Abstract
Glutathione peroxidase 4 (GPX4) and arachidonic acid 15-lipoxygenase (ALOX15) are antagonizing enzymes in the metabolism of hydroperoxy lipids. In spermatoid cells and/or in the male reproductive system both enzymes are apparently expressed, and GPX4 serves as anti-oxidative enzyme but also as a structural protein. In this study we explored whether germ line inactivation of the Alox15 gene might rescue male subfertility induced by heterozygous expression of catalytically silent Gpx4. To address this question we employed Gpx4 knock-in mice expressing the Sec46Ala-Gpx4 mutant, in which the catalytic selenocysteine was replaced by a redox inactive alanine. Because homozygous Gpx4 knock-in mice (Sec46Ala-Gpx4+/+) are not viable we created heterozygous animals (Sec46Ala-Gpx4+/−) and crossed them with Alox15 knock-out mice (Alox15−/−). Male Sec46Ala-Gpx4+/− mice, but not their female littermates, were subfertile. Sperm extracted from the epididymal cauda showed strongly impaired motility characteristics and severe structural midpiece alterations (swollen mitochondria, intramitochondrial vacuoles, disordered mitochondrial capsule). Despite these structural alterations, they exhibited similar respiration characteristics than wild-type sperm. When Sec46Ala-Gpx4+/− mice were crossed with Alox15-deficient animals, the resulting males (Sec46Ala-Gpx4+/−+Alox15−/−) showed normalized fertility, and sperm motility was reimproved to wild-type levels. Taken together these data suggest that systemic inactivation of the Alox15 gene normalizes the reduced fertility of male Sec46Ala-Gpx4+/− mice by improving the motility of their sperm. If these data can be confirmed in humans, ALOX15 inhibitors might counteract male infertility related to GPX4 deficiency.
Keywords: eicosanoid, lipoxygenase pathway, mitochondria, oxidative stress, sperm
Introduction
The redox equilibrium is an important parameter for the regulation of the cellular phenotype, and pro- and anti-oxidative enzymes have been implicated in maintaining the cellular redox homeostasis (1, 2). A disturbed redox equilibrium has been related to the pathogenesis of metabolic, cardiovascular, and neurodegenerative disorders (3–5) and may also play a role in hyperproliferative diseases (6). The redox homeostasis is balanced among other things by the catalytic activities of pro- and anti-oxidative enzymes. Alox15 (12/15-lipoxygenases) and Gpx4 (glutathione peroxidase 4) have previously been identified as important players in this regulatory network (7, 8). Alox15 is capable of oxygenating polyenoic fatty acids to the corresponding hydroperoxides, and thus, it up-regulates the cellular oxidative potential (9, 10). On the other hand, Gpx4 is the only glutathione peroxidase isoform capable of reducing complex hydroperoxy lipids (7), eliminating these peroxides as sources of free radical-induced secondary reactions. Thus, ALOX15 and GPX4 are functional counter players in metabolism of hydroperoxy lipids (11).
Lipoxygenases (LOX)2 are expressed in the testis of various mammals (12–14), but systemic inactivation of the genes encoding for Alox15, Alox12, and Alox5 does not induce major phenotypic alterations unless the animals were challenged otherwise (8, 15). However, more detailed studies on the reproduction of Alox15−/− mice indicated that breeding of these animals was hampered since male Alox15−/− mice turned out to be subfertile (16). Although the molecular basis for this subfertility has not been explored in detail, it was suggested that epididymal sperm maturation, in particular the proximal-distal migration of the cytoplasmic droplet, which contains remnants of the spermatogenic cytoplasma as well as Alox15 (17), was impaired in Alox15-deficient sperm (16).
Homozygous Gpx4-deficient animals die in utero before mouse embryonic day 7.5 (18, 19). Similarly, homozygous mice expressing catalytically inactive Gpx4 variants (Sec46Ala/Ser mutant) do not survive the seventh day of embryogenesis, and systemic Alox15 deficiency did not rescue the lethal phenotype (20). These data suggest that Gpx4 is essential for normal embryogenesis, but its detailed function remains a matter of discussion. Conditional neuron-specific knock-out of Gpx4 expression in adult mice induces neurodegeneration suggesting that embryonic lethality of Gpx4 deficiency might be related to defective brain development (22). This conclusion was consistent with the previous observation indicating that expression silencing of Gpx4 during in vitro mouse embryogenesis induces developmental defects in the central nervous system (23). More recent studies employing conditional knock-out mice, in which expression of the enzyme was selectively inactivated in erythroid precursors, suggest a role of Gpx4 during erythropoiesis (24). These data indicate that embryonic lethality of Gpx4 deficiency in mice might not only be related to developmental defects of the central nervous system. In humans GPX4 expression is reversibly induced by hepatitis C virus to control lipid peroxidation and to increase virion infectivity (25). Naturally occurring truncation mutants (splicing defects, premature stop codon) in the human GPX4 gene cause a rare hereditary neonatally lethal disorder called Sedaghatian spondylometaphyseal dysplasia (26). Patients suffering from this disease are characterized by severe metaphyseal chondrodysplasia with limb shortening, cardiac conduction defects, and central nervous system abnormalities (26). These data are consistent with embryonic lethality of Gpx4-deficient knock-out/knock-in mice (18–21).
In adult mammals, testis is the major organ of Gpx4 expression (27–29), and genetic variants of the human GPX4 gene have been related to male infertility (30). In this organ the Gpx4 protein exhibits a moonlighting character as it functions as glutathione peroxidase but also as a structural protein involved in the formation of the sperm specific mitochondrial capsule (31). Male spermatocyte-specific Gpx4 knock-out mice are infertile (32). Their testes display a decreased number of sperm, and isolated epididymal sperm were unable to fertilize oocytes in vitro. The sperm exhibited reduced forward motility and were characterized by structural abnormalities (32). In this model system expression of the Gpx4 protein was completely blocked, and thus, it was impossible to conclude whether the lack of catalytic activity or the absence of Gpx4 as the structural protein was the major reason for the deleterious effects. To address this question, two different strains of knock-in mice were recently created by independent research groups, which express a catalytically inactive Gpx4 variant (Sec46Ala (20) and Sec46Ser (21)), and male heterozygous allele carriers of the Sec46Ser animals were shown to be subfertile (21). Fertility of Sec46Ala males has not been tested, but similar problems were expected. The major reasons why we preferred the Sec-to-Ala strategy are the redox properties of the introduced amino acid. Because of its aliphatic OH group, there is the principle possibility that the Ser site chain might be oxidized by peroxide substrates to an aldehyde or even to a carboxylic acid. In contrast, the Ala side chain is redox-silent and may not be oxidized by peroxides.
Employing Sec46Ala-Gpx4+/− knock-in mice (20), we here confirmed subfertility of male mice, which are deficient in catalytically active Gpx4. Moreover, we found that systemic expression silencing of Alox15 rescued the subfertility of Sec46Ala-Gpx4+/− mice and reimproved the motility of their sperm. These changes were paralleled by an enhanced respiratory capacity, suggesting an up-regulated energy metabolism of these sperm.
Results
GPx4 and Alox15 Are Expressed in the Male Reproductive System
Gpx4 is high level-expressed in mammalian sperm (27, 31), but little is known on the expression of LOX-isoforms in the male reproductive system (12). More recent data indicated expression of Alox15 in the plasmatic droplet and suggested a role of this enzyme in sperm maturation (17). To confirm expression of both enzymes in the male reproductive system and to quantify their relative expression levels, we first carried out qRT-PCR with sperm RNA extracts prepared from the epididymal cauda and compared the relative copy numbers of their mRNAs in relation to somatic GAPDH mRNA (Table 1). As expected, mRNAs encoding for the cytosolic, the mitochondrial, and the nuclear Gpx4 isoforms were present in large abundance. In contrast, Alox15 mRNA was present in much lower quantities. Next, we purified epididymal caput sperm by Percoll gradient centrifugation and quantified Alox15 mRNA in these cells. Here we obtained 10.4 ± 0.8 copies of Alox15 mRNA per 103 copies of somatic GAPDH and 71 ± 5.1 copies of Alox15 mRNA per 103 copies of sperm-specific GAPDH. Because possible functional alterations of sperm might also be related Alox15 deficiency in other cells of the male reproductive system, we next explored Alox15 expression in testis and epididymal caput. qRT-PCR of testis indicated 3.3 ± 0.3 copies of Alox15 mRNA per 103 copies of somatic GAPDH. qRT-PCR of RNA extracts from the epididymal caput revealed 6.6 ± 1.1 copies of Alox15 mRNA per copies of 103 copies of somatic GAPDH. These data indicate the presence of Alox15 mRNA in both organs, but they also suggest that Alox15, in contrast to Gpx4, is not expressed at high levels. Thus, Alox15 constitutes a low copy gene in the cells of the male reproductive organs. Furthermore, we carried out immunoblotting of protein extracts prepared from purified sperm, testis, and epididymal caput but did not obtain specific Alox15 signals in the appropriate molecular weight range. Finally, we performed immunohistochemistry with our purified sperm preparation employing a homemade anti-Alox15 antibody. Evaluating a large number (>30) of slides, we observed specific Alox15 staining in a few sperm. In these Alox15-positive cells the signal was localized in a microscopic structure, which could be the plasma droplet. However, the majority of the cells were devoid of a specific Alox15 signal. Taken together, these data indicate that Alox15 mRNA is expressed at low levels in testis and epididymis but that the enzyme is only present at low quantities in mature sperm.
