Abstract
Small leucine-rich proteoglycans interact with other extracellular matrix proteins and are important regulators of matrix assembly. Fibromodulin has a key role in connective tissues, binding collagen through two identified binding sites in its leucine-rich repeat domain and regulating collagen fibril formation in vitro and in vivo. Some nine tyrosine residues in the fibromodulin N-terminal domain are O-sulfated, a posttranslational modification often involved in protein interactions. The N-terminal domain mimics heparin, binding proteins with clustered basic amino acid residues. Because heparin affects collagen fibril formation, we investigated whether tyrosine sulfate is involved in fibromodulin interactions with collagen. Using full-length fibromodulin and its N-terminal tyrosine-sulfated domain purified from tissue, as well as recombinant fibromodulin fragments, we found that the N-terminal domain binds collagen. The tyrosine-sulfated domain and the leucine-rich repeat domain both bound to three specific sites along the collagen type I molecule, at the N terminus and at 100 and 220 nm from the N terminus. The N-terminal domain shortened the collagen fibril formation lag phase and tyrosine sulfation was required for this effect. The isolated leucine-rich repeat domain inhibited the fibril formation rate, and full-length fibromodulin showed a combination of these effects. The fibrils formed in the presence of fibromodulin or its fragments showed more organized structure. Fibromodulin and its tyrosine sulfate domain remained bound on the formed fiber. Taken together, this suggests a novel, regulatory function for tyrosine sulfation in collagen interaction and control of fibril formation.
Keywords: collagen, extracellular matrix, fibromodulin, post-translational modification (PTM), protein assembly, tyrosine sulfation, fibrillogenesis
Introduction
Small leucine-rich proteoglycans (SLRPs)3 mediate important interactions with other components in the extracellular matrix of connective tissue, e.g. collagens, which give tissues their specific properties (1). In these proteins, the central leucine-rich repeat (LRR) domain comprises ∼80% of the protein and is composed of 10–12 repeats with leucine residues at conserved locations (2). This domain is flanked by N- and C-terminal motifs (3), containing conserved cysteine residues forming disulfide bonds.
The LRR domains bind to collagen and in many cases modulates collagen fibril formation in vitro (4–6). Indeed, collagen matrix assembly in vivo is altered upon SLRP gene inactivation. Fibromodulin-deficient mice show a larger proportion of thin collagen fiber bundles in weakened tendon and ligaments (7, 8), whereas lumican-deficient mice have fragile skin and opaque cornea with abnormally thick collagen fibrils (9), and keratocan-deficient mice show increased fibril diameters and less organized packing of collagen fibrils in the stroma (10). Aggravated phenotypes are seen in compound knock-out mice, where e.g. the fibromodulin/lumican double knock-out mice shows weak tendons and development of early osteoarthritis, probably due to destabilization of the joints (11, 12).
The N-terminal-extended domains confer unique properties to the different SLRP proteins, e.g. acidic properties with a variable number of tyrosine sulfate residues in the case of fibromodulin, osteoadherin, and lumican (13, 14) or basic with a cluster of Arg or Lys residues in PRELP (proline arginine-rich end LRR protein) (15, 16). Osteoadherin also contains a C-terminal extension carrying up to two additional tyrosine sulfates (13). Tyrosine sulfation is believed to enhance protein interactions, and is critical, e.g. for P-selectin binding to P-selectin glycoprotein ligand-1 in leukocyte extravasation (17). We have previously found that the tyrosine-sulfated N-terminal domain of fibromodulin mimics heparin in interacting with protein domains containing clusters of basic amino acids (18). Heparin and other oversulfated glycosaminoglycans have been shown to bind to different types of collagens (19) and influence collagen fibril formation in vitro (20, 21). This implies that the tyrosine sulfate domain of fibromodulin could interact with collagen. We now show that this domain contributes a third collagen binding site to fibromodulin, that it affects collagen fibril assembly, and that this is dependent on sulfated tyrosine residues.
Results
Fibromodulin Protein Variants
In the present study, we have used a number of different fibromodulin protein variants, purified from tissue or expressed as recombinant proteins. The different proteins are shown schematically in Fig. 1A, and a Coomassie Brilliant Blue-stained SDS-PAGE gel with the proteins is shown in Fig. 1B.
FIGURE 1.
The fibromodulin N-terminal extension binds collagen type I. A, illustration of the different variants of fibromodulin used in experiments. B, Coomassie Brilliant Blue-stained SDS-PAGE showing different variants of fibromodulin. C, solid-phase assay of fibromodulin binding to collagen. Fibromodulin variants (500 nm) were incubated in microtiter plate wells coated with acid-extracted collagen type I (black bars) and blocked with BSA, or control surfaces without collagen (white bars). Bound proteins were detected with antibodies specific for fibromodulin (tissue fibromodulin tFM_19–376 and tFM_19–98), or for His6 tag (recombinant mammalian 293-EBNA expressed rFM_19–376 and rFM_64–376). Data are presented as mean ± S.E. of three replicates from a representative experiment. Independent experiments showed similar results. D–F, surface plasmon resonance assay of fibromodulin binding to pepsin-extracted collagen type I. Pepsin-extracted collagen type I was immobilized on a carboxymethylated surface and D, tissue fibromodulin tFM_19–376. E, recombinant fibromodulin rFM_19–376 expressed in 293 EBNA cells or F, rFM_64–376 were injected at 500 or 250 nm at a flow rate of 35 μl/min and analyzed for interaction in a BIAcore 2000 instrument.
The names of these proteins or protein fragments reflect: 1) the origin of the protein (tissue purified or recombinant), 2) the included residue numbers, and 3) the absence of posttranslational modification when produced in Escherichia coli. Thus, full-length fibromodulin purified from tracheal cartilage is referred to as tFM_19–376 (starting at residue 19 because the signal peptide is cleaved off, and extending to the C terminus, i.e. residue 376), and rFM_19–376 is the corresponding recombinant protein produced in 293-EBNA cells. The recombinant protein rFM_64–376 is a truncated fibromodulin omitting the tyrosine sulfate domain and starting at amino acid Ala64, a previously identified matrix metalloproteinase 13 cleavage site in fibromodulin (22). The tFM_19–98 protein is the isolated tyrosine-sulfated N-terminal fragment of tFM_19–376, whereas its bacterially expressed equivalent was named rFM_19–98_0S to emphasize its lack of post-translational modification. Finally, tFM_33–74 is a tryptic fragment of tFM_19–98 containing the central part of the N-terminal extension with tyrosine sulfate residues.
The Fibromodulin N-terminal Extension Binds Collagen Type I
To determine whether the tyrosine-sulfated N-terminal extension of fibromodulin was involved in collagen interaction, acid-extracted collagen type I from mouse tail tendon was coated onto a plastic surface and the fibromodulin variants tFM_19–376, tFM_19–98, rFM_19–376, or rFM_64–376 were allowed to interact. All the fibromodulin variants showed binding to collagen type I, including the tFM_19–98 fragment (Fig. 1C). To verify and characterize the interaction with collagen type I, tFM_19–376, rFM_19–376, or rFM_64–376 were injected over pepsin-extracted collagen type I immobilized onto a BIAcore C1 surface plasmon resonance chip. The full-length tFM_19–376 (Fig. 1D) and rFM_19–376 (Fig. 1E) as well as the N terminally truncated rFM_64–376 (Fig. 1F) fibromodulin showed binding to the immobilized collagen molecules, confirming the solid phase results. The interactions showed distinct differences in both the association and dissociation phases. In particular the dissociation of full-length tFM_19–376 was much slower than that of truncated rFM_64–376, verifying contributions from both the N-terminal site and the LRR-domain sites in binding to the collagen molecule. This also demonstrates that binding via the N-terminal tyrosine sulfate-rich domain contributes to the overall affinity to collagen (Fig. 1, D and F). As the rFM_64–376 protein is expressed without keratan sulfate chains and can be purified under mild conditions, we compared rFM_19–376, expressed in the same system as the rFM_64–376, to the bovine cartilage derived tFM_19–376 carrying keratan sulfate (14). The two full-length variants showed similar binding to collagen type I (Fig. 1, D and E). As there are several binding sites, both for fibromodulin on the collagen molecule and for collagen on fibromodulin, it was not possible to calculate affinities. The tyrosine-sulfated N-terminal tFM_19–98 protein gave no detectable response in the BIAcore assay, perhaps reflecting charge repulsion from the carboxymethylated surface (data not shown).
