Abstract
Polo-like kinase 1 (Plk1) is a serine/threonine-protein kinase that has been implicated in mitosis, cytokinesis, and smooth muscle cell proliferation. The role of Plk1 in smooth muscle contraction has not been investigated. Here, stimulation with acetylcholine induced Plk1 phosphorylation at Thr-210 (an indication of Plk1 activation) in smooth muscle. Contractile stimulation also activated Plk1 in live smooth muscle cells as evidenced by changes in fluorescence resonance energy transfer signal of a Plk1 sensor. Moreover, knockdown of Plk1 in smooth muscle attenuated force development. Smooth muscle conditional knock-out of Plk1 also diminished contraction of mouse tracheal rings. Plk1 knockdown inhibited acetylcholine-induced vimentin phosphorylation at Ser-56 without affecting myosin light chain phosphorylation. Expression of T210A Plk1 inhibited the agonist-induced vimentin phosphorylation at Ser-56 and contraction in smooth muscle. However, myosin light chain phosphorylation was not affected by T210A Plk1. Ste20-like kinase (SLK) is a serine/threonine-protein kinase that has been implicated in spindle orientation and microtubule organization during mitosis. In this study knockdown of SLK inhibited Plk1 phosphorylation at Thr-210 and activation. Finally, asthma is characterized by airway hyperresponsiveness, which largely stems from airway smooth muscle hyperreactivity. Here, smooth muscle conditional knock-out of Plk1 attenuated airway resistance and airway smooth muscle hyperreactivity in a murine model of asthma. Taken together, these findings suggest that Plk1 regulates smooth muscle contraction by modulating vimentin phosphorylation at Ser-56. Plk1 activation is regulated by SLK during contractile activation. Plk1 contributes to the pathogenesis of asthma.
Keywords: cytoskeleton, excitation-contraction coupling (E-C coupling), intermediate filament, phosphorylation, signal transduction, smooth muscle
Introduction
The vimentin network of smooth muscle attaches to the membrane at the desmosome and connects to the dense bodies in the myoplasm, which enables vimentin filaments to mediate the intercellular and intracellular force transmission in smooth muscle (1–5). Recent studies suggest that vimentin undergoes phosphorylation at Ser-56, which has a role in regulating various cellular functions including smooth muscle contraction (4–9). Vimentin phosphorylation at Ser-56 regulates vimentin depolymerization and the spatial reorientation of vimentin filaments, which modulates the intercellular and intracellular force transmission and contraction in smooth muscle (2–5, 9–15). Vimentin phosphorylation at Ser-56 is catalyzed in part by p21-activated kinase (4, 5, 9), whereas vimentin dephosphorylation at this position is mediated by type 1 protein phosphatase in smooth muscle (16). However, other molecules may also regulate vimentin phosphorylation in smooth muscle.
Polo-like kinase 1 (Plk1)2 is a serine/threonine-protein kinase that has been implicated in mitosis and cytokinesis (17, 18). Our recent studies suggest that Plk1 is indispensable for smooth muscle cell proliferation. Plk1 regulates smooth muscle cell proliferation by controlling the mitogen-activated protein kinase pathway in response to activation of growth factors (19, 20). The role of Plk1 in smooth muscle contraction has not been previously investigated.
Structural analysis reveals that Plk1 is composed of a common N-terminal catalytic domain and a C-terminal regulatory domain with highly conserved sequences named polo-box domain (PBD) and an interdomain linker (18, 21). PBD is observed only in Plk and is involved in an autoregulatory mechanism and binding to substrates (17, 18, 21). In vitro biochemical studies suggest that the activation of Plk1 can be regulated by phosphorylation at Thr-210 (18, 21). When inactive, PBD binds to the catalytic domain, which inhibits the access of substrates to the catalytic domain. Plk1 phosphorylation at Thr-210 may induce the dissociation of PBD from the catalytic domain, increasing kinase activity (17, 18, 21, 22). Plk1 undergoes phosphorylation at Thr-210 during mitosis and cytokinesis, which may regulate its function in the processes (17, 23).
Ste20-like kinase (SLK) is a serine/threonine-protein kinase that has been implicated in spindle orientation and microtubule organization during mitosis (24, 25). The interaction of SLK with Plk1 in human cells is largely unknown.
In this study we find that Plk1 is indispensable for smooth muscle contraction. Plk1 regulates smooth muscle contraction by controlling vimentin phosphorylation at Ser-56. Furthermore, contractile stimulation induces Plk1 phosphorylation at Thr-210 and activation in smooth muscle. SLK is an upstream regulator of Plk1 in smooth muscle.
Results
Contractile Activation Induces Plk1 Phosphorylation at Thr-210 in Smooth Muscle
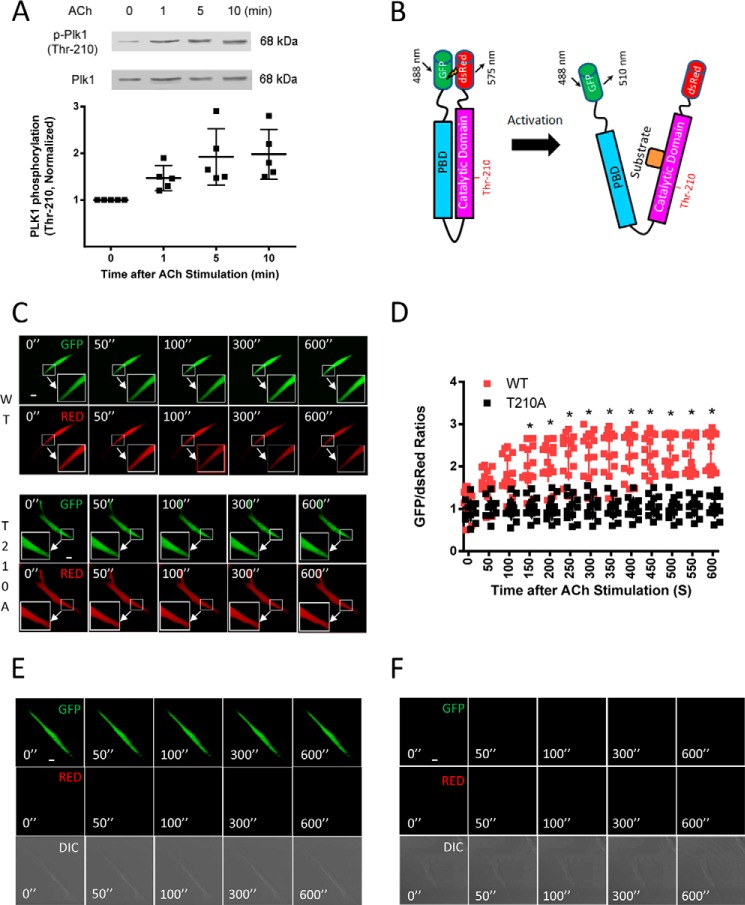
In vitro biochemical studies suggest that Plk1 is activated by phosphorylation at Thr-210 (18, 21). We used immunoblot analysis to determine whether contractile stimulation induces Plk1 phosphorylation at this residue. In unstimulated smooth muscle cells, phosphorylation of Plk1 at Thr-210 was low. Stimulation with acetylcholine (ACh) increased the phosphorylation level of Plk1 at Thr-210 in smooth muscle (Fig. 1A).
FIGURE 1.
