Abstract
Infiltrating monocyte-derived macrophages (M-MΦ) influence stroke-induced brain injury. Although the inflammatory nature of M-MΦ in acute stroke has been well documented, their role during the resolution phase of stroke is less clear. With emerging evidence for the involvement of scavenger receptors in innate immunity, this study addresses an M-MΦ CD36 role in mediating phagocytosis during the recovery phase of stroke. Stroke increases CD36 and TSP-1/2 mRNA levels in the ipsilateral hemisphere at acute (3-day (d)) and recovery (7d) periods. Quantification of total, intracellular, and cell surface CD36 protein levels showed relatively unchanged expression at 3d post-ischemia. At 7d, there was a significant increase in cell surface CD36 (p < 0.05) with a concurrent reduction of intracellular CD36 (p < 0.05) in the ipsilateral hemisphere. Both cell surface and intracellular CD36 were found in whole brain lysates, whereas cell surface CD36 was predominantly detected in isolated brain mononuclear cells, blood monocytes, and peritoneal macrophages, suggesting that cell surface CD36 expressed in the post-ischemic brain originates from the periphery. The stroke-induced CD36 mRNA level correlated with increased expression of lysosomal acid lipase, an M2 macrophage marker. Functionally, higher CD36 expression in M-MΦ is correlated with higher phagocytic indices in post-ischemic brain immune cells. Moreover, pharmacological inhibition of CD36 attenuated phagocytosis in peritoneal macrophages and brain M-MΦ. These findings demonstrate that cell surface CD36 on M-MΦ mediates phagocytosis during the recovery phase in post-stroke brains and suggests that CD36 plays a reparative role during the resolution of inflammation in ischemic stroke.
Keywords: inflammation, macrophage, monocyte, phagocytosis, stroke, CD36, ischemic stroke, resolution
Introduction
Stroke elicits multiple pathological cascades involving inflammation and immunity (1, 2). These processes are tightly coupled with the activation of mononuclear phagocytes, including resident brain microglia and infiltrating peripheral immune cells. Over the past few decades, mechanistic and functional studies addressing stroke-induced inflammation and immunity have largely focused on the acute phase of stroke. Although the persistent presence of monocyte-derived macrophages (M-MΦ)2 in the post-ischemic brain has been linked to acute post-ischemic inflammation, the role of M-MΦ on the subsequent resolution phase of stroke is not clearly defined. The literature suggests that the ischemic environment changes from a toxic pro-inflammatory setting to a more permissive milieu in the resolution phase, allowing inflammation to resolve. Because the ischemic environment influences immune cell function (3), studies have reported conflicting protective and pathological functions of mononuclear phagocytes, suggesting that there are context-dependent roles of M-MΦ in the post-ischemic brain.
CD36 is a highly glycosylated class B scavenger receptor. The expression occurs in monocytes/macrophages and microglia as well as various other cells and tissues, including microvascular endothelial cells, platelets, adipocytes, and the heart (4–8). It has been reported that glycosylation of CD36 is required for trafficking of intracellular CD36 to the cell surface membrane (9). The extent of CD36 glycosylation varies in various steps of monocyte differentiation. Although intracellular CD36 with a lesser degree of glycosylated precursor resides in the subcellular compartment of the secretory pathway, heavily glycosylated CD36 is localized in the extracellular membrane (10). Because of the high affinity of CD36 toward many ligands, including apoptotic cells, thrombospondins (TSPs), fibrillary Amyloid β and oxidized lipids (11–14), the interactions between individual ligands and surface CD36 in specific cell types was shown to elicit diverse physiological and pathological functions. The interactions of CD36 with TSPs in endothelial cells or with fibrillary Amyloid β in microglia produce pro-inflammatory responses (5). As a pattern recognition receptor, CD36 is involved in clearance of cell debris and phagocytosis, which is an important function for tissue repair and inflammation resolution (12, 15).
Although CD36 expression is low in the normal brain, stroke up-regulates its expression, mainly in M-MΦ in the brain (16–18). Genetic deletion and pharmacological intervention studies have shown that CD36 contributes to acute ischemic brain injury (18, 19), apparently mediated by CD36 expressed in endothelial cells (20). On the other hand, hemorrhagic and neonatal stroke studies showed a beneficial side of CD36 through enhancing phagocytic function (21–23). Therefore, these reports of detrimental and beneficial outcomes indicate a context-dependent role for CD36 involving inflammation and resolution. Thus, this study set out to determine the role of M-MΦ CD36 during the recovery phase of stroke and its involvement in phagocytosis at the time of inflammation resolution. Here we report that cell surface CD36 protein expression in M-MΦ increases at the resolution phase of stroke and contributes to phagocytosis in the ischemic brain, demonstrating a post-stroke stage-specific role of CD36 in ischemic stroke.
