Abstract
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ape1/Ref-1) is a multifunctional protein possessing DNA repair, redox control, and transcriptional regulatory activities. Although Ape1/Ref-1 plays multiple roles in the immune system, its functions in helper T (Th) cell activation and differentiation are largely unknown. In this study, the function of Ape1/Ref-1 in Th cell activation was analyzed using an Ape1/Ref-1 redox-specific inhibitor, E3330. When splenocytes from OT-II mice, which are ovalbumin (OVA)-specific T-cell receptor transgenic mice, were activated with OVA in the presence of E3330, the induction of IFN-γ-producing OT-II T cells was significantly increased. In contrast, E3330 did not enhance IFN-γ production from plate-bound anti-CD3 antibody-stimulated CD4+ T cells in the absence of antigen presenting cells (APCs). Furthermore, E3330-pretreated and OVA-pulsed APCs also enhanced the IFN-γ production from OT-II T cells. These results suggested that E3330 enhances Th1 responses by modifying APC function. E3330 did not alter the surface expression of MHC-II or the co-stimulatory molecules CD80 and CD86 on APCs. On the other hand, E3330 up-regulated the IL-12 p35 and p40 gene expression, and IL-12 surface retention, but decreased the IL-12 secretion from Toll-like receptor (TLR) ligand-stimulated APCs. These results were confirmed with Ape1/Ref-1 knockdown experiments. Taken together, our findings indicated that the suppression of Ape1/Ref-1 redox function leads to an increased cell surface retention of IL-12 and enhances Th1 responses. This is the first study to demonstrate that Ape1/Ref-1 modulates the IL-12 production and secretion from APCs and controls Th1 immune responses.
Keywords: cytokine, dendritic cell, differentiation, redox regulation, T helper cells, Ape1/Ref-1, IL-12, antigen presenting cell
Introduction
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ape1/Ref-1)2 is a ubiquitous multifunctional protein that contains a redox control domain and a DNA repair domain (1–3). Ape1/Ref-1 functions in DNA base excision repair by virtue of its apurinic/apyrimidinic endonuclease activity, and in DNA proofreading by its exonuclease activity, and also modulates the DNA binding activity of several transcription factors, including nuclear factor-κB, early growth response protein-1, p53, activator protein (AP)-1, cyclic response element-binding protein, and hypoxia-induced factor-1α (4, 5), via its redox activity.
Ape1/Ref-1 plays several roles in the immune system. The redox activity of Ape1/Ref-1 is involved in CD40-mediated B cell activation through Pax5 transcription factor activation (6), whereas its AP endonuclease activity is essential for immunoglobulin class-switch recombination (7). Moreover, Ape1/Ref-1 modulates TLR2-mediated inflammatory responses in primary keratinocytes by activating nuclear factor-κB and hypoxia-induced factor-1α, resulting in the expression of inflammatory cytokines and chemokines (8). Our group also reported that Ape1/Ref-1 is essential for IL-21-induced ERK1/2 signaling in a mouse pro-B cell line (9). Despite the accumulating evidence that Ape1/Ref-1 plays various roles in the immune system, its function in T cell immune responses has not been thoroughly investigated.
To examine the role of Ape1/Ref-1 in naïve Th cell activation and differentiation, we used a small molecule inhibitor of Ape1-Ref-1 redox activity, (E)-3-(2-[5,6-dimethoxy-3-methyl-1,4-benzoquinonyl])-2-nonylpropenoic acid, known as E3330 (10). E3330 binds Ape1/Ref-1 directly to inhibit its redox activity (11), but does not impede its DNA repair function (12). We found that E3330 significantly increased the differentiation of IFN-γ-producing antigen-specific Th1 cells by up-regulating the IL-12 gene expression and IL-12 cell surface retention, but not the IL-12 secretion from bone marrow-derived DCs (BMDCs). These results were confirmed using Ape1/Ref-1 knock-down BMDCs. Taken together, these findings indicated that the redox function of Ape1/Ref-1 down-regulates Th1 responses through APCs. The mechanism by which the redox activity of Ape1/Ref-1 modulates IL-12 gene activation and cell surface retention is also discussed.
Results
Inhibition of the Redox Function of Ape1/Ref-1 with E3330 Enhances the Induction of IFN-γ-Producing OT-II T Cells Upon OVA Stimulation
To determine the role of the redox function of Ape1/Ref-1 in naïve helper T cell activation and differentiation, splenocytes from OT-II mice were stimulated with OVA and/or Pam3 CSK4 (Pam3) for 3 days in the presence or absence of E3330, and then analyzed by intracellular IFN-γ staining and flow cytometry. Notably, E3330 significantly increased the induction of IFN-γ-producing OT-II T cells upon OVA, or OVA and Pam3 stimulation (Fig. 1, A and B). Secreted IFN-γ in the culture medium, determined by cytometric bead array (CBA) analysis, was also up-regulated in E3330-treated splenocytes stimulated with OVA or OVA/Pam3 (data not shown). These results suggested that the redox function of Ape1/Ref-1 suppresses Th1 immune responses.
FIGURE 1.