Phosphorylation and activation of Plk1 in smooth muscle cells. A, smooth muscle cells were stimulated with ACh (10−4 m) for 1–10 min or left unstimulated. Plk1 phosphorylation at Thr-210 was evaluated by immunoblot analysis. Data are the mean values of experiments from five batches of cell culture. Error bars indicate S.D. B, schematic diagram of Plk1 biosensor. In inactive status, PBD binds to the catalytic domain forming a closed conformation. The closed conformation renders N-terminal DsRed and C-terminal GFP proximity, leading to high levels of FRET and lower GFP/DsRed ratios. Upon activation, Plk1 becomes an open conformation. The distance between the N-terminal DsRed and C-terminal GFP increases, which leads to low FRET signal and higher GFP/DsRed ratios. The open conformation facilitates the recruitment of substrate to the catalytic domain. C, representative images illustrating the effects of ACh stimulation (10−4 m) on emission of GFP and DsRed in HASM cells. The insets are the 4× magnification of the selected areas. WT, wild type Plk1; T210A, T210A Plk1; ”, seconds. D, stimulation with ACh increases the activity of WT Plk1 but not T210A Plk1. Ratios of GFP/DsRed fluorescence were determined for HASM cells in response to ACh stimulation (10−4 m). Increases in GFP/DsRed ratios indicate higher Plk1 activity. Data are the means ± S.D. (n = 15 cells from 4 batches of cell culture for WT Plk1 and 12 cells from 4 batches of cell culture for T210A Plk1). E, the GFP and DsRed emissions of cells expressing GFP-tagged Plk1 were evaluated using a confocal microscope. GFP emission was very high, whereas DsRed signal was not detected. In addition, ACh stimulation did not affect GFP/DsRed emission. Because no DsRed (RED) emission was detected, we failed to calculate the ratio of GFP/DsRed (n = 12 cells from 4 batches of cell culture). DIC, differential interference contrast. F, the emissions of GFP and DsRed of cells expressing DsRed-tagged construct were assessed. Neither GFP nor DsRed was found under the experimental conditions. ACh treatment did not affect the emission of GFP and DsRed. No GFP/DsRed ratios were calculated because of lack of fluorescent signals (n = 14 cells from 4 batches of cell culture). Scale bar: 10 μm.
Effects of ACh on Plk1 Activation in Live Smooth Muscle Cells
To monitor Plk1 activation in live cells, we constructed a fluorescence resonance energy transfer (FRET)-based Plk1 biosensor. We fused DsRed to the N terminus and GFP to the C terminus of Plk1 (Fig. 1B). We then evaluated the effects of contractile stimulation on Plk1 activation in live cells. Human airway smooth muscle (HASM) cells expressing the Plk1 sensor were treated with ACh, and emissions of DsRed and GFP were monitored live using a laser-scanning confocal microscope. Before stimulation, the emission ratios of GFP/DsRed were relatively lower. In response to ACh stimulation, the GFP signal was increased, whereas the DsRed signal was decreased (Fig. 1C). The GFP/DsRed ratios gradually increased during the course of contractile activation (Fig. 1D). These results suggest that contractile stimulation activates Plk1 in live smooth muscle cells.
To assess whether Thr-210 phosphorylation affects Plk1 activation in cells, we generated a T210A Plk1 biosensor (alanine substitution at Thr-210) and evaluated the effects of ACh stimulation on the activation of mutant Plk1. The emission ratios of GFP/dsRed for the T210A Plk1 biosensor were not increased in response to ACh stimulation (Fig. 1, C and D). The results indicate that Thr-210 phosphorylation regulates Plk1 activation in smooth muscle cells.
To further characterize the biosensor, cells expressing single-probe constructs (GFP-tagged construct or DsRed-tagged construct) were treated with ACh, and emission of GFP and DsRed was evaluated using the confocal microscope under the same experimental condition. For cells expressing the GFP construct, GFP emission was high, whereas DsRed emission was undetected. In cells expressing the DsRed construct, neither GFP nor DsRed emission was detected (Fig. 1, E and F). The results validate the selectivity and sensitivity of the biosensor.
Plk1 Is Required for Smooth Muscle Contraction
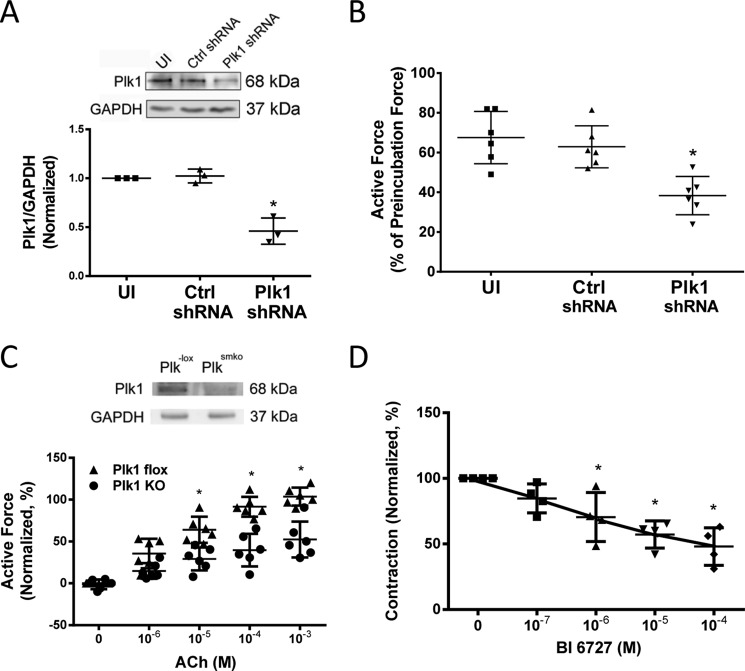
As described earlier, Plk1 is a serine/threonine-protein kinase that has a role in cytokinesis (17, 18) and smooth muscle cell proliferation (19, 20). The role of Plk1 in smooth muscle contraction is largely unknown. To assess its role, we utilized a lentivirus-mediated RNAi approach (26–28) to inhibit the expression of Plk1. Contractile response of human bronchial rings to ACh was evaluated. Bronchial rings were then transduced with lentiviruses encoding control shRNA or Plk1 shRNA. These tissues were incubated in the serum-free medium for 3 days. Contractile force of these tissues was then evaluated. Immunoblot analysis verified the lower expression of Plk1 in tissues transduced with viruses encoding Plk1 shRNA compared with uninfected rings and tissues transduced with viruses for control shRNA (Fig. 2A). More importantly, contractile responses of human bronchial rings were lower in Plk1-deficient tissues than in control tissues (Fig. 2B).
FIGURE 2.
Plk1 is necessary for smooth muscle contraction. A, human bronchial rings were transduced with lentiviruses encoding control shRNA or Plk1 shRNA. These tissues were then incubated in the serum-free medium for 3 days. Immunoblot analysis was used to assess protein expression in tissues. UI, uninfected. *, significantly lower protein ratios of Plk1/GAPDH in tissues transduced with virus encoding Plk1 shRNA than in uninfected tissues and tissues expressing control shRNA (p < 0.05). Data are the mean values of experiments from three donors. Error bars indicate S.D. B, contraction of human bronchial rings to ACh was evaluated, after which they were transduced with lentiviruses as described above. Contractile responses were compared before and after incubation. *, significantly lower contractile force in bronchial rings treated with Plk1 shRNA as compared with uninfected tissues or tissues infected with viruses encoding control shRNA (p < 0.05). Data are the mean values of 6 samples from 3 donors. Error bars indicate S.D. C, tracheal rings of Plk1smko mice and Plk1−lox mice were treated with difference concentration of ACh. Dose response of these rings was then evaluated. Contractile force is normalized to maximal force induced by 10−3 m ACh. Data are the mean values of experiments from six mice/each group. Error bars indicate S.D. *, significantly lower active force in Plk1smko mice than in Plk1−lox at corresponding doses (p < 0.05). Inserted immunoblots show Plk1 protein expression in airway smooth muscle cells from Plk1−lox and Plk1smko mice. Blots are representative of four identical experiments. D, tracheal rings of wild type mice were precontracted with 10 μm ACh. Different concentrations of BI6727 were then imposed to assess the relaxation response. Treatment with the pharmacological inhibitor induced relaxation of tracheal segments precontracted by ACh. Data are the mean values of experiments from four mice. Error bars indicate S.D. *, p < 0.05 versus contraction before the addition of BI6727.