Results
The Acute Pro-inflammatory Milieu Changes to a Less Inflammatory Milieu at 7 Days Post-stroke
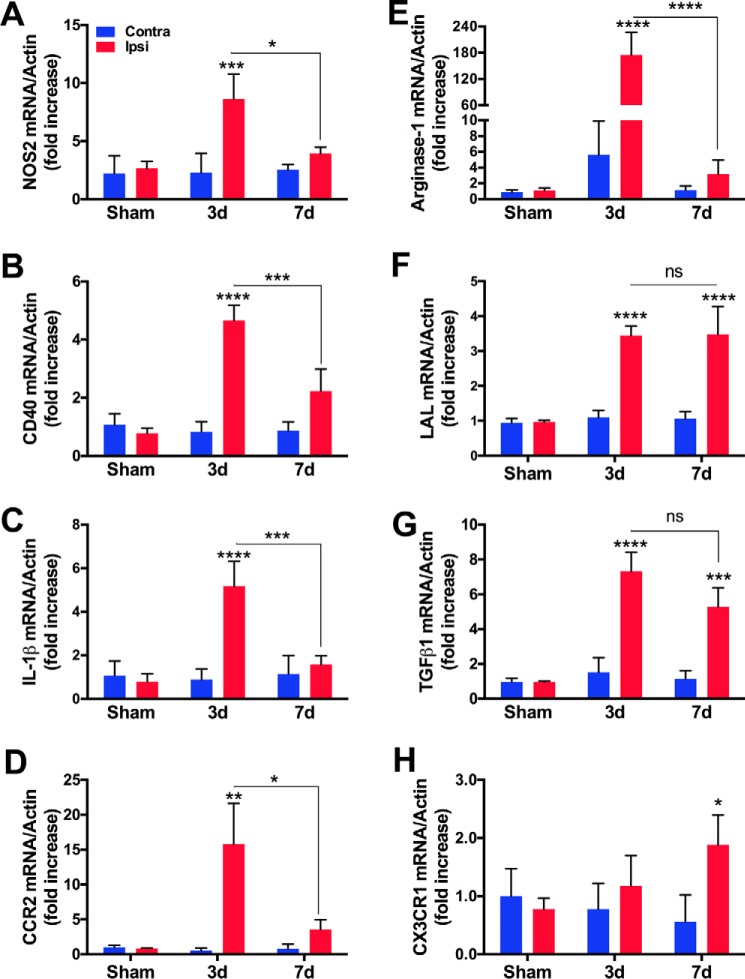
Thirty-minute transient middle cerebral artery occlusion (MCAO) was performed in mice as described previously (19), with a slight modification in the use of monofilament suture. Compared with the heat-blunted monofilament surgical sutures used in the earlier study (19), Teflon-coated 6–0 monofilament sutures (Doccol, Redland, CA) resulted in an ∼25% reduction in mean infarct size at 3 days (d) (∼55 mm3 versus ∼40 mm3, n = 8–10). To investigate changes in the ischemic milieu in the acute inflammatory and subsequent resolution phases of stroke, we first determined gene expression of inflammatory and macrophage phenotype markers in the ischemic brain at 3d post-stroke, when inflammation and infiltration of M-MΦ are greatest, and at 7d post-stroke, when post-stroke edema and inflammation are resolving (24). Expression of the pro-inflammatory (M1) markers NOS2 (inducible nitric oxide synthase, iNOS), CD40, IL-1β, and CCR2 mRNA was increased at 3d post-stroke, and their expression was significantly attenuated at 7d post-stroke (Fig. 1, A–D). Expression of the anti-inflammatory (M2) markers arginase-1, lysosomal acid lipase (LAL), and TGFβ1 was also profoundly increased at 3d post-stroke (Fig. 1, E–G). CX3CR1 gene expression was slightly increased at 3d without statistical significance (Fig. 1H). With the exception of arginase-1 mRNA, which was reduced at 7d, LAL, TGFβ1, and CX3CR1 mRNA levels remained high at 7d. The results demonstrate overlapping expression of M1 and M2 macrophage markers at 3d with a change toward a less inflammatory ischemic milieu associated with M2 polarization at 7d.
FIGURE 1.
Stroke induces pro and anti-inflammatory (M1/M2) makers in the brain. Gene expression of M1/pro-inflammatory markers (A–D) and M2/anti-inflammatory markers (E–H) in sham, 3d, and 7d post-stroke brain. n = 4–7/group, two-way ANOVA (effect of stroke, effect of post-ischemic time) with Bonferroni correction. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant.
