Summary
Statins, widely prescribed as cholesterol‐lowering drugs, have recently been extensively studied for their pleiotropic effects on immune systems, especially their beneficial effects on autoimmune and inflammatory disorders. However, the mechanism of statin‐induced immunosuppression is far from understood. Here, we found that atorvastatin promoted the expansion of myeloid‐derived suppressor cells (MDSCs) both in vitro and in vivo. Atorvastatin‐derived MDSCs suppressed T‐cell responses by nitric oxide production. Addition of mevalonate, a downstream metabolite of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase, almost completely abrogated the effect of atorvastatin on MDSCs, indicating that the mevalonate pathway was involved. Along with the amelioration of dextran sodium sulphate (DSS) ‐induced murine acute and chronic colitis, we observed a higher MDSC level both in spleen and intestine tissue compared with that from DSS control mice. More importantly, transfer of atorvastatin‐derived MDSCs attenuated DSS acute colitis and T‐cell transfer of chronic colitis. Hence, our data suggest that the expansion of MDSCs induced by statins may exert a beneficial effect on autoimmune diseases. In summary, our study provides a novel potential mechanism for statins‐based treatment in inflammatory bowel disease and perhaps other autoimmune diseases.
Keywords: atorvastatin, immunosuppression, murine colitis, myeloid‐derived suppressor cells, nitric oxide
Abbreviations
- CFSE
5,6 carboxyfluorescein diacetate, succinimidyl ester
- CM‐H2DCFDA
5‐(and‐6)‐chloromethyl‐2,7‐dichlorodihydrofluorescein diacetate, acetyl ester
- DSS
dextran sodium sulphate
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- G‐MDSCs
granulocytic MDSCs
- HMG‐CoA
3‐hydroxy‐3‐methylglutaryl coenzyme A
- IFN‐γ
interferon‐γ
- IL‐17
interleukin‐17
- iNOS
inducible nitric oxide synthase
- JAK/STAT
Janus kinase/singal transducer and activator of transcription
- LPMCs
lamina propria mononuclear cells
- MDSCs
myeloid‐derived suppressor cells
- mLN
mesenteric lymph nodes
- M‐MDSCs
monocytic MDSCs
- NADPH
nicotinamide adenine dinucleotide phosphate‐oxidase complex
- PE
phycoerythrin
- PP
Peyer's patches
- ROS
reactive oxygen species
- Th1
T helper type 1
Introduction
Statins are inhibitors of the 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase, a rate‐limiting enzyme that catalyses the conversion of HMG‐CoA to mevalonate in cholesterol synthesis. Statins have been used extensively as cholesterol‐lowering agents and for the treatment of cardiovascular disease. However, accumulating evidence has demonstrated that statins have extensive immunomodulatory and anti‐inflammatory properties in addition to their essential role in lipid lowering.1, 2, 3
In recent years, considerable interest has arisen in statin‐based therapy of autoimmune and inflammatory disorders.1, 2, 4 Studies have found that statin treatment results in an improved clinical outcome in animal models of murine colitis,5, 6, 7, 8, 9 experimental autoimmune encephalomyelitis,10, 11 arthritis12 and systemic lupus erythematosus.13 These beneficial effects are partly attributed to statins' immunomodulatory role in suppressing T helper type 1 and type 17 responses, while promoting T helper type 2 responses.1, 2 Atorvastatin (Lipitor), a current extensively prescribed statin drug, has recently gained much attention in inflammatory bowel disease due to its immunomodulating property.1, 2 In an animal model of acute experimental colitis, atorvastatin significantly inhibited systemic tumour necrosis factor‐α, interleukin‐17 (IL‐17) and IL‐23 levels.5 Several studies also obtained promising results in atorvastatin‐based treatment of Crohn's disease.14, 15, 16 Despite the extensive studies on statin‐mediated immunomodulation and anti‐inflammatory effects, the detailed mechanism is not completely understood.
Myeloid‐derived suppressor cells (MDSCs) are defined as a heterogeneous population of pathologically activated immature cells and exert a negative regulation of the immune responses in cancer, autoimmunity, inflammatory conditions, trauma and infections.17, 18, 19, 20 In mice, MDSCs simultaneously express CD11b and Gr‐1, which can be further divided into two different subsets [monocytic MDSCs (M‐MDSCs) and granulocytic MDSCs (G‐MDSCs)], based on their expression of Ly6C and Ly6G, respectively.21, 22 These two subsets differ in their tissue localization, antigen presentation and immunosuppressive mechanism.22 Human MDSCs are generally defined as HLA‐DR−/low CD11b+ CD33+ cells.23 A variety of cytokines and growth factors [such as granulocyte–macrophage colony‐stimulating factor (GM‐CSF), macrophage colony‐stimulating factor, IL‐6, interferon‐γ (IFN‐γ), IL‐1β and IL‐13] in the tumour environment can induce MDSC expansion or activation by activating certain signalling pathways, especially Janus kinase/singal transducer and activator of transcription (JAK/STAT) pathway.24, 25 MDSCs then display a potent immune suppressive activity towards various immune cells, especially T cells, mainly by the l‐arginine metabolic pathway.17, 18 Recently, a number of studies have shown that MDSCs have beneficial effects on inflammatory bowel disease,26, 27, 28 asthma,29 rheumatoid arthritis,30 multiple sclerosis31 and pregnancy.32 Hence, drugs with few side effects that promote MDSC expansion under these conditions may improve clinical outcomes.