To further evaluate the role of Plk1 in smooth muscle, we also generated smooth muscle conditional Plk1 knock-out mice. Contractile responses of mouse tracheal rings to ACh stimulation were compared between Plk1smko (smooth muscle knock-out of Plk1) mice and Plk1−lox (control) mice. As shown in Fig. 2C, contractile responses of mouse tracheal rings to ACh were lower in Plk1smko mice than in Plk1−lox mice, which was dose-dependent.
We also evaluated acute effects of the Plk1 pharmacological inhibitors BI6727 (29) on airway smooth muscle contraction. Treatment of mouse tracheal rings with BI6727 significantly induced relaxation of tracheal rings precontracted by ACh (Fig. 2D).
Plk1 Regulates Vimentin Phosphorylation at Ser-56 in Smooth Muscle and In Vitro
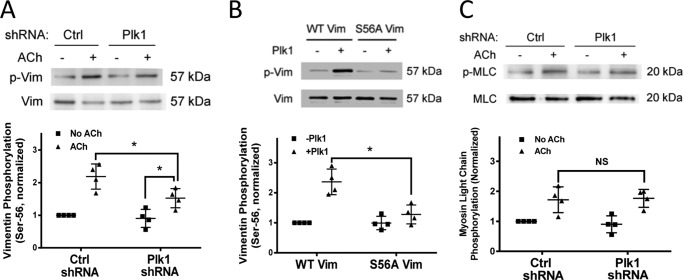
Recent studies suggest that vimentin undergoes phosphorylation at Ser-56, which has a role in regulating various cellular functions including smooth muscle contraction (3, 5–9). To determine whether Plk1 mediates vimentin phosphorylation, we evaluated the effects of Plk1 knockdown on vimentin phosphorylation in smooth muscle cells. In cells treated with control shRNA, acetylcholine stimulation induced vimentin phosphorylation at Ser-56. In contrast, the ACh-induced vimentin phosphorylation was reduced in smooth muscle treated with Plk1 shRNA (Fig. 3A). However, ACh-induced vimentin phosphorylation in Plk1 knockdown cells was higher compared with unstimulated Plk1 knockdown cells (Fig. 3A). The results suggest that Plk1 partially regulates vimentin phosphorylation at this residue in smooth muscle.
FIGURE 3.
Knockdown of Plk1 attenuates the ACh-induced vimentin phosphorylation at Ser-56 without affecting myosin light chain phosphorylation at Ser-19. A, smooth muscle cells expressing control shRNA or Plk1 shRNA were stimulated with ACh (10−4 m, 5 min) or left unstimulated. Vimentin phosphorylation at Ser-56 was evaluated by immunoblot analysis. Data are the mean values of four batches of cell culture. Error bars indicate S.D. (*, p < 0.05). B, purified active Plk1 (20 ng) and 1 μg of wild type vimentin or mutant S56A vimentin were placed in kinase buffer. Vimentin phosphorylation at Ser-56 was determined by immunoblot analysis 30 min after the initiation of the reaction. Vimentin phosphorylation catalyzed by Plk1 was normalized to the level of vimentin phosphorylation in the absence of Plk1. Data are the mean values of four in vitro biochemical experiments. Error bars indicate S.D. (*, p < 0.05). C, myosin light chain (MLC) phosphorylation at Ser-19 in cells transduced with lentivirus encoding control or Plk1 shRNA was assessed by immunoblot analysis. Myosin phosphorylation was similar in cells expressing control shRNA or Plk1 shRNA (NS, not significant). Data are the mean values of four batches of cell culture. Error bars indicate S.D.
We also used the in vitro kinase assay to assess whether Plk1 directly mediates vimentin phosphorylation. Phosphorylation of vimentin at Ser-56 was increased in the presence of Plk1. However, Plk1 did not enhance phosphorylation of S56A mutant vimentin (alanine substitution at Ser-56) (Fig. 3B) (9, 30). The results suggest that Plk1 mediates vimentin phosphorylation at Ser-56 in smooth muscle.
Plk1 Knockdown Did Not Affect Myosin Light Chain Phosphorylation at Ser-19
Because myosin light chain phosphorylation at Ser-19 is known to regulate smooth muscle contraction (12, 31, 32), we evaluated the effects of Plk1 knockdown on myosin light chain phosphorylation at Ser-19. The knockdown of Plk1 did not affect myosin light chain phosphorylation in smooth muscle (Fig. 3C).
Expression of the Plk1 Nonphosphorylatable Mutant (T210A Plk1) Inhibits Smooth Muscle Contraction and Vimentin Phosphorylation without Affecting Myosin Light Chain Phosphorylation
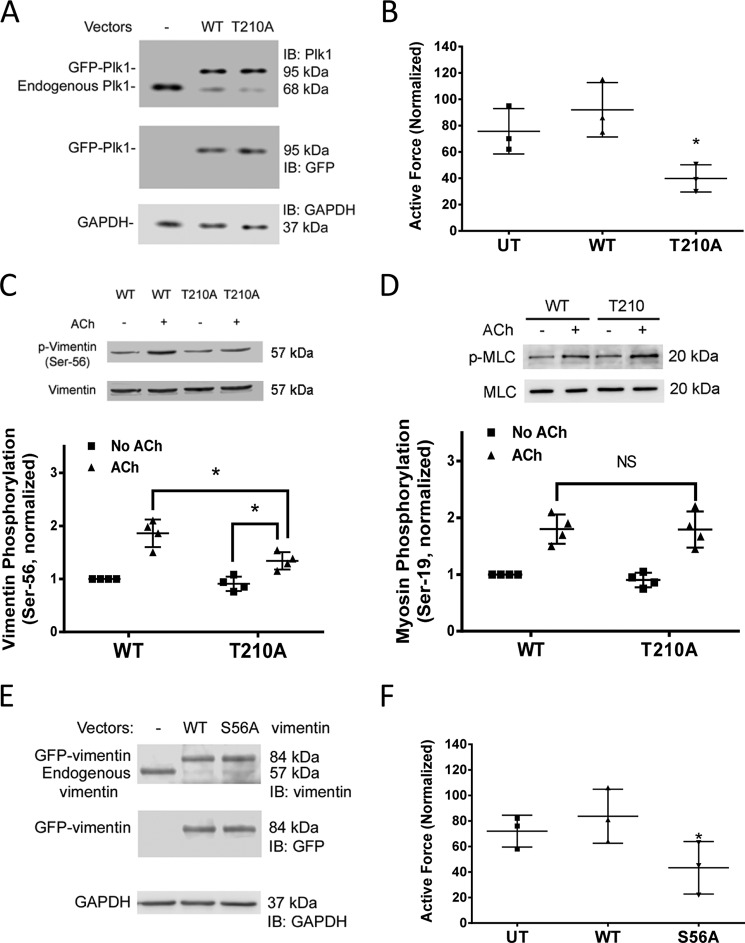
To determine the role of Plk1 phosphorylation at this residue, human bronchial rings were transfected with plasmids encoding T210A Plk1 using the method of reversible permeabilization (26, 28, 33–35). The tissues were cultured in the serum-free medium for 2 days to allow for protein expression. Immunoblot analysis confirmed the expression of the recombinant proteins (Fig. 4A). It was noticed that endogenous Plk1 was reduced in tissues expressing wild type (WT) or T210A Plk1. This could be because exogenous Plk1 mRNA competes with endogenous Plk1 mRNA for the same translational machine (9, 33). Contractile force of these tissues was compared before and after incubation. Contractile force was reduced in tissues expressing mutant T210A Plk1compared with tissues transfected with WT Plk1 (Fig. 4B).
FIGURE 4.
