Abstract
This study continues to explore the plasticity of Toll-like receptor 2 (TLR2) previously described in immune response during Trypanosoma cruzi infection. Here, we have shown that Ly6ChiTLR2hi monocytes were involved in TNF-α and IL-12 production, whereas Ly6CloTLR2hi monocytes were mainly committed to IL-10 and TNF-α production during T. cruzi infection independently of TLR agonist used (i.e. TLR2 or TLR9 agonists). Another difference between the monocyte populations is that the adapter Mal (encoded by TIRAP) has appeared crucial for the cytokine production by Ly6Clo but not by Ly6Chi monocytes. The protein Mal was necessary to induce cytokine synthesis by Ly6Clo monocytes after triggering TLR2 or TLR9. Finally, our data have suggested that TLR2, TLR9, and Mal/TIRAP controlled differentially the emergence of the different TLR2hi monocyte populations in the spleen. In summary, this study highlights the central role of the TLR2/Mal tandem in the distinct activity among the monocyte subsets during T. cruzi infection. Such findings provide a basis for understanding the challenge posed by the use of TLR2 agonist in immunotherapy.
Keywords: cytokine, flow cytometry, infection, inflammation, innate immunity, MAL/TIRAP, monocyte, Toll-like receptor (TLR)
Introduction
TLRs2 play a critical role in the innate immunity by recognizing the pathogen-associated molecular patterns, which may be expressed differentially according to the cell type (1, 2). There is no rule concerning the function of TLRs in cells because the same TLR can assume distinct roles, and different TLRs can assume similar functions (3–5). In the case of Trypanosoma cruzi (the causative agent of Chagas disease), several agonists from membrane and genome have been identified (6–11). As previously demonstrated, despite a differential use of TLR2 and TLR9 by immune cells, both receptors are involved in the establishment of pro-inflammatory response (5). In addition to its role in inflammatory response, TLR2 has also been shown to assume an immunomodulatory function during the first stage of T. cruzi infection (5, 12), which illustrates the plasticity of this receptor. A beneficial role for TLR2 signaling is described in mucosal homeostasis and defense against specific pathogens, whereas TLR2 signaling via other pathogens or after endogenous triggering is correlated with a more severe phenotype in infectious or inflammatory disease. Recently, the differential effects of TLR2 activation on distinct outcomes, including the influence over the expression of additional TLRs or PRRs on cells, has been reviewed by van Bergenhenegouwen et al. (13).
Mal (MyD88 adaptor-like), encoded by the gene TIRAP (Toll-interleukin 1 receptor domain-containing adaptor protein), was initially described as a signaling adaptor protein leading to NF-κB activation downstream of TLR4 (14, 15) and TLR2 (16, 17). A role for Mal as a “bridging adaptor” has since been established with Mal recruited to the plasma membrane where it facilitates MyD88 (myeloid differentiation primary response gene 88) delivery to activated TLRs. Interestingly, Mal has been consistently associated with an immunoregulatory function (18, 19). More specifically, studies from different groups have involved Mal in IL-10 production in several models (20–22). Recently, Ní Cheallaigh et al. have suggested a role for Mal outside the TLR system (23).
To have a comprehensive overview of the functional consequence of TLR2 activation during T. cruzi infection, we have focused on monocytes that express TLR2. In mice, the monocyte subsets can be categorized by differences in the expression of Ly6C as classic (Ly6Chi) or non-classic (Ly6Clo) (24, 25). The relationship between them is a matter of debate (26) addressing whether Ly6Chi monocytes give rise to Ly6Clo monocytes (27) or whether monocyte subsets arise independently of each other (28). The strategy of using TLR2 as cell marker has permitted us to characterize splenic LyC6hiTLR2hi and Ly6CloTLR2hi populations as functional monocytes that produced pro and anti-inflammatory cytokines, respectively, and to define that they appeared independently in the spleen during T. cruzi infection. Further, we were able to show that Mal was associated with the cytokine production by Ly6CloTLR2hi monocytes, but not by LyC6hiTLR2hi monocytes, after triggering TLR2 or TLR9.
Results
Characterization of Two Splenic Monocyte Populations Expressing High Levels of TLR2 during the Acute Phase of T. cruzi Infection
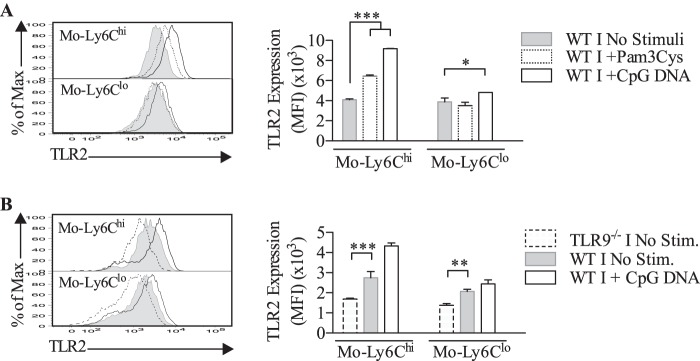
We sought to define different splenic subpopulations of monocytes by using TLR2 as a marker. Gated CD11b+MHCII+ cells corresponded to the principal splenic population expressing elevated TLR2 levels (Fig. 1A). When Ly6C and CD11c were used as additional markers, two distinct subtypes have emerged within CD11bhiMHCII+TLR2hi: Ly6Chi and Ly6Clo populations. The different surface markers used here allowed us to appoint CD11bhiCD11c+MCHII+Ly6Chi/loTLR2hi cells as monocytes after excluding the other myeloid cells. We verified that Ly6ChiTLR2hi and Ly6CloTLR2hi populations were producers of cytokines (e.g. TNF-α) when stimulated with Pam3Cys, a TLR2 agonist (Fig. 1A).
FIGURE 1.