TABLE 1.
Expression of Gpx4 and Alox15 mRNA in cauda sperm of wild-type mice
Sperm were isolated from the epididymal cauda, and total RNA was extracted. Synthesis of cDNA and amplification of the mRNAs encoding for the Gpx4 isoforms and for Alox15 was carried out as described under “Materials and Methods.” mGpx4, mitochondrial Gpx4; cGpx4, cytosolic Gpx4; nGpx4, nuclear Gpx4.
| mRNA copy numbers/1000 GAPDH mRNA copies |
||||
|---|---|---|---|---|
| mGpx4 | cGpx4 | nGpx4 | Alox15 | |
| Mean ± S.D. | 4022 ± 874 | 7772 ± 1528 | 775 ± 8 | 2.0 ± 0.4 |
Male Sec46Ala-Gpx4+/− Knock-in Mice Are Subfertile, and Their Sperm Exhibit Impaired Motility Characteristics
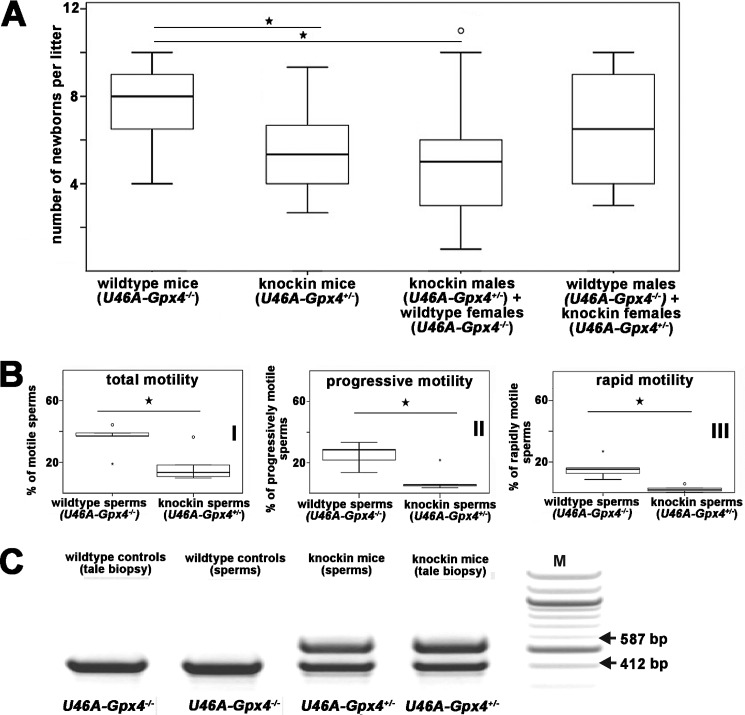
It has been reported before that mice that heterozygously express a catalytically inactive variant of Gpx4 (Sec46Ser-Gpx4+/− knock-in mice) are subfertile (21), and here we confirmed these data using a similar in vivo knock-in mouse model (20). When we intercrossed Sec46Ala-Gpx4+/− mice, we observed significantly lower litter sizes when compared with intercrossing wild-type controls. When we crossed male Sec46Ala-Gpx4+/− knock-in mice with wild-type females, we observed similar alterations (Fig. 1A). In contrast, similar litter sizes were found when female Sec46Ala-Gpx4+/− knock-in mice were crossed with male wild-type individuals. These data confirm that heterozygous deficiency of catalytically active Gpx4 impairs male fertility.
FIGURE 1.
Fertility of wild-type (Sec46Ala−/−) and heterozygous Gpx4 knock-in mice (Sec46Ala-Gpx4+/−) and motility of sperm extracted from the epididymal cauda. A, male and female individuals of different genotype were mated, and the sizes of the different litters (number of newborns) were quantified. Because of the early lethality of homozygous Gpx4 Sec46Ala knock-in embryos, the litter sizes (number of embryos) were corrected according to Mendelian genetics. Stars indicate significant (p < 0.05) differences between the experimental groups. Sec46Ala−/− × Sec46Ala−/−: 36 matings with 269 newborns; Sec46Ala+/− x Sec46Ala+/−: 9 matings with 35 newborns, 47 (Mendel's law); Sec46Ala−/− (males) × Sec46Ala+/− (females): 18 matings with 115 newborns, Sec46Ala+/− (males) × Sec46Ala−/− (females): 50 matings, 257 newborns. B, sperm extracted from the epididymal cauda were analyzed for their motility characteristics using a computer-assisted system (see “Materials and Methods”). According to their motility, sperm were classified into 3 groups: I, motile sperm; II, sperm with progressive movement (slow and rapid forward movement, circular movement); III, sperm with rapid forward movement. For each genotype, five mice were included in the motility analyses. C, sperm were extracted from the epididymal cauda of male Sec46Ala-Gpx4+/− mice and were genotyped by allele specific genomic PCR as described under “Materials and Methods.” U46A represents Sec46Ala exchange. M, mass markers. o, outlier.
Next we compared the motility characteristics of sperm extracted from the epididymal cauda of Sec46Ala-GPx4+/− and wild-type mice. For this purpose sperm were classified in motile and immotile sperm, and the motile sperm were further subclassified in sperm with total (any signs of movement) and progressive (rapid and slow forward movement, circular movement) as well as rapid forward movement. The shares of sperm with total, progressive, and rapid motility were significantly lower in Sec46Ala-Gpx4+/− mice when compared with wild-type animals (Fig. 1B).
Sperm of Sec46Ala-Gpx4+/− Mice Express Wild-type and Mutant Alleles in a 1:1 Ratio
Sperm are haploid cells that undergo genome reduction during meiosis. To explore whether sperm carrying the wild-type allele (functional Gpx4) or the mutant allele (dysfunctional Gpx4) are similarly treated during spermatogenesis, we genotyped the sperm isolated from the epididymal cauda of Sec46Ala-Gpx4+/− mice. As shown in Fig. 1C, we found a 1:1 ratio of the Sec46Ala-Gpx4+/− and the Sec46Ala-Gpx4−/− alleles. These data indicate that sperm carrying the inactive mutant allele are not removed during spermatogenesis.
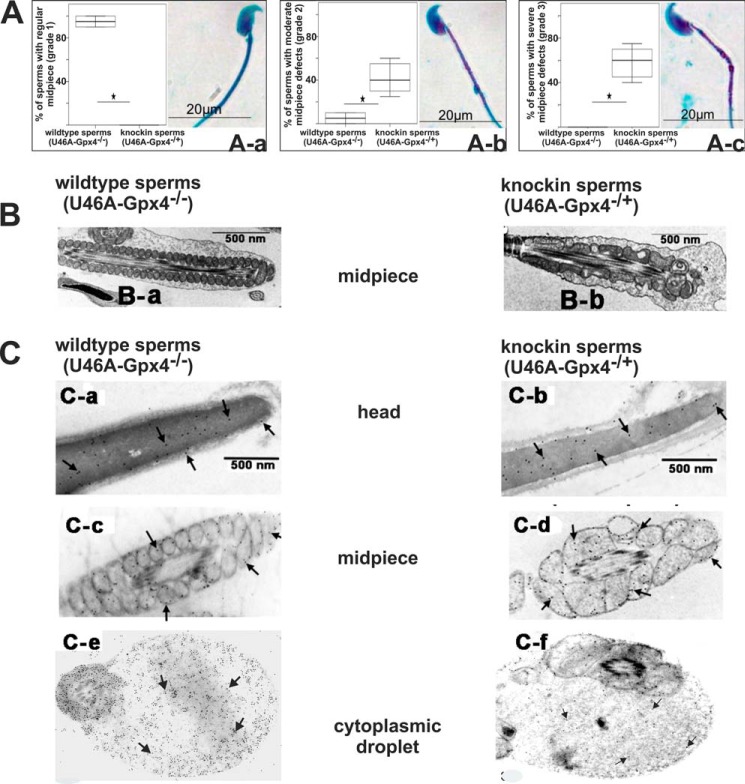
Deficiency of Catalytically Active Gpx4 Induces Structural Midpiece Alterations
To explore the basis for the impaired motility of Gpx4-deficient sperm, we quantified the structural alterations employing a microscopic scoring system that classifies all sperm in three categories: Grade 1 sperm (normal shape), no midpiece granulation and smooth plasma membranes; Grade 2 (minor alterations), moderate midpiece granulation and rough plasma membranes; Grade 3, severe midpiece granulation and discontinued plasma membrane. When we determined the relative share of grade 1 sperm (normal appearance) in wild-type controls, we found that >90% of the sperm exhibited a “normal” morphological phenotype (Fig. 2Aa). In contrast, in Sec46Ala-Gpx4+/− mice we did not detect grade 1 sperm at all. In fact, it was possible to conclude the genotype of the animals simply by looking at the morphological characteristics of the sperm. Grade 2 (Fig. 2Ab) and grade 3 (Fig. 2Ac) sperm were virtually absent in wild-type controls but together amounted to >90% in Sec46Ala-Gpx4+/− mice.
FIGURE 2.