The Tyrosine Sulfate-rich Domain of Fibromodulin Modulates Collagen Fibril Formation in Vitro and Is Retained on the Fibers
Fibrillogenesis of pepsin-extracted collagen in the presence of the tFM_19–98 fragment resulted in a shortened lag phase compared with fibrillogenesis of collagen alone (Fig. 2, A and B) and the effect on lag-phase was dose dependent (Fig. 5, A and C). The tFM_19–98 fragment had a very minor effect on the rate of turbidity increase (Hill slope), whereas the plateau level was unchanged (Fig. 2B). In contrast, adding the rFM_64–376 fragment to the collagen fibrillogenesis assay resulted in a slightly prolonged lag phase, along with a minor decrease in the rate of turbidity increase and a slightly delayed t50 (Fig. 2, A and B). As previously observed (5), tissue extracted full-length fibromodulin (tFM_19–376) slowed down the turbidity increase and lowered the plateau level in the fibrillogenesis assays, as did recombinant full-length rFM_19–376 (Fig. 2, A and B). Notably, both full-length fibromodulin proteins consistently shortened the lag phase, to the same extent as tFM_19–98 or more (Fig. 2, A and B). This shortening of the lag phase was reproducibly observed with several batches of full-length fibromodulin purified from different types of cartilage tissue (data not shown).
FIGURE 2.

Fibromodulin variants affect collagen fibril formation in vitro and are retained on the fibers. A, pepsin-extracted collagen type I was allowed to form fibrils at 37 °C, measuring turbidity at 405 nm over time in the absence (PBS) or presence of tissue-extracted or recombinant fibromodulin variants tFM_19–376, tFM_19–98, rFM_19_376, or rFM_64–376. For clarity, error bars (S.E.) are shown for every 4th data point. B, comparison of collagen fibril formation reactions (shown in panel A) in the presence of fibromodulin variants to collagen alone (PBS), expressed as graphs of lag phase length (tLag), inflection point (t50), final plateau level, and Hill slope of four-parameter sigmoidal curve fits. Data are shown as mean ± S.E. of six replicates from a representative experiment; *, p < 0.1; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 by one-way ANOVA and Tukey's multiple comparison test. C, after completion of the fibrillogenesis assay, collagen fibrils were collected by centrifugation. Samples taken before centrifugation (T), and supernatant (S) and pellet (P) after centrifugation were analyzed by SDS-PAGE and Western blot analysis for fibromodulin. D, collagen type I fibrils formed in vitro in the presence of fibromodulin tFM_19–376, tFM_19–98, or rFM_64–376 were visualized by negative staining electron microscopy and bound fibromodulin variants were detected with a gold-labeled antibody. E, acid-extracted collagen type I from mouse tail tendon (20 μg/ml) in PBS or in the presence of tissue-derived tFM_19–98 (0.29 μm) was allowed to form fibrils at 28 °C and turbidity was measured at 405 nm over time. F, lag phase length (tLag) from four-parameter sigmoidal curve fits of the collagen fibril formation reaction shown in panel E.
FIGURE 5.

Tyrosine sulfation is critical for the tFM_19–98 fragment effects on collagen fibril formation. A, pepsin-extracted collagen type I in PBS was allowed to form fibrils at 37 °C and turbidity was measured at 405 nm over time in the presence of tissue-derived tFM_19–98 (0, 0.7, or 3.6 μm). Error bars (S.E.) are shown for every 4th data point. B, fibrillogenesis of pepsin-extracted collagen type I in PBS or in the presence of bacterially expressed non-sulfated rFM_19–98_0S at 0, 0.4, 0.7, 1.4, 3.6, and 7.3 μm. Error bars (S.E.) are shown for every 5th data point. C, comparison of collagen fibril formation reactions (shown in panels A and B) in the presence of the tissue-derived tFM_19–98 (black bars) or bacterially expressed rFM_19–98_0S (gray bars) fragments or collagen alone (0, white bars) expressed as graphs of lag phase length (tLag), inflection point (t50), final plateau level, and Hill slope of four-parameter sigmoidal curve fits. Data are shown as mean ± S.E. of six replicates from a representative experiment. D, fibrillogenesis assay without or in the presence of the tFM_33–74 trypsin-generated fragment at 1, 2, or 4 μm. E, comparison of collagen fibril formations (shown in panel D) in the presence of the tFM_33–74 fragment (black bars), expressed as graphs of lag phase length (tLag), inflection point (t50), final plateau level and Hill slope of four-parameter sigmoidal curve fits. Data are shown as mean ± S.E. of five replicates from a representative experiment; *, p < 0.1; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 by one-way ANOVA and Tukey's multiple comparison test.
To determine whether the different fibromodulin variants remained bound to collagen fibrils, the presence of tFM_19–376, tFM_19–98, or rFM_64–376 was evaluated in pelleted collagen fibers and supernatants, respectively, after overnight fibril formation in the presence of the proteins. There was an approximately equal amount of the rFM_64–376 in the supernatant and pellet, whereas almost all tFM_19–376 and most tFM_19–98 was detected in the pellet (Fig. 2C). The presence of tFM_19–376, tFM_19–98, and rFM_64–376 on fibers of collagen type I after 24 h of incubation was verified by negative staining EM, using a gold-labeled fibromodulin-specific antibody (Fig. 2D). No staining was seen in the absence of fibromodulin (not shown).
To verify that the tyrosine-sulfated fibromodulin domain had an effect also on collagen with retained telopeptides, we performed a fibril formation assay using acid-extracted tail tendon collagen from 3-week-old mice. Due to the rapid polymerization of this collagen, the assay was performed at lower temperature (28 °C) and with lower concentrations of collagen and tFM_19–98. As shown in Fig. 2, E and F, tFM_19–98 shortened the polymerization lag phase without an effect on the plateau level or slope of the turbidity curve.
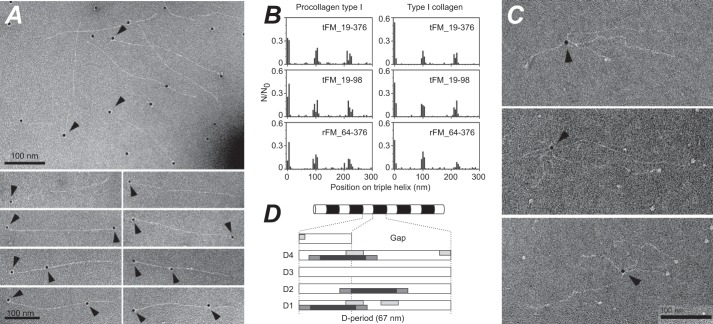
The Fibromodulin Tyrosine Sulfate-rich and LRR Domains Bind Separately to Collagen Monomers at Three Shared Binding Regions
Three fibromodulin binding sites along the collagen molecule were identified using gold-labeled antibody detection and negative staining EM (Fig. 3A). In a few cases, binding was observed to both termini of pepsin-treated collagen molecules (Fig. 3A). Full-length fibromodulin (tFM_19–376), the tyrosine sulfate-rich domain (tFM_19–98), and the LRR domain (rFM_64–376) showed similar binding patterns on pro-collagen type I, with one site at the N-terminal of the collagen triple helical part and additional sites at distances of 100 and 220 nm from the N-terminal, respectively (Fig. 3B, left). In parallel experiments with pepsin-treated collagen type I, the same binding sites were found (Fig. 3B, right). Neither the pepsin-extracted collagen, nor the procollagen, showed any reactivity to the anti-fibromodulin antiserum (not shown). The resolution of the electron microscopy method, when employing a gold-tagged antibody to detect binding, is 20 ± 5 nm, corresponding to about 70 amino acids along the triple helix, which does not allow conclusions on whether binding is to identical sites on the collagen molecule for all three fibromodulin variants. We did, however, find simultaneous binding of two individual fibromodulin molecules to separate binding sites on the same collagen molecule (Fig. 3A).