Roles of Plk1 phosphorylation at Thr-210 and vimentin Ser-56 phosphorylation in smooth muscle contraction. A, representative immunoblots (IB) illustrating the expression of WT Plk1 or T210A Plk1 mutant. Extracts of human bronchial tissues transduced with plasmids encoding WT Plk1 or T210A Plk1 mutant were immunoblotted with antibodies against Plk1, GFP, or GAPDH. GFP-Plk1 was detected in the extracts of tissues transduced with plasmid for WT or T210A Plk1. Blots are representative of experiments from three donors. B, contraction of human bronchial rings was evaluated, after which they were transduced with the plasmids as described under “Experimental Procedures.” Contractile responses were compared before and after incubation. *, significantly lower contractile force in bronchial rings expressing T210A Plk1 as compared with tissues transfected with WT Plk1 or untransfected (UT) tissues (p < 0.05). Data are the mean values of experiments from three donors. Error bars indicate S.D. C, cells expressing WT or T210A Plk1 were stimulated with ACh (10−4 m, 5 min) or left unstimulated. Vimentin phosphorylation at Ser-56 in the cells was evaluated using immunoblot analysis. Data are the mean values of experiments from four batches of cell culture. Error bars indicate S.D. (*, p > 0.05). D, myosin light chain (MLC) phosphorylation at Ser-19 in cells expressing WT or T210A Plk1 was assessed by immunoblot analysis. Myosin phosphorylation was similar in cells expressing WT or T210A Plk1 (NS, not significant). Data are the mean values of experiments from four batches of cell culture. Error bars represent S.D. E, representative immunoblots showing the expression of WT and S56A vimentin. Extracts of human bronchial tissues transduced with plasmids encoding WT or S56A vimentin were probed with antibodies against vimentin, GFP, or GAPDH. Blots are representative of experiments from three donors. F, contraction of tissues expressing S56A vimentin is reduced as compared with tissues treated with WT vimentin or UT tissues. Data are the mean values of experiments from three donors. Error bars indicate S.D.
We then determined the effects of mutant T210A Plk1 on vimentin phosphorylation at Ser-56 and myosin light chain phosphorylation. The ACh-induced vimentin phosphorylation was reduced in smooth muscle cells expressing Plk1T210A (Fig. 4C). In addition, ACh-induced vimentin phosphorylation in cells expressing T210A Plk1 was higher compared with basal vimentin Ser-56 phosphorylation in cells expressing T210A Plk1 (Fig. 4C). This indicates that vimentin phosphorylation at Ser-56 is regulated in part by Plk1 phosphorylation (activation). However, myosin light chain phosphorylation at Ser-19 was comparable in cells expressing WT or mutant Plk1 (Fig. 4D).
To determine the role of vimentin phosphorylation at Ser-56 in contraction, we introduced WT vimentin or S56A vimentin (alanine substitution at Ser-56) (9, 30) into human bronchial rings by reversible permeabilization. Immunoblot analysis verified the expression of the recombinant proteins (Fig. 4E). Moreover, endogenous vimentin was reduced in tissues treated with WT or S56A vimentin. This could be because exogenous vimentin mRNA competes with endogenous vimentin mRNA for the same translational machine (9, 33). More importantly, contractile force of tissues treated with S56A vimentin was reduced as compared with tissues expressing WT vimentin (Fig. 4F).
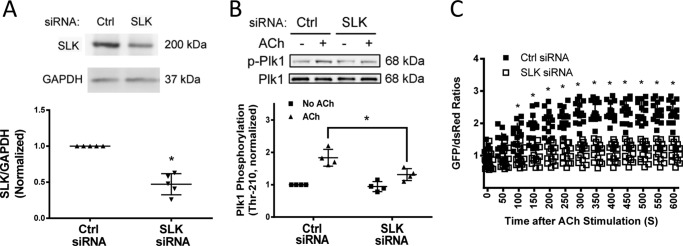
Knockdown of SLK Inhibits Plk1 Phosphorylation and Activation
SLK is a serine/threonine-protein kinase that has been implicated in spindle orientation and microtubule organization during mitosis (24, 25). To determine whether SLK has a role in regulating Plk1, smooth muscle cells were transfected with siRNA against SLK. Immunoblot analysis verified SLK knockdown in cells (Fig. 5A). SLK knockdown attenuated Plk1 phosphorylation at Thr-210 upon ACh stimulation (Fig. 5B). The emission ratios of GFP/DsRed for Plk1 biosensor was also reduced in SLK knockdown cells (Fig. 5C). The results suggest that SLK mediates Plk1 activation in smooth muscle during contractile activation.
FIGURE 5.
Knockdown of SLK inhibits the ACh-induced phosphorylation and activation of Plk1. A, extracts of smooth muscle cells transfected with control siRNA or SLK siRNA for 2 days were immunoblotted with antibodies against SLK or GAPDH. *, significantly lower protein ratios of SLK/GAPDH in cells treated with SLK siRNA than in cells treated with control siRNA (p < 0.05). Data are the mean values of experiments from five batches of cell culture. Error bars indicate S.D. B, smooth muscle cells transfected with control or SLK siRNA for days were stimulated with ACh (10−4 m, 5 min) or left unstimulated. Plk1 phosphorylation at Thr-210 was evaluated by immunoblot analysis. Data are the mean values of experiments from four batches of cell culture. Error bars indicate S.D. (*, p < 0.05). C, cells treated with control or Plk1 siRNA were transfected with plasmids for Plk1 sensor. These cells were stimulated with ACh (10−4 m) 2 days after transfection. The fluorescence of GFP/DsRed was captured live using a laser confocal microscope. The ratios of GFP/DsRed fluorescence were determined using the method described under “Experimental Procedures.” Values represent the means ± S.D. (n = 14 cells from 4 batches of each cell culture). *, Significantly different at each time point between cells treated with control siRNA and SLK KD cells (p < 0.05).
Conditional Knock-out of Plk1in Smooth Muscle Attenuates HDM-induced Airway Resistance and Contraction of Tracheal Rings
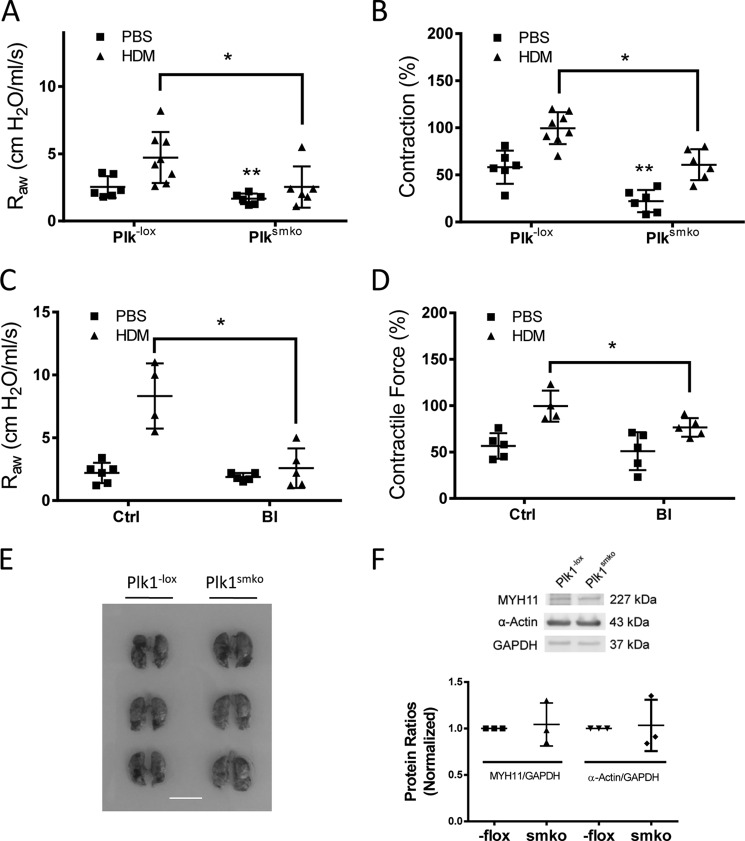
Asthma is characterized by airway hyperresponsiveness, which is largely attributed to hyperreactivity of airway smooth muscle (36, 37). We used the HDM-induced animal model of asthma to determine whether Plk1 in smooth muscle has a role in airway hyperresponsiveness. Briefly, Plk1−lox and Plk1smko mice were exposed to HDM for 6 weeks. Airway resistance in response to methacholine (MCh) inhalation was measured using the FlexiVent system (27, 37). HDM exposure induced a higher response to MCh inhalation in Plk1−lox mice as compared with Plk1−lox mice treated with PBS. However, airway resistance induced by MCh was lower in Plk1smko mice exposed to HDM than in Plk1−lox mice treated with HDM (Fig. 6A). In addition, airway resistance in Plk1smko mice treated with PBS was also lower than that in Plk1−lox mice treated with PBS (Fig. 6A).