Structural alterations induced by heterozygous knock-in of the catalytically inactive Gpx4-mutant Sec46Ala. A, sperm extracted from the epididymal cauda were stained according to the Spermac® method (see “Materials and Methods”) and classified into three different groups according to morphological appearance of their midpiece in light microscopy. a, Grade 1 sperm: no granulation in the midpiece (normal sperm); b, Grade 2 sperm: moderate granulation in the midpiece; c, Grade 3 sperm: severe granulation in the midpiece. B, electron microscopy of grade 1 (a) and grade 3 (b) sperm. Grade 1 sperm are characterized by condensed mitochondria and a lack of intramitochondrial vacuoles and regular mitochondrial capsules. Grade 3 sperm have swollen mitochondria with intramitochondrial vacuoles and disturbed structure of the mitochondrial capsule. C, immunogold staining of different parts of epididymal cauda sperm indicating the intracellular localization of Gpx4 in the head (a and b), the midpiece (c and d), and the cytoplasmic droplet (e and f). Immunopositive material is indicated by the small black dots. U46A represents Sec46Ala exchange.
Next, we explored the midpiece alterations by transmission electron microscopy (Fig. 2B). Here we found that the mitochondria of wild-type sperm are present as condensed organelles, which are well ordered within the mitochondrial capsule (Fig. 2Ba). In contrast, in Sec46Ala-Gpx4+/− sperm the mitochondria are swollen and contain intramitochondrial vacuoles, and the mitochondrial capsule was not well ordered (Fig. 2Bb). Similar alterations have been described for the sperm of spermatocyte-specific Gpx4-knock-out mice (32).
Previous reports have suggested that different Gpx4 isoforms (nuclear, mitochondrial, cytoplasmic) are expressed in different parts of sperm (33). To compare the subcellular distribution of Gpx4 in wild-type and Sec46Ala-Gpx4+/− mice, we carried out immunogold electron microscopy employing a monoclonal anti-Gpx4 antibody, which does not differentiate between the different isoforms (34). Here we found a similar distribution pattern of wild-type Gpx4 and its Sec46Ala-mutant in the head (Fig. 2C, a and b) and the midpiece of cauda sperm (Fig. 2C, c and d). Moreover, Gpx4 protein was also detected in large amounts in the plasma droplet of caput sperm (Fig. 2C, e and f). This is a remarkable finding because the cytoplasmic droplet is the major source of spermatoid Alox15 (16). Although immune electron microscopy is not a strongly quantitative method, staining intensity was not significantly different when we compared sperm of wild-type control animals (Sec46Ala-Gpx4−/−; Fig. 2, C, a, c, and e) with those of Sec46Ala-Gpx4+/− mice (Fig. 2, C, b, d, and f).
Homozygous Inactivation of the Alox15 Gene Rescues the Subfertile Phenotype of Heterozygous Sec46Ala-Gpx4 Knock-in Mice
Gpx4 and Alox15 are counter-players in the metabolism of hydroperoxy lipids, and experiments with LOX inhibitors revealed that functional defects induced by Gpx4 deficiency might be antagonized when Alox15 pathway is inhibited (22,35). To test whether systemic inactivation of the Alox15 gene is capable of rescuing the subfertile phenotype of male Sec46Ala-Gpx4+/− individuals, we crossed Sec46Ala-Gpx4+/− mice with Alox15−/− animals to obtain Sec46Ala-Gpx4+/−+Alox15−/− individuals. These mice do not express Alox15 any more, as indicated by previous activity assays (20).
Next, male and female Sec46Ala-Gpx4+/−+Alox15−/− mice were intercrossed (52 matings), and we quantified the percentage of successful mating. Here we found that under our mating conditions 25% of mating resulted in pregnancy (Fig. 3A). In contrast, we only observed 11% pregnancies when male and female Sec46Ala-Gpx4+/−+Alox15+/+ mice were mated (Fig. 3A). Next, we compared the litter sizes of the resulting pregnancies. As indicated in Fig. 3B the litter sizes of heterozygous Sec46Ala mice with wild-type alleles in the Alox15 locus were significantly lower than those obtained for mice with Alox15 deficiency on Sec46Ala-Gpx4+/− background. Taken together these data indicate that functional inactivation of the Alox15 gene appears to rescue the subfertility of male Sec46Ala-Gpx4+/− mice.
FIGURE 3.
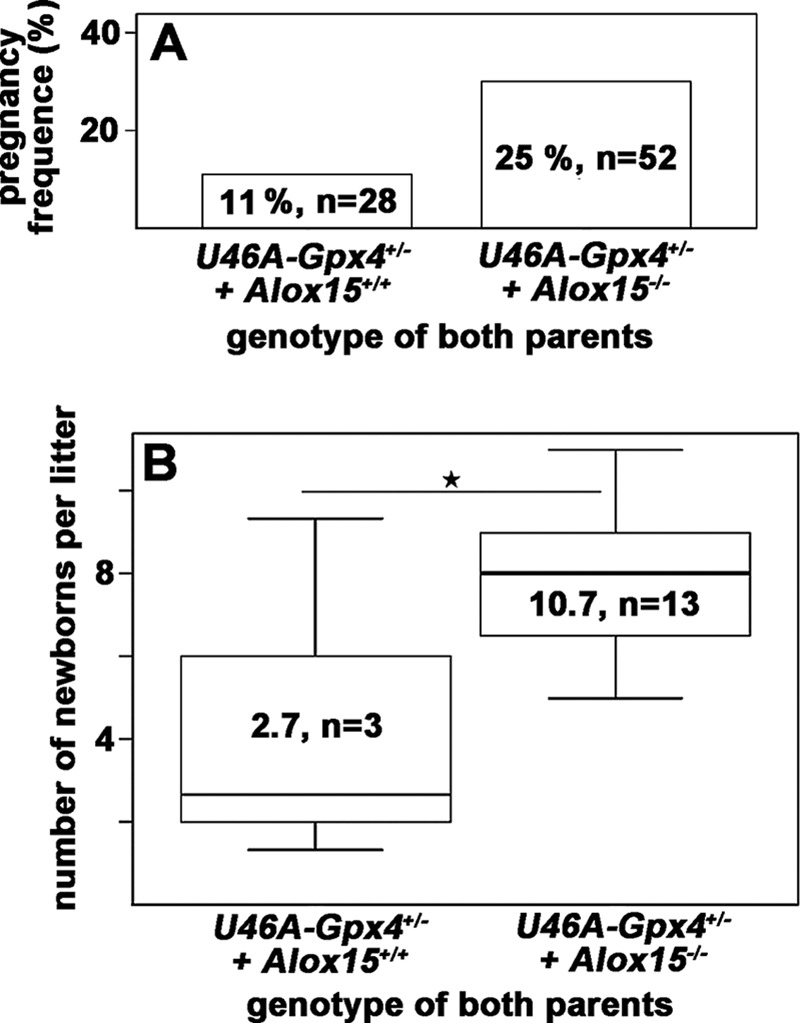
Systemic inactivation of the Alox15 gene rescues subfertility of Sec46Ala-Gpx4+/− mice. Male Sec46Ala-Gpx4+/−+Alox15+/+ and Sec46Ala-Gpx4+/−+Alox15−/− mice were mated with female individuals of the same genotype. As suitable measures for male fertility, the percentage of successful mating (matings resulting in pregnancies, panel A) and the litter sizes (panel B) were quantified. Stars indicate significant (p < 0.05) differences between the experimental groups.
Impact of Alox15 Deficiency on Functional and Structural Characteristics of Sec46Ala-Gpx4+/− Sperm
As indicated in Fig. 2, Sec46Ala-Gpx4+/− cauda sperm exhibit impaired motility characteristics when compared with wild-type controls. In contrast, we did not find significant motility differences when wild-type animals were compared with Alox15 knock-outs (data not shown). However, when the Alox15 knock-out was crossed into the Sec46Ala+/− Gpx4 background, we observed reimprovement of the impaired motility characteristics (Fig. 4). Total motility of Sec46Ala-Gpx4+/− sperm, which was significantly lower than that of wild-type sperm (Fig. 2), was significantly improved by homozygous Alox15-deficiency (Fig. 4A). Similar pictures were observed for progressively (Fig. 4B) and rapidly moving sperm (Fig. 4C). When we compared the motility characteristics of epididymal cauda sperm of Sec46Ala-Gpx4+/−+Alox15−/− mice with those of animals carrying two wild-type alleles at both gene loci (U46A-Gpx4−/−+Alox15+/+), we observed a significantly higher total motility of the genetically modified sperm (54% for Sec46Ala-Gpx4+/−+Alox15−/− mice versus 37 % for U46A-Gpx4−/−+Alox15+/+ mice, p < 0.05). These data suggest that Alox15 knock-out does not only compensate, but over-compensates the defective sperm motility induced by functional Gpx4 deficiency.
FIGURE 4.