Here, we found that atorvastatin, a widely prescribed drug in the statin family, remarkably promotes mouse G‐MDSC expansion both in vitro and in vivo. Furthermore, the atorvastatin‐induced G‐MDSCs can attenuate murine acute and chronic colitis. Hence, our study provides a novel immunomodulation pathway of statins in inflammatory bowel disease and other autoimmune disorders.
Materials and methods
Mice
C57BL/6 and BALB/c mice were purchased from the Laboratory Animal Centre of Sun Yat‐Sen University. Rag1 −/− mice (B6.129S7‐Rag1 tm1Mom/JNju) were purchased from the Model Animal Research Centre of Nanjing University. All mice were housed under specific pathogen‐free conditions and used at an age of 6–8 weeks. All mouse experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat‐Sen University (approval No. IACUC‐DB‐16‐0507).
Reagents and antibodies
RPMI‐1640, fetal bovine serum, 2‐mercaptoethanol, penicillin, streptomycin, 5‐(and‐6)‐chloromethyl‐2,7‐dichlorodihydrofluorescein diacetate, acetyl ester (CM‐H2DCFDA) and 5,6 carboxyfluorescein diacetate, succinimidyl ester (CFSE) were obtained from Invitrogen (Grand Island, NY). Dextran sodium sulphate (DSS) (MW 36 000–50 000) was obtained from MP Biomedicals (Solon, OH). Recombinant murine GM‐CSF, IL‐6 and human GM‐CSF and IL‐6 were purchased from Peprotech (Oak Park, CA). NW‐hydroxy‐nor‐arginine and l‐NG‐monomethyl‐arginine (l‐NMMA) were obtained from Cayman Chemical (Ann Arbor, MI). Atorvastatin calcium salt trihydrate, N‐acetyl‐l‐cysteine, l‐arginine, cucurbitacin I hydrate (JSI‐124), DMSO, castor oil and mevalonolactone were purchased from Sigma‐Aldrich (St Louis, MO). The following anti‐mouse antibodies were purchased from eBioscience (San Diego, CA): CD3e‐phycoerythrin (PE), CD4‐PE‐Cy5, CD45RB‐PE, CD8a‐PE, Gr‐1‐PE‐Cy5, CD11b‐PE‐Cy7, CD11c‐PE‐Cy5 and their corresponding isotype antibodies. Anti‐human antibodies: CD11b‐FITC, CD33‐PE and HLA‐DR‐allophycocyanin were also purchased from eBioscience. Ly6G‐PE‐Cy7, Ly6C‐PE and CD11b‐FITC were obtained from BD Biosciences (San Jose, CA). MHC Class II‐PE and the mouse myeloid‐derived suppressor cell isolation kit were from Miltenyi Biotec (Auburn, CA).
Generation of mouse MDSCs in vitro
To generate MDSCs in vitro, 2 million bone marrow cells from the mouse femurs and tibias were cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum, 20 ng/ml GM‐CSF, 50 μm 2‐mercaptoethanol, with or without 20 ng/ml IL‐6, as described previously.33, 34 The cultures were kept at 37° in a 5% CO2‐humidified atmosphere in 24‐well plates. Media were half changed on day 3. Cells were analysed by flow cytometry on day 5.
T‐cell proliferation assay
T‐cell proliferation was performed by CFSE dilution as previously described.34 Briefly, sorted CD3+ T cells containing CD4+ T cells and CD8+ T cells from BALB/c mice were labelled with CFSE (2 μm) (Invitrogen), stimulated with anti‐mouse CD3 (5 μg/ml) coated plates and soluble anti‐mouse CD28 (1 μg/ml) antibody (eBioscience) and cultured alone or co‐cultured with allogeneic MDSCs (from C57BL/6 mice) at different ratios for 3 days. Cells were then stained with CD4‐PE‐Cy5 and CD8a‐PE antibodies, and T‐cell proliferation was analysed by flow cytometry.
Atorvastatin treatment
For atorvastatin administration in vivo, C57BL/6 female mice (6–8 weeks) were injected intraperitoneally with 50 mg/kg/day atorvastatin dissolved in 50 μl 5% DMSO/castor oil. Controls received the vehicle (5% DMSO/castor oil, 50 μl) via the same route. The same dose of atorvastatin for mice was used as described earlier.9, 35 Mouse weight was monitored daily and mice were killed at day 12.