FIGURE 6.
Knock-out or inhibition of Plk1 diminishes airway resistance and contractile response of tracheal rings from HDM-exposed mice. A, Plk1−lox and Plk1smko mice were exposed to HDM for 6 weeks. Airway resistance (RAW) in response to inhalation of 80 mg/kg MCh was then measured. The data represent the means ± S.D. (*, p < 0.05). **, significantly lower airway resistance in Plk1smko mice treated with PBS than in Plk1−lox mice treated with PBS (p < 0.05). n = 6–8 mice/each group. B, the contractile response of tracheal rings from these mice was determined by using an in vitro organ bath system. Contractile force is normalized to the mean maximal force of rings from HDM-treated Plk1−lox mice. The data represent the means ± S.D. (*, p < 0.05). **, significantly lower tracheal contraction of Plk1smko mice treated with PBS than Plk1−lox mice treated with PBS (p < 0.05). n = 6–8 mice/each group. C, C57BL/6J mice were exposed to HDM in the presence or absence of the Plk1 inhibitor BI6727 as described under “Experimental Procedures.” Intranasal instillation of BI6727 inhibits RAW in mice treated with HDM. The data represent the means ± S.D. (*, p < 0.05, n = 4–6 mice/group). D, treatment with the Plk1 inhibitor BI6727 attenuates HDM-sensitized tracheal contraction ex vivo. Contractile force is normalized to maximal force of rings from HDM- and vehicle-treated mice. Error bars indicate S.D. (*, p < 0.05, n = 4–5 mice/group). E, the size of lungs from Plk1smko mice is similar to that from Plk1−lox mice. Shown are images of lungs from 3 Plk1−lox mice and 3 Plk1smko mice. Scale bar, 1 cm. F, blots of tracheal smooth muscle tissues from Plk1−lox mice and Plk1smko mice were probed with antibodies against smooth muscle myosin heavy chain 11 (MYH11), α-actin. and GAPDH. Protein ratios of MYH11/GAPDH and α-actin/GAPDH from Plk1smko mice are normalized to those from Plk1−lox mice. The data represent the means ± S.D. (NS, not significant, n = 3 mice/group).
We also assessed the effects of Plk1 knock-out on airway smooth muscle hyperreactivity in vitro. Contractile force in isolated tracheal rings from HDM-treated Plk1−lox mice was greater compared with naïve Plk1−lox mice. However, active force of isolated tracheal rings from HDM-treated Plk1smko mice was reduced compared with HDM-treated Plk1−lox mice (Fig. 6B). Moreover, tracheal contraction of Plk1smko mice treated with PBS was reduced as compared with Plk1−loxmice treated with PBS (Fig. 6B).
Furthermore, we evaluated the effects of the Plk1 inhibitor BI6727 on airway resistance and contraction of isolated tracheal rings. Treatment with BI6727 attenuated the HDM-induced increase in airway resistance and airway smooth muscle hyperreactivity (Fig. 6, C and D).
Conditional Knock-out of Plk1 Does Not Affect the Size of Animal Lungs and Expression of α-Actin and Myosin Heavy Chain
Because Plk1 conditional knock-out attenuates airway resistance without allergen exposure (Fig. 6A), this raises a possibility that Plk1 knock-out could impair lung development, which results in smaller lungs. To test this, we compared the lung size of Plk1−lox mice and Plk1smko mice. The lung size of Plk1smko mice was similar to that of Plk1−lox mice (Fig. 6E). Furthermore, the expression of α-actin and myosin heavy chain in tracheal smooth muscle was comparable between Plk1−lox mice and Plk1smko mice (Fig. 6F). These results suggest that Plk1 conditional knock-out does not affect lung development and contractile protein expression in smooth muscle.
Discussion
Plk1 is a serine/threonine-protein kinase that has been implicated in cytokinesis, mitosis (17, 18), and smooth muscle cell proliferation (19, 20). The role of Plk1 in smooth muscle contraction has not been previously investigated. In this study, knockdown of human airway smooth muscle inhibited the agonist-induced contraction. Furthermore, smooth muscle conditional knock-out of Plk1 in mice also attenuated tracheal ring contraction. To the best of our knowledge, this is the first evidence to suggest that Plk1 is necessary for smooth muscle contraction.
Contractile activation induces myosin light chain phosphorylation at Ser-19, initiating cross-bridge cycling (31, 32). In addition, actin filament polymerization transpires in smooth muscle upon agonist stimulation, which promotes the force transmission between the contractile units and the extracellular matrix (12, 19, 33, 38–41). Furthermore, vimentin phosphorylation at Ser-56 occurs in response to contractile stimulation, facilitating reorganization of the vimentin network and intercellular/intercellular force transmission (1, 2, 4, 5). Thus, myosin may serve as an “engine” for smooth muscle contraction, whereas the actin cytoskeleton and the vimentin network may function as a “transmission system” in smooth muscle (1, 5, 26, 27). Actin dynamics in smooth muscle is largely regulated by protein tyrosine phosphorylation. Because Plk1 is a serine/threonine kinase (17, 21), we have assessed the role of Plk1 in vimentin phosphorylation and myosin light chain phosphorylation. Knockdown of Plk1 diminished the agonist-induced vimentin phosphorylation at Ser-56 but not myosin light chain phosphorylation at Ser-19. The results suggest that Plk1 regulates smooth muscle contraction by controlling vimentin phosphorylation.
Vimentin undergoes phosphorylation at Ser-56 in various cell types including smooth muscle cells/tissues (4–9). In smooth muscle cells, ∼65% of total vimentin gets phosphorylated at Ser-56 (30). Vimentin phosphorylation at Ser-56 affects the spatial reorientation of vimentin filaments, which may promote the intercellular and intracellular mechanical transduction (1, 5, 9, 10, 30). In addition, vimentin phosphorylation at this position regulates the redistribution of Crk-associated substrate, which indirectly modulates actin dynamics and contraction in smooth muscle (4, 5, 41, 42).
Plk1 undergoes phosphorylation at Thr-210 during cell division and stimulation of smooth muscle cells with growth factors (17, 20, 23). In the present study, ACh stimulation induced Plk1 phosphorylation at Thr-210, suggesting Plk1 activation during contractile activation. Furthermore, we provide the first evidence that contractile stimulation activated Plk1 in live smooth muscle cells by using the Plk1 biosensor. The results also provide direct evidence that contractile stimulation is able to induce conformational changes of Plk1 from a “closed” structure to an “open” conformation. When in closed state, PBD binds to the catalytic domain, which inhibits the access of substrates to the catalytic domain. Plk1 phosphorylation at Thr-210 may induce the dissociation of PBD from the catalytic domain, increasing kinase activity (17, 18, 21, 22). More importantly, the introduction of T210A Plk1 inhibited vimentin phosphorylation and contraction without affecting myosin light chain phosphorylation. Therefore, Plk1 phosphorylation at Thr-210 is critical for vimentin phosphorylation at Ser-56 and force development in smooth muscle.