E3330 enhances the induction of IFN-γ-producing OT-II T cells upon OVA stimulation. Splenocytes from OT-II mice were treated with or without 50 μm E3330 for 1 h and then stimulated with OVA and/or Pam3 for 72 h. The cells were re-stimulated with PMA, ionomycin, and brefeldin A for the last 4 h of culture and then stained for the cell surface expression of CD3 and CD4 and intracellular expression of IFN-γ. A, flow cytometry profiles of the CD3+/CD4+ cells. The percentages of IFN-γ positive cells are indicated. These results are representative of three independent experiments. B, scatter plots showing the percentages of IFN-γ-producing CD3+/CD4+ T cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
E3330 Has No Effect on the Anti-CD3/Anti-CD28 Antibody-mediated Induction of IFN-γ-producing CD4+ T Cells in the Absence of APCs
The above findings prompted us to investigate whether E3330 acts directly on T cells. To address this question, we used anti-CD3 antibodies to stimulate CD4+ T cells in the absence of APCs. Purified CD4+ T cells were activated with plate-bound anti-CD3 antibodies and soluble anti-CD28 antibodies, and then cultured in the presence or absence of E3330. E3330 had no significant effect on the induction of IFN-γ-producing T cells in the absence of APCs (Fig. 2, A and B), suggesting that E3330 may affect APCs, but not T cells, during Th1 cell differentiation.
FIGURE 2.
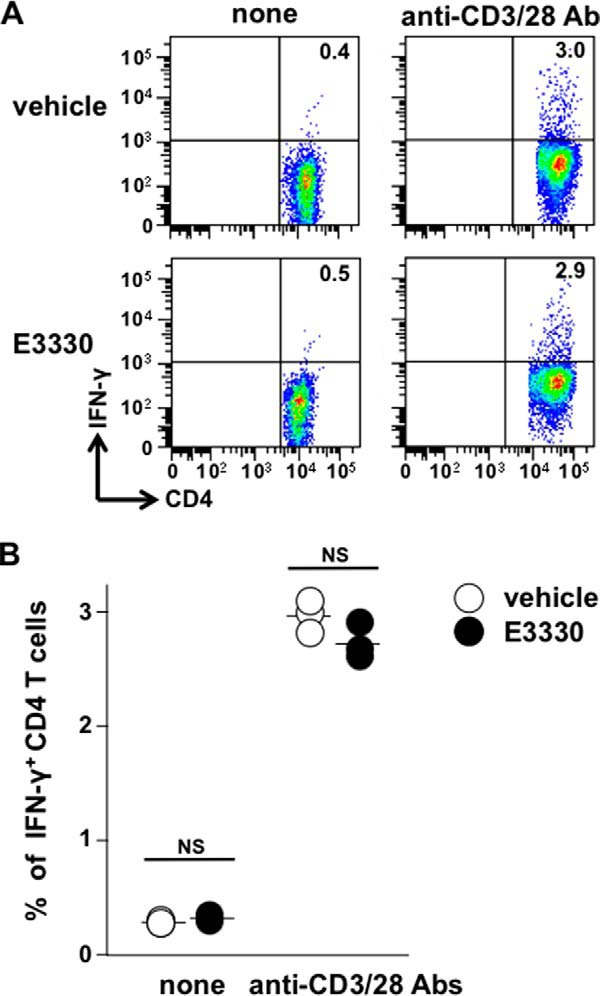
E3330 has no effect on the induction of IFN-γ-producing T cells upon anti-CD3/anti-CD28 antibody stimulation in the absence of APCs. Purified CD4+ T cells from C57BL/6J mouse spleens were stimulated with or without plate-bound anti-CD3 Ab and soluble anti-CD28 Ab in the presence or absence of 50 μm E3330 for 72 h. The cells were re-stimulated with PMA, ionomycin, and brefeldin A for the last 4 h of culture and then stained for the cell surface expression of CD3 and CD4 and intracellular expression of IFN-γ. A, flow cytometry profiles of the CD3+/CD4+ cells, representative of three independent experiments. B, scatter plots showing the percentages of IFN-γ-producing CD3+/CD4+ T cells. NS, not significant.
E3330 Enhances the Induction of OT-II IFN-γ-producing T Cells by Altering the Function of Antigen Presenting Cells
To test the hypothesis that E3330 modulates OT-II T cell differentiation through effects on APCs, splenic DCs or BMDCs were pretreated with E3330 and pulsed with OVA or OVA-(323–339) peptide, respectively. The E3330 was then washed away, and the pretreated DCs were co-cultured with the OT-II T cells. The E3330-pretreated OVA- or OVA peptide-pulsed DCs induced more IFN-γ-producing CD4+ OT-II T cells than did the control OVA or OVA peptide-pulsed DCs (Fig. 3, A and B). These results further suggested that E3330 enhances Th1 responses by altering APC function.
FIGURE 3.
E3330 enhances the induction of IFN-γ-producing OT-II T cells by altering antigen presenting cells. Adherent splenocytes (A) or BMDCs prepared as described under “Experimental Procedures” (B) were pretreated with or without E3330 for 1 h and then stimulated with or without OVA (100 μg/ml) (A) or OVA-(323–339) peptide (10 μg/ml) (B) and cultured for 24 h. The pretreated cells were washed with PBS to remove the E3330 and then co-cultured with OT-II T cells for 72 h. The OT-II T cells were re-stimulated with PMA, ionomycin, and brefeldin A for the last 4 h of culture and then stained for the cell surface expression of CD3 and CD4 and intracellular expression of IFN-γ. Flow cytometry profiles of the CD3+/CD4+ cells are representative of two (A) and three (B) independent experiments. The percentages of the IFN-γ positive cells are indicated.