Glycosylated Cell Surface CD36 Is Up-regulated in Brains 7 Days Post-stroke
CD36 transcription occurs in a feed-forward manner in the presence of ligands (25, 26). We first investigated expression of CD36 and the ligands thrombospondin 1 and 2 (TSP-1 and TSP-2) in the ischemic brain. CD36 and TSP-1/2 mRNA levels were low in sham animals and in the contralateral hemispheres of stroke mice. Stroke significantly increased CD36 and TSP-1/2 mRNA levels in the ipsilateral hemisphere at 3d and 7d (Fig. 2A). Next we determined CD36 protein levels in brain tissue. Precursor intracellular CD36 protein is synthesized and glycosylated in the endoplasmic reticulum and transported to the cell surface in monocytes (10). Total glycosylated CD36 protein levels were low in brain homogenates from naïve mice (data not shown) and from contralateral hemispheres of stroke mice but markedly elevated in stroke hemispheres at 7d (Fig. 2B). The addition of PNGase F, a deglycosylating enzyme, to brain homogenates resulted in 53-kDa nascent CD36 protein (Fig. 2B). CD36 KO mice did not have corresponding glycosylated or deglycosylated CD36 bands. We determined whether intracellular (74 kDa) or cell surface (bracket between 90–105 kDa) CD36 (10) was present in the ischemic brain at the acute (3d) and resolution (7d) phases of stroke. Despite increased CD36 mRNA levels at 3d, intracellular and cell surface CD36 protein levels were relatively low in sham mice and the contralateral hemispheres of stroke mice. Stroke moderately increased cell surface CD36 expression at 3d without statistical significance. At 7d, stroke significantly increased cell surface CD36 protein levels with a concomitant reduction of intracellular CD36 protein levels (Fig. 2C). The findings indicate redistribution of intracellular CD36 to cell surface CD36 at 7d post-stroke.
FIGURE 2.
Stroke increases cell surface CD36 protein expression at 7 days post-stroke. A, gene expressions of CD36, TSP-1, and TSP-2 in the brain in sham and 3d and 7d after stroke. n = 6–8/group, Student's t test, *, p < 0.05; **, p < 0.01; ***, p < 0.001; Contra versus Ipsi. B, identification of glycosylated CD36 in the presence of PNGase F (deglycosylation enzyme). Brain homogenates (2 μg) were obtained from naïve CD36 KO mice and 7d post-stroke wild-type (10× backcrossed with C57BL/6) mice. C, intracellular and cell surface CD36 protein levels in tissue homogenates (2 μg) obtained from 3d and 7d post-stroke or sham brains. n = 5, two-way ANOVA Bonferroni correction. *, p < 0.05. C, Contra; I, Ipsi.
Cell Surface CD36 Is Predominantly Expressed in [CD45]Hi Mononuclear Cells
Cell surface CD36 is thought to play a role in innate immunity by clearing debris and apoptotic bodies, which are critical processes for wound repair and remodeling. After observing elevated cell surface CD36 expression at 7d post-stroke, we investigated the source of cell surface CD36 in the ischemic brain during this resolution phase. There was no detectible cell surface CD36 protein in isolated immune cells from naïve brains and contralateral hemispheres; only the weak intracellular CD36 protein was observed (Fig. 3A). Compared with ischemic brain lysates containing both forms of CD36, mononuclear cells isolated from ischemic brains had only cell surface CD36 (Fig. 3A). Peripheral blood monocytes and peritoneal macrophages also expressed only cell surface CD36 (Fig. 3B), indicating that the source of cell surface CD36 expressed in the ischemic brain comes from infiltrating M-MΦ. To address which mononuclear subsets in the ischemic brain express cell surface CD36, the surface antigens CD45 and CD11b were used to distinguish [CD45]Hi/CD11b+ M-MΦ from [CD45]Low/CD11b+-resident microglia in the ischemic brain. We found that the CD36+ cells are a predominantly [CD45]Hi-expressing population in the stroke hemisphere at 7d (Fig. 3C), further supporting that the CD36+ cells in the ischemic brain are M-MΦ and not resident microglia.
FIGURE 3.
Cell surface CD36 is predominantly expressed in [CD45]Hi mononuclear cells. A, CD36 protein expression in whole tissue homogenates (2 μg) and isolated immune cell lysates (2 μg) from 7d post-stroke brain. B, CD36 protein expression in peripheral monocytes and peritoneal macrophages in naïve WT mice. C, flow cytometry analysis of CD45+/CD11b+ subsets in CD36+ populations in brain immune cells at 7d post-stroke. Immune cells from CD36 KO mice were used for negative control. n = 8, two-way ANOVA Bonferroni correction. #, p < 0.05, [CD45]Low versus [CD45]Hi. **, p < 0.01, Contra versus Ipsi of [CD45]Hi. C, Contra; I, Ipsi; N, naive; M, protein marker.
CD36 Expression Is Associated with M2 Polarization and Enhanced Phagocytosis
Phagocytosis is a salient feature of alternatively activated M2 macrophages (27, 28). CD36 and LAL are crucial for fatty acid oxidation, which is required for M2 macrophage polarization (29). There was increased CD36 and LAL mRNA expression at 7d, and the transcriptions were significantly correlated in the stroke hemisphere but not in the contralateral hemisphere (Fig. 4A). To address the function of the mature form of cell surface CD36, phagocytic activity was determined in brain immune cells. Compared with the contralateral hemisphere, phagocytic activity in immune cells from the ipsilateral hemisphere was significantly elevated (Fig. 4B). To address the involvement of CD36 in M-MΦ in phagocytosis, brain immune cells isolated from stroke animals at 7d with small (Fig. 4C, red), medium (Fig. 4C, blue), and large (Fig. 4C, green) infarct (n = 2 each) were pooled and divided into two to assess CD36 protein expression and phagocytic activity in the same sample. Neither phagocytic activity nor CD36 protein was detected in the cells from the contralateral hemispheres. In the ipsilateral hemispheres, higher cell surface CD36 protein expression in brain immune cells correlated with higher phagocytic indices (Fig. 4C). The results showed that cell surface CD36 is associated with M2 polarization and phagocytosis in the brain during the post-stroke resolution phase.