FIGURE 3.
Fibromodulin binds to collagen monomers. A, negative staining EM of pepsin-extracted collagen type I monomers in the presence of tFM_19–98, detected by a gold-labeled specific antibody against fibromodulin. B, histogram of the distribution of fibromodulin variants on pro-collagen type I (left panel, 0 nm represents the N terminus), or pepsin-extracted collagen type I (right panel). C, negative staining EM of procollagen type I in the presence of tissue fibromodulin tFM_19–376. Arrowheads mark simultaneous binding of fibromodulin to two separate procollagen type I monomers. Fibromodulin was detected with a gold-labeled specific antibody. D, schematic showing a collagen fiber (upper panel) with alternating overlap (white) and gap (black) zones, as seen by negative staining EM. In the lower panel, the identified fibromodulin binding sites are drawn to scale (20 nm black bar ± 5 nm dark gray bar) on the five overlapping collagen regions in a D-period. Light gray bars represent fibromodulin binding sites previously reported using peptides from collagen types II and III toolkits (34).
An informative observation from EM studies was that the full-length fibromodulin could bind two collagen molecules at the same time (Fig. 3C), showing that the two binding sites in the LRR and the tyrosine sulfate domain of fibromodulin can concomitantly bind different collagen molecules, which could be important in networking and assembly. The constructs representing the isolated N-terminal or LRR domains did not mediate such cross-linking (data not shown).
As shown in Fig. 2D, the fibromodulin constructs bound to the formed fibers, predominantly at the borders between overlap and gap regions. This is in agreement with the mapping of collagen binding sites (Fig. 3B) on the collagen fibril D-period, as illustrated in Fig. 3D.
The Tyrosine Sulfate-rich Domain Directs and Arranges the Collagen Monomers into Highly Organized Fibril Structures
To study the effects of the N-terminal and LRR domains of fibromodulin on collagen fibril formation, samples were collected from a fibrillogenesis experiment and visualized by negative staining electron microscopy. At time point 0 the collagen monomers were aggregated in the presence of all of the fibromodulin variants, resulting in engagement of 7–11 collagen chains (11–16 nm) compared with collagen alone, where no such aggregates could be detected (Table 1 and Fig. 4A, 0h). After 3 h incubation at 37 °C, all samples contained a second population of thicker fibers, in addition to the fibril population of 10–16 nm in diameter. In the presence of tFM_19–98 or rFM_64–376, this second population of fibrils was thicker with more variable diameter compared with the sample in the presence of full-length fibromodulin (tFM_19–376) or PBS. The latter two showed more uniform thinner diameter fibrils, but with differences in fibril maturation. As judged from the fibrillar cross-striation, samples containing tFM_19–376, tFM_19–98, or rFM_64–376 already showed a highly organized structure compared with the control reaction (Table 1 and Fig. 4A, 3h). In samples taken after 24 h of incubation at 37 °C, no fibrils of the 10–16-nm populations remained (Table 1). In all samples, the fibrils present had wider diameters and showed mature cross-striation (Table 1 and Fig. 4A, 24h). Measurement of collagen, precipitated by centrifugation (20,800 × g for 30 min) after 24 h of fibril formation, was in general agreement with this, with a minor decrease in hydroxyproline in the pellet of the rFM_64–376 samples, and no differences seen in the tFM_19–376 or tFM_19–98 samples (Fig. 4B).
TABLE 1.
Fibromodulin effects on collagen fibril formation
Collagen type I was incubated at 37 °C to form fibrils, in the presence of PBS or different variants of fibromodulin. Samples were taken after 0, 3, and 24 h incubation and analyzed by negative staining electron microscopy for diameter of present fibrils and cross-striation. Data are presented as mean ± S.D.
| Time | PBS | tFM_19–98 | rFM_64–376 | tFM_19–376 | |
|---|---|---|---|---|---|
| 0 h | Cross-striation | 0% | 0% | 0% | 0% |
| Diameter | 16 (±5 nm) | 14 (±3 nm) | 11 (±3 nm) | ||
| 3 h | Cross-striation | 27% | 68% | 79% | 82% |
| Diameter | 10 (±3 nm) | 16 (±5 nm) | 14 (±3 nm) | 10 (±3 nm) | |
| 42 (±15 nm) | 75 (±50 nm) | 63 (±40 nm) | 39 (±10 nm) | ||
| 24 h | Cross-striation | 83% | 77% | 96% | 94% |
| Diameter | 91 (±30 nm) | 82 (±20 nm) | 109 (±30 nm) | 71 (±10 nm) |
FIGURE 4.

Fibromodulin variants affect collagen fibril assembly. A, pepsin-extracted collagen type I was incubated at 37 °C to form fibrils in the presence of PBS or fibromodulin variants. Samples were taken at 0, 3, and 24 h and visualized by negative staining EM. Scale bar, 100 nm. B, hydroxyproline content measured in the pellet after 24 h of fibril formation with or without fibromodulin variants. Data are expressed as percentage of the sum of hydroxyproline in the pellet and supernatant and mean ± S.E. of three replicates is shown; *, p < 0.1, one-way ANOVA and Tukey's multiple comparison test.
Tyrosine O-Sulfation Is Critical for Fibromodulin Shortening of the Fibrillogenesis Lag Phase
To investigate the role of sulfated tyrosine residues in collagen fibril formation, a recombinant fragment was produced in E. coli (rFM_19–98_0S). This fragment corresponds exactly to the tFM_19–98 amino acid sequence, but contains no post-translational modifications, i.e. sulfation and cysteine disulfide bridge formation. Unlike the sulfated fragment from tissue-extracted protein, rFM_19–98_0S showed no binding to basic domains (18), even though it contains a number of acidic amino acids contributing to a negative charge at neutral pH. In solid phase assays, the purified rFM_19–98_0S fragment showed binding to collagen (data not shown), but adding the fragment in a collagen fibrillogenesis assay only resulted in a minor shortening of the fibril formation lag phase with no effects on the rate of fibrillogenesis or plateau turbidity (Fig. 5, B and C). In contrast, the corresponding fragment from tissue-derived fibromodulin had a significant and dose-dependent effect on both the lag phase and t50 (Fig. 5, A and C), confirming the involvement of post-translational modifications.
To verify that the effects on collagen fibril formation were dependent on sulfated tyrosine residues and not the cysteine-loop structure, a shorter fragment of the tyrosine-rich region, lacking the cysteine-loop, was generated by trypsin digestion of tFM_19–98 as described (18). Addition of this tFM_33–74 fragment, in the same molar range as the tFM_19–98 fragment, to the in vitro collagen fibril formation assay, shortened the lag phase and accelerated the fibrillogenesis process in a dose-dependent manner (Fig. 5, D and E). This clearly shows that the sulfated tyrosine residues are responsible for the effect on collagen fibril formation.