E3330 Has No Effect on the Expression of Co-stimulatory Molecules but Enhances IL-12 Expression on the Cell Surface of BMDCs
Next, we explored the mechanism by which E3330 modulated the APC function. In addition to the MHC-II-presented antigenic peptide, the B7 co-stimulatory molecules CD80 and CD86 expressed on APCs provide indispensable activation signals to naïve CD4+ T cells (13). The treatment of BMDCs with E3330 alone had no significant effect on the cell surface expression of these molecules (Fig. 4). Although the TLR ligand LPS or Pam3 clearly up-regulated the expression of CD80 and CD86, and up-regulated MHC-II to a lesser extent, the addition of E3330 had no further effect on the expression of these molecules (Fig. 4).
FIGURE 4.
E3330 has no effect on the TLR ligand-induced expression of CD80, CD86, or MHC-II on BMDCs. BMDCs were pretreated with or without E3330 (50 μm) for 1 h and then stimulated with Pam3 (20 μg/ml) or LPS (100 ng/ml), as shown at the left. After a 24-h incubation, the expression of CD80 (left), CD86 (middle), and MHC-II (right) on CD11c+ DCs was examined by flow cytometry. The black line histograms depict specific Ab staining, whereas the shaded histograms represent background staining with isotype-matched control Abs. The mean fluorescent intensity values of the specific Ab staining are indicated in the histograms. These flow cytometry profiles are representative of four independent experiments.
Next, we examined the effect of E3330 on the TLR ligand-stimulated expression of cytokines in BMDCs. Although treating BMDCs with LPS or Pam3 increased the secretion of IL-6, IL-12, and TNF-α, the addition of E3330 did not further enhance the secretion of these cytokines. On the contrary, E3330 had a mild, but statistically significant inhibitory effect on the LPS-induced IL-12 level (Fig. 5). Because IL-12 plays a key role in Th1 differentiation, we further investigated the effect of E3330 on IL-12 expression. Notably, we found that E3330 significantly enhanced the Pam3-induced mRNA expression of Il12a and Il12b, which, respectively, encode the p35 and p40 subunits of IL-12 (Fig. 6). Taken together, these results suggested that E3330 enhances the IL-12 mRNA expression, but suppresses the IL-12 protein secretion. IL-12p70 is reported to be localized to the cell surface membrane before its secretion (14, 15). We therefore examined the cell surface IL-12 by flow cytometry analysis. As expected, E3330 significantly up-regulated the surface expression of IL-12 on BMDCs (Fig. 7, A and B).
FIGURE 5.

E3330 does not enhance the TLR ligand-stimulated cytokine secretion from BMDCs. BMDCs were pretreated with or without E3330 (50 μm) for 1 h and then stimulated with Pam3 (20 μg/ml) or LPS (100 ng/ml) for 48 h. After stimulation, cytokines in the culture supernatants were analyzed by CBA as described under “Experimental Procedures.” The cytokine levels are shown as the scatter plots. **, p < 0.01; NS, not significant.
FIGURE 6.

E3330 enhances the Pam3-stimulated IL-12 gene expression in BMDCs. BMDCs were pretreated with or without E3330 (50 μm) for 1 h and then stimulated with Pam3 (20 μg/ml) for 24 h. The cells were harvested, and the IL-12 mRNA levels were analyzed by quantitative real-time RT-PCR as described under “Experimental Procedures.” The IL-12 mRNA levels in E3330-pretreated BMDCs were normalized to the DMSO (vehicle)-pretreated control levels. *, p < 0.05.
FIGURE 7.

E3330 enhances IL-12 expression on the cell surface of BMDCs. BMDCs were pretreated with or without E3330 (50 μm) for 1 h and then stimulated with Pam3 (20 μg/ml) or LPS (100 ng/ml) for 48 h and stained for the cell surface expression of IL-12. A, flow cytometry profiles of CD11c+ DCs, representative of four independent experiments. B, scatter plots showing the percentages of IL-12 positive CD11c+ BMDCs. *, p < 0.05; ***, p < 0.001.
A genetically engineered membrane-bound form of IL-12 is reported to be active (16, 17). To confirm that the membrane-bound IL-12 retains its cytokine function, we stimulated splenic CD4+ T cells with various concentrations of plate-bound IL-12 and plate-bound anti-CD3 and anti-CD28 antibodies. The solid-phase IL-12 promoted the differentiation of CD4+ T cells to IFN-γ-producing Th1 cells in a dose-dependent manner (Fig. 8). These results strongly suggested that instead of secreted, soluble IL-12, the IL-12 expressed on the APC surface enhanced Th1 differentiation.
FIGURE 8.

Solid-phase IL-12 promotes the induction of IFN-γ-producing CD4+ T cells. Purified CD4+ T cells were stimulated with various concentrations of plate-bound IL-12, anti-CD3 Ab, and anti-CD28 Ab for 72 h in the presence of IL-2 (10 units/ml). The cells were re-stimulated with PMA, ionomycin, and brefeldin A for the last 4 h of culture and then stained for the cell surface expression of CD3 and CD4 and intracellular expression of IFN-γ. A, flow cytometry profiles of the CD3+/CD4+ cells, representative of two independent experiments. B, representative graph showing the percentages of IFN-γ-producing CD3+/CD4+ T cells. Horizontal axis shows the IL-12 concentration used for pretreating the plates.