Induction of DSS colitis
Murine acute colitis was induced by giving 2·5% DSS (MW 36 000–50 000; MP Biomedicals, Solon, OH) dissolved in drinking water as previously described.36 Atorvastatin (50 mg/kg/day) or vehicle was injected intraperitoneally for 3 days. After that, mice were injected daily with atorvastatin (atorvastatin alone and DSS + atorvastatin group) or vehicle (the control and DSS alone group). Control mice (n = 6) received drinking water without DSS. Mice were killed at day 10. For chronic DSS colitis induction, mice were given 2% DSS for 7 days followed by 14 days of drinking water.37 This cycle was repeated three times. Atorvastatin or vehicle was injected intraperitoneally for 3 days before each DSS administration and lasted for 12 days. Mice were killed at day 55. For the induction of DSS‐induced chronic colitis in Rag1 −/− mice, mice were given 2% DSS for 5 days followed by 5 days of drinking water.38, 39 This cycle was repeated three times. Atorvastatin or vehicle was injected intraperitoneally for 3 days before DSS administration and lasted to the end. All the mice were killed at day 33.
To evaluate the colitis severity, animals were monitored daily for loss of body weight, daily stool consistency, haematochezia as previously described.6, 37 Disease activity index was determined as the combined scores of (i) weight loss, (ii) stool consistency, and (iii) bleeding divided by 3. Each score was obtained as follows: by change in weight (0, ≤ 1%; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, ≥ 15%), haemoccult positivity (0, negative; 2, positive; 4, gross bleeding), and stool consistency (0, normal; 2, loose stools; 4, diarrhoea). Colon length as an indirect marker of inflammation was also recorded. After removing the caecum, the rest of the colon was cut into proximal and distal halves. Tissue was fixed in 3% buffered formalin, paraffin‐embedded, sectioned, and stained with haematoxylin & eosin for histological examination and scored as described previously.37
Flow cytometry and sorting
Single cell suspensions were stained with specific fluorescein‐conjugated antibodies and the proportions of different cell populations were obtained by a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). All data were analysed by flowjo software (Tree Star, Ashland, OR). For flow cytometric sorting, cultured bone marrow cells or mouse splenocytes were stained with anti‐mouse Gr‐1 and CD11b antibodies and CD11b+ Gr‐1high G‐MDSCs were isolated by cell sorting on a FACS Aria cell sorter (BD, Mountain View, CA). For isolation of T cells, mouse splenocytes were stained with CD3e‐PE antibody and CD3+ T cells were isolated by flow cytometric sorting. The purity of all sorted cells was 97–98%.
Adoptive transfer experiments
Adoptive transfer of G‐MDSCs was carried out as described previously.27, 40 Briefly, G‐MDSCs (Gr‐1high Ly6G+, 97–98% purity) or control cells with the same phenotype were enriched using an MDSC isolation kit (Miltenyi Biotec) from the spleen of atorvastatin‐treated mice or control mice using two LS columns, followed by tail‐vein injection (2 million cells) into each mouse on day 2 post‐DSS treatment. After that, the same numbers of G‐MDSCs were injected intravenously into recipient mice every 4 days. Mice were killed at day 10.
The T‐cell transfer model of colitis was performed following the procedures described previously.41 Briefly, Rag1 −/− mice were injected intraperitoneally with 5 × 105 fluorescence‐activated cell sorted CD4+ CD45RBhigh T cells from C57BL/6 mice (purity 97–98%), 5 × 105 CD4+ CD45RBhigh T cells + 5 × 105 G‐MDSCs (Gr‐1high Ly6G+) or 5 × 105 control cells with the same phenotype from the spleens of mice after atorvastatin or vehicle treatment. After that, the same number of G‐MDSCs and control cells were transfered into recipient mice every 4 days and lasted for 2 weeks. Mice were killed at 8 weeks post‐injection when they had lost 15–20% of their original body weight.
NO production
The assay was performed following the same procedures described previously.42
Reactive oxygen species production
Reactive oxygen species (ROS) level was measured by flow cytometry using CM‐H2DCFDA (Invitrogen) following procedures described earlier.34
Quantative real‐time PCR
Quantitative real‐time RT‐PCR was performed as described earlier.43 Briefly, total RNA was extracted with Trizol (Invitrogen) according to the manufacturer's protocols, and cDNA was synthesized using a SuperScript III reverse transcriptase kit (Qiagen, Valencia, CA). Quantitative RT‐PCR was performed in triplicate with SYBR Green (TakaRa, Otsu, Japan) with gene‐specific primers (see Supplementary material, Table S1).
Human peripheral blood mononuclear cells isolation and generation of human MDSCs in vitro
Human peripheral blood mononuclear cells from healthy volunteer donors were isolated by Ficoll centrifugation as described earlier,41 followed by culturing the cells in the presence of 10 ng/ml GM‐CSF and IL‐6 to generate human MDSCs as described previously.44
Statistics
Statistical analysis was performed by graphpad prism Version 5.0a (GraphPad Software Inc., San Diego, CA, USA) and spss 17.0 (SPSS Inc., Chicago, IL, USA). Differences between the two groups were assessed by unpaired Student's t‐tests and one‐way analysis of variance, followed by Dunnett's post hoc test, was used to compare the differences between more than two groups. P < 0·05 was considered significant.