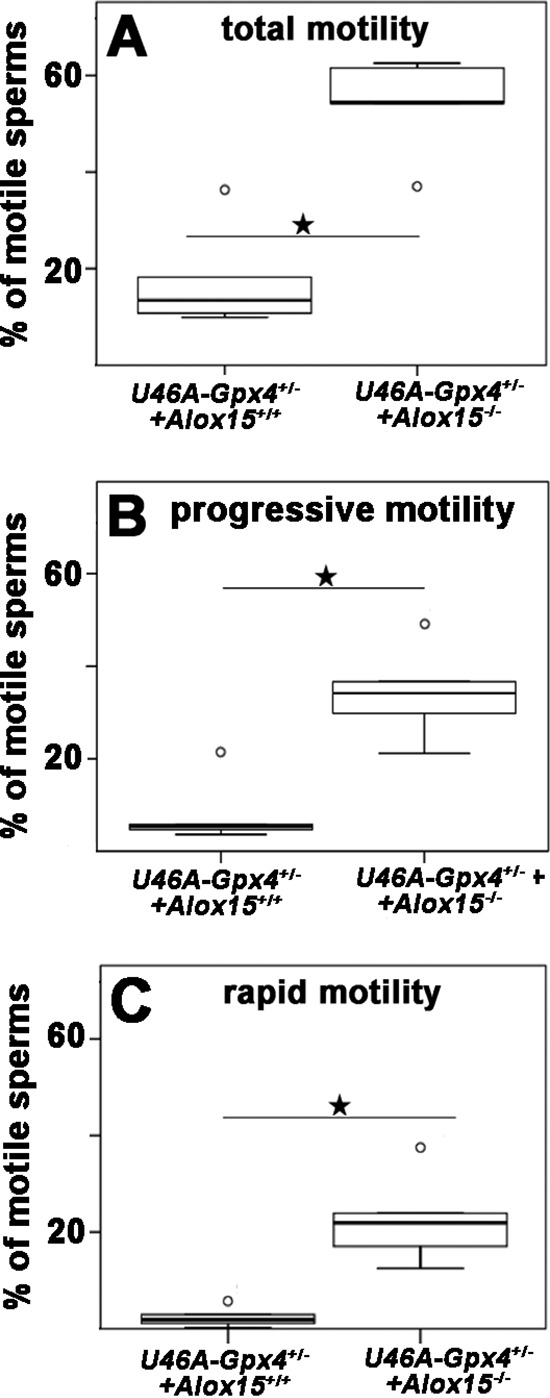
Systemic inactivation of the Alox15 gene normalized the impaired motility characteristics of sperm induced by heterozygous knock-in of the dysfunctional Gpx4 allele (Sec46Ala-Gpx4+/−). Sperm were extracted from the epididymal cauda of male Sec46Ala-Gpx4+/−+Alox15+/+ and Sec46Ala-Gpx4+/−+Alox15−/− mice, and their motility characteristics were determined as described under “Materials and Methods.” For this image the percentage of total (panel A), progressive (panel B), and rapid motility (panel C) was quantified. For each genotype the sperm of five individuals were evaluated. Stars indicate significant (p < 0.05) differences between the experimental groups.
To explore whether reimprovement of sperm motility may be paralleled by reversal of the structural alterations induced by Gpx4-deficiency, we quantified the morphological differences of the sperm as described in Fig. 2 for the Sec46Ala-Gpx4+/− mice. Here we found that normalization of the sperm motility by Alox15-knock-out was not paralleled by complete reversal of structural and ultrastructural alterations induced by Gpx4 deficiency. In fact, for many Sec46Ala-Gpx+/−+Alox15−/− sperm we observed similar ultrastructural features (similar degree of midpiece granulation, roughening of the plasma membrane, swollen mitochondria, disordered alignment of the mitochondria) as in the Sec46Ala-Gpx4+/− sperm (Fig. 5, A, a–c). These structural alterations are also observed by electron microscopy (Fig. 5B, I and II). On the other hand, we also observed normal-looking sperm produced by Sec46Ala-Gpx4+/−+Alox15−/− animals (Fig. 5BIII). These data indicate a high degree of structural heterogeneity, which is also indicated by interindividual variations in the box plots (Fig. 5A).
FIGURE 5.
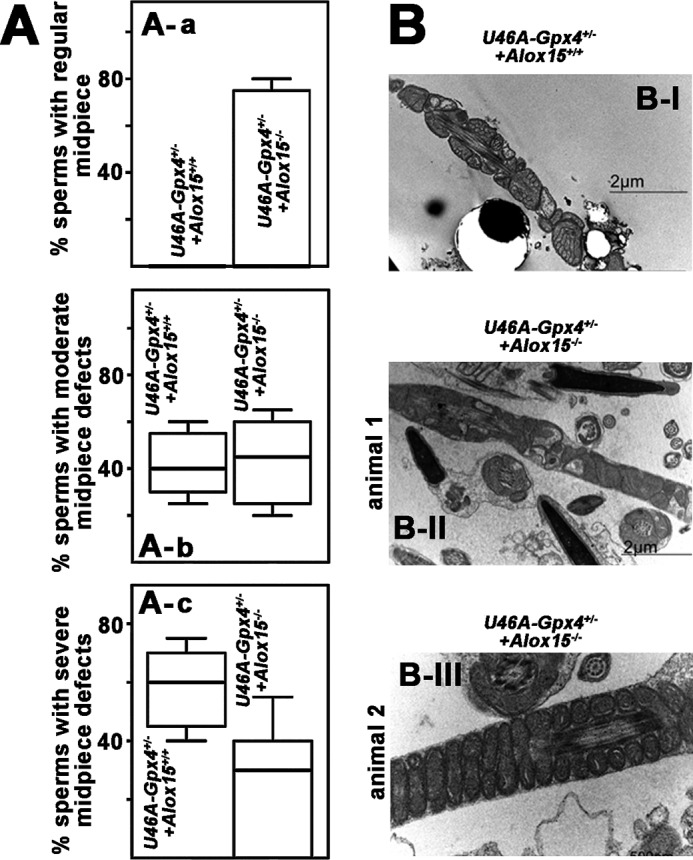
Morphological alterations induced by heterozygous expression of catalytically silent Gpx4 are partially reversed by Alox15 knock-out. A, the structure of sperm extracted from the epididymal cauda of male Sec46Ala-Gpx4+/−+Alox15+/+ and Sec46Ala-Gpx4+/−+Alox15−/− mice were compared by light microscopy as described in the legend to Fig. 2. Sperm were classified as Grade 1 (normal structure, a, grade 2 (slightly disturbed structure, b), and grade 3 (severely disturbed structure, c) sperm. The relative shares of the different sperm classes are indicated by the box plots. For each genotype, five mice were measured. B, electron micrograph of Sec46Ala-Gpx4+/−+Alox15+/+ sperm with severe structural alterations (I). Among Sec46Ala-Gpx4+/−+Alox15−/− sperm we observed a high degree of structural diversity. Here we found normal looking sperm (III) but also cells with a severely disrupted structure (II). Because of the high degree of interindividual variations, there were no significant differences between the two groups of animals (A, a–c).
Inactivation of the Alox15 Gene Impacts the Energy Metabolism of Sperm
The motility of sperm is closely related to the energy metabolism of these cells as ATP is required for movement of the tail. To explore whether the increase in cell motility induced by inactivation of the Alox15 gene (Fig. 4) is related to an altered energy supply, we quantified cellular respiration of sperm isolated from Sec46Ala-Gpx4+/−+Alox15−/− animals and compared it with that of Sec46Ala-Gpx4+/−+Alox15+/+. Here we found that despite the structural alterations induced by heterozygous knock-in of a dysfunctional Gpx4 allele (Fig. 2), the cauda sperm of Sec46Ala-Gpx4+/−+Alox15+/+ mice and of Sec46Ala-Gpx4−/−+Alox15+/+ mice show similar basal oxygen consumption rates (Fig. 6A). The addition of inhibitors of the respiratory chain (antimycin as inhibitor of complex 3, rotenone as inhibitor of complex 1) completely abolished the oxygen uptake, indicating the mitochondrial respiratory chain as source for the oxygen uptake measured (Fig. 6A). These data suggest that the observed structural alterations of the mitochondria (Fig. 2) do not significantly impact cellular respiration of the sperm. In contrast, when the Alox15 gene was inactivated on the Gpx4-deficient background, a significantly augmented (almost doubling) basal oxygen consumption rate was measured (Fig. 6, A and B), suggesting an up-regulation of the energy metabolism. Up-regulated cellular respiration indicates a more intense energy metabolism (higher ATP synthesis), providing an explanation for improved sperm motility (Fig. 4).
FIGURE 6.
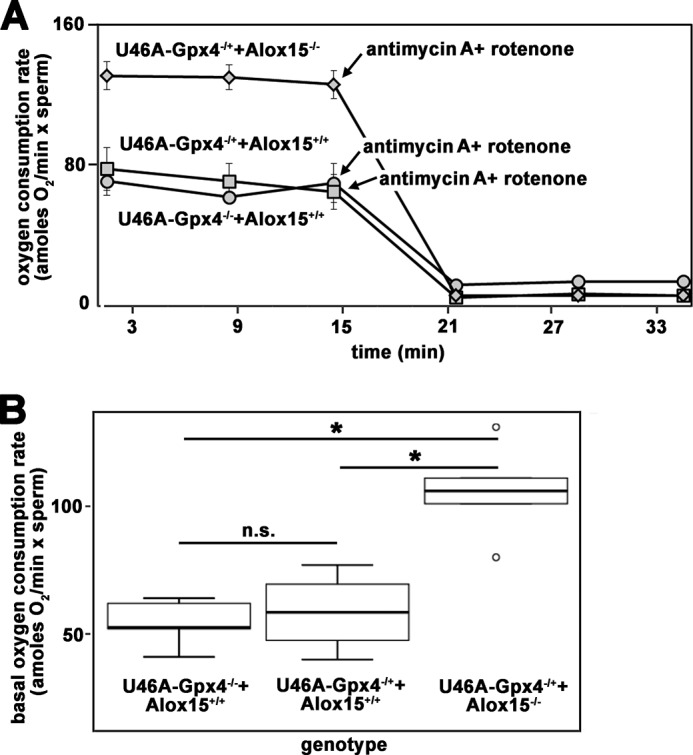
Cellular respiration of epididymal cauda sperm prepared from mice of different genotype. Sperm were prepared from the epididymal cauda of mice with different genotypes and employed for in vitro respiration studies as indicated under “Materials and Methods.” The measured oxygen consumption was normalized to the number of cells. A, the basal oxygen consumption rate was assayed over a time period of 16 min, and then inhibitors of the mitochondrial respiratory chain (antimycin A, rotenone) were added to block cellular respiration. Representative examples of the respiration curves for each genotype are shown. B, statistical evaluation of the repetitive oxygen uptake studies (n = 4–5 for each genotype). n.s., not significant. Stars indicate significant (p < 0.05) differences between the experimental groups; o, outlier.