The serine/threonine kinase SLK has been implicated in spindle orientation and microtubule organization during mitosis (24, 25). SLK may directly catalyze Plk1 phosphorylation at Thr-210 as evidenced by mass spectrometry analysis (43). Here, we showed that knockdown of SLK inhibited Plk1 phosphorylation at Thr-210 and activation in response to contractile activation. Thus, SLK is an upstream regulator of Plk1 in smooth muscle.
Abnormal airway smooth muscle contraction contributes to the pathogenesis of airway hyperresponsiveness, a cardinal characteristic of asthma (36, 37). IL-13 has been implicated in the hyperactivity of airway smooth muscle (44). In addition, c-Abl tyrosine kinase has been implicated in the pathogenesis of airway hyperresponsiveness (19, 37). Here, conditional knock-out of Plk1 or inhibition of Plk1 attenuated the allergen-induced airway hyperresponsiveness and airway smooth muscle hyperreactivity. These results suggest that Plk1 plays a critical role in the pathogenesis of airway hyperresponsiveness.
In summary, we unveiled a novel mechanism that is indispensable for smooth muscle contraction. In response to contractile activation, Plk1 is phosphorylated at Thr-210 and activated, which subsequently mediates vimentin phosphorylation at Ser-56. The phosphorylation of vimentin may facilitate intracellular and intercellular force transduction and smooth muscle contraction. Plk1 phosphorylation is regulated by SLK (Fig. 7).
FIGURE 7.

Role and regulation of Plk1 in smooth muscle. Contractile stimulation induces phosphorylation and activation of Plk1 mediating vimentin phosphorylation at Ser-56, which may facilitate intracellular/intercellular force transduction and smooth muscle contraction. Plk1 activation is mediated by SLK.
Experimental Procedures
Cell Culture
HASM cells were prepared from human bronchi and adjacent tracheas obtained from the International Institute for Advanced Medicine (26–28, 33, 45). Human tissues were non-transplantable and consented for research. This study was approved by the Albany Medical College Committee on Research Involving Human Subjects. Briefly, muscle tissues were incubated for 20 min with dissociation solution (130 mm NaCl, 5 mm KCl, 1.0 mm CaCl2, 1.0 mm MgCl2, 10 mm Hepes, 0.25 mm EDTA, 10 mm d-glucose, 10 mm taurine, pH 7, 4.5 mg of collagenase (type I), 10 mg of papain (type IV), 1 mg/ml BSA, and 1 mm dithiothreitol). All enzymes were purchased from Sigma. The tissues were then washed with Hepes-buffered saline solution (10 mm Hepes, mm 130 NaCl, 5 mm KCl, 10 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 0.25 mm EDTA, 10 mm taurine, pH 7). The cell suspension was mixed with Ham's F-12 medium supplemented with 10% (v/v) fetal bovine serum (FBS) and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin). Cells were cultured at 37 °C in the presence of 5% CO2 in the same medium. The medium was changed every 3–4 days until cells reached confluence, and confluent cells were passaged with trypsin/EDTA solution (9, 10, 45, 46). Smooth muscle cells within passage 5 were used for the studies.
Immunoblot Analysis
Cells were lysed in SDS sample buffer composed of 1.5% dithiothreitol, 2% SDS, 80 mm Tris-HCl, pH 6.8, 10% glycerol, and 0.01% bromphenol blue. The lysates were boiled in the buffer for 5 min and separated by SDS-PAGE. Proteins were transferred to nitrocellulose membranes. The membranes were blocked with bovine serum albumin or milk for 1 h and probed with the use of primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific). Proteins were visualized by enhanced chemiluminescence (Thermo Fisher Scientific) using the LAS-4000 Fuji Image System or GE Amersham Biosciences Imager 600 system.
Total Plk1 antibody (1:1000) was purchased from EMD Millipore (#05-844, L/N 2477015). Anti-SLK (1:250) was purchased from Santa Cruz Biotechnology (#sc-79068, L/N B2410). Anti-Plk1 and anti-SLK were validated by using corresponding knockdown cells. Phospho-vimentin (S56) antibody (1:500) was produced as previously described (9, 30) and validated by examining molecular weight of detected bands and higher intensity in stimulated cells. Vimentin antibody (1:2000) was purchased from BD Biosciences (#550515, L/N 3214517) and validated by examining molecular weight of detected bands. Phospho-Plk1 (T210) antibody (1:500) was purchased from Cell Signaling (#9062S, L/N 1) and validated by assessing molecular weight of detected bands and higher intensity in stimulated cells. Antibody against phospho-myosin light chain (Ser-19, 1:250) was purchased from Santa Cruz Biotechnology (sc-19849-R, L/N B2411) and validated by assessing molecular weight of detected bands and higher intensity in stimulated cells. Anti-myosin light chain (1:2000) was a gift of Dr. Gunst (34, 47) and validated by assessing molecular weight of detected bands. GFP antibody (1:3000) was purchased from Life Technologies (#G10362, L/N1696193) and validated by assessing molecular weight of detected bands. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:10,000) was purchased from Ambion (AM4300, L/N 1311029) and validated by assessing molecular weight of detected bands. MYH11 antibody (1:250) was purchased from Santa Cruz Biotechnology (#sc-6956, L/N C1815) and validated by examining molecular weight of detected bands. Finally, vendors have provided data sheet to show that antibodies were validated by positive controls. The levels of proteins were quantified by scanning densitometry of immunoblots (Fuji Multigauge Software or GE IQTL software). The luminescent signals from all immunoblots were within the linear range.
Virus-mediated RNAi
For Plk1 knockdown (KD), lentiviruses encoding Plk1 shRNA (sc-36277-V) or control shRNA (sc-108080) were purchased from Santa Cruz Biotechnology. HASM cells were infected with control shRNA lentivirus or Plk1 shRNA lentivirus for 12 h. They were then cultured for 3–4 days. Positive clones expressing shRNAs were selected by puromycin. Immunoblot analysis was used to determine the expression levels of Plk1 in these cells. Plk1 KD cells and cells expressing control shRNA were stable at least five passages after initial infection.
In Vitro Kinase Assay
Active Plk1 were purchased from EMD Millipore. Recombinant vimentin was produced by our laboratory as previously described (9). Active Plk1 (40 ng) and 1 μg of vimentin were placed in 20 μl of kinase buffer containing 20 mm HEPES, pH 7.5, 60 mm NaCl, 2 mm MgCl2, 5 mm EGTA, and 100 μm ATP. The kinase reaction mix was incubated at 30 °C for 30 min and stopped by the addition of the SDS sample buffer (9, 35). The samples were boiled for 5 min and separated by SDS-PAGE followed by membrane transfer. The membrane was probed with phospho-vimentin antibody, stripped, and reprobed with vimentin antibody.
Mutagenesis, Plasmid Purification, and Cell Transfection
T210A Plk1 (alanine substitution at Thr-210) was generated by using QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) as previously described (20). The template plasmid pcDNA3 3×FLAG-Plk1 was kindly provided by Dr. David Stern (Yale University). To construct Plk1 biosensor, Plk1 cDNA was produced by PCR using pcDNA3 3×FLAG-Plk1. The sequence of 5′-primer was 5′-CTCGAGCTATGAGTGCTGCAGTGACTGC-3′. The sequence of 3′-primer was 5′-GAATTCTTTAGGAGGCCTTGAGACGGTTGC-3′. The resulting product was ligated with pDsRed cDNA (Clontech), which was then subcloned into pEGFP (Clontech) followed by bacterial transformation. Plasmids were purified by using the Pureklink Quick Plasmid Miniprep kit (Invitrogen). DNA sequencing was performed by Genewiz Life Sciences.
For SLK knockdown, SLK siRNA (sc-76514) and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. Cell transfection was performed using the siRNA transfection reagent (Santa Cruz, sc-29528).