E3330 Enhances the Pam3-induced Activity of p38 MAPK, an Upstream Regulator of IL-12 Gene Expression
To gain insight into the intracellular signaling mechanisms that mediate the E3330-enhanced activation of IL-12 genes in BMDCs, we focused on the mitogen-activated protein kinase (MAPK) signaling pathway, one of the most conserved signaling pathways in mammalian cells. MAPKs consist of three major subgroups: the p38 MAPKs, extracellular-regulated kinases (ERKs), and c-Jun N-terminal kinases (JNKs). The MAPKs play various roles in regulating Th1/Th2 balance (18), and p38 MAPK is specifically involved in the regulation of IL-12 gene expression (19). Therefore, we investigated the effect of E3330 on p38 MAPK phosphorylation in BMDCs, and found that E3330-pretreated BMDCs exhibited enhanced p38 MAPK phosphorylation after Pam3 stimulation (Fig. 9A). This finding indicated that the redox function of Ape1/Ref-1 might inhibit the TLR ligand-induced p38 MAPK activation and IL-12 gene expression in APCs.
FIGURE 9.

E3330 treatment or Ape1/Ref-1 knockdown enhances the Pam3-stimulated p38 MAPK activation in BMDCs. BMDCs were pretreated with or without E3330 (50 μm) for 1 h (A) or infected with control or Ape1/Ref-1 knockdown shRNA expressing lentivirus as described under “Experimental Procedures” (B), and then stimulated with Pam3 (20 μg/ml) for the indicated time periods. Whole cell extracts were analyzed by immunoblotting with an anti-phospho-p38 MAPK Ab (upper panels) and anti-p38 MAPK Ab (lower panels). These data are representative of three independent experiments.
Ape1/Ref-1 Knockdown Enhances the TLR Ligand-induced IL-12 Cell Surface and mRNA Expression in BMDCs
To confirm the function of Ape1/Ref-1 in APCs, we performed Ape1/Ref-1 knockdown experiments with lentiviral vectors expressing Ape1/Ref-1-specific short hairpin RNA (shRNA). First, we examined the knockdown efficiency of the Ape1/Ref-1 shRNA by monitoring the Ape1/Ref-1 protein levels in the virally infected BMDCs with an immunoblot assay. BMDCs infected with the Ape1/Ref-1 shRNA-encoding virus expressed lower levels of Ape1/Ref-1 protein on days 5 to 8 after infection, whereas BMDCs infected with control shRNA virus expressed similar levels of Ape1/Ref-1 as the uninfected control cells (Fig. 10A). The same membrane probed with an anti-actin antibody showed a similar expression of actin in each sample. The Ape1/Ref-1 knockdown BMDCs also expressed enhanced membrane-associated IL-12 (Fig. 10, B and C) and IL-12 transcript levels after Pam3 stimulation (Fig. 10D).
FIGURE 10.
Ape1/Ref-1 knockdown enhances the Pam3-stimulated IL-12 surface expression and IL-12 gene expression in BMDCs. A, uninfected (none) or Ape1/Ref-1 shRNA (Ape1 sh) or control shRNA (cont. sh) expressing lentivirus-infected BMDCs were analyzed by immunoblotting using an anti-Ape1/Ref-1 Ab (upper panel) and an anti-actin Ab (lower panel). B and C, lentivirus-infected BMDCs were stimulated with Pam3 (20 μg/ml) for 48 h and stained for IL-12 surface expression. B, flow cytometry profiles of the CD11c+ BMDC, representative of four independent experiments. C, scatter plots showing the percentages of IL-12 positive CD11c+ BMDCs. *, p < 0.05; ***, p < 0.001. D, lentivirus-infected BMDCs were stimulated with Pam3 (20 μg/ml) for 24 h. The cells were then harvested and the IL-12 mRNA levels were analyzed by real-time quantitative RT-PCR. The IL-12 mRNA values were normalized to that of the untreated (vehicle) control virus-infected cells. **, p < 0.01; NS, not significant.
Ape1/Ref-1 Knockdown Enhances the TLR Ligand-stimulated p38 MAPK Activation in BMDCs
To confirm the inhibitory effect of the redox function of Ape1/Ref-1 on p38 MAPK signaling in BMDCs, we analyzed the p38 MAPK activation in Ape1/Ref-1 knockdown BMDCs. Similar to the results obtained with the E3330-pretreated BMDCs, the p38 MAPK activation was enhanced in the Ape1/Ref-1 knockdown BMDCs upon Pam3 stimulation (Fig. 9B).
Discussion
Here we demonstrated that Ape1/Ref-1 modulated the helper T cell activation and differentiation through the modification of APC functions. The redox activity of Ape1/Ref-1 may regulate IL-12 production in BMDCs by two different mechanisms, one of which involves the transcriptional inhibition of both Il12a and Il12b, possibly through the inhibition of p38 MAPK activity, whereas the other involves the activation of IL-12 release from the cell surface.
E3330, a specific inhibitor of the redox activity of Ape1/Ref-1, or the knockdown of Ape1/Ref-1 enhanced the TLR ligand-induced p38 MAPK activity and both IL-12 gene expressions. Inhibition of p38 MAPK activity using its specific inhibitor SB203580 reduced the E3330-enhanced IL-12 gene activations.3 This result further supports the idea that inhibiting the redox activity of Ape1/Ref-1 enhanced the p38 MAPK activation, thereby enhancing the IL-12 gene activations.