Variations in Tyrosine Sulfation Have Minor Influence on Collagen Fibrillogenesis
In bovine fibromodulin, nine of the N-terminal tyrosine residues are O-sulfated to varying degrees (13). Thus, in anion exchange chromatography, the N-terminal endoproteinase LysC fragment of tFM_19–376 elutes in six sharp peaks that contain identical protein cores but differ in the distribution and amount of O-sulfate (18). The sulfation variant fragments can either be pooled (as the tFM_19–98 preparation) or kept separated (peak 1–6) (Fig. 6A, inset). These different fractions were previously shown to have different affinity for heparin binding domains (18). To investigate if variations in tyrosine sulfation also affected the assembly of collagen monomers, the different peaks were added at equimolar concentration in the fibrillogenesis assay. Because O-sulfation changes the UV absorbance of the tyrosine residues, the concentration of the tFM_19–98 fragment or individual peaks was assayed by quantitative mass spectrometry.
FIGURE 6.

Effects of variation in sulfation pattern on collagen fibrillogenesis. A, pepsin-extracted collagen type I in PBS was allowed to form fibrils at 37 °C and turbidity was measured at 405 nm over time. Subpopulations of tissue-extracted tFM_19–98 that differ in the amount and distribution of tyrosine sulfation (Mono Q ion exchange peaks 1 to 6) were isolated by ion exchange chromatography (A, inset) and included at 1.4 μm in the fibrillogenesis reaction. Error bars (S.E.) are shown for every 2nd data point. B, comparison of collagen fibril formation reactions (shown in panel A) in the presence of the Mono Q peaks of tFM_19–98, expressed as graphs of time of lag phase length (tLag), inflection point (t50), final plateau level, and Hill slope of five-parameter sigmoidal curve fits. Data are shown as mean ± S.E. of five replicates in one experiment; *, p < 0.1, **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 by one-way ANOVA and Tukey's multiple comparison test.
All individual peak fractions shortened the fibril formation lag phase, and all except the least charged fraction (peak 1) increased the final plateau level (Fig. 6, A and B). Combining the 6 different peak fractions in the same relative volume proportions as in the tFM_19–98 pool also gave strong influence on the lag phase (data not shown). No significant effects were seen on the rate of turbidity increase. Taken together, this indicates that the degree of sulfation, rather than a specific sulfation pattern, is a critical parameter regarding the influence on the fibril formation.
Discussion
The small leucine-rich proteins, e.g. fibromodulin, decorin, asporin and lumican, are known to influence the fibril formation of collagens and in some of the cases the functional interaction sites have been identified (23–26). Studies on fibromodulin-deficient mice showed abnormal fused collagen fibrils in tendon and ligaments, resulting in weaker tendons and development of osteoarthritis due to instability of the joints (7, 8). These data clearly indicate that fibromodulin has a crucial role in the formation of proper collagen fibers in tissues where it is present. Tyrosine O-sulfation in the N-terminal domain of fibromodulin (13) has been reported to mimic heparin in binding to clusters of basic amino acids (18), and heparin and heparin-like over-sulfated glycosaminoglycans are known to bind to collagen and have an effect on the fibril formation in vitro (20, 21). In view of this, we searched for a similar function of this negatively charged domain of fibromodulin.
The isolated tyrosine sulfate-rich N-terminal domain of tissue-extracted fibromodulin (tFM_19–98) indeed bound collagen type I coated onto a plastic surface, as did full-length tissue-extracted fibromodulin (tFM_19–376) and N-terminally truncated recombinant fibromodulin (rFM_64–376). Previous studies have shown that tissue-extracted full-length fibromodulin inhibits collagen fibril formation in vitro (5). In the present study, collagen fibrillogenesis was also assayed in the presence of the isolated N-terminal tyrosine sulfate-rich domain. In this case the lag phase was shortened in a dose-dependent manner. This result is in line with reports on collagen fibril formation in the presence of negatively charged glycosaminoglycans such as CS-E or over-sulfated CS from the proteoglycan perlecan, which were shown to enhance the fibril formation of collagen type I in vitro and direct the formation of highly, organized fibrils (21). As expected, full-length tyrosine-sulfated fibromodulin inhibited the rate of turbidity increase and the final turbidity level in the collagen fibrillogenesis assay. In contrast to previous studies (5), we also observed a reproducible shortening of the lag phase by full-length tyrosine-sulfated fibromodulin. For comparison, rFM_64–376, a protein representing the LRR domain of fibromodulin, i.e. lacking the N-terminal tyrosine sulfate domain but containing the two previously described collagen interaction sites (27, 28), was generated and expressed in a mammalian system. As expected, this truncated protein did not shorten the collagen fibrillogenesis lag phase (Fig. 2, A and B). Although the fibromodulin effects on collagen fibril formation clearly depend on contributions from both the tyrosine sulfate domain and the LRR domain, the effects of the individual domains are not simply additive. This probably reflects the complex interactions between fibromodulin and collagen, as discussed below, and is supported by the different binding kinetics of the LRR domain and full-length fibromodulin. Nevertheless, this suggests a mechanism for regulating the activity by varying the degree of tyrosine sulfation, e.g. in different tissues, but whether this is the case remains unclear.
We next investigated the binding sites for the different domains of fibromodulin on the collagen molecule and the fully formed fibers. Surprisingly, the N-terminal tyrosine-sulfated extension and the LRR domain both bound to the same three areas of the procollagen I molecule: one binding site in the N-terminal, one binding site at ∼100 nm, and another site at ∼220 nm from the N terminus. The same binding pattern was seen with pepsin-extracted collagen type I and in a few cases binding to the C-terminal was observed. Whether the fibromodulin tyrosine sulfate domain and LRR domain bind the exact same sites on the collagen molecule, or whether specific sites for the two domains exist within the mapped sites/regions, cannot be distinguished by EM and needs to be clarified using complementary methods. All the fibromodulin binding sites found are in line with the study by Viola et al. (29), where binding of fibromodulin to cyanogen bromide (CNBr)-generated fragments of collagen-type I was studied. Thus, our N-terminal, 100 and 220 nm binding sites are contained within the α2(I) CB4, α1(I) CB8, and α1(I) CB7 fibromodulin binding CNBr fragments. In the quarter staggered model of collagen molecule alignment in fibrils (30), the mapped sites were located around the borders of overlap and gap regions (Fig. 3D).
This suggests a model where fibromodulin may cross-bridge between collagen molecules and direct the assembly of the fibrils. This hypothesis is supported by EM data, where such complexes were found, notably only in the samples containing full-length fibromodulin. These complexes contained one fibromodulin molecule binding to two collagen molecules at either the 100 or 220 nm binding sites. This result shows that a fibromodulin molecule can bind two separate tropocollagen molecules via its N-terminal domain and either of its central or C-terminal sites, bringing the collagen molecules together for assembly.
The collagen telopeptide regions have been shown to accelerate the rate of fibril assembly and affect the form of the resulting fibrils (31, 32). Fibromodulin binding to the N-terminal region of the collagen molecule could possibly interfere with telopeptide function. The tFM_19–98 protein, however, shortened the fibril assembly lag phase both of acid-extracted collagen (retaining the telopeptides) and pepsin-treated collagen (without telopeptides).