Results
Atorvastatin promotes MDSC expansion in vitro
To investigate whether atorvastatin influences MDSC expansion, we first cultured mouse bone marrow cells in the presence of GM‐CSF with or without IL‐6 as described previously.33 After 5 days, phenotype was evaluated by flow cytometry. Atorvastatin significantly increased the frequency of MDSCs (CD11b+ Gr‐1+) from 34·9 to 57·4% (Fig. 1a); the total number of cultured cells was not affected. Further, we analysed the subset of MDSCs and data showed that the CD11b+ Ly6G+ Ly6C−/low G‐MDSC population, not CD11b+ Ly6G− Ly6Chigh M‐MDSCs, was significantly elevated by atorvastatin (Fig. 1a). Meanwhile, a dramatic reduction of CD11c+ MHC‐II+ dendritic cells (DCs) frequency was observed upon atorvastatin administration (Fig. 1a), indicating a deficiency in myeloid differentiation.
Figure 1.
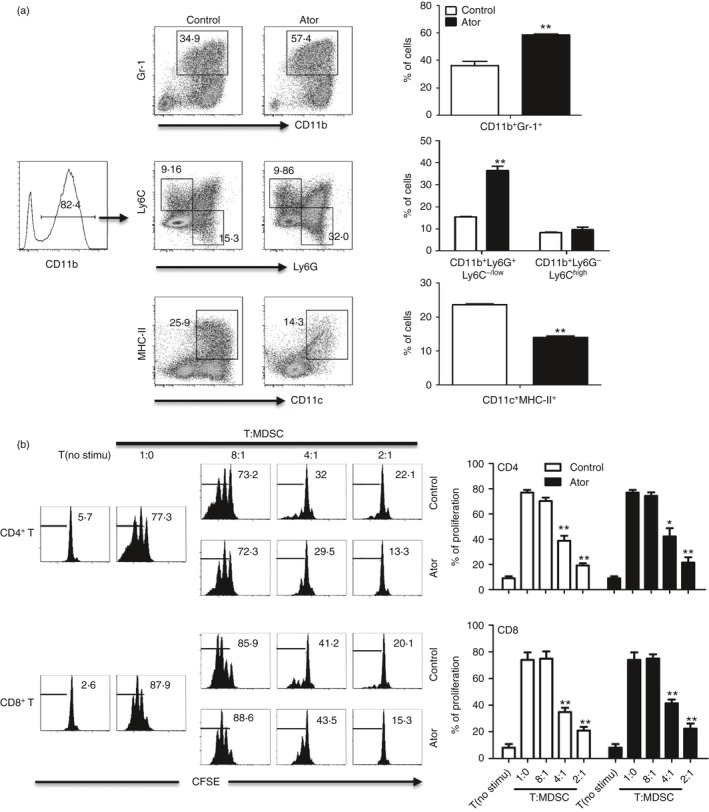
Atorvastatin enhanced the expansion of myeloid‐derived suppressor cells (MDSCs) in vitro. (a) Mouse bone marrow (BM) cells were cultured in the presence of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (20 ng/ml) and 5 μm atorvastatin. DMSO was used as control. The frequency of MDSCs (CD11b+ Gr‐1+), MDSCs subsets, and dendritic cells (DCs) (CD11c+ MHC‐II+) were measured by flow cytometry analysis. Representative data from a single experiment (left), as well as mean + SEM of three independent experiments (right) are shown. (b) The suppressive function of atorvastatin‐derived and control granulocytic (G‐) MDSCs (CD11b+ Gr‐1high) was determined. C57BL/6 mouse BM cells were cultured in the presence of GM‐CSF and interleukin‐6 (IL‐6) (20 ng/mL) for 5 days with atorvastatin or DMSO control. G‐MDSCs (CD11b+ Gr‐1high) were purified by flow cytometric sorting. Allogeneic CD3+ T cells containing CD4+ and CD8+ T cells (from BALB/c mice) were stimulated with anti‐CD3/CD28 antibodies, and co‐cultured with isolated G‐MDSCs at different ratios for 3 days. CD4+ and CD8+ T‐cell proliferation was determined by CFSE dilution. Unstimulated T cells were used as a negative control. Representative data from a single experiment (left), as well as mean+SEM of three independent experiments (right) are shown. Ator, atorvastatin. *P < 0·05, **P < 0·01.
Based on the fact that MDSCs can dampen T‐cell immune responses under pathological conditions,17 we next evaluated the suppressive activity of atorvastatin‐induced MDSCs. Mouse bone marrow cells were cultured in the presence of GM‐CSF and IL‐6 with or without atorvastatin treatment in vitro for 5 days. As CD11b+ Gr‐1high MDSCs correspond to G‐MDSCs (see Supplementary material, Fig. S1), we sorted CD11b+ Gr‐1high MDSCs and co‐cultured with CFSE‐labelled allogeneic T cells at different ratios; CD4+ and CD8+ T‐cell proliferation was determined after 3 days. Atorvastatin‐derived MDSCs exerted a strong suppressive activity towards T‐cell proliferation, but no difference was observed when compared with control (Fig. 1b). Together, these results suggest that atorvastatin could promote the expansion of MDSCs in vitro.