Because Ape1/Ref-1 is a multifunctional protein, it may regulate TLR signaling through molecules other than the p38 MAPK. For example, Ape1/Ref-1 is known to be required for AP-1 activity. Interestingly, TLR signaling-induced c-Fos expression and AP-1 activity appear to negatively regulate both IL-12 gene activations (20). Therefore, we analyzed the TLR ligand stimulation-induced activity of ERK1/2, which are upstream signaling molecules of c-Fos and AP-1. However, E3330 had no effect on this ERK1/2 activity (data not shown), suggesting that the inhibition of Ape1/Ref-1 by E3330 mainly affects the p38 MAPK signaling in the BMDCs.
IL-12 has both secreted and membrane-bound forms (14, 15). Given the divergent effects of E3330 on cell surface retention and secretion of IL-12 from BMDCs, we hypothesized that the inactivation of the redox activity of Ape1/Ref-1 may inhibit shedding of IL-12 from the cell surface. IL-6 receptor, which shares sequence homology with IL-12p40 (21), is shed from the cell surface by a disintegrin and metalloprotease 17 (ADAM17), a member of the ADAM metalloprotease family that was originally identified as TACE (TNF-α converting enzyme), or ADAM10 (22, 23). Therefore, a similar mechanism may be involved in IL-12 shedding from the cell surface. Thus, the inactivation of the redox activity of Ape1/Ref-1 may inhibit extracellular proteases, such as ADAM family enzymes, resulting in the accumulation of IL-12 on the cell surface. Further studies will be required to clarify the mechanism by which Ape1/Ref-1 controls the release of IL-12 from the cell surface.
Cell surface-expressed IL-12 appears to be active, given that a genetically engineered membrane-bound form of IL-12 is biologically active (16, 17), and naïve Th cell activation requires cell-cell interaction with APCs via an immunological synapse. Furthermore, our finding that plate-bound IL-12 functions as a Th1-inducing cytokine strongly supports this possibility. Although we do not know whether the cell surface-expressed IL-12 is more effective for promoting the differentiation of Th1 cells than secreted IL-12, it is likely to be responsible for Th1 cell differentiation under physiological conditions.
The Ape1/Ref-1 redox function-specific inhibitor E3330 has therapeutic potential for many tumors (24). Our finding that the inhibition of the redox function of Ape1/Ref-1 enhances the IFN-γ-producing Th1 response may reveal one of the mechanisms behind the E3330-induced anti-tumor activity in vivo. Ape1/Ref-1 redox suppression by small molecule inhibitors, such as E3330, may be a useful therapeutic approach for inducing anti-tumor immunity.
Experimental Procedures
Mice
C57BL/6J mice were purchased from CLEA Japan. OT-II transgenic mice (OT-II mice) (25), which express T cell receptor (TCR) α and β chains that recognize the MHC class II Ib-restricted OVA peptide (residues 323–339) in a C57BL/6J background, were kindly provided by Dr. W. Heath (WEHI, Melbourne, Australia). The mice were bred at the animal facilities of Yamagata University, Faculty of Medicine, under specific pathogen-free conditions and were used for experiments at 6–12 weeks of age. All of the animal experiments were approved by the Animal Experiment Committee of Yamagata University, Faculty of Medicine.
Reagents
E3330 was prepared as described previously (26). The following reagents were used in this study: the synthetic OVA-(323–339) peptide (ISQAVHAAHAEINEAGR, AnaSpec), synthetic triacylated lipoprotein-TLR1/2 ligand, Pam3CSK4 (InvivoGen), recombinant murine GM-CSF, recombinant murine IL-12 and recombinant human IL-2 (PeproTech), phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS, Escherichia coli serotype 0127:B8), ionomycin calcium salt from Streptomycin conglobates, protease inhibitor mixture, phosphatase inhibitor mixture II, and phosphatase inhibitor mixture III (Sigma), and BD GolgiPlugTM Protein Transport Inhibitor (brefeldin A, BD Biosciences).
Preparation of Splenocytes and Isolation of CD4+ T Cells
Mouse spleen was homogenized with a glass homogenizer. The cell suspensions were passed through nylon mesh, and the cells were suspended in RPMI 1640 medium containing l-glutamine and 25 mm HEPES and supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), 50 μm 2-mercaptoethanol, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (complete RPMI 1640 medium). CD4+ T cells were isolated using BDTM IMag anti-mouse CD4 particles (BD Biosciences) according to the manufacturer's instructions. To activate CD4+ T cells with Abs, purified CD4+ T cells were treated with anti-CD3ϵ (plate-bound, 10 μg/ml; catalog number 100314, lot number B133074, BioLegend) and anti-CD28 (2 μg/ml; catalog number 16-0281-85, lot number E06391-1637, eBioscience) Abs.
To investigate whether solid-phase IL-12 functions as a cytokine, we pre-treated culture plates with recombinant murine IL-12 for 2 h at 37 °C. The IL-12 was washed out completely, and the plates were further coated with anti-CD3ϵ and anti-CD28 Abs. After rinsing these Abs, purified splenic CD4+ T cells were cultured in the pretreated plates in the presence of IL-2 (10 units/ml).
Preparation of BMDCs in Vitro
BMDCs were prepared as previously described (27). Briefly, bone marrow cells were isolated from the femurs and tibias of 5–10-week-old mice by flushing with PBS. The red blood cells were lysed using ACK buffer, and the cells were cultured in bacterial dishes in complete RPMI 1640 medium containing 10 ng/ml of murine GM-CSF. The medium was replaced every 2 or 3 days, and the BMDCs that were generated after 6–8 days of culture were used in experiments.