Two splenic monocyte populations characterized as Ly6Clo and Ly6Chi express high levels of TLR2 during the acute phase of T. cruzi infection. A, gating strategy for defining Ly6Clo and Ly6Chi TLR2hi monocytes in spleen from healthy (non-infected (NI)) or infected (I) wild-type (C57Bl/6 WT) mice. The interpolations (CD11b versus MHCII/TLR2) allowed selecting a population CD11bhiMHCII+TLR2hi that was subgated on Ly6C versus CD11c. TLR2 expression and TNF-α production by Ly6Chi/lo monocytes were evaluated. B, representative dot plots with individual frequency values of Ly6Chi and Ly6Clo monocyte populations at 0, 4, and 7 days post-infection. C, frequency of CD11bhiMHCII+TLR2hi monocytes within splenic cells at 0, 4, and 7 days after infection. D, number of Ly6Chi/loTLR2hi monocytes within 106 splenic cells (acquired events) at 0, 4, and 7 days after infection. E and F, levels of expression of TLR2 on Ly6Chi/lo monocytes (MFI), and number of Ly6Chi/loTLR2hiTNF-α+ monocytes after triggering TLR2 with Pam3Cys at 0, 4, and 7 days post-infection. The results are displayed as means ± S.D. of four animals representing three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by the ANOVA and Bonferroni post-test. Mo, monocyte.
Next, we have quantified the evolution of these monocyte populations expressing TLR2hi in the spleen during infection. As presented in the Fig. 1 (B and C), an increase of the total population of TLR2hi monocytes was observed, indicating a biological relevance of these cells in the first days of T. cruzi infection. However, differences appeared between Ly6ChiTLR2hi and Ly6CloTLR2hi populations regarding cell number and proportion during the infection (Fig. 1, B and D). At first, the low frequency of Ly6CloTLR2hi cells encountered in the spleen from non-infected mice contrasted with the high number of Ly6ChiTLR2hi monocytes present in this organ. Interestingly, after 7 days of infection, the presence of monocytes was more marked for Ly6CloTLR2hi than Ly6ChiTLR2hi population (82% versus 18%, respectively) (Fig. 1B). Furthermore, although the increase of Ly6CloTLR2hi monocytes appeared continuous during infection, a decrease of Ly6ChiTLR2hi population was observed after 7 days of infection (Fig. 1D). The number of Ly6ChiTLR2hi cells correlated with the evolution of TLR2 expression on these cells, indicating that the increased Ly6ChiTLR2hi population observed was due to an augment of TLR2 expression on Ly6Chi cells already present (Fig. 1E). Interestingly, the variation of TLR2 expression directly influenced the number of Ly6Chi cells producing TNF-α after stimulation with TLR2 agonist (Pam3Cys) (Fig. 1F). By contrast, no significant modulation of TLR2 levels was detected within Ly6Clo monocytes during infection, suggesting that the high levels of TLR2 represented an intrinsic characteristic of these monocytes (Fig. 1E).
The Role of TLR2 and TLR9 in the Modulation of Splenic TLR2hi Monocyte Number during T. cruzi Infection
As previously described, the evolution of the number of Ly6ChiTLR2hi and Ly6CloTLR2hi splenic cells was different during infection. Therefore, we sought to define the factors able to influence this event, and we focused on TLR2 and TLR9 previously reported as important in T. cruzi infection (5, 9). In the first set of experiments, total splenic cells were incubated in vitro with TLR2 or TLR9 agonists. As shown in the Table 1, an increase of the number of Ly6ChiTLR2hi monocytes has been observed in spleen cell culture from infected and non-infected mice in the presence of TLR agonists, indicating they may equally influence the Ly6Chi population. By contrast, the effect of TLR2 or TLR9 agonists led to a slight diminution of Ly6CloTLR2hi cell number that may correspond to a down-regulation of TLR2 expression or cell death.
TABLE 1.
Effect of TLR agonists on the number of Ly6ChiTLR2hi and Ly6CloTLR2hi monocytes from 7 days infected (I) mice
WT monocytes were cultured with TLR2 (Pam3Cys 1 μg/ml) or/plus TLR9 (CpG DNA 1 μg/ml) agonists for 12 h. The number of cells from non-infected (NI) mice was normalized as 1N for Ly6Chi or 1n for Ly6Clo monocytes (1N ≈ 4000 cells/106 events; 1n ≈ 1500 cells/106 events). The data are representative of three independent experiments (means ± S.D. of four animals).
| Mo-Ly6ChiTLR2hi (X N) |
Mo-Ly6CloTLR2hi (X n) |
|||
|---|---|---|---|---|
| NI | I (7days) | NI | I (7days) | |
| No stimuli | 1.00 ± 0.13 | 1.18 ± 0.04a | 1.00 ± 0.92 | 10.0 ± 0.40b |
| With Pam3Cys | 1.69 ± 0.13b | 1.54 ± 0.17c | 1.04 ± 0.45 | 8.7 ± 0.50d |
| With CpG DNA | 1.29 ± 0.15a | 1.52 ± 0.18c | 1.02 ± 0.46 | 8.5 ± 0.15d |
| With Pam3Cys + CpG DNA | 1.52 ± 0.18b | 1.63 ± 0.23c | 0.96 ± 0.48 | 7.6 ± 0.30d |
a p < 0.05 compared with non-infected, unstimulated cells.
b p < 0.001 compared with non-infected, unstimulated cells.
c p < 0.001 compared with infected, unstimulated cells (bold type).
d p < 0.05 compared with infected, unstimulated cells (bold type) by the ANOVA and Bonferroni post-tests.
To confirm the importance of the TLR pathway on regulation of monocyte populations, we compared the number of TLR2hi monocytes in infected spleen from TLR9−/−, TLR2−/−, and WT mice (Table 2). In the absence of TLR2 or TLR9, a significant reduction of Ly6Chi cell number was observed. Importantly, the number of Ly6Clo cells was unchanged in the absence of TLR2 or TLR9. This corroborated with the results presented in the Table 1. These findings suggest that the up-regulation of Ly6ChiTLR2hi number is under the control of TLR2 and TLR9 during T. cruzi infection.
TABLE 2.
Comparison of the number of splenic Ly6ChiTLR2hi and Ly6CloTLR2hi monocytes from WT versus deficient mice at 7 days post-infection (means ± S.D.)
