Google Scholar]
- 56.Adams ES, Mesterton-Gibbons M. 1995. The cost of threat displays and the stability of deceptive communication. J. Theor. Biol. 175, 405–421. ( 10.1006/jtbi.1995.0151) [DOI] [Google Scholar]
- 57.Wilson RS, Angilletta MJ Jr. 2015. Dishonest signaling during aggressive interactions: theory and empirical evidence. In Animal signaling and function: an integrative approach (eds Irschick DJ, Briffa M, Podos J), pp. 205–228. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- 58.Akçay Ç, Campbell SE, Beecher MD. 2014. Individual differences affect honest signalling in a songbird. Proc. R. Soc. B 281, 20132496 ( 10.1098/rspb.2013.2496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bywater CL, Wilson RS. 2012. Is honesty the best policy? Testing signal reliability in fiddler crabs when receiver-dependent costs are high. Funct. Ecol. 26, 804–811. ( 10.1111/j.1365-2435.2012.02002.x) [DOI] [Google Scholar]
- 60.Botero CA, Pen I, Komdeur J, Weissing FJ. 2010. The evolution of individual variation in communication strategies. Evolution 64, 3123–3133. ( 10.1111/j.1558-5646.2010.01065.x) [DOI] [PubMed] [Google Scholar]
- 61.Tibbetts EA. 2013. The function, development, and evolutionary stability of conventional signals of fighting ability. Adv. Study Behav. 45, 49–80. ( 10.1016/B978-0-12-407186-5.00002-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the supplementary material.