Flow Cytometry Analysis
Intracellular cytokine staining and flow cytometry analysis of T cells were performed as described previously (28). T cells were stimulated with 25 ng/ml of PMA and 1 μm ionomycin in the presence of 1 μg/ml of brefeldin A for the last 4 h of culture, followed by cell surface FITC-anti-CD3 (1:50; catalog number 100204, lot number B172039, BioLegend) and PE-Cy7-anti-CD4 (1:50; catalog number 100422, lot number B196264, BioLegend) Ab staining for 30 min on ice, and permeabilization in 100 μl of BD Cytofix/Cytoperm solution (BD Biosciences) for 20 min at 4 °C. The permeabilized cells were washed with BD Perm/Wash buffer (BD Biosciences) and incubated with an APC-anti-IFN-γ (1:50; catalog number 505810, lot number B149785, BioLegend) or APC-rat IgG1 kappa isotype-matched control (1:50; catalog number 400412, lot number B191545, BioLegend) Ab for 30 min at 4 °C. These cells were washed with BD Perm/Wash buffer and resuspended in the same buffer. To analyze the MHC-II, CD80, and CD86 expression, BMDCs were stained with PerCP-Cy5.5-anti-CD11c (1:50; catalog number 117327, lot number B190178, BioLegend), APC-anti-MHC-II (1:50; catalog number 107613, lot number B191784, BioLegend), or APC-rat IgG1κ isotype-matched control (1:50; catalog number 400412, lot number B191545, BioLegend) and FITC-anti-CD80 (1:50; catalog number 104705, lot number B172060, BioLegend), FITC-anti-CD86 (1:50; catalog number 105109, lot number B154233, BioLegend), or FITC-rat IgG2aκ isotype-matched control (1:50; catalog number 557228, lot number 0000035937, BD Biosciences) Abs. To analyze the cell surface IL-12 expression, BMDCs were stained with PerCP-Cy5.5-anti-CD11c (1:50; catalog number 117327, lot number B190178, BioLegend), APC-anti-IL-12 p40 (1:50; catalog number 505205, lot number B170873, BioLegend), or APC-rat IgG1κ isotype-matched control (1:50; catalog number 400412, lot number B191545, BioLegend) Abs. These cells were washed and resuspended in 3% (v/v) FCS-0.2 mg/ml NaN3-PBS. Cells were analyzed using a FACSCanto II flow cytometer (BD Biosciences). The data were analyzed with FlowJo software (version 7.6.1, Tree Star).
CBA Analysis
Cytokines in the cell culture supernatants were analyzed by CBA (BD Biosciences) as described previously (29). A customized set of fluorescent anti-cytokine multiplex microbeads was obtained together with recombinant standards with which to prepare a standard curve for each cytokine. The assays were performed on a FACSCanto II flow cytometer, and the data were analyzed using FCAP Array software (version 1.0, BD Bioscience).
Quantitative Real-time RT-PCR Analysis
Total RNA isolation and quantitative RT-PCR were performed as described previously (30). Thermo-cycling was performed using a LightCycler (Roche Molecular Systems). The IL-12 p35 and p40 mRNA levels were expressed relative to the corresponding β-actin mRNA level. The primer sets used in the real-time RT-PCR were as follows: mouse IL-12 p35 sense, 5′-ATGTGTCAATCACGCTACCTCC-3′ and antisense, 5′-GAAGAAGTCTCTCTAGTAGCCAGG-3′; mouse IL-12 p40 sense, 5′-GGGACATCATCAAACCAGACCC-3′ and antisense, 5′-GCCTTTGCATTGGACTTCGG-3′; mouse β-actin sense, 5′-TGACAGGATGCAGAAGGAGA-3′ and antisense, 5′-GCTGGAAGGTGGACAGTGAG-3′.
Cell Extraction and Immunoblot Analysis
The cell extraction and immunoblot analysis were performed as described previously with some modifications (31). The cells were suspended in whole cell extraction buffer (10 mm phosphate buffer (pH 7.4), 1 mm EDTA, 400 mm KCl, 10% (v/v) glycerol, 1% (v/v) protease inhibitor mixture, 1% (v/v) phosphatase inhibitor mixture II and III, 5 mm NaF, 1 mm DTT, and 1 mm PMSF) and subjected to three freeze-thaw cycles. The extracted proteins were separated by SDS-PAGE and then transferred to Immun-blot PVDF membranes (Bio-Rad Laboratories). The membranes were blocked with 5% (w/v) nonfat milk in TBS supplemented with 0.05% (w/v) Tween 20, and then incubated with anti-phospho-p38 MAPK (Thr180/Tyr182, 1:1,000; catalog number 9211, lot number 6, Cell Signaling Technology) or anti-APE-1 (1:20,000; catalog number NB100–116, lot number C-3, Novus Biologicals) Abs, followed by incubation with HRP-conjugated anti-rabbit IgG (1:2,000; catalog number 7074, Cell Signaling Technology) or HRP-conjugated anti-mouse IgG (1:10,000; catalog number 7076, Cell Signaling Technology) secondary Abs, respectively. To reprobe the membranes, they were soaked in stripping buffer (62.5 mm Tris-HCl (pH 6.7), 2% (w/v) SDS, 100 mm 2-mercaptoethanol) and incubated for 30 min at 55 °C. The membranes were reprobed with anti-p38 MAPK (1:1,000; catalog number sc535, lot number G2304, Santa Cruz Biotechnology) or anti-actin (2 μg/ml; catalog number sc8432, lot number L0403, Santa Cruz Biotechnology) Abs, followed by incubation with HRP-conjugated anti-rabbit IgG (1:2,000; catalog number 7074, Cell Signaling Technology) or HRP-conjugated anti-mouse IgG (1:2,000; catalog number 7076, Cell Signaling Technology) secondary Abs, respectively. The signals were visualized using the ECL Prime detection system (Amersham Biosciences).