Inactivation of the Alox15 Gene Does Not Alter the Degree of Membrane Oxygenation of Sec46Ala-Gpx4+/− Sperm
Because Alox15 and Gpx4 are antagonizing enzymes in the metabolism of hydroperoxy lipids (11), because Gpx4 functions as endogenous inhibitor of Alox15 (36), and because the expression of both enzymes is inversely regulated (37), we assumed that the oxidation degree of the membrane lipids might be increased in Gpx4 deficiency. In contrast, this measure should be down in Alox15−/− mice. To test this hypothesis we prepared the membrane lipids from sperm extracted from the epididymal cauda of Sec46Ala-Gpx4−/−+Alox15+/+ mice (homozygous wild type in both gene loci), Sec46Ala-Gpx4+/−+Alox15+/+ mice (heterozygous mutant in the Gpx4 locus and homozygous wild type in the Alox15 locus), and Sec46Ala-Gpx4+/−+Alox15−/− mice (heterozygous mutant in the Gpx4 locus and homozygous mutant in the Alox15 locus) and quantified the degree of oxidation of the membrane lipids. For this purpose the membrane lipids were extracted and subjected to alkaline hydrolysis, and the hydrolysis mixtures were analyzed by HPLC for the content of non-oxygenated and oxygenated polyunsaturated fatty acids. The oxygenation degree of membrane lipids was defined as relative share of the oxygenated polyenoic fatty acids (PUFA), which is given in % of the total PUFA. A typical HPLC trace of sperm-hydrolyzed lipid extract is shown in Fig. 7A. Here the oxygenated PUFAS are quantified recording the chromatogram at 235 nm (upper trace). In contrast, the non-oxygenated PUFAs were followed, recording the absorbance at 210 nm (lower trace). When we compared the oxygenation degree of the sperm membrane lipids extracted from mice with different genotypes we did not find significant differences (Fig. 7B). Thus, genetic manipulation of Gpx4 and Alox15 genes did not appear to impact the degree of oxygenation of the membrane lipids. However, it should be stressed that the oxygenation degree of sperm lipids is surprisingly high as red cell membranes have lower oxygenated PUFA/PUFA ratios, ranging between 0.1 and 1% (38, 39).
FIGURE 7.
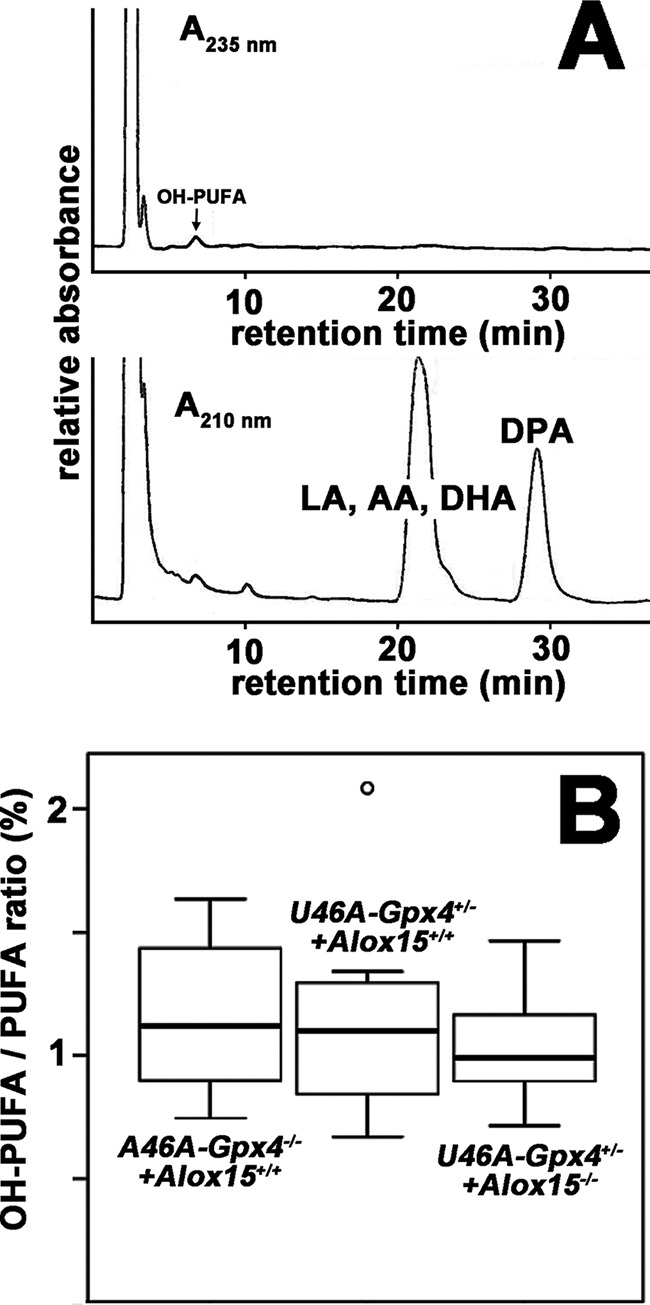
Systemic inactivation of the Alox15 gene did not impact the oxygenation degree of sperm membrane lipids. Sperm were prepared from the epididymal cauda of male Sec46Ala-Gpx4+/−+Alox15+/+ and Sec46Ala-Gpx4+/−+Alox15−/− mice, and the total membrane lipids were extracted. After alkaline hydrolysis of the ester lipids under an argon atmosphere, aliquots of the hydrolysates were analyzed by RP-HPLC for the quantification of the oxygenated and non-oxygenated PUFAs. A, representative RP-HPLC indicating the presence of hydroxylated and non-hydroxylated polyenoic fatty acids in the total membrane lipids. The chromatographic scales were calibrated by injecting known amounts of 13-HODE (hydroxylated fatty acid), arachidonic acid, and linoleic acid. LA, linoleic acid; AA, arachidonic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid. B, statistic evaluation of the hydroxy fatty acid/fatty acid ratios of the sperm total lipids. For each genotype, sperm from eight mice were analyzed.
Discussion
As a peroxidase (40), Gpx4 is a member of the large family of peroxide reducing enzymes that employs glutathione as electron donor. In mammals, Gpx4 is a selenoprotein, and germ line knock-out of the corresponding gene leads to early embryonic lethality (19, 32). A similar lethal phenotype was recently reported for homozygous knock-in mice, which express a catalytically inactive mutant of Gpx4 (20, 21). In these animals the catalytically active selenocysteine was mutated to redox-silent Ala (20) or Ser (21). Both types of heterozygous knock-in mice were viable and fertile, and adult individuals did not show obvious phenotypic alterations (20). However, more detailed studies on the breeding behavior of Sec46Ser-Gpx4+/− mice indicated impaired male fertility (21). In the present study we confirmed the subfertility of heterozygous male knock-in mice expressing a catalytically inactive Gpx4 variant in a different genetically modified mouse strain (Sec46Ala-Gpx4+/− mice). Moreover, we found that female fertility (not explored in previous studies) was not impacted by heterozygous expression of mutant Gpx4. Although we did not quantify the copulation frequency during synchronized mating, there was no evidence suggesting a differential mating behavior of Sec46Ala-Gpx4+/− males and corresponding wild-type controls.
The major finding of this study is that systemic inactivation of the Alox15 gene reversed the defective functional phenotype of Sec46Ala-Gpx4+/− sperm. This functional rescue is somewhat surprising for several reasons. Functional inactivation of the Alox15 gene in mice carrying two wild-type alleles in the Gpx4 locus induced subtle subfertility (16), and we confirmed these findings in our study (data not shown). Although the mechanistic basis for this observation has not been clarified, Alox15-deficient males had significantly more epididymal sperm with retained cytoplasmic droplet (16). Moreover, the cytoplasmic droplets of wild-type sperm had a smooth appearance and contained mostly empty membrane vesicles. In contrast, the cytoplasmic droplet of Alox15-deficient sperm contained functional mitochondria, which are targeted for exocytosis. Moreover, epithelial lesions, phagocytosis-like figures, and missing or aberrant apical blebs were observed in the epididymal caput of Alox15-deficient males (16). From these data, the authors concluded that the process of epididymal sperm maturation and proximal-to-distal migration of the cytoplasmic droplet is altered in Alox15-deficient mice. Thus, Alox15-deficient mice are characterized by normal spermatogenesis but altered epididymal sperm maturation. However, the molecular basis for the developmental role of Alox15 remains elusive. As an oxidizing enzyme, it might contribute to Gpx4 polymerization during the formation of the mitochondrial capsule, which is an essential step in spermatogenesis. Interestingly, in preliminary experiments we found that sperm of Alox15-deficient mice form significantly less insoluble Gpx4 polymers, as indicated by immunoblotting, which is consistent with a role of Alox15 in Gpx4 polymerization. However, more detailed studies are required to clarify the molecular basis for this observation.