FIGURE 4.

Increased CD36 expression is associated with M2 polarization and enhanced phagocytosis. A, CD36 and LAL mRNA levels in brain homogenates and correlation between CD36 and LAL gene expression in the brain 7d post-stroke. B, phagocytosis in isolated brain immune cells at 7d post-stroke. Immune cells incubated with fluorescent beads at 4 °C were used as a negative control. The phagocytic activity is expressed as a phagocytosis index calculated by [number of fluorescent+ cells]×[mean fluorescence intensity]. n = 11/group. C, brain immune cells were isolated from the brain and divided into two. A phagocytic assay was performed in cells, and CD36 Western blotting was performed in immune cell lysate (1 μg). *, p < 0.05. C, Contra; I, Ipsi.
Inhibition of CD36 Attenuates Phagocytosis in M-MΦ
To address the importance of CD36 in phagocytosis, phagocytic activity was determined in the presence of neutralizing anti-CD36 antibody or salvianolic acid B (SAB), a specific CD36 inhibitor (30). Treatment of peritoneal macrophages with neutralizing anti-CD36 antibody reduced macrophage phagocytic activity (Fig. 5A). Macrophages treated with SAB also reduced phagocytosis, and the effect was dose-dependent (Fig. 5B). There was a profound attenuation of intracellular fluorescent signals in macrophages treated with 200 μm SAB, indicative of reduced phagocytosis (Fig. 5C). Furthermore, SAB reduced phagocytosis in brain immune cells isolated from 7d post-stroke brain (Fig. 5D). The findings demonstrate that cell surface M-MΦ CD36 contributes to phagocytosis during the resolution phase of ischemic stroke.
FIGURE 5.
Inhibition of CD36 reduces phagocytosis in mononuclear phagocytes. A, effect of anti-CD36 antibody on phagocytosis in mouse peritoneal macrophages (n = 7–8/dose). B, effect of SAB on phagocytosis in mouse peritoneal macrophages (n = 20/dose). C, visualization of phagocytosis in vehicle-treated (0 μm) and 200 μm SAB-treated peritoneal macrophages. D, effect of SAB on brain immune cells isolated from ipsilateral hemispheres of 7d post-stroke mice (n = 5–10/dose). One-way ANOVA Bonferroni correction. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus 0 μg/ml anti-CD36 antibody or 0 μm SAB.
Discussion
The literature indicates that infiltrating M-MΦ affect CNS inflammation and injury. In addition, there is increasing awareness that peripheral immunity regulates the resolution of inflammation. With a limited current understanding of the events that underlie the transition from the post-stroke inflammation phase to the resolution phase, this study addresses the involvement of CD36 in the resolution of post-stroke inflammation. As an innate immune receptor expressed in peripheral monocytes/macrophages, key findings from this study include the presence of two distinct forms of CD36 protein, intracellular and cell surface CD36, in the post-ischemic brain and a specific increase of cell surface CD36 in M-MΦ during the 7d resolution phase. Functionally, increased cell surface CD36 expression is associated with features of M2 macrophage polarization and increased phagocytosis in post-stroke brains during the resolution phase.
As a multifunctional receptor, various functions of CD36 have been observed, depending on the expressing cell type and the stroke milieu. Previous studies have implicated that the damaging/pro-inflammatory nature of CD36 is associated with the acute setting (≤3d post-stroke) (16, 17, 19). However, the oldest and most conserved function of CD36 is phagocytosis by mononuclear phagocytes that interact with phosphatidylserine moieties of apoptotic cells (11). The engulfment of apoptotic cells suppresses inflammation and polarizes macrophages to the alternatively activated M2 phenotype (31, 32). In neonatal stroke, a beneficial role of CD36 by enhancing phagocytosis has been reported (23), suggesting a more permissive and less inflammatory ischemic milieu in stroke during early development. Other studies have shown an association between increased CD36 and enhanced hematoma resolution in hemorrhagic stroke (21, 22) and that CD36 plays a role in inflammation resolution after permanent stroke (33), likely reflecting the presence of apoptotic cells that interact with CD36 for phagocytosis. Thus, the functions of CD36 are likely dictated by the ligands available in the ischemic milieu, depending on developmental stages of stroke (neonatal versus adult), types of stroke (ischemic versus hemorrhagic), and specific post-stroke stages (acute inflammatory versus late recovery phases). Consistent with this notion, we observed the anti-inflammatory nature of CD36 during the resolution phase of stroke in adult mice. Significant increases in cell surface CD36 protein expression at 7d but not at 3d support the role of CD36 in phagocytosis at the time of resolution. Several gene analyses at 7d also demonstrated reduced expression of pro-inflammatory mediators with increased anti-inflammatory molecules compared with the 3d time point, supporting the notion that changes occur in the ischemic milieu as inflammation resolves.