All three fibromodulin variants tested were retained on the formed fibers but the N terminally truncated rFM_64–376 was present to a lower extent on the fibers compared with the two variants containing the N-terminal tyrosine sulfate-rich domain (Fig. 2, C and D). Further evidence that the fibromodulin N-terminal domain is important for the assembly and fusion of collagen molecules was shown by measuring hydroxyproline content in the pellet after fibrillogenesis in the presence of the variants of fibromodulin. Even though tFM_19–376, and to some extent rFM_64–376, inhibited the fibril formation based on the measure of turbidity, more of the total collagen was found in the formed fibrils when full-length tFM_19–376 fibromodulin or tFM_19–98 was present, compared with when rFM_64–376 lacking the tyrosine sulfate domain was present (Fig. 4B). In surface plasmon resonance experiments, where collagen monomers were immobilized on a sensor chip and the fibromodulin variants were allowed to interact under continuous flow, differences in the association and dissociation were observed when comparing tFM_19–376 and rFM_64–376. The rFM_64–376 showed a faster association to the immobilized collagen but also dissociated rapidly from the collagen at post-injection. In contrast, the tFM_19–376 was retained at the surface during the post-injection dissociation phase and injection of alkali solution was required to dissociate this molecule from the collagen surface. This indicates a high affinity interaction, although KD values could not be calculated due to the multiple different interaction sites on the two molecules. Despite the clear interaction observed in the solid phase assay, EM and collagen pull-down experiments, injecting the tyrosine-sulfated tFM_19–98 fragment over collagen in the BIAcore system did not result in any response, perhaps reflecting charge repulsion from the carboxymethylated sensor chip.
Fibromodulin-deficient mice have a larger population of thinner collagen fibers with irregular and rough surface in tendon (8). Tendon collagen from these mice is more resistant to non-proteolytic extraction due to increased and altered cross-linking. It was shown that the C-telopeptide lysines in the extracted collagen were more oxidized to aldehydes (33) and we therefore speculated that fibromodulin inhibits lysyl-oxidase-mediated collagen cross-linking by protecting the collagen from the enzyme. Recently, Kalamajski et al. (34) showed an interaction between the N-terminal domain of fibromodulin and lysyl oxidase, which strengthens this hypothesis. In the same paper, by using triple-helical collagen toolkit peptides, binding to collagen was mapped to sites corresponding to the N-terminal and 220-nm sites, but no direct binding was observed to the 100-nm site observed by us (Fig. 3D). This may reflect the different collagens used. The toolkit peptides correspond to the homotrimeric collagen types II and III sequences, whereas heterotrimeric collagen type I was used in this study. Interestingly, the collagen toolkit peptides did not completely block fibromodulin binding to immobilized collagen type I, even when combining both the cross-linking site and protease site peptides (34), suggesting the presence of an additional fibromodulin binding site. A further difference is that in the present study, the interaction was assayed with the collagen in solution, as opposed to immobilized in solid phase assays, which could explain the discrepancy observed for the 100-nm site on collagen type I.
Tissue-extracted fibromodulin molecules will all contain the same protein core, but the N-terminal tyrosine residues will have a different amount and distribution of the O-sulfate post-translational modification. By mass spectrometry, as many as 9 of 10 tyrosine residues in the bovine fibromodulin sequence have been identified to contain this modification (13). The distribution of the tyrosine O-sulfation modifications in the N-terminal domain of fibromodulin remains elusive due to a lack of methods to study individual modified tyrosine residues. Nevertheless, separation of the N-terminal tFM_19–98 fragment into distinct peaks based on net charge shows that individual fibromodulin molecules in the tissue are differently substituted with O-sulfate and therefore differ in charge and properties. A previous study showed preferably binding of peak 4 to proteins with basic domains (18), but little difference in effect on collagen fibril formation was seen for the subfractionated tFM_19–98 with different O-sulfation patterns. Further attempts to subfractionate the individual six peaks by ion-exchange chromatography at lower pH demonstrated inhomogeneity of the individual peaks, which could explain why no particular peak stood out (18). Elucidating the amount and distribution of sulfate on the fragment will be of great interest for the understanding of its different biological functions. It is possible that a specific sulfation pattern with strong impact on the formation of collagen fibrils exists, but this remains to be identified.
Taken together, these results show that the tyrosine sulfate domain of fibromodulin contributes to its high affinity binding to collagen and influences the arrangement of the collagen molecules in the early fibrillogenesis phase. This likely leads to changes in affinity or accessibility for the other binding domains of fibromodulin or collagen, which control the lateral growth and fusion of the fibrils and result in well defined, highly organized collagen fibers.
Previously described protein effects on collagen fibrillogenesis are generally inhibitory. One exception is the extracellular matrix protein dermatopontin (also known as tyrosine-rich acidic matrix protein, TRAMP), which shortens the lag phase and accelerates in vitro collagen fibril formation (35). Dermatopontin knock-out mice also show abnormal collagen fibril organization (36). Interestingly, dermatopontin is tyrosine sulfated (37), although it remains unclear if this contributes to the effect of this protein on collagen fibril formation. Furthermore, like fibromodulin, several other members of the class II SLRP family have clustered sulfated tyrosine residues in their N-terminal domains (13). Thus, whereas fibromodulin is the first case where a role for tyrosine sulfate in collagen fibril formation has been directly demonstrated, this appears likely to be a general function for this post-translational modification.
Experimental Procedures
Materials
Pepsin extracted collagen type I from bovine skin (PureCol) was from Advanced BioMatrix, San Diego, CA. A His6 tag specific antibody (ab18184) was from Abcam (Cambridge, MA). TMB ELISA substrate kit (34021) was from Thermo Scientific Pierce (Rockford, IL). The N-terminal tyrosine sulfate-rich domain of fibromodulin (tFM_19–98 and tFM_33–74) and peaks of the tFM_19–98 from separation by ion exchange chromatography were prepared as described (18). Recombinant full-length fibromodulin (rFM_19–376), expressed in human embryonic kidney 293-EBNA cells, was prepared as described (27). Tissue-extracted full-length fibromodulin (tFM_19–376) was purified from bovine tracheal cartilage (38). The stable isotope-labeled peptide, SpikeTidesTM_TQL (pyr-Q)YEEDSHWWFQFLR, was from JPT Peptide Technologies, Germany.
Purification of Procollagen Type I
Human fibroblasts (HFL1, ATCC, Manassas, VA) were cultured in Dulbecco's MEM (DMEM), 10% FCS to pre-confluence. Medium was exchanged to DMEM with 50 μg/ml of ascorbic acid, 40 μg/ml of β-aminoproprionitrile and collected after a 24-h incubation (39). Collected medium was cooled to 4 °C and 5 mm N-ethylmaleimide, 1 mm EDTA, and Complete protease inhibitor (Roche Diagnostics, GmbH) was added. The medium was centrifuged at 1000 × g for 5 min at 4 °C. Ammonium sulfate was added to the supernatant to a final concentration of 20% (w/v) and the sample was centrifuged at 50,000 × g for 30 min at 4 °C. The pellet was dissolved in PBS, 1 mm EDTA and stored at 4 °C.
Acid Extraction of Collagen Type I from Mouse Tail Tendon
For solid phase assay, 6-week-old mice were sacrificed and the tail tendons were dissected and washed 5 min in each solution as follows: PBS (0.137 mm NaCl, 26.8 mm KCl, 10 mm Na2HPO4, pH 7.4), acetone, 70% isopropyl alcohol and PBS. After the washes, the tendons were incubated for 48 h in 16.7 mm acetic acid followed by homogenization. The sample was centrifuged at 50,000 × g for 15 min and the supernatant containing extracted collagen was stored at 4 °C. All steps were performed at 4 °C. For collagen fibrillogenesis assay, 3-week-old mice were sacrificed and the tail tendons were dissected and incubated for 20 h in 0.5 m acetic acid, 5 mm EDTA. The sample was centrifuged at 11,872 × g for 30 min. Extracted collagen in the supernatant was precipitated by adding NaCl to 0.9 m and collected by centrifugation as above. The pellet was resolved in 0.5 m acetic acid and dialyzed against 12 mm HCl and stored at 4 °C. All steps were performed at 4 °C.