The number of monocytes in the spleen from non-infected (NI) mice was normalized as 1N for Ly6Chi or 1n for Ly6Clo populations (1N ≈ 4000 cells/106 events; 1n ≈ 1500 cells/106 events). The data are representative of two independent experiments means ± S.D. of four animals).
| Mo-Ly6ChiTLR2hi (X N) | Mo-Ly6CloTLR2hi (X n) | |
|---|---|---|
| WT NI | 1.00 ± 0.13 | 1.0 ± 0.9 |
| WT I | 1.18 ± 0.04a | 10.0 ± 0.4b |
| TLR2−/− Ic | 0.59 ± 0.14d | 11.0 ± 0.8 |
| TLR9−/− I | 0.70 ± 0.06d | 11.0 ± 1.2 |
| IL-12−/− I | 1.00 ± 0.18 | 5.1 ± 1.1e |
| Mal/TIRAP−/− I | 0.41 ± 0.03e | 7.2 ± 0.2d |
a p < 0.05 compared with WT, non-infected cells.
b p < 0.001 compared with WT, non-infected cells.
c Cells from TLR2−/− mice were evaluated only by the interpolation (CD11c versus Ly6C).
d p < 0.01 compared with WT, infected cells (bold type).
e p < 0.001 compared with WT, infected cells (bold type) by the ANOVA and Bonferroni post-tests.
Furthermore, we evaluated how the pro-inflammatory response that was crucial for the survival of T. cruzi infected mice could affect the evolution of monocyte numbers during the initial phase of infection (Table 2). For this purpose, mice deficient in IL-12 were infected, and the splenic monocyte populations were analyzed. A significant diminution of Ly6Clo population was observed in the absence of IL-12 without alteration in the Ly6Chi cells. This led to the conclusion that the up-regulation of Ly6CloTLR2hi number is under the control of IL-12 during T. cruzi infection.
The Role of TLR2 and TLR9 in the Modulation of TLR2 Expression on Monocytes during T. cruzi Infection
Considering that TLR2 and TLR9 agonists were capable of modulating the number of Ly6Chi/loTLR2hi monocytes during infection, we verified whether these variations were due to a differential level of TLR2 expression. As shown in the Fig. 2, the stimulation with TLR9 agonist induced a dramatic increase of TLR2 expression in Ly6Chi monocytes, whereas the TLR9 agonist effect was more discrete on Ly6Clo population, inducing a slight increase of TLR2 expression. The incubation of monocytes with TLR2 agonist affected TLR2 expression in Ly6Chi without impact on Ly6Clo cells (Fig. 2A). These data clearly pointed out a differential influence of TLR agonists on monocyte populations from infected mice, confirming the results presented in Tables 1 and 2.
FIGURE 2.
TLR agonists impact on TLR2 expression in splenic Ly6Chi monocytes in the early stage of T. cruzi infection. A, effect of TLR2 (Pam3Cys, 1 μg/ml) and TLR9 (CpG DNA, 1 μg/ml) agonists on the TLR2 expression in monocytes from 7 days infected WT mice after culture for 10 h. B, effect of TLR9 deficiency on the TLR2 expression in monocytes in the same conditions. The data represent three independent experiments (means ± S.D. of four animals). *, p < 0.05; **, p < 0.01; ***, p < 0.001 by the ANOVA and Bonferroni post-test.
To validate the involvement of TLR9 in the modulation of TLR2 expression, the level of TLR2 was evaluated in monocytes from infected mice deficient in TLR9. According to the data presented in the Fig. 2B, the absence of TLR9 significantly affected TLR2 expression in Ly6Chi cells, which correlated with the effect of the TLR9 agonist on this population. A moderate impact of TLR9 deficiency on TLR2 expression in Ly6Clo monocytes was observed.
Evaluation of the Capacity of Ly6Clo and Ly6Chi TLR2hi Monocytes to Produce Cytokine after Triggering TLR2 or TLR9
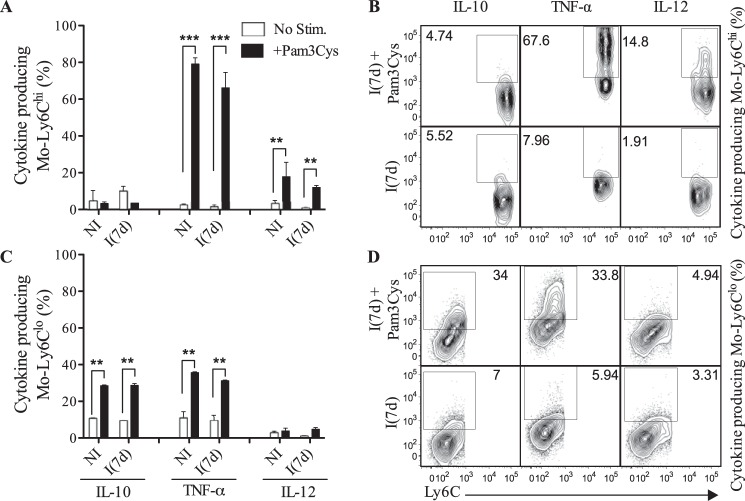
After showing that both TLR2hi monocyte populations were controlled differentially by TLR agonists, we sought to define the capacity of these cells to produce cytokine in the presence of TLR agonists. For this purpose, splenic TLR2hi monocytes were stimulated in vitro with TLR2 and TLR9 agonists and were marked with antibodies against intracellular cytokines. The frequencies of IL-10+, TNF-α+, and IL-12+ cells were shown in the Fig. 3 (A and B to Ly6Chi and C and D to Ly6Clo cells). The intracellular staining showed a significant increase in the percentage of Ly6CloIL-10+ and Ly6CloTNF-α+ cells after TLR2 stimulation at 7 days post-infection. Indeed, approximately 40% of Ly6Clo cells were committed to IL-10 and TNF-α production. No significant synthesis of IL-12 by these cells was observed in the same conditions (Fig. 3, C and D). Notably, a high number of Ly6ChiTLR2hi cells from non-infected and infected spleens were involved in TNF-α synthesis (∼80%) and in less extend in IL-12 production (∼20%) after stimulation with Pam3Cys, but, importantly, these cells were unable to produce IL-10 under stimulation with TLR2 agonist (Fig. 3, A and B). This clearly evidenced functional differences between monocyte populations. Further, splenic monocytes were stimulated with TLR9 agonist with similar results as shown in the Fig. 4. Like in the presence of TLR2 agonist, TLR9 agonist induced an increased Ly6ChiIL-12+ cell number (Fig. 4A) and a significant increase in Ly6CloIL-10+ population (Fig. 4B). Interestingly, the presence of TLR9 agonist led to an up-regulation of Ly6Chi/loTNF-α+ monocytes. According to these data, the functional monocyte population was characterized as TLR2hi, and the cytokine pattern produced by Ly6Clo or Ly6Chi monocytes appeared different and independent of the type of TLR agonist.