Knockdown of Ape1/Ref-1 Using an shRNA Lentivirus Vector
An Ape1/Ref-1-shRNA-containing plasmid pLKO.1-puro-Ape1/Ref-1 shRNA (NM_009687.1–1033s1c1) and pLKO.1-puro-Non-Mammalian shRNA control plasmid (shc002) were purchased from Sigma. The lentiviral packaging plasmids pRSV-Rev, pMD.G, and pMDLg/pRRE were kindly provided by Dr. N. Tanaka (Division of Immunology, Miyagi Cancer Center Research Institute, Natori, Japan). The three viral packaging plasmids and the Ape1/Ref-1 shRNA or shRNA control plasmid were transfected into 293T cells using the calcium-phosphate method. The culture supernatants were recovered, and the viral titers were assessed. BMDCs were incubated with the viral supernatants at an multiplicity of infection of 2 with 8 μg/ml of Polybrene for 8 h. Thirty-two hours after infection, 2 μg/ml of puromycin (Sigma) was added to the culture medium and the cells were further cultured for 7 days to select the virally infected BMDCs.
Statistical Analysis
The statistical analysis of pairwise comparisons between two or more samples was performed by Student's t test using the R software (version 3.2.2). p values <0.05 were considered statistically significant.
Author Contributions
N. Akhter conducted most of the experiments, analyzed the results, and wrote most of the paper. Y. T. conducted experiments on the flow cytometry analysis. H. N. and A. A. conducted cell culture experiments and BMDCs establishment. N. I. conducted experiments using OTII mice. N. Asao prepared E3330 and H. A. conceived the idea for the project, conducted the knockdown experiments, and wrote the paper with N. Akhter.
Acknowledgment
We thank Dr. N. Tanaka for the gifts of the retrovirus packaging plasmids.
This work was supported in part by Grant-in-Aid for Scientific Research (C) 22590432 and a grant from the Seizankai Medical Welfare Group and the Nanotechnology Platform Program (Molecule and Material Synthesis) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The authors declare that they have no conflicts of interest with the contents of this article.
A. Nasrin and H. Asao, unpublished observations.
- Ape1/Ref-1
- apurinic/apyrimidinic endonuclease1/redox factor-1
- OVA
- ovalbumin
- APCs
- antigen presenting cells
- Th1
- T helper cell type 1
- TLR
- Toll-like receptor
- DC
- dendritic cell
- E3330
- (E)-3-(2-[5,6-dimethoxy-3-methyl-1,4-benzoquinonyl])-2-nonylpropenoic acid
- BMDC
- bone marrow-derived DC
- Pam3
- Pam3CSK4
- PMA
- phorbol 12-myristate 13-acetate
- OT-II T cells
- CD4+ T cells of OT-II mice
- CBA
- cytometric bead array
- ADAM
- a disintegrin and metalloprotease.
References
- 1. Robson C. N., and Hickson I. D. (1991) Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 19, 5519–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demple B., Herman T., and Chen D. S. (1991) Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc. Natl. Acad. Sci. U.S.A. 88, 11450–11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xanthoudakis S., and Curran T. (1992) Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 11, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tell G., Quadrifoglio F., Tiribelli C., and Kelley M. R. (2009) The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid. Redox Signal. 11, 601–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhakat K. K., Mantha A. K., and Mitra S. (2009) Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid. Redox Signal. 11, 621–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merluzzi S., Moretti M., Altamura S., Zwollo P., Sigvardsson M., Vitale G., and Pucillo C. (2004) CD40 stimulation induces Pax5/BSAP and EBF activation through a APE/Ref-1-dependent redox mechanism. J. Biol. Chem. 279, 1777–1786 [DOI] [PubMed] [Google Scholar]
- 7. Masani S., Han L., and Yu K. (2013) Apurinic/apyrimidinic endonuclease 1 is the essential nuclease during immunoglobulin class switch recombination. Mol. Cell. Biol. 33, 1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee H. M., Yuk J. M., Shin D. M., Yang C. S., Kim K. K., Choi D. K., Liang Z. L., Kim J. M., Jeon B. H., Kim C. D., Lee J. H., and Jo E. K. (2009) Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J. Immunol. 183, 6839–6848 [DOI] [PubMed] [Google Scholar]
- 9. Juliana F. M., Nara H., Onoda T., Rahman M., Araki A., Jin L., Fujii H., Tanaka N., Hoshino T., and Asao H. (2012) Apurinic/apyrimidinic endonuclease1/redox factor-1 (Ape1/Ref-1) is essential for IL-21-induced signal transduction through ERK1/2 pathway. Biochem. Biophys. Res. Commun. 420, 628–634 [DOI] [PubMed] [Google Scholar]
- 10. Shimizu N., Sugimoto K., Tang J., Nishi T., Sato I., Hiramoto M., Aizawa S., Hatakeyama M., Ohba R., Hatori H., Yoshikawa T., Suzuki F., Oomori A., Tanaka H., Kawaguchi H., Watanabe H., and Handa H. (2000) High-performance affinity beads for identifying drug receptors. Nat. Biotechnol. 18, 877–881 [DOI] [PubMed] [Google Scholar]