It has been challenging to distinguish infiltrating M-MΦ from resident microglia in the ischemic brain because of overlapping expression of common lineage markers. Related to CD36 expression in these two cell types, we provide experimental evidence that the primary source of cell surface CD36 in the post-ischemic brain is infiltrating M-MΦ, not resident microglia. This was based on the observation that isolated immune cells from stroke hemispheres express cell surface CD36, whereas the expression was absent in the contralateral hemisphere (Fig. 2C). Additionally, cell surface CD36 is only present in blood monocytes and peritoneal macrophages, supporting the idea that cell surface CD36 is from the periphery. The presence of intracellular CD36 in whole brain lysates but not in isolated brain immune cells (Fig. 3A) indicates that intracellular CD36 expression occurs in cells other than mononuclear phagocytes. In our previous bone marrow transplantation study, in which wild-type mice received bone marrow from CD36 KO mice, we found an absence of stroke-induced CD36 expression in the ipsilateral hemisphere at acute phase (3d) (17), confirming that CD36 expressed in ischemic brains primarily comes from infiltrating M-MΦ. Conventionally, [CD45]Low/CD11b+ and [CD45]Hi/CD11b+ cells in the post-stroke brain have been considered to represent resident microglia and M-MΦ, respectively. Because we observed that cell surface CD36+ immune cells were predominantly in the [CD45]Hi/CD11b+ subset, our study further supports the peripheral origin of cell surface CD36 and demonstrates the importance of M-MΦ in mediating CD36 function in the post-stroke brain.
Macrophages polarize to either pro-inflammatory M1 or anti-inflammatory M2 phenotypes, depending on environmental stimuli. Although the expression of M1 and M2 markers may occur on a continuum, M2 polarization of macrophages is associated with enhanced phagocytic ability and a more permissive environment for tissue repair. We observed increased expression of pro-inflammatory mediators and M1 phenotype markers at 3d, whereas expression was reduced with increased and/or sustained expression of M2 markers at 7d (Fig. 1). Relevant to the M2 phenotype, this study addressed the importance of CD36 in M2 macrophage polarization. Considering the fact that M2 macrophages depend on fatty acid oxidation for energy, another study demonstrated that CD36 is critical for fatty acid uptake and that LAL is critical for subsequent lipolysis to sustain adequate metabolism for the M2 phenotype (29). In agreement, we found increased expression of CD36 in the brain 7d post-stroke, with a corresponding increase of LAL expression (Fig. 4A). A CD36 inhibitor reduced phagocytosis in peritoneal macrophages and 7d post-stroke brain immune cells (Fig. 5), further supporting the necessary role of CD36 in mediating phagocytosis.
In summary, this study reveals the presence of intracellular and cell surface CD36 in the ischemic brain. Importantly, there is increased cell surface CD36 expression in M-MΦ during the recovery phase in post-stroke brains, and CD36-expressing M-MΦ are crucial for mediating phagocytosis. Besides its inflammatory nature in acute ischemic stroke, CD36 has a reparative role through phagocytosis during the resolution phase of stroke, indicating that the role of CD36 in cerebral ischemia depends on the context and timescale of injury. Modulating CD36 as a therapeutic strategy should be carefully considered for specific post-stroke phases.
Experimental Procedures
Animals
The use of animals and procedures were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University and in accordance with the Institutional Animal Care and Use Committee, National Institutes of Health, and Animal Research: Reporting of in Vivo Experiments (ARRIVE) guidelines. The mice were bred at the institute's animal facility, which maintained temperature, humidity, and a 12-h light/dark cycle. A maximum of five mice were housed in a cage with an individual ventilating system and irradiated bedding (1/8-inch Bed O's Cobs, The Anderson, Maumee, OH). Sterilized food (PicoLab rodent diet 5053, LabDiet, St. Louis, MO) and water were freely accessible in their cages. Experiments were performed in 10- to 11-week-old male CD36WT and CD36 KO mice. Both genotypes were backcrossed 10 times with C57BL/6 (99.9% C57BL/6 background). Mice were subjected to 30-min MCAO and randomized for 3d or 7d post-stroke survival. Inclusion and exclusion criteria for mice were based on the severity of ischemia. Animals exhibiting cerebral blood flow (CBF) reduction greater than 80% during MCAO and CBF restoration greater than 80% by 10 min after reperfusion were included in the study.
Transient MCAO
Thirty-minute MCAO in mice was performed according to methods described previously (19) with the following modification. Heat-blunted monofilament surgical sutures used in the earlier study were replaced with 6–0 monofilament surgical sutures coated with Teflon (Doccol) (24, 34, 35). CBF was continuously monitored for 10 min prior to stroke, during 30 min MCAO, and for 10 min during reperfusion using laser-doppler flowmetry (Periflux System 5010, Perimed AB, Järfälla, Sweden). The core body temperature of the animals was maintained at 37 ± 0.5 °C during the entire procedure by a thermocouple-regulated temperature controller (Digi Sense R/S, Cole-Parmer, Vermon Hills, IL).
Tissue Section Strategy
Brains were excised, frozen, and sectioned using an unbiased stereological sampling strategy to reflect the MCA territory in both hemispheres as described previously (35). The brain tissue was sectioned and collected from each hemisphere to determine mRNA and protein levels.