FIGURE 3.
Differential patterns of cytokine synthesis by Ly6Chi and Ly6Clo monocytes after triggering TLR2 during the acute phase of T. cruzi infection. A and C, frequencies of IL-10-, TNF-α-, or IL-12-producing Ly6Chi or Ly6Clo monocytes from healthy (NI) or infected (I(7d)) WT mice after culture with Pam3Cys (1 μg/ml) for 12 h. B and D, representative dot plots of the frequency of individual data values. The data are representative of three independent experiments (means ± S.D. of four animals). **, p < 0.01; ***, p < 0.001 by the analysis of means using Student's t test.
FIGURE 4.
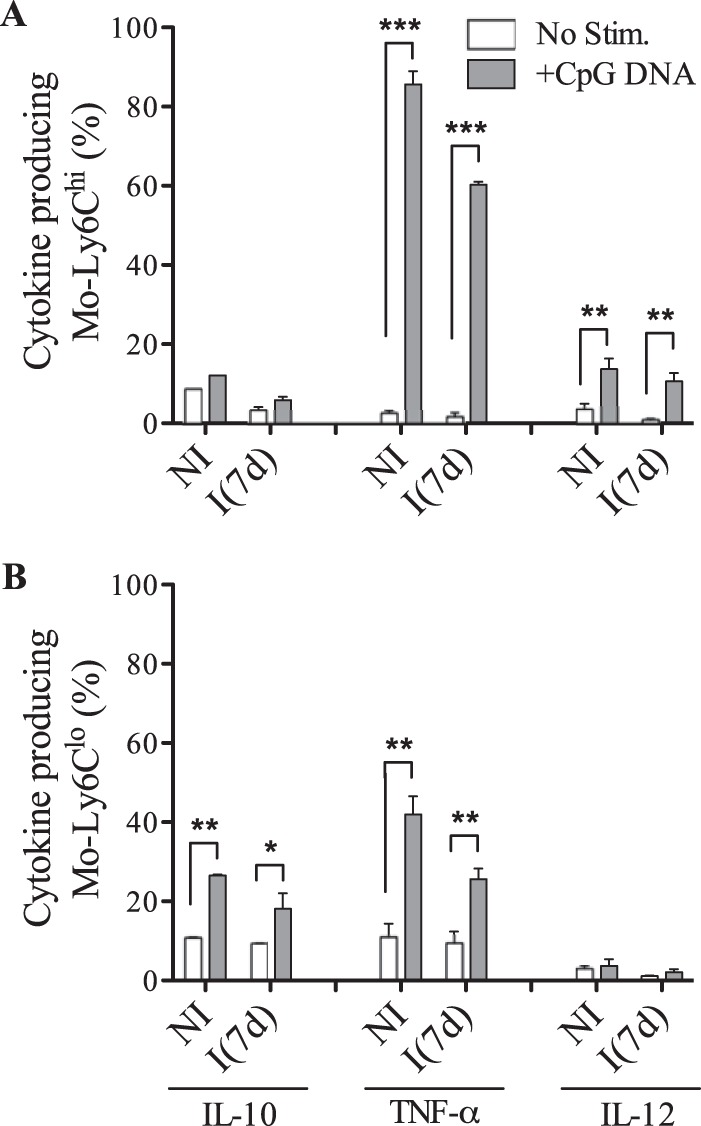
Differential patterns of cytokine synthesis by Ly6Chi and Ly6Clo monocytes after triggering TLR9 during the acute phase of T. cruzi infection. A and B, frequencies of IL-10-, TNF-α-, or IL-12-producing Ly6Chi or Ly6Clo monocytes from healthy (NI) or infected (I(7d)) WT mice after culture with CpG DNA (1 μg/ml) for 12 h. The data are representative of three independent experiments (means ± S.D. of four animals). *, p < 0.05; **, p < 0.01; ***, p < 0.001 by the analysis of means using Student's t test.
To support the notion that the monocyte response was cell context-dependent and not TLR context-dependent, we quantified the amount of cytokine being produced per cell (median fluorescence intensity (MFI)) after incubation with the different TLR agonists (Fig. 5). When we analyzed the individual capacity of TLR2hi monocytes to produce cytokine in response to TLR agonist, Ly6Chi cells were shown to be high producers of IL-12 in the presence of TLR2 or TLR9 agonists (Fig. 5A), whereas Ly6Clo cells were specialized in IL-10 production after triggering TLR2 or TLR9 (Fig. 5B). Both populations shared the capacity of synthesizing TNF-α independently of TLR agonist type. These data show a specialization of these monocyte subpopulations.
FIGURE 5.

Amount of cytokine produced per Ly6Chi/loTLR2hi monocytes after incubation with TLR agonists. A, amount of IL-12 and TNF-α being produced by WT Ly6Chi cells from infected mice after incubation with Pam3Cys (1 μg/ml) or CpG DNA (1 μg/ml) for 12 h. B, amount of IL-10 and TNF-α being produced by WT Ly6Clo cells from infected mice after incubation with Pam3Cys (1 μg/ml) or CpG DNA (1 μg/ml) for 12 h. The data expressed as MFI (mean of the cytokine level/cells) represent three independent experiments (means ± S.D. of four animals). ***, p < 0.001 by the ANOVA and Bonferroni post-test.