Expression and Purification of the N-Terminally Truncated (Ala64-Ile376) Fibromodulin Protein (rFM_64–376)
Bovine fibromodulin cDNA in plasmid pET27b(+) (27) was amplified using the primers (5′-GCTTACGGCTCTCCACC-3′ and 5′-GGATCCTTTCAGATCTCGATGAAG-3′), corresponding to nucleotides 249–1191 with a flanking BamHI site on the C-terminal primer. The generated fragment was ligated into the PvuII and BamHI sites of the expression vector pCEP4-TAGZyme (40). Transfection of the construct into 293-EBNA cells (Invitrogen), selection with hygromycin, and purification of the secreted protein from culture medium by Ni2+ chelation affinity chromatography was as previously described (41). Fractions were analyzed by SDS-PAGE and bands of interest were cut out from the gel and analyzed by in-gel trypsin digestion and MALDI-TOF MS (42). Fractions containing the expressed truncated fibromodulin were pooled and stored at 4 °C with 0.02% sodium acid present.
Expression and Purification of Un-sulfated rFM_19–98_0S in E. coli
Bovine fibromodulin cDNA was used as a template to amplify the N-terminal domain with the primers (5′-AAGCAATATGAGGAAGAC-3′ and 5′-CAATCTCAAGTACCTGCCCC-3′), corresponding to nucleotides 249–1191 with flanking NdeI and HindIII sites. The generated fragment was ligated into the NdeI and HindIII sites of the expression vector pET28a (Novagen), the sequence identity was confirmed, and the construct was transformed into the protein expression system RosettaTM (DE3) E. coli (Novagen). The transfected cells were grown in 2× YT medium with kanamycin sulfate (50 μg/ml), and after isopropyl 1-thio-β-d-galactopyranoside induction, the cells were harvested by centrifugation and lysed in 6 m guanidine-HCl, 0.01 m Tris, 7 mm N-ethylmaleimide (pH 8). The cell suspension was passed 10 times through a 0.8-mm injection needle to make the sample less viscous and the supernatant was collected by centrifugation. The recombinant protein was purified by Ni2+ affinity onto a HisTrapTM HP column (GE Healthcare, Sweden), equilibrated in the lysis buffer. Unbound sample was removed by sequential washing with 10 column volumes of lysis buffer, 50 mm Tris-HCl (pH 8), 50 mm Tris-HCl (pH 8) in 60% isopropyl alcohol and 50 mm Tris-HCl (pH 8). Bound material was eluted in 3 column volumes of 1 m imidazole, 0.5 m NaCl, 50 mm Tris-HCl (pH 8). The eluate was analyzed by SDS-PAGE Tris/Tricine and fractions containing the N-terminal fragment of fibromodulin were pooled, diluted with 50 mm Tris-HCl (pH 8) to a final concentration of 0.15 m NaCl, and enzymatically digested with endoproteinase LysC overnight at 37 °C to remove the His tag. The digest was applied onto a reverse phase column Source 5 (GE Healthcare Life Science) and bound fragments were eluted and fractionated by a 20-column volume gradient of 0–100% acetonitrile in 0.1% TFA. The fractions were analyzed by MALDI-TOF MS to identify polypeptides present. Defined fractions were pooled, lyophilized, and dissolved in PBS.
Solid Phase Binding Assay
Acid-extracted collagen type I from mouse-tail tendon, 50 μg/ml in 16 mm acetic acid, was coated overnight onto a 96-well Microtest plate (MaxiSorb C96, Nunc, Denmark). Coated wells were rinsed three times with 10 mm HEPES, 0.135 m NaCl (pH 7.4) (HBS) and the remaining sites were blocked by a 1-h incubation in 3% BSA in HBS. All wells were washed three times with HBS followed by incubation with 500 nm of either tFM_19–376 or tFM_19–98, both purified from bovine cartilage, or recombinant mammalian-expressed rFM_19–376 or rFM_64–376 in HBS, 0.05% Tween 20 (HBS-T), 0.3% BSA for 1 h. After washing three times with HBS-T, wells containing tFM_19–376 or tFM_19–98 were incubated for 1 h with an affinity purified rabbit antibody specific for fibromodulin (38) in HBS-T, 0.3% BSA. Wells containing His-tagged rFM_19–376 or rFM_64–376 were incubated with a mouse antibody specific for the His6 tag in the same buffer. Unbound antibodies were removed by three washes of HBS-T and wells were incubated for 1 h with horseradish peroxide-conjugated secondary antibodies specific to rabbit or mouse immunoglobulins in HBS-T, 0.3% BSA. Bound secondary antibodies were detected by use of the TMB ELISA detection kit. All incubation steps and washes were performed at room temperature.
Surface Plasmon Resonance Interaction Studies
Pepsin-extracted type I collagen from bovine skin (PureCol) was immobilized by amine coupling to a level of 300 resonance units on a C1 sensorchip, according to the manufacturer's instructions (GE Healthcare, Uppsala, Sweden). A control surface without added protein was prepared in parallel by activating and deactivating the surface in an adjacent flow cell. Samples (130 μl) of tissue fibromodulin tFM_19–376, recombinant fibromodulin rFM_19–376 or rFM_64–376, at 250 or 500 nm in 10 mm HEPES (pH 7.5), 135 mm NaCl, 0.05% Surfactant P20, were injected over the surface at 35 μl/min and the association and dissociation recorded in a BIAcore 2000 instrument. The surfaces were regenerated with 0.02 mm NaOH. Data were analyzed using BIAevaluation 3.1 software (GE Healthcare).
In Vitro Collagen Fibrillogenesis Assay
Working solutions containing tFM_19–376 (35 μg/ml, 0.85 μm), rFM_19–376 (35 μg/ml, 0.85 μm), rFM_64–376 (35 μg/ml, 0.99 μm), tFM_19–98 (7 or 35 μg/ml, 0.73 or 3.6 μm), the different peaks of tFM_19–98 (35 μg/ml, 0.73 μm), rFM_19–98_0S (3.5, 7, 14, 35, or 70 μg/ml, 0.365, 0.73, 1.4, 3.6, or 7.3 μm), tFM_33–74 (1, 2, or 4 μm, 4.37, 8.75, or 17.5 μg/ml), or PBS in fibrillogenesis buffer (20 mm HEPES, 150 mm NaCl, pH 7.4) were prepared. Pepsin-extracted collagen type I from bovine skin (PureCol) in 0.012 m HCl was added to the working solutions at a final concentration of 250 μg/ml (0.9 μm). For neutralization of the collagen solution, an equal amount of 0.012 m sodium hydroxide was added. Working solution, containing PBS was used as buffer blank. Aliquots (250 μl) of the mixed samples were transferred to Microtest plate 96-well, F (Sarstedt, Germany) with 4 to 6 replicates for each condition, overlaid with 10 μl of mineral oil and incubated at 37 °C in a FluoStar OptimaTM microplate reader (BMG Labtech, Germany). Changes in absorbance due to light scattering were monitored at 405 nm over time, with data collection every 4th min.
Data were analyzed using the GraphPad Prism® 6.0 software package. An average of the buffer blank sample sets were subtracted from all datasets to subtract the background noise level and the curves for each well were baseline normalized by subtracting the lowest reading value in the individual data set. After log-transforming the x axis data (time), a four parameter sigmoidal curve was fit to the transformed data. For the data presented in Fig. 6, a five-parameter sigmoidal curve fit was used. The curve fit was set to start at measure point 5 and end at the plateau level. For each individual reaction, the plateau turbidity, inflection point (t50), and Hill slope parameters were obtained from the fitted curve and the lag phase length (tLag) was calculated as the time when a tangent line through the inflection point of the sigmoidal curve intersected with the x axis (y = 0).