Isolation of Brain Immune Cells, Peripheral Macrophages, and Blood Monocytes
Mice were anesthetized with isoflurane and pentobarbital and then perfused with ice-cold heparinized PBS to remove blood. Brains were removed, and the hemispheres were separated and placed into ice-cold Hanks' balanced salt solution (Life Technologies) without Ca2+ and Mg2+. The tissue was enzymatically and mechanically dissociated with MACS® neural tissue dissociation kits with papain (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the instructions of the manufacturer. The dissociated tissue was used to obtain brain homogenates or isolated brain immune cells. Brain immune cells were isolated according to methods described previously (36). Cells were centrifuged by discontinuous Percoll PLUS (GE Healthcare) gradients and collected from 37–70% interphases. Peritoneal macrophages were prepared by peritoneal lavage as described previously (24). The adherent cells were used to obtain macrophage protein lysates. Blood monocytes were prepared according to a method published previously with minor modifications (35). After collecting total blood cells, red blood cells were lysed using RBC lysing buffer (Sigma-Aldrich, St. Louis, MO), and cells were sequentially applied to discontinuous Percoll (GE Healthcare) gradients and collected from 37–70% interphases.
Flow Cytometry Analysis
Isolated brain immune cells were counted by trypan blue staining. 0.5–1 × 105 cells were preincubated with mouse BD Fc Block (anti-CD16/CD32, ≤1 μg/106 cells in 100 μl, BD Biosciences) and then incubated with 10% FBS in PBS at 4 °C for 15 min. Cells were then stained with antibodies against CD11b (Phycoerythrin-Cyanine 7 (PE-Cy7) rat anti-mouse CD11b, 1:400, 552850), CD45 (Phycoerythrin (PE) rat anti-mouse CD45, 1:1,000, 553081), and CD36 (Allophycocyanin (APC) mouse anti-mouse CD36, 1:200, 562744) in 1% FBS in PBS at 4 °C for 1 h. After washing with PBS, cells were immediately analyzed using a flow cytometer (Accuri C6, BD Biosciences). Unstained cells and stained cells with the corresponding isotype controls (PE-Cy7 rat IgG2b, κ isotype control, 552849; PE rat IgG2b, κ isotype control, 553989; APC mouse IgA, κ isotype control, 562140) were used for negative controls. The specificity of CD36 signal in brain immune cells was confirmed using CD36 KO mice. All antibodies and isotype controls used for flow cytometry were purchased from BD Biosciences.
Real-time RT-PCR Analysis
Relative mRNA levels were quantified with real-time RT-PCR using fluorescent TaqMan technology as described previously (35). Total RNA from brain tissues or cell line cultures was reverse-transcribed using QuantiTech reverse transcription kits (Qiagen, Valencia, CA). Gene-specific PCR primers and probes were obtained as TaqMan predeveloped assay reagents for gene expression (Life Technologies), including NOS2 (iNOS, Mm00440485_m1), CD40 (Mm00441891_m1), IL-1β (Mm00434228_m1), Arginase-1 (Mm00475988_m1), TGFβ1 (Mm01178820_m1), CD36 (Mm00432398_m1), TSP-1 (Mm01335418_m1), TSP-2 (Mm01279240_m1), LAL (Mm00498820_m1), CCR2 (Mm00438270_m1) and β-actin (Mm00607939_s1). β-Actin was used as an internal control for sample normalization. The PCR reaction was performed using FastStart Universal Probe Master Mix (Roche) in an Applied Biosystems 7500 fast real-time PCR system (Life Technologies). Gene expression was presented as the β-actin normalized value according to the following formula: value = 2(β-actin threshold cycle − target gene threshold cycle).
Western Blotting Analysis
Brain tissue was homogenized, and cells were lysed in lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with 1 mm PMSF and complete Mini protease inhibitor and Phospho Stop (Roche Applied Science). Tissue and cell lysates were centrifuged, and supernatants were used for Western blotting analyses. Protein concentration was determined, and the indicated amount of total protein from brain tissue or cells was separated on NuPAGE 4–12% BisTris gels (Thermo Fisher, Waltham, MA) and then transferred to polyvinylidene fluoride membranes (Bio-Rad). Membranes were incubated in blocking buffer (Li-Cor, Lincoln, NE) for 1 h, followed by anti-mouse goat CD36 (1:1,000, AF2519, lot VYQ0108041, R&D Systems, Minneapolis, MN) or anti-broad spectrum of species, including anti-mouse goat β-actin (1:10,000, sc-1615, lot B2206, Santa Cruz Biotechnology) antibody in blocking buffer at 4 °C. Membranes were washed with Tris-buffered saline containing 0.05% Tween 20 followed by incubation with appropriate secondary antibodies conjugated with Alexa Fluor 680 rabbit anti-goat (1:5,000, A21088, Thermo Fisher) or IRDye 800CW donkey anti-mouse (1:10,000, 926-32212, Li-Cor) in blocking buffer at room temperature for 1 h. Protein bands were visualized using the Odyssey imaging system (Li-Cor). Protein quantification was done using ImageJ, and CD36 protein levels were normalized by β-actin. The specificity of CD36 protein bands was confirmed using tissues from CD36 KO mice. To identify deglycosylated CD36 protein, protein lysates were incubated with denaturing buffer (New England Biolabs Inc., Ipswich, MA) at 100 °C for 10 min and then with PNGase F and 1% Nonidet P-40 (New England Biolabs Inc.) at 37 °C for 1 h. Western blotting was performed on the deglycosylated lysates to visualize naïve deglycosylated CD36.