Atorvastatin promotes MDSCs accumulation in vivo
Next, we investigated whether atorvastatin can induce MDSC accumulation in vivo. C57BL/6 mice were administered intraperitoneally with atorvastatin or vehicle control. After 12 days, the frequency of MDSCs in spleen, bone marrow, liver and peripheral blood was determined by flow cytometry. We found consistently higher levels of CD11b+ Ly6G+ Ly6C−/low cells in these tissues from mice treated with atorvastatin than in those from control mice, whereas no differences in the frequency of CD11b+ Ly6G− Ly6Chigh cells were observed (Fig. 2a). Previous studies have demonstrated that CD11b+ Gr‐1+ cells from naive mice are not immunosuppressive and are regarded as immature myeloid cells.45, 46 To determine whether the accumulated population of CD11b+ Ly6G+ Ly6C−/low cells were G‐MDSCs, we performed a T‐cell proliferation assay and found that this subset from atorvastatin‐treated mouse spleen were immunosuppressive, whereas the cells from control mice were non‐suppressive (Fig. 2b). Moreover, we also found that CD11b+ Gr‐1high cells from liver, peripheral blood mononuclear cells, but not bone marrow were immunosuppressive in atorvastatin groups, but were non‐suppressive in control groups (see Supplementary material, Fig. S2). These observations indicated that atorvastatin induces G‐MDSC accumulation in vivo.
Figure 2.
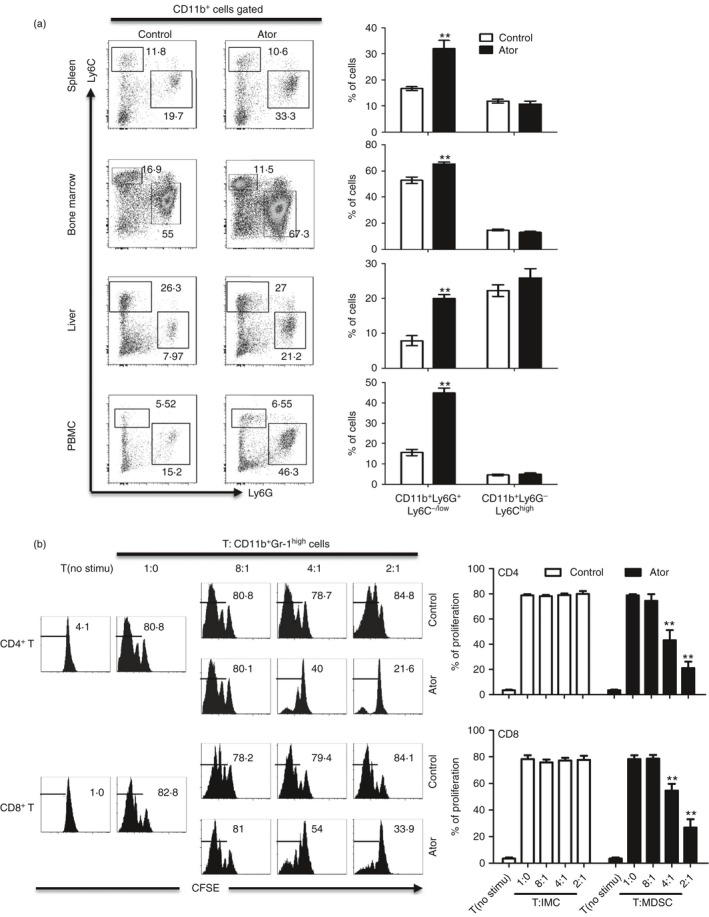
Atorvastatin promoted granulocytic myeloid‐derived suppressor cell (G‐MDSC) accumulation in vivo. (a) C57BL/6 mice (6–8 weeks) were injected intraperitoneally with atorvastatin at a dose of 50 mg/kg/day in a volume of 50 μl 5% DMSO/castor oil (n = 6). Control mice received the vehicle (5% DMSO/castor oil) via the same route (n = 6). Mice were killed after 12 days and different tissues were obtained. Proportions of MDSC subsets in the spleen, bone marrow (BM), liver and peripheral blood mononuclear cells (PBMCs) were measured by flow cytometry. Representative data from a single mouse (left), as well as the mean + SEM from all mice (right) are shown. (b) The suppressive function of G‐MDSCs (CD11b+ Gr‐1high) from the spleen of mice treated with atorvastatin. Unstimulated T cells were used as a negative control. Representative data from a single experiment (left), as well as mean + SEM of three independent experiments (right) are shown. **P < 0·01.