Impact of Mal/TIRAP Deficiency on the Inflammatory Response of TLR2hi Monocytes during Infection
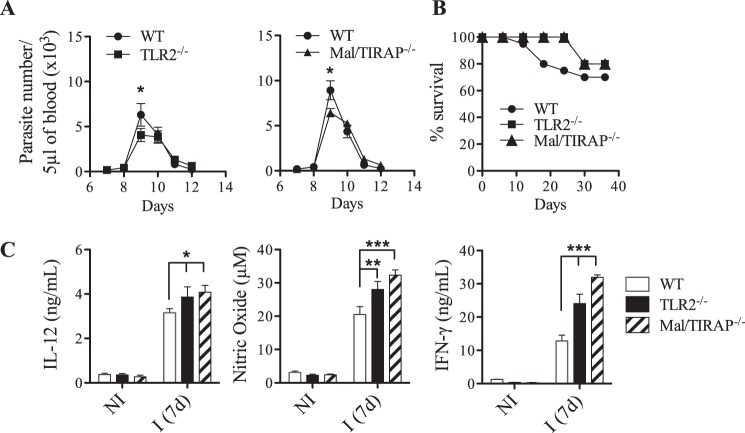
As revealed by our data, TLR agonists differentially influence Ly6Clo and Ly6Chi monocytes in term of number, TLR2 expression, and inflammatory properties. Also, different patterns of cytokine release have been detected in monocyte populations after triggering TLRs, suggesting the use of distinct signaling pathways by these cells. Because Mal/TIRAP, the adapter downstream of TLR2, has been consistently associated with an immune regulatory function, we have investigated its role in our infection model. According to the data presented in the Fig. 6 (A and B), the absence of Mal did not significantly affect parasitemia and mortality of infected mice, indicating that this adapter was not required to mount the host defense response. The same has been previously published concerning TLR2 (9, 12). Further, an increased inflammatory response illustrated by an augment of IL12p40, IFN-γ, and NO was associated with the absence of Mal as shown in the Fig. 6C. When we focused on monocytes, the deficiency in Mal affected the number of splenic Ly6Chi cells in a more pronounced way than the other monocyte population (Table 2). To evaluate the role of Mal in the cytokine synthesis by monocytes, we have primarily verified that TLR2 expression was unaffected in Mal−/− monocytes as presented in the Fig. 7A. This has allowed us to compare the cytokine production by WT and Mal−/− monocytes in the presence of TLR agonists. As shown in the Fig. 7B, the absence of Mal affected the Ly6Clo cell capacity to produce TNF-α after stimulation with TLR2 or TLR9 agonists. By contrast, the production of TNF-α by Mal−/−Ly6Chi monocytes remained intact in response to TLR2 or TLR9 agonists. Further, our results provided evidence of the connection between Mal and IL-10 production by Ly6Clo monocytes. The absence of Mal impeded IL-10 synthesis by these cells (Fig. 7C). In summary, the differential use of Mal by each subtype of monocyte was independent of the TLR stimuli, suggesting that Mal may be a key molecule to predefine the function of monocytes.
FIGURE 6.
Effect of TLR2 and Mal/TIRAP deficiencies on host resistance to T. cruzi infection. A and B, male, 8-week-old WT, TLR2−/−, or Mal/TIRAP−/− mice were infected with 100 forms of the Y strain of T. cruzi. Parasitemia (A) and host survival (B) were assessed daily. (means ± S.D. of seven animals). C, cytokine levels in spleen cell culture from either control (NI) or infected (I) C57Bl/6 WT, TLR2−/−, and Mal/TIRAP−/− mice were evaluated 7 days post-infection. The data are representative of two independents experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by the analysis of means using Student's t test for parasitemia and by using the ANOVA and Bonferroni post-test for the cytokine.
FIGURE 7.
Evaluation of cytokine synthesis by Ly6Chi/lo monocytes from WT and Mal/TIRAP deficient mice after triggering TLR. A, levels of expression of TLR2 on Mal/TIRAP−/−Ly6Chi/lo monocytes (MFI). B, frequencies and individual representative dot plots of TNF-α producing Ly6Chi/lo monocytes from infected WT and Mal/TIRAP−/− mice after incubation with Pam3Cys (1 μg/ml) or CpG DNA (1 μg/ml) by 12 h. C, frequencies and individual representative dot plots of IL-10 producing Ly6Clo monocytes from infected mice after incubation with Pam3Cys (1 μg/ml) or CpG DNA (1 μg/ml) by 12 h. The data are representative of two independents experiments (means ± S.D. of four animals). ***, p < 0.001, by the analysis of means using the ANOVA and Bonferroni post-test.
Discussion
Most pathogens possess different TLR agonists, which expand the possibility of interactions between the immune cells in the different lymphoid organs and so brings more complexity to the dissecting of host immune response (4). The simultaneous activation of two or more TLRs represents the real situation during T. cruzi infection (5, 9). As previously published, TLR2 appears to play a dual role during T. cruzi infection inducing a pro-inflammatory response in macrophage but controlling TLR9-dependent IL-12 production by DCs (5, 12). Therefore, it appeared relevant to explore the role of TLR2 in monocytes during T. cruzi infection. In some reports, the presence of the TLR2 on monocyte has been assimilated to an inflammatory marker (29–31), but this covers only one aspect of TLR2 activity.
The present study has shown that monocytes constituted the cell population expressing the highest levels of TLR2 in the spleen. Previous studies have related a high level of TLR2 on monocytes in different models of infection in human (32–34) and mice (35). According to our data, whereas the elevated TLR2 expression may constitute an intrinsic characteristic of Ly6Clo cells, it appears that TLR2 and TLR9 modulate TLR2 expression on resident Ly6Chi cells directly reflecting on the Ly6ChiTLR2hi population cell size. This provides new insights into the cooperation between TLRs during innate immune response. By contrast, TLR2 and TLR9 agonists have no or little influence on Ly6CloTLR2hi cell number in the spleen. Such data led to the following hypothesis: Ly6ChiTLR2hi and Ly6CloTLR2hi cells emerge independently in the spleen that is supported by the fact that both populations were differentially modulated after incubation in vitro with TLR2 and TLR9 agonist or in the absence of TLR2 or TLR9 during infection. Our data corroborated the conclusions of other groups claiming that the maturation of monocyte subsets occurs separately before migration in a differentiated stage toward secondary lymphoid organs, like spleen (36, 37).
Importantly, a correlation between the increased TLR2 expression and the production of inflammatory cytokines by the Ly6ChiTLR2hi cells suggests that the high levels of TLR2 could provide a molecular signature for activated Ly6Chi monocytes. Others have previously reported an up-regulation of TLR2 expression on blood monocytes in patients with Kawasaki disease or sepsis, underlining that TLR2 may serve as an inflammatory marker and be responsible for the immunopathogenesis in different situations (30, 35, 38).