- 11. Kelley M. R., Luo M., Reed A., Su D., Delaplane S., Borch R. F., Nyland R. L. 2nd, Gross M. L., and Georgiadis M. M. (2011) Functional analysis of novel analogues of E3330 that block the redox signaling activity of the multifunctional AP endonuclease/redox signaling enzyme APE1/Ref-1. Antioxid. Redox Signal. 14, 1387–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou G. M., Luo M. H., Reed A., Kelley M. R., and Yoder M. C. (2007) Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood 109, 1917–1922 [DOI] [PubMed] [Google Scholar]
- 13. Lenschow D. J., Walunas T. L., and Bluestone J. A. (1996) CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 [DOI] [PubMed] [Google Scholar]
- 14. Fan X., Sibalic V., Niederer E., and Wüthrich R. P. (1996) The proinflammatory cytokine interleukin-12 occurs as a cell membrane-bound form on macrophages. Biochem. Biophys. Res. Commun. 225, 1063–1067 [DOI] [PubMed] [Google Scholar]
- 15. Quinones M., Ahuja S. K., Melby P. C., Pate L., Reddick R. L., and Ahuja S. S. (2000) Preformed membrane-associated stores of interleukin (IL)-12 are a previously unrecognized source of bioactive IL-12 that is mobilized within minutes of contact with an intracellular parasite. J. Exp. Med. 192, 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji J., Li J., Holmes L. M., Burgin K. E., Yu X., Wagner T. E., and Wei Y. (2004) Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12. J. Gene Med. 6, 777–785 [DOI] [PubMed] [Google Scholar]
- 17. Chakrabarti R., Chang Y., Song K., and Prud'homme G. J. (2004) Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA). Vaccine 22, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 18. Re F., and Strominger J. L. (2001) Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276, 37692–37699 [DOI] [PubMed] [Google Scholar]
- 19. Feng G. J., Goodridge H. S., Harnett M. M., Wei X. Q., Nikolaev A. V., Higson A. P., and Liew F. Y. (1999) Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163, 6403–6412 [PubMed] [Google Scholar]
- 20. Liu J., Cao S., Kim S., Chung E. Y., Homma Y., Guan X., Jimenez V., and Ma X. (2005) Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Curr. Immunol. Rev. 1, 119–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gearing D. P., and Cosman D. (1991) Homology of the p40 subunit of natural killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin-6 receptor. Cell 66, 9–10 [DOI] [PubMed] [Google Scholar]
- 22. Althoff K., Reddy P., Voltz N., Rose-John S., and Müllberg J. (2000) Shedding of interleukin-6 receptor and tumor necrosis factor α: contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur. J. Biochem. 267, 2624–2631 [DOI] [PubMed] [Google Scholar]
- 23. Matthews V., Schuster B., Schütze S., Bussmeyer I., Ludwig A., Hundhausen C., Sadowski T., Saftig P., Hartmann D., Kallen K. J., and Rose-John S. (2003) Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 278, 38829–38839 [DOI] [PubMed] [Google Scholar]
- 24. Fishel M. L., and Kelley M. R. (2007) The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol. Aspects Med. 28, 375–395 [DOI] [PubMed] [Google Scholar]
- 25. Barnden M. J., Allison J., Heath W. R., and Carbone F. R. (1998) Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 [DOI] [PubMed] [Google Scholar]
- 26. Luo M., Delaplane S., Jiang A., Reed A., He Y., Fishel M., Nyland R. L. 2nd, Borch R. F., Qiao X., Georgiadis M. M., and Kelley M. R. (2008) Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid. Redox Signal. 10, 1853–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu W., Ventevogel M. S., Knilans K. J., Anderson J. E., Oldach L. M., McKinnon K. P., Hobbs M. M., Sempowski G. D., and Duncan J. A. (2012) Neisseria gonorrhoeae suppresses dendritic cell induced, antigen-dependent CD4 T cell proliferation. PLOS ONE 7, e41260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onoda T., Rahman M., Nara H., Araki A., Makabe K., Tsumoto K., Kumagai I., Kudo T., Ishii N., Tanaka N., Sugamura K., Hayasaka K., and Asao H. (2007) Human CD4+ central and effector memory T cells produce IL-21: effect on cytokine-driven proliferation of CD4+ T cell subsets. Int. Immunol. 19, 1191–1199 [DOI] [PubMed] [Google Scholar]
- 29. Takeda Y., Kaneda K., Jimma F., Shiobara N., Saniabadi A. R., and Wakabayashi I. (2013) Suppression of Th1 cytokine production by a peptide derived from C4b. Inflamm. Res. 62, 951–959 [DOI] [PubMed] [Google Scholar]
- 30. Araki A., Nara H., Rahman M., Onoda T., Li J., Juliana F. M., Jin L., Murata K., Takeda Y., and Asao H. (2013) Role of interleukin-21 isoform in dextran sulfate sodium (DSS)-induced colitis. Cytokine. 62, 262–271 [DOI] [PubMed] [Google Scholar]
- 31. Asao H., and Fu X. Y. (2000) Interferon-γ has dual potentials in inhibiting or promoting cell proliferation. J. Biol. Chem. 275, 867–874 [DOI] [PubMed] [Google Scholar]