In fibrillogenesis samples prepared for EM, the reactions were prepared at 7 times lower concentrations and incubated at 37 °C in quartz cuvettes in a water-jacket cuvette holder in a Beckman DU-640 spectrophotometer and samples were taken at different time points (0, 3, and 24 h). In fibrillogenesis of acid-extracted collagen type I from mouse tail tendon, the reactions were prepared at 20 μg/ml of collagen with or without tFM_19–98 (0.29 μm) and incubated at 28 °C in quartz cuvettes in a water-jacket cuvette holder in a Beckman DU-640 spectrophotometer.
Gold Labeling of Fibromodulin-specific Polyclonal Antibody
Collodial gold was prepared as described (43). AuCl4 was reduced by KSCN to give a particle diameter of 5 ± 1 nm. The gold particle solution was added at a molar ratio of 1 gold to 1.1 affinity purified antifibromodulin antibody.
Negative Staining Electron Microscopy
The collagen type I fibril structures in samples from ongoing fibrillogenesis reactions were prepared for electron microscopy (EM) after 0, 3, or 24 h incubation. Collagen was incubated with tFM_19–376, rFM_64–376, or tFM_19–98, or without added fibromodulin. Fibromodulin variants binding pro-collagen, pepsin-extracted collagen type I monomers, or collagen fibrils were visualized by the gold-labeled specific antibody against fibromodulin and analyzed by negative staining and EM (43). Quantitation of immunogold complexes was carried out as previously described in detail (44). For each experiment 500 individual particles were evaluated and the relative frequency (N/No) along the collagen molecules was determined.
Quantitation of tFM_19–98 Protein
Because O-sulfation affects tyrosine UV absorbance, the tFM_19–98 subpopulations with varying sulfation pattern were quantified by mass spectrometry. Ten pmol of a stable isotope-labeled peptide, (pyr-Q)YEEDSHWWFQFLR, was mixed with either the unfractionated tFM_19–98 sample, rFM_19–98_0S, or the tFM_19–98 fractionated peaks. The mixed samples were digested with trypsin and aliquots analyzed using the TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific, Waltham, MA) equipped with an EASY nano-LC system (Thermo Scientific, Waltham, MA) as described (45). Data were analyzed using the Skyline-daily software (MacCoss Lab Software, University of Washington (46)).
Hydroxyproline Assay
Collagen type I fibril formation in vitro assay was mixed as described above. Duplicate samples were incubated for 24 h at 37 °C in 2-ml microtubes with screw caps (Sarstedt, Germany). The samples were centrifuged at 20,800 × g for 30 min at room temperature, and supernatants were transferred to fresh microtubes with screw caps. Hydroxyproline content in pellets was assayed as described (47).
Binding of Fibromodulin Variants to Collagen Type I Fibrils
In vitro collagen fibrillogenesis assay in the presence of tissue fibromodulin tFM_19–376 (0.24 μm), tFM_19–98 (1 μm) or rFM_64–376 (1 μm) was performed as described above but in excess of collagen (3.8 μm). After incubation for 20 h at 37 °C, one set of samples was centrifuged (20,800 × g, 30 min, room temperature), and one set was kept as a non-centrifuged control. Supernatants were collected and pellets washed once with fibrillogenesis buffer. Supernatant and pellets from centrifugation and the controls were dissolved in SDS-PAGE sample buffer with reducing agent, separated on 4–12% SDS-PAGE (NOVEX NuPAGE Bis-Tris gel, Life Technologies), and transferred to a nitrocellulose membrane (Thermo Scientific, Germany). Fibromodulin proteins tFM_19–376, tFM_19–98, or rFM_64–376 were detected by a fibromodulin-specific polyclonal rabbit antibody (38) and visualized by horseradish peroxide-conjugated secondary swine anti-rabbit antibody. All samples were done in duplicate.
Statistical Analysis
All comparisons of multiple means were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test, using the GraphPad Prism® 6.0 software.
Author Contributions
V. T. and A. A. designed the research. V. T. performed the research with M. M., P. O., and S. K. V. T., M. M., P. O., S. K., and A. A. analyzed the data. V. T. and A. A. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank the late Prof. Dick Heinegård for stimulating discussions, continuous support, and initiation of this project. Mass spectrometers were funded by the Crafoord and the Inga-Britt and Arne Lundberg foundations.
This work was supported by grants from the Swedish Research Council, King Gustaf V's 80-years fund, the Crafoord Foundation, the Swedish Rheumatism association, the Greta and Johan Kock Foundation, and the Alfred Österlund Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
- SLRP
- small leucine-rich proteoglycan
- CS
- chondroitin sulfate
- HBS
- HEPES-buffered saline
- LRR
- leucine rich-repeat
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ANOVA
- analysis of variance.
References
- 1. Iozzo R. V., and Schaefer L. (2015) Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsushima N., Ohyanagi T., Tanaka T., and Kretsinger R. H. (2000) Super-motifs and evolution of tandem leucine-rich repeats within the small proteoglycans: biglycan, decorin, lumican, fibromodulin, PRELP, keratocan, osteoadherin, epiphycan, and osteoglycin. Proteins 38, 210–225 [DOI] [PubMed] [Google Scholar]
- 3. Park H., Huxley-Jones J., Boot-Handford R. P., Bishop P. N., Attwood T. K., and Bella J. (2008) LRRCE: a leucine-rich repeat cysteine capping motif unique to the chordate lineage. BMC Genomics 9, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bengtsson E., Mörgelin M., Sasaki T., Timpl R., Heinegård D., and Aspberg A. (2002) The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 277, 15061–15068 [DOI] [PubMed] [Google Scholar]
- 5. Hedbom E., and Heinegård D. (1989) Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J. Biol. Chem. 264, 6898–6905 [PubMed] [Google Scholar]
- 6. Rada J. A., Cornuet P. K., and Hassell J. R. (1993) Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp. Eye Res. 56, 635–648 [DOI] [PubMed] [Google Scholar]
- 7. Gill M. R., Oldberg Å., and Reinholt F. P. (2002) Fibromodulin-null murine knee joints display increased incidences of osteoarthritis and alterations in tissue biochemistry. Osteoarthritis Cartilage 10, 751–757 [DOI] [PubMed] [Google Scholar]
- 8. Svensson L., Aszódi A., Reinholt F. P., Fässler R., Heinegård D., and Oldberg Å. (1999) Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 274, 9636–9647 [DOI] [PubMed] [Google Scholar]
- 9. Chakravarti S., Magnuson T., Lass J. H., Jepsen K. J., LaMantia C., and Carroll H. (1998) Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 141, 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu C. Y., Birk D. E., Hassell J. R., Kane B., and Kao W. W. (2003) Keratocan-deficient mice display alterations in corneal structure. J. Biol. Chem. 278, 21672–21677 [DOI] [PubMed] [Google Scholar]
- 11. Ezura Y., Chakravarti S., Oldberg Å., Chervoneva I., and Birk D. E. (2000) Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 151, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jepsen K. J., Wu F., Peragallo J. H., Paul J., Roberts L., Ezura Y., Oldberg Å., Birk D. E., and Chakravarti S. (2002) A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J. Biol. Chem. 277, 35532–35540 [DOI] [PubMed] [Google Scholar]
- 13. Önnerfjord P., and Heathfield T. F., and Heinegård D. (2004) Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J. Biol. Chem. 279, 26–33 [DOI] [PubMed] [Google Scholar]