Atorvastatin‐derived MDSCs inhibited T‐cell responses by nitric oxide production
It is well known that l‐arginine and its metabolic products are important for the suppressive function of MDSCs under certain conditions.17, 18 To investigate the functional mechanism of atorvastatin‐derived MDSCs, we firstly analysed mRNA expression of the genes related to MDSCs by quantitative RT‐PCR.34 Intriguingly, we observed that the expression of inducible nitric oxide synthase (iNOS) was significantly increased, whereas no effect was observed for other genes, including all the components of the nicotinamide adenine dinucleotide phosphate‐oxidase complex (NADPH) (Fig. 3a). We next added different inhibitors of l‐arginine metabolism into MDSCs and T‐cell co‐culture systems. As expect, addition of the iNOS inhibitor l‐NMMA almost completely abrogated the suppressive effect of atorvastatin‐derived G‐MDSCs (Fig. 3b), accompanied by a clear lower level of NO content by l‐NMMA treatment (Fig. 3c). No effect of l‐NMMA on cultured T cells alone was observed, indicating that the suppression was mediated through MDSCs (data not shown). Moreover, there was no difference in ROS level between MDSCs and immature myeloid cells from the spleen and bone marrow of mice treated with atorvastatin and vehicle, respectively (Fig. 3d). These results support that atorvastatin‐derived MDSCs suppress T‐cell in an NO‐dependent manner.
Figure 3.
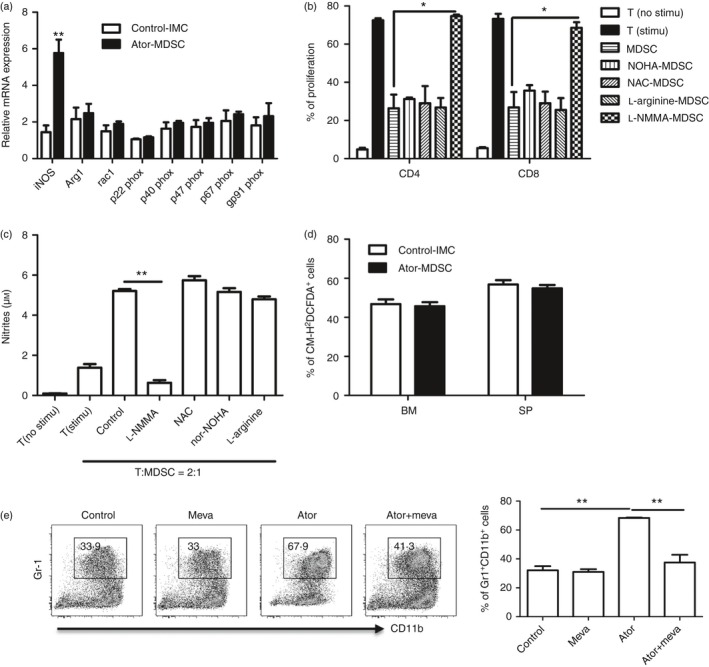
Atorvastatin‐derived granulocytic myeloid‐derived suppressor cells (G‐MDSCs) inhibited T‐cell responses by nitric oxide production. (a) G‐MDSCs or immature myeloid cells (IMC) were isolated from the spleens of mice treated with atorvastatin or vehicle control. The expression of l‐arginine metabolizing enzymes was measured by quantitative RT‐PCR. (b) Effect of different inhibitors on the function of atorvastatin‐derived G‐MDSCs was evaluated by T‐cell proliferation assay. CD3+ T cells containing CD4+ and CD8+ T cells from BALB/C mice were stimulated with anti‐CD3/CD28 antibodies and co‐cultured with allogeneic MDSCs isolated from the spleen of mice treated with atorvastatin at a 2 : 1 ratio for 3 days with treatments as indicated. (c) The culture supernatant was collected for NO content determination. NW‐hydroxy‐nor‐arginine (nor‐NOHA) (100 μm): arginase inhibitor; l‐arginine (1 mm); l‐NG‐monomethyl‐arginine (l‐NMMA (100 μm): iNOS inhibitor; N‐acetyl‐l‐cysteine (NAC) (1 mm): reactive oxygen species (ROS) inhibitor. (d) ROS production in MDSCs was measured by flow cytometry. CD11b+ Gr‐1high cells were gated and the percentage of CM‐H2DCFDA + cells is shown. (e) Effect of mevalonate on the MDSCs expansion mediated by atorvastatin. Mouse bone marrow cells were cultured in the presence of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) + interleukin‐6 (IL‐6) with treatments as indicated. After 5 days, the frequency of MDSCs was evaluated by flow cytometry. Meva (100 μm), mevalonolactone. Representative data from a single experiment (left) are shown. For all the experiments, mean + SEM of three independent experiments is shown. *P < 0·05, **P < 0·01.
Statins block cholesterol synthesis by inhibiting the HMG‐CoA reductase, which catalyses the conversion of HMG‐CoA to mevalonic acid.1 To determine whether the effect of atorvastatin on MDSC expansion was dependent on the mevalonate pathway, we asked whether mevalonate could reverse atorvastatin's effect. We found that the addition of mevalonate at the initial culture of bone marrow cells in the presence of atorvastatin prevented MDSC expansion (Fig. 3e). These results confirmed that the expansion of MDSCs caused by atorvastatin was a consequence of the inhibition of HMG‐CoA reductase.