In the absence of IL-12, a reduction of Ly6Clo cell population was observed that is in accord with the anti-inflammatory role of these cells, producers of IL-10. Interestingly, the lack of Mal mainly affected the inflammatory Ly6Chi cell number that may be linked to the higher inflammatory response observed in Mal−/− mice. Altogether, these data clearly demonstrated that the inflammatory environment controls the emergence of Ly6Clo or Ly6Chi cell population in the spleen. As is well known, the primary role of monocytes is considered to sense the environment to adapt the immune response.
A new concept has emerged from our data: the cytokine pattern exhibited by Ly6CloTLR2hi or Ly6ChiTLR2hi monocytes was cell context-dependent and TLR context-independent. Whatever TLR agonist used, monocyte subpopulations produce the same cytokine pattern. Ly6Chi monocytes were involved in TNF-α and IL-12 production, which agrees with others studies defining these cells as pro-inflammatory cells (35, 39, 40). On the other hand, Ly6Clo population was committed with TNF-α and IL-10 synthesis. Such results have put into question the fact that the same agonist can induce pro- or anti-inflammatory cytokines. These findings have led us to compare the signaling pathways used by both cell populations after triggering TLR2 or TLR9.
According to our data, Mal was required for the TNF-α production by Ly6Clo monocytes, but not by Ly6Chi monocytes in response to TLR2 or TLR9 agonists. In addition, it was possible to connect the IL-10 production by Ly6Clo cells with the requirement of Mal, which reinforces the idea that Mal plays an immunoregulatory role during T. cruzi infection, as was observed in homeostasis and in other diseases (18). This correlated with the phenotype of infected Mal−/− mice able to mount a host defense response and inducing higher levels of, IL-12, NO, and IFN-γ. The mechanism involved in the differential recruitment of Mal by both monocyte populations has not been elucidated but may result from differential expression of this adapter in monocytes. Because Mal plays a role as a key upstream of transcription factors, it may also influence the expression of other proteins in Ly6Clo cells, like FIZZ1, and arginase-1, that classically represent markers of M2-monocyte/macrophage polarization (41) that in its turn may directly impact on cytokine pattern release by cells. Another explanation that may be advanced about the differential use of Mal by the monocyte populations concerns the possible interaction of co-receptor associated with TLR9 or/and TLR2 in one population but not in the other, which may differentially activate downstream signaling pathways.
Furthermore, our results indicate that TLR2 response did not require Mal in Ly6Chi cells. This parallels a study where Mal has been described as dispensable when the interactions between TLR2 and its agonist are prolonged or enhanced (42). This may be achieved by using elevated concentration of TLR2 agonist (43) or may be by high level of TLR2 expression, as in Ly6ChiTLR2hi cells. Our finding that Mal was required for cytokine production by Ly6Clo cells in response to the TLR9 agonist suggests that this adaptor plays a role in signaling from endosomal receptors. In the same way, Bonham et al. (44) have reported that Mal is required for signaling downstream of endosomal TLRs in response to viral nucleic acids including TLR9 agonist. It is possible that Mal may assume a role outside the conventional TLR signaling pathway to turn the Ly6Clo cell into cytokine producer. Through these data, we confirmed that the cytokine pattern exhibited by monocytes was TLR context-independent and differentially engaged the responsibility of adapter protein like Mal/TIRAP.
Few studies using infection models have included a comparative analysis of the role of the different monocyte subsets. Therefore, our data bring new knowledge concerning the relative contributions of Ly6Chi versus Ly6Clo cells in a model of acute infection. Further, we have shown the requirement of Mal in monocyte biology independently of the type of TLR engaged. More studies will be necessary to better understand the TLR2/Mal axis and to predict the effect of TLR2 therapy.
Experimental Procedures
Ethics Statement
All experiments involving animals were in accordance with guidelines set forth by the American Association for Laboratory Animal Science and with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation and Federal Law 11.794 (October 8, 2008). All protocols developed for this work were approved by the Institutional Animal Care and Use Committee on Ethics of Animal Experimentation (Comitê de Ética em Experimentação Animal) from Universidade Federal de Minas Gerais (protocol 83/2012).
Mice
The wild-type (C57Bl/6 WT) and deficient animals in IL-12, TLR2, TLR9, and Mal/TIRAP were provided by the central animal facility of Centro de Pesquisas René Rachou or by the particular animal house of Dr. Sérgio Costa Oliveira (Universidade Federal de Minas Gerais) and kept in microisolators in heated animal room in Centro de Pesquisas René Rachou, Oswaldo Cruz Foundation (Belo Horizonte, Minas Gerais, Brazil). TLR2−/− and TLR9−/− mice were generated by Dr. Shizuo Akira at Osaka University (Osaka, Japan). All deficient animals were backcrossed with C57Bl/6 for at least eight generations.
Reagents
They were obtained from Sigma-Aldrich except for those indicated hereafter. The CpG DNA (TCGACGTTTGGATCGGT) sequence is similar to one encountered in the T. cruzi genome and was synthetized in a phosphorothioate backbone and purchased from Coley pharmaceutical group (Wellesley, MA) (10). Pam3Cysk was obtained from InvivoGen.
Experimental T. cruzi Infection
The mice were infected i.p. with 100 bloodstream trypomastigote forms of T. cruzi Y strain and euthanized 4–7 days after infection. Parasitemia levels were evaluated by counting parasites in 5 μl of blood from the tail vein, and mortality was assessed daily. Aliquots of the supernatants from splenic cells were collected after 48 and 72 h of culture for respective measurements of nitrite (nitric oxide) by the Griess reaction, IL-12/IL-23p40, and IFN-γ with Duoset ELISA kits from R&D Systems Inc. (Minneapolis, MN).