FRET Analysis
HASM cells cultured in glass-bottom dishes were transfected with plasmids encoding the Plk1 sensor using the FuGENE HD transfection reagent kit (Promega) and analyzed 2 days after transfection by using a laser scanning confocal microscope (Zeiss 510 Meta, C-Apochromat 40×/1.2 watt corr, objective). Briefly, cells were excited at wavelength of 488 nm, and emission of DsRed and GFP was simultaneously collected every 50 s. Exposure time was between 1 s and 3 s. Appropriate microscope setting (laser power level, detector gain, and amplifier gain) was used to minimize potential bleed-through. The same microscope setting was used for the experiments. For quantification of FRET efficiency, region of interest (ROI) of cells were positioned, and the fluorescent intensity of each channel was measured by Zeiss Analysis software. GFP/DsRed fluorescent ratios were used to assess the FRET efficiency.
Measurement of Human Bronchial Ring Contraction
The study was approved by Albany Medical College Institutional Review Board. Bronchial rings (diameter, 5 mm) were prepared from human lungs obtained from the International Institutes for Advanced Medicine (see above). Bronchial rings were placed in physiological saline solution (PSS) at 37 °C in a 25-ml organ bath and attached to a Grass force transducer that had been connected to a computer with A/D converter (Grass). For lentivirus-mediated RNAi in tissues, the thin epithelium layer of human bronchial rings was removed by using forceps. They were then transduced with lentivirus encoding Plk1 shRNA or control shRNA for 3 days. Force development in response to ACh (100 μm, 10 min) activation was compared before and after lentivirus transduction. For biochemical analysis, human tissues were frozen using liquid nitrogen and pulverized as previously described (3, 4, 26).
We used reversible permeabilization (34, 35, 47) to introduce the constructs of WT or mutant Plk1 into human bronchi. Briefly, contractile responses of human tissues were determined, after which they were placed in 0.5-ml tubes and incubated successively in each of the following solutions: Solution 1 (at 4 °C for 120 min) containing 10 mm EGTA, 5 mm Na2 ATP, 120 mm KCl, 2 mm MgCl2, and 20 mm TES; Solution 2 (at 4 °C overnight) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 2 mm MgCl2, 20 mm TES, and 10 μg/ml plasmids; Solution 3 (at 4 °C for 30 min) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 10 mm MgCl2, and 20 mm TES; Solution 4 (at 22 °C for 60 min) containing 110 mm NaCl, 3.4 mm KCl, 0.8 mm MgSO4, 25.8 mm NaHCO3, 1.2 mm KH2PO4, and 5.6 mm dextrose. Solutions 1–3 were maintained at pH 7.1 and aerated with 100% O2. Solution 4 was maintained at pH 7.4 and aerated with 95 %O2, 5% CO2. After 30 min in Solution 4, CaCl2 was added gradually to reach a final concentration of 2.4 mm. The tissues were then incubated in a CO2 incubator at 37 °C for 2 days in DMEM containing 5 mm Na2ATP, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml plasmids.
Animals and Measurement of Airway Resistance
All animal protocols were reviewed and approved by the Institutional Animal Care and Usage Committee of Albany Medical College. To generate smooth muscle conditional Plk1 knock-out mice (Plk1smko mice), Plk1−lox mice (from the Toronto Centre for Phenogenomics; genetic background, C57BL/6N) were crossed with B6.Cg-Tg (Myh11-cre,-EGFP) 2Mik/J mice (from The Jackson Laboratory, genetic background, C57BL/6J). Plk1−lox, Cre-, and Plk1smko mice (6–8 weeks old, male and female) were randomly exposed to HDM extracts (50 μg of Dermatophagoides pteronyssinus, Greer) or PBS (control) intranasally for 5 days followed by every other day exposures weekly for 5 weeks. Airway resistance in these mice was then assessed on day 43.
To measure airway resistance, mice were anesthetized with intraperitoneal injection of ketamine/xylazine mixture (320 mg/kg), tracheotomized, and connected to the FlexiVent system (SCIREQ, Montreal, Canada). Mice were mechanically ventilated at 150 breaths/min with a tidal volume of 10 ml/kg and a positive end-expiratory pressure of 3.35 cm H2O. After baseline measurements, mice were challenged with MCh aerosol for 10 s at different doses. Airway resistance was measured for each mouse after inhalation of the aerosol. Contractile response was then determined. This study was approved by the Institutional Committee of Animal Care and Usage of Albany Medical College.
To assess the effects of inhibitors, C57BL/6J mice were exposed to HDM extracts or PBS intranasally for 5 days followed by every other day exposures weekly for 5 weeks. In addition, animals were intranasally instilled with 10 mg/kg BI6727 (Santa Cruz Biotechnology, #sc-364432, L/N H1513) or PBS for 1 h before HDM instillation and 5 h after HDM instillation for last 2 weeks. Airway resistance in these mice was then assessed on day 43.
Each animal experiment was performed on monthly basis. The results were substantiated by repetition by at least two researchers. Animals were randomly treated with PBS or HDM. Animal experiments associated with the inhibitor were blindly performed. The inhibitor and vehicle control were coded and blinded to researchers. Animal experimental data were excluded if anesthesia was not appropriate, which can be detected by the FlexiVent software.
Assessment of Tracheal Ring Contraction
Mice were euthanized by intraperitoneal injection of euthanasia solution (VEDCO, 0.1 ml/25g). All experimental protocols were approved by the Institutional Animal Care and Usage Committee. A segment of tracheas (4–5 mm in length) was immediately removed and placed in PSS containing 110 mm NaCl, 3.4 mm KCl, 2.4 mm CaCl2, 0.8 mm MgSO4, 25.8 mm NaHCO3, 1.2 mm KH2PO4, and 5.6 mm glucose. The solution was aerated with 95 %O2, 5% CO2 to maintain a pH of 7.4. Two stainless steel wires were passed through the lumen of tracheal rings. One of the wires was connected to the bottom of organ baths, and the other was attached to a Grass force transducer that had been connected to a computer with A/D converter (Grass). Tracheal segments were then placed in PSS at 37 °C. At the beginning of each experiment, 0.5 g of passive tension was applied to tracheal rings. After 60 min of equilibrium they were stimulated with 80 mm KCl repeatedly until contractile responses and passive tension reached a steady state. Contractile force in response to acetylcholine was then measured.
Statistical Analysis
All statistical analysis was performed using Prism 6 software (GraphPad Software, San Diego, CA). Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. p < 0.05 was considered to be significant.
Author Contributions
J. L. performed physiological and biochemical experiments and drafted the manuscript. R. W. carried out cellular and biochemical studies. O. J. G., A. C. R., B. D. G., and S. J. performed the mouse experiments. G. L. and S. J. performed HASM cell experiments and molecular experiments. D. T. conceived and coordinated the study and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Rachel A. Cleary for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-110951, HL-113208, and HL-130304 (NHLBI; to D. D. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Plk1
- polo-like kinase 1
- ACh
- acetylcholine
- DsRed
- red fluorescent protein from Discosoma sp.
- HASM
- human airway smooth muscle
- HDM
- house dust mite
- PBD
- polo-box domain
- SLK
- ste20-like kinase
- PSS
- physiological saline solution
- TES
- N-tris (hydroxymethyl)methyl-2-aminoethane sulfonic acid
- MCh
- methacholine.