Atorvastatin‐derived MDSCs attenuate DSS‐induced acute colitis
Previous studies have demonstrated that statins have therapeutic benefits for the treatment in animal models of autoimmune disease, including inflammatory bowel disease.1, 5, 9 Intriguingly, a number of studies have shown beneficial effects of MDSCs in treating inflammatory bowel disease (IBD).26, 27, 28, 47, 48, 49 We therefore speculated that atorvastatin‐derived G‐MDSCs may reduce the severity of murine experimental colitis. To this end, we firstly performed atorvastatin administration during DSS‐induced acute colitis (Fig. 4a). Consistent with previous studies,9 our results showed that administration of atorvastatin significantly reduced the severity of murine colitis as assessed by body weight loss (Fig. 4b), colon length (Fig. 4c) and disease activity index (Fig. 4d) compared with the DSS control group. Histological analysis also showed an improvement in colonic histology upon atorvastatin co‐treatment (Fig. 4e,f). In addition, we observed the accumulation of MDSCs in mouse spleen, mesenteric lymph nodes (mLN), Peyer's patches (PP) and colonic lamina propria mononuclear cells (LPMCs) after DSS administration (Fig. 4g). More importantly, the frequency of MDSCs (CD11b+ Gr‐1+) from DSS‐atorvastatin co‐treated mice was higher in spleen, mLN, PP and LPMCs than that from DSS control mice (Fig. 4g). Further, we found that the elevation of MDSCs was mainly derived from the G‐MDSC subset (Fig. 4h, and see Supplementary material, Fig. S3). These observations suggest that the G‐MDSCs induced by atorvastatin may contribute to the improvement of murine colitis induced by atorvastatin.
Figure 4.
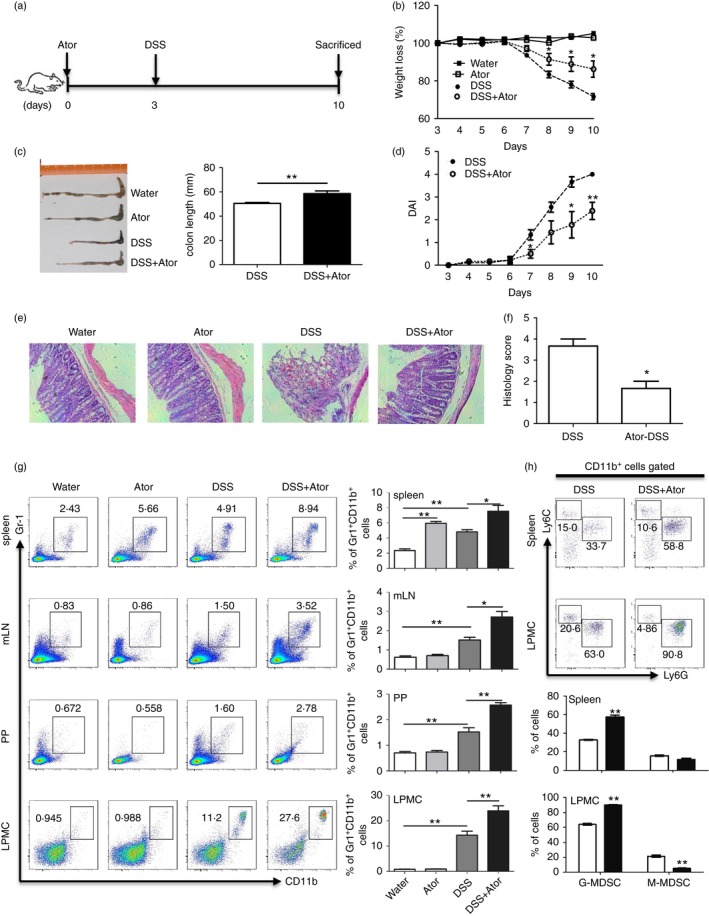
Atorvastatin attenuated dextran sodium sulphate (DSS) ‐induced murine colitis with myeloid‐derived suppressor cell (MDSC) expansion. (a–f) C57BL/6 mice were injected intraperitoneally with atorvastatin (50 mg/kg/day). At day 3, mice were given water containing 2·5% DSS or drinking water (a). Mice were killed at day 10 for evaluation of colitis severity: weight loss (b), the colon length (c), disease activity index (DAI) (d), colon histology (100 ×) (e) and histology score (f). (g) Proportions of MDSCs (CD11b+ Gr‐1+) in different tissues of mice were evaluated by flow cytometry. Representative graph (left) and statistical graph (right) are shown (n = 6). (h) MDSC subsets in mouse spleen and colon lamina propria mononuclear cells (LPMCs) were evaluated by flow cytometry. For (b) and (d), the DSS+Ator group was compared with the DSS group. *P < 0·05, **P < 0·01.