Flow Cytometric Assays and Analysis
Spleens were harvested from 6–8-week-old C57Bl/6 or deficient mice and macerated in RPMI 1640 medium. The cell suspensions were centrifuged for 10 min at 700 × g at 4 °C, and the red blood lysis was performed using ACK lysing buffer by 10 min/4 °C. After assays with 5 × 106 cells/tube, the cells were stained with mAb specific to CD11b-PE-Cy7, MHCII-APC, Ly6C-eFluor 450, and CD11c-Alexa Fluor 488 (eBioscience) for immunophenotyping. Surface staining was performed in the dark for 30 min at 4 °C in staining buffer. The data were collected by the cell analyzing LSRFortessa (BD Biosciences, Immunocytometry Systems) using BD FACSDivaTM software (BD Biosciences) and analyzed with FlowJo software (Tree Star). The results were presented as frequency (%), number of cells (# of cells/106 events), and levels of TLR2 expression or cytokine production (MFI).
Analysis of TLR2 Expression on Mouse Splenic Cells
To evaluate TLR2 expression, total splenic cells were stimulated with CpG DNA (1 μg/ml) and Pam3Cys (1 μg/ml) for 10 h. Then specific mAb anti-TLR2-PE (eBioscience) was added together with the antibodies for immunophenotyping mentioned above. After surface staining, the cells were washed twice with staining buffer followed by fixation in 1% paraformaldehyde. These assays were realized in the absence of the protein transport inhibitor brefeldin A (Sigma-Aldrich). Cells lacking TLR2 were used as a control for antibody binding; this strategy allowed us to identify the positive versus negative populations.
Analysis of Cytokines Production by Mouse Splenic Cells
To evaluate intracellular cytokine staining, total splenic cells were stimulated with CpG DNA (1 μg/ml) or Pam3Cys (1 μg/ml) for 12 h in the presence of 10 μg/ml of brefeldin A. After immunophenotyping by labeling surface markers, the cells were permeabilized according to the kit instructions (Fix/Perm kit; BD Biosciences), and specific antibodies were added for IL-10-PE (BD Biosciences, Pharmingen), IL-12-PE, or TNF-α-PE (eBioscience). Then the cells were washed twice with staining buffer followed by fixation in 1% paraformaldehyde.
Statistical Analysis
Analysis was performed using GraphPad Prism software. The statistical significance of the data were determined by an unpaired Student's test or a two-way analysis of variance (ANOVA) with Bonferroni post-test. The experiments were realized with averages ± S.D. of four animals, and p values are shown with asterisks: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Author Contributions
C. R. and H. D. G. conceived and designed the experiments, performed the experiments, analyzed the data, and wrote the paper. S. M. F. M. and A. M. G. contributed reagents/materials/analysis tools.
Acknowledgments
We thank the Program for Technological Development in Tools for HealthPDTIS-FIOCRUZ for the use of its facilities. We are grateful to Jasper Katz and the entire Tree Star group for the offer of FlowJo Software. We express our thanks to Dr. Policarpo Sales Jr. (FIOCRUZ) for the support in the experimental infection. We are grateful to Dr. Ricardo Tostes Gazzinelli for technical support and for providing laboratory facilities.
The authors declare that they have no conflicts of interest with the contents of this article.
- TLR
- Toll-like receptor
- ANOVA
- analysis of variance
- MFI
- median fluorescence intensity.
References
- 1. Takeda K., and Akira S. (2004) Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34, 73–82 [DOI] [PubMed] [Google Scholar]
- 2. Takeda K., Kaisho T., and Akira S. (2003) Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 [DOI] [PubMed] [Google Scholar]
- 3. Kawai T., and Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 4. Trinchieri G., and Sher A. (2007) Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 [DOI] [PubMed] [Google Scholar]
- 5. Gravina H. D., Antonelli L., Gazzinelli R. T., and Ropert C. (2013) Differential use of TLR2 and TLR9 in the regulation of immune responses during the infection with Trypanosoma cruzi. PLoS One 8, e63100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almeida I. C., Camargo M. M., Procópio D. O., Silva L. S., Mehlert A., Travassos L. R., Gazzinelli R. T., and Ferguson M. A. (2000) A highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 19, 1476–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campos M. A., Almeida I. C., Takeuchi O., Akira S., Valente E. P., Procópio D. O., Travassos L. R., Smith J. A., Golenbock D. T., and Gazzinelli R. T. (2001) Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167, 416–423 [DOI] [PubMed] [Google Scholar]
- 8. Oliveira A.-C., Peixoto J. R., de Arruda L. B., Campos M. A., Gazzinelli R. T., Golenbock D. T., Akira S., Previato J. O., Mendonça-Previato L., Nobrega A., and Bellio M. (2004) Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 173, 5688–5696 [DOI] [PubMed] [Google Scholar]
- 9. Bafica A., Santiago H. C., Goldszmid R., Ropert C., Gazzinelli R. T., and Sher A. (2006) Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 177, 3515–3519 [DOI] [PubMed] [Google Scholar]
- 10. Bartholomeu D. C., Ropert C., Melo M. B., Parroche P., Junqueira C. F., Teixeira S. M., Sirois C., Kasperkovitz P., Knetter C. F., Lien E., Latz E., Golenbock D. T., and Gazzinelli R. T. (2008) Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J. Immunol. 181, 1333–1344 [DOI] [PubMed] [Google Scholar]
- 11. Caetano B. C., Carmo B. B., Melo M. B., Cerny A., dos Santos S. L., Bartholomeu D. C., Golenbock D. T., and Gazzinelli R. T. (2011) Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J. Immunol. 187, 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ropert C., and Gazzinelli R. T. (2004) Regulatory role of Toll-like receptor 2 during infection with Trypanosoma cruzi. J. Endotoxin Res. 10, 425–430 [DOI] [PubMed] [Google Scholar]
- 13. van Bergenhenegouwen J., Plantinga T. S., Joosten L. A., Netea M. G., Folkerts G., Kraneveld A. D., Garssen J., and Vos A. P. (2013) TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J. Leukoc. Biol. 94, 885–902 [DOI] [PubMed] [Google Scholar]
- 14. Fitzgerald K. A., Palsson-McDermott E. M., Bowie A. G., Jefferies C. A., Mansell A. S., Brady G., Brint E., Dunne A., Gray P., Harte M. T., McMurray D., Smith D. E., Sims J. E., Bird T. A., and O'Neill L. A. (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413, 78–83 [DOI] [PubMed] [Google Scholar]