On heterozygous Sec46Ala+/− background, systemic inactivation of the Alox15 gene induced re-improvement of the impaired fertility, which was induced by functional Gpx4 deficiency. The morphological alterations induced by heterozygous Gpx4 deficiency (modified midpiece structure, swollen mitochondria, disordered mitochondrial capsule) were detected in both epididymal caput and epididymal cauda sperm (data not shown). In fact, there was no significant difference in the degree of morphological alterations between these two sperm maturation stages. On the other hand, the functional defects (lower sperm motility) were only observed in the cauda sperm (data not shown). Thus, there is no 1:1 translation of the morphological alterations induced by Gpx4 deficiency into the functional characteristics of the sperm. Although the detailed molecular mechanisms for the maturation-dependent sperm dysfunction remains to be investigated, the most probable explanation is that the morphological defects, which are already visible at earlier stages of spermatogenesis, are not sufficiently severe to cause functional alterations at early developmental stages. However, when sperm mature, the morphological changes might become functionally more relevant as the alterations exceed the compensation capacity of the sperm.
As pro- and anti-oxidative enzymes Alox15 and Gpx4 have been implicated as counter-players in the regulation of the cellular redox homeostasis (1, 41, 42) and a number of in vitro, ex vivo, and in vivo studies suggested a functional interplay between the two enzymes (22, 35, 43, 44). In some of these studies inhibitors of Alox15 were used to silence the catalytic activity of the enzyme. Unfortunately, the use of such compounds is problematic for the reasons below.
Lacking Isoform Specificity
In humans six different LOX isoforms including ALOX15 have been identified, but for most commercially available LOX inhibitors the isoform specificity has not been tested in detail. Recently, a systematic study was carried out in which all rat 12-lipoxygenating LOX isoforms were overexpressed in HEK cells, and an array of commercially available LOX inhibitors was tested (45). The data obtained indicate that frequently employed LOX inhibitors (nordihydroguaiaretic acid, cinnamyl-3,4-dihydroxy-a-cyanocinnamate, A861, baicalein, PD146176) only exhibited a low degree of isoform specificity. If these inhibitors are used in vivo it is virtually impossible to puzzle out which LOX isoform may be responsible for an observed biological effect.
Variable Ortholog Specificity
The orthologs of human ALOX15 in mice and rats exhibit different catalytic characteristics. For instance, human ALOX15 oxygenates arachidonic acid to 15-hydroperoxy arachidonic acid (46, 47). In contrast, the mouse (48) and rat (49, 50) orthologs catalyze dominant arachidonic acid 12-lipoxygenation. Similarly, human and rat ALOX15 exhibits differential sensitivity toward LOX inhibitors. PD146176, which has been suggested as isoform-specific inhibitor of rabbit and human ALOX15 (51) did not at all inhibit rat Alox15.
Off-target Effects
Many LOX inhibitors, such as nordihydroguaiaretic acid, propyl gallate, baicalein, and cinnamyl-3,4-dihydroxy-a-cyanocinnamate are catechols and, thus, may function as anti-oxidants. Thus, in addition of being LOX inhibitors they impact cellular redox homeostasis by distinct mechanisms. For in vivo studies it is difficult to discriminate which of the two functions (LOX inhibition versus antioxidant function) is the major reason for an observed biological effect. Similar effects may be discussed for non-catecholic LOX inhibitors, which may be biotransformed to catechols. To avoid misinterpretations and to improve reliability inhibitor studies should always be confirmed by supplementing loss-of-function strategies.
Naturally occurring mutations in the human GPX4 gene have been related to the pathogenesis of oligoasthenozoospermia (30) and to Sedaghatian-type spondylometaphyseal dysplasia (26). Here we report in a murine system that defects in the Gpx4 gene can be compensated by inactivation of Alox15 expression. If this is also the case in humans, ALOX15 inhibitors might constitute potential drugs for such diseases. Unfortunately, for the time being no ALOX15 inhibitor has been approved for clinical use, and thus, it is impossible to test this therapeutic concept.
Materials and Methods
Chemicals
The chemicals were from the following sources. HPLC standards of 5(±)-HETE, 12(±)-HETE, and 15(±)-HETE were from Cayman Chemical (distributed by Spi Bio, Montigny le Bretonneux, France), sodium borohydride was from Life Technologies, HPLC solvents were from J. T. Baker or VWR International GmbH (Darmstadt, Germany), and oligonucleotide synthesis was performed at BioTez (Berlin, Germany). Other chemicals were purchased from Invitrogen.
Animals and Breeding
All the mice were bred and maintained in a specific pathogen free animal facility according to the FELASA recommendation with food and water ad libitum. All animal experiments were performed in compliance with the German animal welfare law and have been approved by the institutional committee on animal experimentation. Alox15 knock-out mice (strain name: B6.129S2-Alox15tm1Fun/J) were obtained from The Jackson Laboratory (Bar Harbor, ME) (48) and backcrossed for seven generations into black six background.
PCR Genotyping of the Gpx4 and Alox15 Locus
For routinely genotyping, mouse-tail biopsies were used. To genotype the Gpx4 and the Alox15 loci, genomic PCR was carried out employing allele-specific primer combinations. PCR was performed with the MyTaqTMRed Mix (Bioline, Luckenwalde, Germany) according to the vendor's instructions. The primers used to detect the Gpx4 wild type or Gpx4 Sec46Ala mutant allele were as follows: forward, 5′-GACAGATGGCTCTGGACCTGGGTG-3′; reverse, 5′-TAATCTGGCGTGGTAGGGGCAGAC-3′). The wild-type band is 412 bp long, and the Sec46Ala mutant band is 587 bp long, which included a residual LoxP/FRT sequence after neo deletion. Heterozygous allele carriers have two bands, whereas homozygous allele carriers would either have the lower (homozygous wild type) or the higher (homozygous mutant) band. For genotyping the Alox15 locus, the primer sequences were recommended by The Jackson Laboratory: oIMR9535-mutant reverse (5′-GGG AGG ATT GGG AAG ACA AT-3′), oIMR9711-common (5′-GGC TGC CTG AAG AGG TAC AG-3′), and oIMR9712 wild-type reverse (5′-CCA TAG ACG AGA CCA GCA CA-3′). The wild-type band is 200 bp, and the knock-out band is 417 bp. Homozygous allele carriers only show the upper band. The same method was employed to genotype the sperm extracted from the epididymis.
Cross-breeding Studies
Three- to six-month-old mice, wild-type mice ((Sec46Ala-Gpx4−/−+Alox15+/+), heterozygous Sec46Ala knock-in mice (Sec46Ala-Gpx4+/−+Alox15+/+), and heterozygous Sec46Ala knock-in mice with Alox15-deficient background (Sec46Ala-Gpx4+/−+Alox15−/−)), were mated as described previously (20). All mice were genotyped by genomic PCR employing allele-specific primer combinations.
DNA Preparation
Genomic DNA was prepared from mouse-tail biopsies or cauda sperm using the Invisorb Spin Tissue Mini kit (Berlin, Germany) extraction kit and employed for genomic PCR-based genotyping.
Sperm Extraction and Morphological Analysis
After euthanasia, testes and epididymes were isolated and kept at 4 °C. Both testes and epididymides were freed from surrounding tissue and blood vessels. Each testis was dissected from the epididymis and weighed. One testis per male was fixed in Bouin's solution, and histological sections were prepared and stained by conventional hematoxylin-eosin staining.
For sperm extraction epididymal caput and epididymal cauda were minced in M199-k composed of M199 (Sigma M7528) supplemented with 1 mm sodium pyruvate, 14 mm sodium lactate, and 0.4% (w/v) BSA (Merck) at room temperature (20–23 °C). After 5 min of incubation, the suspension was filtered through 30-μm nylon mesh (Partec, Goerlitz, Germany). For morphological analysis, droplets of a 5-μl sperm suspension were spread on a slide, fixed, and stained with the Spermac® stain kit (Minitüb, Tiefenbach, Germany). After Spermac® staining acrosomes and midpieces appeared dark green, nuclei appeared red and the equatorial region was colored light green.
Percoll Gradient Sperm Purification
Freshly prepared sperm from the epididymal caput were centrifuged at 500 × g for 5 min and resuspended in 1 ml of PBS. This suspension was then placed on top of a 30–45% discontinuous Percoll gradient. The sample was centrifuged for 30 min at 1500 × g, and the resulting upper layer, which contained contaminating cell debris and somatic cells, was removed. The purified sperm were isolated from the lower layer and washed by centrifugation.
RNA Extraction and qRT-PCR of Gpx4 and Alox15 mRNA
Total RNA was extracted from the sperm using the NucleoSpin RNA II Kit (Macherey-Nagel, Düren, Germany). Synthesis of the cDNAs was performed with 1–3 μg of the total RNA preparations using RevertAid™ Premium First Strand cDNA Synthesis kit (Schwerte, Germany). Quantitative real-time PCR was carried out with a Rotor Gene 3000 (Corbett Research, Mortlake, Australia) using the SensiMixTMSYBR PCR Kit (Bioline). GAPDH, Alox15, and Gpx4 isoform-specific amplification employed here are described in Refs. 20 and 23). The primers used to detected sperm-specific GAPDH were as follows: forward (5′-AGCTAGAGAGCTGACAGTGGGTAT-3′) and reverse (5′-CCACCACTAGTTGTCCATTCTTATGT-3′).