Phagocytosis Assay
The phagocytic activity was determined according to a method described previously (37) with a minor modification in the number of cells and microspheres. Brain immune cells or peritoneal macrophages were plated on 24-well plates (1 or 2 × 105 cells/well, respectively) and incubated at 37 °C with 5% CO2 for 1 h. After washing non-adherent cells, CD36 antibody (324205, Novus Biologicals, Littleton, CO) was added to peritoneal macrophages for 1 h. Different concentrations of SAB were added to cells for 1 h for brain immune cells or overnight for peritoneal macrophages. Fluorescent microspheres (1 μm, yellow-green (505/515), F-13081, Molecular Probes, Eugene, OR) were added to peritoneal macrophages (1 × 105) or isolated brain immune cells (1 × 106) and incubated at 37 °C with 5% CO2 for 3 h or 24 h, respectively. After discarding non-phagocytosed beads by washing multiple times with PBS, cells were harvested and resuspended in PBS and analyzed by flow cytometer. Cells incubated at 4 °C with beads were used as negative controls. For visualization of phagocytosis, cells were analyzed by fluorescent microscopy (Nikon Eclipse TS100, Nikon, Melville, NY).
Statistical Analysis
Statistical analyses were performed using Student's t test for comparison between two groups. One-way or two-way ANOVA followed by post hoc Bonferroni's multiple comparison tests were used for multiple group comparisons. Correlation analyses between gene expressions were made using linear regression. Differences were considered statistically significant at p < 0.05 in all analyses.
Author Contributions
M. S. W. performed experiments, analyzed the data, and wrote the manuscript. J. Y. performed experiments and wrote the manuscript. C. B. generated the animal model of stroke. S. C. designed the study and wrote the manuscript.
This work was supported by National Institutes of Health Grants R01HL82511, R01NS077897, and R01NS095359 (to S. C.) and the Burke Foundation (to S. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- M-MΦ
- monocyte-derived macrophage(s)
- TSP
- thrombospondin
- MCAO
- middle cerebral artery occlusion
- d
- day(s)
- LAL
- lysosomal acid lipase
- SAB
- salvianolic acid B
- CBF
- cerebral blood flow
- PE
- phycoerythrin
- ANOVA
- analysis of variance
- Contra
- contralateral
- Ipsi
- ipsilateral
- iNOS
- inducible nitric oxide synthase
- Cy7
- cyanine 7
- APC
- allophycocyanin
- ARRIVE
- Animal Research: Reporting of in Vivo Experiments.
References
- 1. Benakis C., Garcia-Bonilla L., Iadecola C., and Anrather J. (2014) The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell Neurosci. 8, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lo E. H., Dalkara T., and Moskowitz M. A. (2003) Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4, 399–415 [DOI] [PubMed] [Google Scholar]
- 3. Fumagalli S., Perego C., Pischiutta F., Zanier E. R., and De Simoni M. G. (2015) The ischemic environment drives microglia and macrophage function. Front. Neurol. 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coraci I. S., Husemann J., Berman J. W., Hulette C., Dufour J. H., Campanella G. K., Luster A. D., Silverstein S. C., and El-Khoury J. B. (2002) CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to β-amyloid fibrils. Am. J. Pathol. 160, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiménez B., Volpert O. V., Crawford S. E., Febbraio M., Silverstein R. L., and Bouck N. (2000) Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 6, 41–48 [DOI] [PubMed] [Google Scholar]
- 6. Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., and Goldstein G. (1983) Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 78, 83–99 [DOI] [PubMed] [Google Scholar]
- 7. Bordessoule D., Jones M., Gatter K. C., and Mason D. Y. (1993) Immunohistological patterns of myeloid antigens: tissue distribution of CD13, CD14, CD16, CD31, CD36, CD65, CD66 and CD67. Br. J. Haematol. 83, 370–383 [DOI] [PubMed] [Google Scholar]
- 8. Van Nieuwenhoven F. A., Verstijnen C. P., Abumrad N. A., Willemsen P. H., Van Eys G. J., Van der Vusse G. J., and Glatz J. F. (1995) Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem. Biophys. Res. Commun. 207, 747–752 [DOI] [PubMed] [Google Scholar]
- 9. Navazo M. D., Daviet L., Savill J., Ren Y., Leung L. L., and McGregor J. L. (1996) Identification of a domain (155–183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J. Biol. Chem. 271, 15381–15385 [DOI] [PubMed] [Google Scholar]
- 10. Alessio M., De Monte L., Scirea A., Gruarin P., Tandon N. N., and Sitia R. (1996) Synthesis, processing, and intracellular transport of CD36 during monocytic differentiation. J. Biol. Chem. 271, 1770–1775 [DOI] [PubMed] [Google Scholar]
- 11. Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., Hoff H. F., Salomon R. G., and Hazen S. L. (2002) Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 277, 38503–38516 [DOI] [PubMed] [Google Scholar]