- 15. Horng T., Barton G. M., and Medzhitov R. (2001) TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 [DOI] [PubMed] [Google Scholar]
- 16. Horng T., Barton G. M., and Flavell R. A., and Medzhitov R. (2002) The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420, 329–333 [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., and Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-B promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 [DOI] [PubMed] [Google Scholar]
- 18. Aviello G., Corr S. C., Johnston D. G., O'Neill L. A., and Fallon P. G. (2014) MyD88 adaptor-like (Mal) regulates intestinal homeostasis and colitis-associated colorectal cancer in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G769–G778 [DOI] [PubMed] [Google Scholar]
- 19. Bernard N. J., and O'Neill L. (2013) a. Mal, more than a bridge to MyD88. IUBMB Life 65, 777–786 [DOI] [PubMed] [Google Scholar]
- 20. Ladhani S. N., Davila S., Hibberd M. L., Heath P. T., Ramsay M. E., Slack M. P., Pollard A. J., and Booy R. (2010) Association between single-nucleotide polymorphisms in Mal/TIRAP and interleukin-10 genes and susceptibility to invasive Haemophilus influenza serotype b infection in immunized children. Clin. Infect. Dis. 51, 761–767 [DOI] [PubMed] [Google Scholar]
- 21. Mellett M., Atzei P., Jackson R., and O'Neill L. A., and Moynagh P. N. (2011) Mal mediates TLR-induced activation of CREB and expression of IL-10. J. Immunol. 186, 4925–4935 [DOI] [PubMed] [Google Scholar]
- 22. Choi Y. J., Jung J., Chung H. K., Im E., and Rhee S. H. (2013) PTEN regulates TLR5-induced intestinal inflammation by controlling Mal/TIRAP recruitment. FASEB J. 27, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ní Cheallaigh C., Sheedy F. J., Harris J., Muñoz-Wolf N., Lee J., West K., McDermott E. P., Smyth A., Gleeson L. E., Coleman M., Martinez N., Hearnden C. H., Tynan G. A., Carroll E. C., Jones S. A., et al. (2016) A common variant in the adaptor Mal regulates Interferon gamma signaling. Immunity 44, 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.-L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., and Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rose S., Misharin A., and Perlman H. (2012) A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., and Ley K. (2010) Development of monocytes, macrophages and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varol C., Landsman L., Fogg D. K., Greenshtein L., Gildor B., Margalit R., Kalchenko V., Geissmann F., and Jung S. (2007) Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sunderkötter C., Nikolic T., Dillon M. J., Van Rooijen N., Stehling M., Drevets D. A., and Leenen P. J. (2004) Van subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 [DOI] [PubMed] [Google Scholar]
- 29. Dasu M. R., Devaraj S., Park S., and Jialal I. (2010) Increased Toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33, 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin I. C., Kuo H. C., Lin Y. J., Wang F. S., Wang L., Huang S. C., Chien S. J., Huang C. F., Wang C. L., Yu H. R., Chen R. F., and Yang K. D. (2012) Augmented TLR2 expression on monocytes in both human Kawasaki disease and a mouse model of coronary arteritis. PLoS One 7, e38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mukherjee R., Kanti Barman P., Kumar Thatoi P., Tripathy R., Kumar Das B., and Ravindran B. (2015) Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci. Rep. 5, 13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flo T. H., Halaas O., Torp S., Ryan L., Lien E., Dybdahl B., Sundan A., and Espevik T. (2001) Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69, 474–481 [PubMed] [Google Scholar]
- 33. Sabroe I., Jones E. C., Usher L. R., Whyte M. K., and Dower S. K. (2002) Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168, 4701–4710 [DOI] [PubMed] [Google Scholar]
- 34. Heggelund L., Müller F., Lien E., Yndestad A., Ueland T., Kristiansen K. I., Espevik T., Aukrust P., and Frøland S. S. (2004) Increased expression of Toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin. Infect Dis. 39, 264–269 [DOI] [PubMed] [Google Scholar]
- 35. Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A. T., Friedlander G., Mack M., Shpigel N., Boneca I. G., Murphy K. M., Shakhar G., Halpern Z., and Jung S. (2012) Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 [DOI] [PubMed] [Google Scholar]
- 36. Hettinger J., Richards D. M., Hansson J., Barra M. M., Joschko A.-C., Krijgsveld J., and Feuerer M. (2013) Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14, 821–830 [DOI] [PubMed] [Google Scholar]
- 37. Richards D. M., Hettinger J., and Feuerer M. (2013) Monocytes and macrophages in cancer: development and functions. Cancer Microenviron. 6, 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shmuel-Galia L., Aychek T., Fink A., Porat Z., Zarmi B., Bernshtein B., Brenner O., Jung S., and Shai Y. (2016) Neutralization of pro-inflammatory monocytes by targeting TLR2 dimerization ameliorates colitis. EMBO J. 35, 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin S. L., Castaño A. P., Nowlin B. T., Lupher M. L. Jr., and Duffield J. S. (2009) Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 [DOI] [PubMed] [Google Scholar]
- 40. Rivollier A., He J., Kole A., Valatas V., and Kelsall B. L. (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jablonski K. A., Amici S. A., Webb L. M., Ruiz-Rosado J. D., Popovich P. G., Partida-Sanchez S., and Guerau-de-Arellano M. (2015) Novel markers to delineate murine M1 and M2 macrophages. PLoS One 10, e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cole L. E., Laird M. H., Seekatz A., Santiago A., Jiang Z., Barry E., Shirey K. A., and Fitzgerald K. A., and Vogel S. N. (2010) Phagosomal retention of Francisella tularensis results in TIRAP/Mal-independent TLR2 signaling. J. Leukoc. Biol. 87, 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kenny E. F., Talbot S., Gong M., Golenbock D. T., Bryant C. E., and O'Neill L. A. (2009) MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J. Immunol. 183, 3642–51 [DOI] [PubMed] [Google Scholar]
- 44. Bonham K. S., Orzalli M. H., Hayashi K., Wolf A. I., Glanemann C., Weninger W., Iwasaki A., Knipe D. M., and Kagan J. C. (2014) A promiscuous lipid-binding protein diversifies the subcellular sites of Toll-like receptor signal transduction. Cell 156, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]