References
- 1. Garrod D. R., Merritt A. J., and Nie Z. (2002) Desmosomal adhesion: structural basis, molecular mechanism, and regulation. Mol. Membr. Biol. 19, 81–94 [DOI] [PubMed] [Google Scholar]
- 2. Small J. V., and Gimona M. (1998) The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiol. Scand. 164, 341–348 [DOI] [PubMed] [Google Scholar]
- 3. Wang R., Li Q., and Tang D. D. (2006) Role of vimentin in smooth muscle force development. Am. J. Physiol. Cell Physiol. 291, C483–C489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang R., Li Q. F., Anfinogenova Y., and Tang D. D. (2007) Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L240–L248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang D. D. (2008) Invited review: intermediate filaments in smooth muscle. Am. J. Physiol. Cell Physiol 294, C869–C878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee K. Y., Liu L., Jin Y., Fu S. B., and Rosales J. L. (2012) Cdk5 mediates vimentin Ser56 phosphorylation during GTP-induced secretion by neutrophils. J. Cell. Physiol. 227, 739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thaiparambil J. T., Bender L., Ganesh T., Kline E., Patel P., Liu Y., Tighiouart M., Vertino P. M., Harvey R. D., Garcia A., and Marcus A. I. (2011) Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 129, 2744–2755 [DOI] [PubMed] [Google Scholar]
- 8. Liu T., Ghamloush M. M., Aldawood A., Warburton R., Toksoz D., Hill N. S., Tang D. D., and Kayyali U. S. (2014) Modulating endothelial barrier function by targeting vimentin phosphorylation. J. Cell. Physiol. 229, 1484–1493 [DOI] [PubMed] [Google Scholar]
- 9. Li Q. F., Spinelli A. M., Wang R., Anfinogenova Y., Singer H. A., and Tang D. D. (2006) Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 281, 34716–34724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Q. F., Spinelli A. M., and Tang D. D. (2009) Cdc42GAP, reactive oxygen species, and the vimentin network. Am. J. Physiol. Cell Physiol. 297, C299–C309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q. F., and Tang D. D. (2009) Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am. J. Physiol. Cell Physiol. 297, C1424–C1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang D. D., and Anfinogenova Y. (2008) Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J. Cardiovasc. Pharmacol. Ther. 13, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green K. J., Getsios S., Troyanovsky S., and Godsel L. M. (2010) Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2, a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Loop F. T., Schaart G., Langmann H., Ramaekers F. C., and Viebahn C. (1995) Rearrangement of intercellular junctions and cytoskeletal proteins during rabbit myocardium development. Eur. J. Cell Biol. 68, 62–69 [PubMed] [Google Scholar]
- 15. Delva E., Tucker D. K., and Kowalczyk A. P. (2009) The desmosome. Cold Spring Harb. Perspect. Biol. 1, a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J., Wang R., and Tang D. D. (2016) Vimentin dephosphorylation at Ser-56 is regulated by type 1 protein phosphatase in smooth muscle. Respir. Res. 17, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petronczki M., Lénárt P., and Peters J. M. (2008) Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev. Cell 14, 646–659 [DOI] [PubMed] [Google Scholar]
- 18. Barr F. A., Silljé H. H., and Nigg E. A. (2004) Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell. Biol. 5, 429–440 [DOI] [PubMed] [Google Scholar]
- 19. Tang D. D. (2015) Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir. Res. 16, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang S., and Tang D. D. (2015) Plk1 regulates MEK1/2 and proliferation in airway smooth muscle cells. Respir. Res. 16, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J., Shen C., Wang T., and Quan J. (2013) Structural basis for the inhibition of Polo-like kinase 1. Nat. Struct. Mol. Biol. 20, 1047–1053 [DOI] [PubMed] [Google Scholar]
- 22. Jang Y. J., Ma S., Terada Y., and Erikson R. L. (2002) Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem. 277, 44115–44120 [DOI] [PubMed] [Google Scholar]
- 23. Archambault V., and Glover D. M. (2009) Polo-like kinases: conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10, 265–275 [DOI] [PubMed] [Google Scholar]
- 24. Zhapparova O. N., Fokin A. I., Vorobyeva N. E., Bryantseva S. A., and Nadezhdina E. S. (2013) Ste20-like protein kinase SLK (LOSK) regulates microtubule organization by targeting dynactin to the centrosome. Mol. Biol. Cell 24, 3205–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Machicoane M., de Frutos C. A., Fink J., Rocancourt M., Lombardi Y., Garel S., Piel M., and Echard A. (2014) SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation. J. Cell Biol. 205, 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang T., Cleary R. A., Wang R., and Tang D. D. (2013) Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J. Biol. Chem. 288, 20713–20722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang R., Cleary R. A., Wang T., Li J., and Tang D. D. (2014) The association of cortactin with profilin-1 is critical for smooth muscle contraction. J. Biol. Chem. 289, 14157–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang T., Wang R., Cleary R. A., Gannon O. J., and Tang D. D. (2015) Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction. J. Biol. Chem. 290, 8913–8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudolph D., Steegmaier M., Hoffmann M., Grauert M., Baum A., Quant J., Haslinger C., Garin-Chesa P., and Adolf G. R. (2009) BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res. 15, 3094–3102 [DOI] [PubMed] [Google Scholar]
- 30. Tang D. D., Bai Y., and Gunst S. J. (2005) Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem. J. 388, 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamm K. E., and Stull J. T. (1989) Regulation of smooth muscle contractile elements by second messengers. Annu. Rev. Physiol 51, 299–313 [DOI] [PubMed] [Google Scholar]
- 32. Somlyo A. V., Khromov A. S., Webb M. R., Ferenczi M. A., Trentham D. R., He Z. H., Sheng S., Shao Z., and Somlyo A. P. (2004) Smooth muscle myosin: regulation and properties. Philos. Trans. R. Soc. Lond B. Biol. Sci. 359, 1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang T., Cleary R. A., Wang R., and Tang D. D. (2014) Glia maturation factor-γ phosphorylation at Tyr-104 regulates actin dynamics and contraction in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 51, 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu Y., and Gunst S. J. (2015) Vasodilator-stimulated Phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism. J. Biol. Chem. 290, 11403–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anfinogenova Y., Wang R., Li Q. F., Spinelli A. M., and Tang D. D. (2007) Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ. Res. 101, 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amrani Y., Tliba O., Deshpande D. A., Walseth T. F., Kannan M. S., and Panettieri R. A. Jr. (2004) Bronchial hyperresponsiveness: insights into new signaling molecules. Curr. Opin. Pharmacol. 4, 230–234 [DOI] [PubMed] [Google Scholar]
- 37. Cleary R. A., Wang R., Wang T., and Tang D. D. (2013) Role of Abl in airway hyperresponsiveness and airway remodeling. Respir. Res. 14, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gunst S. J., and Zhang W. (2008) Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 295, C576–C587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim H. R., Gallant C., Leavis P. C., Gunst S. J., and Morgan K. G. (2008) Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am. J. Physiol. Cell Physiol. 295, C768–C778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rembold C. M., Tejani A. D., Ripley M. L., and Han S. (2007) Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am. J. Physiol. Cell Physiol. 293, C993–C1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang D. D. (2009) p130 Crk-associated substrate (CAS) in vascular smooth muscle. J. Cardiovasc. Pharmacol. Ther. 14, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jia L., and Tang D. D. (2010) Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am. J. Physiol. Cell Physiol. 299, C630–C637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson T. M., Antrobus R., and Johnson L. N. (2008) Plk1 activation by Ste20-like kinase (Slk) phosphorylation and polo-box phosphopeptide binding assayed with the substrate translationally controlled tumor protein (TCTP). Biochemistry 47, 3688–3696 [DOI] [PubMed] [Google Scholar]
- 44. Tliba O., Deshpande D., Chen H., Van Besien C., Kannan M., Panettieri R. A. Jr, and Amrani Y. (2003) IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br. J. Pharmacol. 140, 1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang R., Mercaitis O. P., Jia L., Panettieri R. A., and Tang D. D. (2013) Raf-1, actin dynamics, and Abl in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 48, 172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cleary R. A., Wang R., Waqar O., Singer H. A., and Tang D. D. (2014) Role of c-Abl tyrosine kinase in smooth muscle cell migration. Am. J. Physiol. Cell Physiol. 306, C753–C761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang Y., Zhang W., and Gunst S. J. (2011) Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J. Biol. Chem. 286, 3630–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]