- 12. El Khoury J. B., Moore K. J., Means T. K., Leung J., Terada K., Toft M., Freeman M. W., and Luster A. D. (2003) CD36 mediates the innate host response to β-amyloid. J. Exp. Med. 197, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kieffer N., Nurden A. T., Hasitz M., Titeux M., and Breton-Gorius J. (1988) Identification of platelet membrane thrombospondin binding molecules using an anti-thrombospondin antibody. Biochim. Biophys. Acta 967, 408–415 [DOI] [PubMed] [Google Scholar]
- 14. Adams J. C. (2004) Functions of the conserved thrombospondin carboxy-terminal cassette in cell-extracellular matrix interactions and signaling. Int. J. Biochem. Cell Biol. 36, 1102–1114 [DOI] [PubMed] [Google Scholar]
- 15. Moore K. J., El Khoury J., Medeiros L. A., Terada K., Geula C., Luster A. D., and Freeman M. W. (2002) A CD36-initiated signaling cascade mediates inflammatory effects of β-amyloid. J. Biol. Chem. 277, 47373–47379 [DOI] [PubMed] [Google Scholar]
- 16. Kim E., Febbraio M., Bao Y., Tolhurst A. T., Epstein J. M., and Cho S. (2012) CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann. Neurol. 71, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim E., Tolhurst A. T., Qin L. Y., Chen X. Y., Febbraio M., and Cho S. (2008) CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J. Neurosci. 28, 4661–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho S., and Kim E. (2009) CD36: a multi-modal target for acute stroke therapy. J. Neurochem. 109, 126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho S., Park E. M., Febbraio M., Anrather J., Park L., Racchumi G., Silverstein R. L., and Iadecola C. (2005) The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J. Neurosci. 25, 2504–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia-Bonilla L., Racchumi G., Murphy M., Anrather J., and Iadecola C. (2015) Endothelial CD36 Contributes to postischemic brain injury by promoting neutrophil activation via CSF3. J. Neurosci. 35, 14783–14793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao X., Sun G., Ting S. M., Song S., Zhang J., Edwards N. J., and Aronowski J. (2015) Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem. 133, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao X., Sun G., Zhang J., Strong R., Song W., Gonzales N., Grotta J. C., and Aronowski J. (2007) Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann. Neurol. 61, 352–362 [DOI] [PubMed] [Google Scholar]
- 23. Woo M. S., Wang X., Faustino J. V., Derugin N., Wendland M. F., Zhou P., Iadecola C., and Vexler Z. S. (2012) Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann. Neurol. 72, 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim E., Tolhurst A. T., and Cho S. (2014) Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J. Neuroinflammation 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagy L., Tontonoz P., Alvarez J. G., Chen H., and Evans R. M. (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93, 229–240 [DOI] [PubMed] [Google Scholar]
- 26. Tontonoz P., Nagy L., Alvarez J. G., Thomazy V. A., and Evans R. M. (1998) PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 [DOI] [PubMed] [Google Scholar]
- 27. Bohlson S. S., O'Conner S. D., Hulsebus H. J., Ho M. M., and Fraser D. A. (2014) Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 5, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rszer T. (2015) Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang S. C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A. M., Beatty W., Love-Gregory L., Lam W. Y., O'Neill C. M., Yan C., Du H., Abumrad N. A., Urban J. F. Jr., Artyomov M. N., et al. (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bao Y., Wang L., Xu Y., Yang Y., Wang L., Si S., Cho S., and Hong B. (2012) Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 223, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ren Y., and Savill J. (1998) Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563–568 [DOI] [PubMed] [Google Scholar]
- 32. Savill J., Dransfield I., Gregory C., and Haslett C. (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 [DOI] [PubMed] [Google Scholar]
- 33. Ballesteros I., Cuartero M. I., Pradillo J. M., de la Parra J., Pérez-Ruiz A., Corbí A., Ricote M., Hamilton J. A., Sobrado M., Vivancos J., Nombela F., Lizasoain I., and Moro M. A. (2014) Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARγ and 5-LO-dependent pathways. J. Leukocyte Biol. 95, 587–598 [DOI] [PubMed] [Google Scholar]
- 34. Qin L., Kim E., Ratan R., Lee F. S., and Cho S. (2011) Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J. Neurosci. 31, 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim E., Yang J., Beltran C. D., and Cho S. (2014) Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 34, 1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardona A. E., Huang D., Sasse M. E., and Ransohoff R. M. (2006) Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 1, 1947–1951 [DOI] [PubMed] [Google Scholar]
- 37. de Haas A. H., Boddeke H. W., Brouwer N., and Biber K. (2007) Optimized isolation enables ex vivo analysis of microglia from various central nervous system regions. Glia 55, 1374–1384 [DOI] [PubMed] [Google Scholar]