The experimental raw data were analyzed with the Rotor-Gene Monitor software (version 4.6). To generate standard curves for quantification of gene expression levels, specific amplicons were used as external standards for each target cDNA. All RNA preparations were analyzed at least in triplicate, and the means ± S.D. are given.
Sperm Motility Assays
To evaluate the sperm motility, the filtered sperm suspension was diluted with prewarmed (38 °C) M199-k medium to a density of 10–50 × 106 cells/ml, and 5 μl of the suspension was added in a prewarmed Makler chamber (Sefi-Medical Instruments, Haifa, Israel). Sperm motility was analyzed by the computer-assisted sperm analysis system AndroVision (Minitüb, Tiefenbach, Germany) under an AXIO Scope A1 microscope (Zeiss, Oberkochen, Germany) equipped with negative phase contrast optics and a video camera Basler avA1000–100gc (Basler AG, Germany). For each field, 100 pictures were recorded at 100 Hz, and 8 fields with 20–50 spermatozoa per field were evaluated per sample. The percentage of motile sperm was evaluated. Motile sperm showed local and progressive movement. Progressive sperm represent cells with any kind of circular or forward movement. Rapid sperm move faster than 80 μm/s.
Electron Microscopy and Immunogold Staining
For electron microscopy, sperm were fixed in 3% (w/v) glutaraldehyde, washed in PBS (pH 7.2), and fixed again in 2% osmium tetroxide. Dehydration of the fixed preparations was performed in ethanol, and samples were embedded in EPON 812 before preparation of ultrathin sections and staining with uranyl acetate and lead citrate. For immune electron microscopy, sperm were fixed in Karnovsky's solution (Serva, Heidelberg, Germany) washed in PBS, dehydrated in ethanol, and embedded in LR white resin. Ultrathin sections were incubated at room temperature with a 1:2000 dilution of our monoclonal antihuman Gpx4 antibody (34). After 1 h of incubation, sections were washed with a few drops of PBS and then treated with a commercial goat anti-mouse IgG antibody (1:40 dilution), which was labeled with 12 nm of colloidal gold. After 45 min at room temperature the sections were washed with PBS and water. Electron microscopy was carried out with FEI TecnaiSpiritBT device (120 kV; FEI Deutschland GmbH).
Measurements of Sperm Respiration
For quantification of sperm respiration, the epididymal cauda was minced in Hepes-buffered modified Tyrode's medium (mT-H: 131.89 mm NaCl, 2.68 mm KCl, 0.49 mm MgCl2·6H2O, 0.36 mm NaH2PO4·2H2O, 20 mm Hepes, 5.56 mm glucose, 1.80 mm CaCl2) that was supplemented with 4 mg/ml fatty acid-free bovine serum albumin at room temperature (52). After 5 min at 37 °C the suspension was filtered through a 30-μm nylon mesh (Partec). The sperm suspension was centrifuged for 2 min at 500 × g, the supernatant was removed, and sperm were resuspended in 1 ml mT-H. Sperm were counted with a Neubauer chamber. Four to five individuals of the different mouse strains were employed to quantify the respiration characteristics. For respiration measurements, 2.5 × 10−5 sperm were seeded on an XFp cell culture miniplate that had been coated with concanavalin A to ensure proper sperm adhesion. Two wells were left without cells to perform background correction. After 1 min, the plate was centrifuged for 2 min at 1200 × g. This centrifugation was repeated, changing the plate orientation to ensure an even adhesion of cells to the bottom of the well. The supernatant was removed, 175 μl of mT-H medium were added, and sperm adhesion was checked by microscope. Then the plate was placed in the instrument. An XFp extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) was used. Oxygen uptake measurements were carried out at 37 °C for ∼16 min. Then rotenone (1 μm final concentration) and antimycin A (1 μm final concentration) were added to block mitochondrial respiration. The oxygen uptake was normalized to the sperm number present in the incubation sample. Basal mitochondrial respiration was calculated by subtracting the oxygen uptake measured after the addition of rotenone and antimycin A from the overall oxygen uptake determined during the first 16 min of the measuring period.
Degree of Oxygenation of Membrane Lipids
Sperm were prepared as described above with the exception that prewarmed PBS was used instead of M199-k medium. Cells were washed, and the total sperm lipids were extracted according to the Bligh/Dyer method (53). The solvent was evaporated, the remaining lipids were reconstituted in 0.35 ml of anaerobic methanol, and 0.075 ml of anaerobic 40% KOH was added. The ester lipids were then hydrolyzed under an argon atmosphere (20 min at 60 °C), and the samples were cooled on ice and neutralized by the addition of 0.075 ml of acetic acid. Precipitate was spun down, and aliquots of the clear supernatant were injected to RP-HPLC. HPLC was performed on a Shimadzu LC-20 instrument recording simultaneously light absorbance at 235 nm (detection of hydroxy PUFAs) and 210 nm (detection of PUFAs). A Nucleodur C18 Gravity column (Macherey-Nagel; 250 × 4 mm, 5 μm particle size) was used, and fatty acid derivatives were eluted with a solvent system consisting of methanol/water/acetic acid (85/15/0.05 by volume) at a flow rate of 1 ml/min. The chromatographic scale was calibrated by injecting known amounts (6-point calibrations for each compound) of 13-HODE, linoleic acid, and arachidonic acid.
Statistics
Statistical analyses were carried out with SPSS 23 (IBM New York) software package, and the results are presented as medians. Non-parametric tests, most frequently the Mann-Whitney U test, was carried out. Two-tailed significances were accepted at p < 0,05. Within the box plots the black vertical lines indicate the median. The 25th and the 75th percentile are visualized as upper and lower box limits.
Author Contributions
S. H. B. carried out most of the experiments and contributed to writing the paper. M. R. carried out lipid analysis and contributed to writing the paper. S. R. R. performed genotyping of animals and sperms and contributed to writing the paper. K. M., S. E., D. V., and C. F. contributed to characterization of sperm motility and performed electron microscopy analysis. H. K. contributed to study design, drafted the manuscript, supervised the study, and contributed to sperm lipid analysis. A. B. contributed to study design and writing the paper and performed the respiration measurements of the sperm.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grants GRK1673 (to H. K.) and Ku961/11-1 (to H. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- LOX
- lipoxygenase
- qRT
- quantitative real-time
- PUFA
- polyenoic fatty acid
- HETE
- hydroxyeicosatetraenoic acid
- RP
- reverse phase.