- 14. Antonsson P., Heinegård D., and Oldberg Å. (1991) Posttranslational modifications of fibromodulin. J. Biol. Chem. 266, 16859–16861 [PubMed] [Google Scholar]
- 15. Bengtsson E., Neame P. J., Heinegård D., and Sommarin Y. (1995) The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissues. J. Biol. Chem. 270, 25639–25644 [DOI] [PubMed] [Google Scholar]
- 16. Bengtsson E., Aspberg A., Heinegård D., Sommarin Y., and Spillmann D. (2000) The amino-terminal part of PRELP binds to heparin and heparan sulfate. J. Biol. Chem. 275, 40695–40702 [DOI] [PubMed] [Google Scholar]
- 17. Moore K. L. (2003) The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 278, 24243–24246 [DOI] [PubMed] [Google Scholar]
- 18. Tillgren V., Önnerfjord P., Haglund L., and Heinegård D. (2009) The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins, including bioactive factors. J. Biol. Chem. 284, 28543–28553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. San Antonio J. D., Karnovsky M. J., Gay S., Sanderson R. D., and Lander A. D. (1994) Interactions of syndecan-1 and heparin with human collagens. Glycobiology 4, 327–332 [DOI] [PubMed] [Google Scholar]
- 20. Öbrink B. (1973) The influence of glycosaminoglycans on the formation of fibers from monomeric tropocollagen in vitro. Eur. J. Biochem. 34, 129–137 [DOI] [PubMed] [Google Scholar]
- 21. Kvist A. J., Johnson A. E., Mörgelin M., Gustafsson E., Bengtsson E., Lindblom K., Aszódi A., Fässler R., Sasaki T., Timpl R., and Aspberg A. (2006) Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J. Biol. Chem. 281, 33127–33139 [DOI] [PubMed] [Google Scholar]
- 22. Heathfield T. F., Önnerfjord P., Dahlberg L., and Heinegård D. (2004) Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J. Biol. Chem. 279, 6286–6295 [DOI] [PubMed] [Google Scholar]
- 23. Kalamajski S., Aspberg A., and Oldberg Å. (2007) The decorin sequence SYIRIADTNIT binds collagen type I. J. Biol. Chem. 282, 16062–16067 [DOI] [PubMed] [Google Scholar]
- 24. Schönherr E., Hausser H., Beavan L., and Kresse H. (1995) Decorin-type I collagen interaction: presence of separate core protein-binding domains. J. Biol. Chem. 270, 8877–8883 [DOI] [PubMed] [Google Scholar]
- 25. Keene D. R., San Antonio J. D., Mayne R., McQuillan D. J., Sarris G., Santoro S. A., and Iozzo R. V. (2000) Decorin binds near the C terminus of type I collagen. J. Biol. Chem. 275, 21801–21804 [DOI] [PubMed] [Google Scholar]
- 26. Kalamajski S., Aspberg A., Lindblom K., Heinegård D., and Oldberg Å. (2009) Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem. J. 423, 53–59 [DOI] [PubMed] [Google Scholar]
- 27. Kalamajski S., and Oldberg Å. (2007) Fibromodulin binds collagen type I via Glu-353 and Lys-355 in leucine-rich repeat 11. J. Biol. Chem. 282, 26740–26745 [DOI] [PubMed] [Google Scholar]
- 28. Kalamajski S., and Oldberg Å. (2009) Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J. Biol. Chem. 284, 534–539 [DOI] [PubMed] [Google Scholar]
- 29. Viola M., Bartolini B., Sonaggere M., Giudici C., Tenni R., and Tira M. E. (2007) Fibromodulin interactions with type I and II collagens. Connect. Tissue Res. 48, 141–148 [DOI] [PubMed] [Google Scholar]
- 30. Olsen B. R. (1963) Electron microscope studies on collagen: II. mechanism of linear polymerization of tropocollagen molecules. Zeitschrift fur Zellforschung und mikroskopische Anatomie (Vienna, Austria: 1948) 59, 199–213 [DOI] [PubMed] [Google Scholar]
- 31. Helseth D. L. Jr., and Veis A. (1981) Collagen self-assembly in vitro: differentiating specific telopeptide-dependent interactions using selective enzyme modification and the addition of free amino telopeptide. J. Biol. Chem. 256, 7118–7128 [PubMed] [Google Scholar]
- 32. Prockop D. J., and Fertala A. (1998) Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides: demonstration that assembly is driven by specific binding sites on the monomers. J. Biol. Chem. 273, 15598–15604 [DOI] [PubMed] [Google Scholar]
- 33. Kalamajski S., Liu C., Tillgren V., Rubin K., Oldberg Å., Rai J., Weis M., and Eyre D. R. (2014) Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J. Biol. Chem. 289, 18873–18879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalamajski S., Bihan D., Bonna A., Rubin K., and Farndale R. W. (2016) Fibromodulin interacts with collagen cross-linking sites and activates lysyl oxidase. J. Biol. Chem. 291, 7951–7960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacBeath J. R., Shackleton D. R., and Hulmes D. J. (1993) Tyrosine-rich acidic matrix protein (TRAMP) accelerates collagen fibril formation in vitro. J. Biol. Chem. 268, 19826–19832 [PubMed] [Google Scholar]
- 36. Takeda U., Utani A., Wu J., Adachi E., Koseki H., Taniguchi M., Matsumoto T., Ohashi T., Sato M., and Shinkai H. (2002) Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis. J. Invest. Dermatol. 119, 678–683 [DOI] [PubMed] [Google Scholar]
- 37. Forbes E. G., Cronshaw A. D., MacBeath J. R., and Hulmes D. J. (1994) Tyrosine-rich acidic matrix protein (TRAMP) is a tyrosine-sulphated and widely distributed protein of the extracellular matrix. FEBS Lett. 351, 433–436 [DOI] [PubMed] [Google Scholar]
- 38. Heinegård D., Larsson T., Sommarin Y., Franzén A., Paulsson M., and Hedbom E. (1986) Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J. Biol. Chem. 261, 13866–13872 [PubMed] [Google Scholar]
- 39. Freiberger H., Grove D., Sivarajah A., and Pinnell S. R. (1980) Procollagen I synthesis in human skin fibroblasts: effect on culture conditions on biosynthesis. J. Investig. Dermatol. 75, 425–430 [DOI] [PubMed] [Google Scholar]
- 40. Geng H., Nandakumar K. S., Pramhed A., Aspberg A., Mattsson R., and Holmdahl R. (2012) Cartilage oligomeric matrix protein specific antibodies are pathogenic. Arthritis Res. Ther. 14, R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Day J. M., Olin A. I., Murdoch A. D., Canfield A., Sasaki T., Timpl R., Hardingham T. E., and Aspberg A. (2004) Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J. Biol. Chem. 279, 12511–12518 [DOI] [PubMed] [Google Scholar]
- 42. Lorenzo P., Aspberg A., Önnerfjord P., Bayliss M. T., Neame P. J., and Heinegård D. (2001) Identification and characterization of asporin. a novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J. Biol. Chem. 276, 12201–12211 [DOI] [PubMed] [Google Scholar]
- 43. Baschong W., and Wrigley N. G. (1990) Small colloidal gold conjugated to Fab fragments or to immunoglobulin G as high-resolution labels for electron microscopy: a technical overview. J. Electron Microsc. Tech. 14, 313–323 [DOI] [PubMed] [Google Scholar]
- 44. Engel J., and Furthmayr H. (1987) Electron microscopy and other physical methods for the characterization of extracellular matrix components: laminin, fibronectin, collagen IV, collagen VI, and proteoglycans. Methods Enzymol. 145, 3–78 [DOI] [PubMed] [Google Scholar]
- 45. Müller C., Khabut A., Dudhia J., Reinholt F. P., Aspberg A., Heinegård D., and Önnerfjord P. (2014) Quantitative proteomics at different depths in human articular cartilage reveals unique patterns of protein distribution. Matrix Biol. 40, 34–45 [DOI] [PubMed] [Google Scholar]
- 46. MacLean B., Tomazela D. M., Shulman N., Chambers M., Finney G. L., Frewen B., Kern R., Tabb D. L., Liebler D. C., and MacCoss M. J. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stegemann H., and Stalder K. (1967) Determination of hydroxyproline. Clin. Chim. Acta 18, 267–273 [DOI] [PubMed] [Google Scholar]