References
- 1. Olsen L. F., Issinger O. G., and Guerra B. (2013) The Yin and Yang of redox regulation. Redox Rep. 18, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bocci V., and Valacchi G. (2013) Free radicals and antioxidants: how to reestablish redox homeostasis in chronic diseases? Curr. Med. Chem. 20, 3397–3415 [DOI] [PubMed] [Google Scholar]
- 3. Karunakaran U., and Park K. G. (2013) A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes Metab. J. 37, 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Marchi E., Baldassari F., Bononi A., Wieckowski M. R., and Pinton P. (2013) Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxid. Med. Cell Longev. 2013, 564961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese V., Cornelius C., Mancuso C., Lentile R., Stella A. M., and Butterfield D. A. (2010) Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 610, 285–308 [DOI] [PubMed] [Google Scholar]
- 6. de Oliveira M. F., Amoêdo N. D., and Rumjanek F. D. (2012) Energy and redox homeostasis in tumor cells. Int. J. Cell Biol. 2012, 593838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brigelius-Flohé R., and Maiorino M. (2013) Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303 [DOI] [PubMed] [Google Scholar]
- 8. Haeggström J. Z., and Funk C. D. (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111, 5866–5898 [DOI] [PubMed] [Google Scholar]
- 9. Ivanov I., Kuhn H., and Heydeck D. (2015) Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 573, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., and Walther M. (2010) Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503, 161–174 [DOI] [PubMed] [Google Scholar]
- 11. Kühn H., and Borchert A. (2002) Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic. Biol. Med. 33, 154–172 [DOI] [PubMed] [Google Scholar]
- 12. Grossman S., Shahin I., and Sredni B. (1979) Rat testis lipoxygenase-like enzyme: characterization of products from linoleic acid. Biochim. Biophys. Acta 572, 293–297 [DOI] [PubMed] [Google Scholar]
- 13. Schön I., Sofer Y., Cojacaru M., and Grossman S. (1989) Arachidonic acid metabolism by perfused ram testis. Int. J. Biochem. 21, 7–13 [DOI] [PubMed] [Google Scholar]
- 14. Reddy G. P., Prasad M., Sailesh S., Kumar Y. V., and Reddanna P. (1992) The production of arachidonic acid metabolites in rat testis. Prostaglandins 44, 497–507 [DOI] [PubMed] [Google Scholar]
- 15. Funk C. D., Chen X. S., Johnson E. N., and Zhao L. (2002) Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid. Mediat. 68, 303–312 [DOI] [PubMed] [Google Scholar]
- 16. Moore K., Lovercamp K., Feng D., Antelman J., Sutovsky M., Manandhar G., van Leyen K., Safranski T., and Sutovsky P. (2010) Altered epididymal sperm maturation and cytoplasmic droplet migration in subfertile male Alox15 mice. Cell Tissue Res. 340, 569–581 [DOI] [PubMed] [Google Scholar]
- 17. Fischer K. A., Van Leyen K., Lovercamp K. W., Manandhar G., Sutovsky M., Feng D., Safranski T., and Sutovsky P. (2005) 15-Lipoxygenase is a component of the mammalian sperm cytoplasmic droplet. Reproduction 130, 213–222 [DOI] [PubMed] [Google Scholar]
- 18. Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., and Nakagawa Y. (2003) Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 305, 278–286 [DOI] [PubMed] [Google Scholar]
- 19. Yant L. J., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J. G., Motta L., Richardson A., and Prolla T. A. (2003) The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 [DOI] [PubMed] [Google Scholar]
- 20. Brütsch S. H., Wang C. C., Li L., Stender H., Neziroglu N., Richter C., Kuhn H., and Borchert A. (2015) Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the Alox15 gene does not rescue such knock-in mice. Antioxid. Redox Signal. 22, 281–293 [DOI] [PubMed] [Google Scholar]
- 21. Ingold I., Aichler M., Yefremova E., Roveri A., Buday K., Doll S., Tasdemir A., Hoffard N., Wurst W., Walch A., Ursini F., Friedmann Angeli J. P., and Conrad M. (2015) Expression of a Catalytically inactive mutant form of glutathione peroxidase 4 (Gpx4) confers a dominant-negative effect in male fertility. J. Biol. Chem. 290, 14668–14678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seiler A., Schneider M., Förster H., Roth S., Wirth E. K., Culmsee C., Plesnila N., Kremmer E., Rådmark O., Wurst W., Bornkamm G. W., Schweizer U., and Conrad M. (2008) Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 [DOI] [PubMed] [Google Scholar]
- 23. Borchert A., Wang C. C., Ufer C., Schiebel H., Savaskan N. E., and Kuhn H. (2006) The role of phospholipid hydroperoxide glutathione peroxidase isoforms in murine embryogenesis. J. Biol. Chem. 281, 19655–19664 [DOI] [PubMed] [Google Scholar]
- 24. Canli Ö., Alanku Y. B., Grootjans S., Vegi N., Hültner L., Hoppe P. S., Schroeder T., Vandenabeele P., Bornkamm G. W., and Greten F. R. (2016) Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood 127, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brault C., Lévy P., Duponchel S., Michelet M., Sallé A., Pécheur E. I., Plissonnier M. L., Parent R., Véricel E., Ivanov A. V., Demir M., Steffen H. M., Odenthal M., Zoulim F., and Bartosch B. (2016) Glutathione peroxidase 4 is reversibly induced by HCV to control lipid peroxidation and to increase virion infectivity. Gut 65, 144–154 [DOI] [PubMed] [Google Scholar]
- 26. Smith A. C., Mears A. J., Bunker R., Ahmed A., MacKenzie M., Schwartzentruber J. A., Beaulieu C. L., Ferretti E., FORGE Canada Consortium, Majewski J., Bulman D. E., Celik F. C., Boycott K. M., and Graham G. E. (2014) Mutations in the enzyme glutathione peroxidase 4 cause Sedaghatian-type spondylometaphyseal dysplasia. J. Med. Genet. 51, 470–474 [DOI] [PubMed] [Google Scholar]
- 27. Ursini F., Heim S., Kiess M., Maiorino M., Roveri A., Wissing J., and Flohé L. (1999) Dual function of the selenoprotein PHGPx during sperm maturation. Science 285, 1393–1396 [DOI] [PubMed] [Google Scholar]
- 28. Dreher I., Schmutzler C., Jakob F., and Köhrle J. (1997) Expression of selenoproteins in various rat and human tissues and cell lines. J. Trace Elem. Med. Biol. 11, 83–91 [DOI] [PubMed] [Google Scholar]
- 29. Roveri A., Casasco A., Maiorino M., Dalan P., Calligaro A., and Ursini F. (1992) Phospholipid hydroperoxide glutathione peroxidase of rat testis: gonadotropin dependence and immunocytochemical identification. J. Biol. Chem. 267, 6142–6146 [PubMed] [Google Scholar]
- 30. Diaconu M., Tangat Y., Böhm D., Kühn H., Michelmann H. W., Schreiber G., Haidl G., Glander H. J., Engel W., and Nayernia K. (2006) Failure of phospholipid hydroperoxide glutathione peroxidase expression in oligoasthenozoospermia and mutations in the PHGPx gene. Andrologia 38, 152–157 [DOI] [PubMed] [Google Scholar]
- 31. Roveri A., Ursini F., Flohé L., and Maiorino M. (2001) PHGPx and spermatogenesis. Biofactors 14, 213–222 [DOI] [PubMed] [Google Scholar]
- 32. Imai H., Hakkaku N., Iwamoto R., Suzuki J., Suzuki T., Tajima Y., Konishi K., Minami S., Ichinose S., Ishizaka K., Shioda S., Arata S., Nishimura M., Naito S., and Nakagawa Y. (2009) Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 284, 32522–32532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfeifer H., Conrad M., Roethlein D., Kyriakopoulos A., Brielmeier M., Bornkamm G. W., and Behne D. (2001) Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 15, 1236–1238 [PubMed] [Google Scholar]
- 34. Borchert A., Küttner G., Giessmann E., Wang C. C., Wessner H., Volkmer R., Höhne W., and Kuhn H. (2010) Defining the immunoreactive epitope for the monoclonal anti-human glutathione peroxidase-4 antibody anti-hGPx4 Mab63–1. Immunol. Lett. 133, 85–93 [DOI] [PubMed] [Google Scholar]
- 35. Schneider M., Wortmann M., Mandal P. K., Arpornchayanon W., Jannasch K., Alves F., Strieth S., Conrad M., and Beck H. (2010) Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia 12, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schnurr K., Belkner J., Ursini F., Schewe T., and Kühn H. (1996) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 271, 4653–4658 [DOI] [PubMed] [Google Scholar]
- 37. Schnurr K., Borchert A., and Kuhn H. (1999) Inverse regulation of lipid-peroxidizing and hydroperoxyl lipid-reducing enzymes by interleukins 4 and 13. FASEB J. 13, 143–154 [DOI] [PubMed] [Google Scholar]
- 38. Kuhn H., Belkner J., Wiesner R., and Brash A. R. (1990) Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J. Biol. Chem. 265, 18351–18361 [PubMed] [Google Scholar]
- 39. Kühn H., Belkner J., and Wiesner R. (1990) Subcellular distribution of lipoxygenase products in rabbit reticulocyte membranes. Eur. J. Biochem. 191, 221–227 [DOI] [PubMed] [Google Scholar]
- 40. Flohé L., and Ursini F. (2008) Peroxidase: a term of many meanings. Antioxid. Redox Signal. 10, 1485–1490 [DOI] [PubMed] [Google Scholar]
- 41. Forman H. J., Ursini F., and Maiorino M. (2014) An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 73, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Passaia G., and Margis-Pinheiro M. (2015) Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 234, 22–26 [DOI] [PubMed] [Google Scholar]
- 43. Conrad M., Sandin A., Förster H., Seiler A., Frijhoff J., Dagnell M., Bornkamm G. W., Rådmark O., Hooft van Huijsduijnen R., Aspenström P., Böhmer F., and Ostman A. (2010) 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. U.S.A. 107, 15774–15779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tobaben S., Grohm J., Seiler A., Conrad M., Plesnila N., and Culmsee C. (2011) Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 18, 282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gregus A. M., Dumlao D. S., Wei S. C., Norris P. C., Catella L. C., Meyerstein F. G., Buczynski M. W., Steinauer J. J., Fitzsimmons B. L., Yaksh T. L., and Dennis E. A. (2013) Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. FASEB J. 27, 1939–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kühn H., Barnett J., Grunberger D., Baecker P., Chow J., Nguyen B., Bursztyn-Pettegrew H., Chan H., and Sigal E. (1993) Overexpression, purification and characterization of human recombinant 15-lipoxygenase. Biochim. Biophys. Acta 1169, 80–89 [DOI] [PubMed] [Google Scholar]
- 47. Sloane D. L., Dixon R. A., Craik C. S., and Sigal E. (1991) Expression of cloned human 15-lipoxygenase in eukaryotic and prokaryotic systems. Adv. Prostaglandin Thromboxane Leukot. Res. 21A, 25–28 [PubMed] [Google Scholar]
- 48. Sun D., and Funk C. D. (1996) Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 271, 24055–24062 [PubMed] [Google Scholar]
- 49. Watanabe T., and Haeggström J. Z. (1993) Rat 12-lipoxygenase: mutations of amino acids implicated in the positional specificity of 15- and 12-lipoxygenases. Biochem. Biophys. Res. Commun. 192, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 50. Pekárová M., Kuhn H., Bezáková L., Ufer C., and Heydeck D. (2015) Mutagenesis of triad determinants of rat Alox15 alters the specificity of fatty acid and phospholipid oxygenation. Arch. Biochem. Biophys. 571, 50–57 [DOI] [PubMed] [Google Scholar]
- 51. Sendobry S. M., Cornicelli J. A., Welch K., Bocan T., Tait B., Trivedi B. K., Colbry N., Dyer R. D., Feinmark S. J., and Daugherty A. (1997) Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. Br. J. Pharmacol. 120, 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tourmente M., Villar-Moya P., Rial E., and Roldan E. R. (2015) Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 290, 20613–20626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bligh E. G., and Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]