For further confirmation, we transferred atorvastatin‐derived G‐MDSCs in vivo or immature myeloid cells into naive C57BL/6 mice to see whether these cells can attenuate DSS‐induced colitis (Fig. 5a). As expected, the frequency of G‐MDSCs in mouse spleen and peripheral blood mononuclear cells, but not M‐MDSCs, dramatically increased after transferring G‐MDSCs (Fig. 5b). Results indicated that adoptive transfer of atorvastatin‐derived G‐MDSCs, but not the corresponding control cells from naive mice, attenuated murine experimental colitis as characterized by less body weight loss (Fig. 5c), lower disease activity index (Fig. 5d), longer colon length (Fig. 5e) and less colon inflammation (Fig. 5f,g) compared with DSS control mice. In addition, the level of G‐MDSCs in mouse spleen and LPMCs was much higher in the DSS+Ator‐MDSCs group than in the control group (Fig. 5h). These observations support that atorvastatin‐derived G‐MDSCs attenuate DSS‐induced acute colitis.
Figure 5.
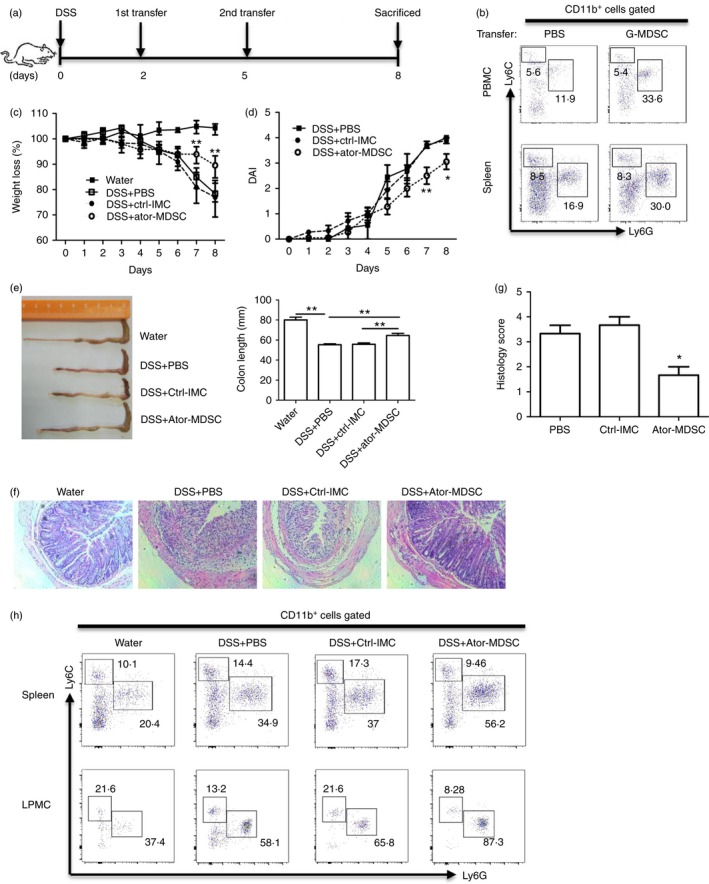
Atorvastatin‐derived granulocytic myeloid‐derived suppressor cells (G‐MDSCs) ameliorated dextran sodium sulphate (DSS) ‐induced murine colitis. (a) The experimental design. Enriched G‐MDSCs (2 million) from the spleen of mice treated with atorvastatin were injected into recipient mice via the tail vein. At day 8, mice were killed for examination. (b) Analysis of MDSC subsets in peripheral blood mononuclear cells (PBMCs) and spleens of mice without DSS treatment after G‐MDSC transfer. The murine colitis severity was evaluated as in Fig. 4: weight loss (c), disease activity index (DAI) (d), the colon length (e), representative colon histology (100 ×) (f) and histology score (g). (h) The representative flow cytometry plots of MDSC subsets (with CD11b+ cells pre‐gated) in spleen and colon lamina propria mononuclear cells (LPMCs) for all four groups. For (c) and (d), DSS+Ator‐MDSC group was compared with the DSS+Ctrl‐IMC group. Ctrl, control; Ator, atorvastatin. *P < 0·05, **P < 0·01.
Atorvastatin‐derived MDSCs attenuate chronic colitis
The main feature of atorvastatin‐derived G‐MDSCs is their immunosuppressive function towards T cells.17 Although some evidence shows that T lymphocytes may be a driving factor in DSS‐induced acute colitis,50, 51 chronic DSS colitis is typically augmented by T lymphocytes.37, 52 Therefore, we next investigated a chronic DSS colitis model (Fig. 6a). Consistent with the acute colitis results, atorvastatin administration attenuated chronic DSS colitis characterized by the amelioration in body weight loss (Fig. 6b), disease activity index (Fig. 6c), colon length (Fig. 6d) and colon inflammation (Fig. 6e). Notably, we also found that G‐MDSC level in mouse spleen, mLN, PP and colon LPMCs from the DSS+atorvastatin group was much higher than that from the DSS group. Analysis of MDSC subsets showed a significant enhancement of G‐MDSCs. Hence, our studies suggest that atorvastatin‐induced MDSCs may mediate the beneficial effects of atorvastatin in chronic colitis by inhibiting T‐cell responses. To support our speculation, we next carried out the DSS‐induced chronic colitis in Rag1 −/− mice, which have no T cells and B cells. As expected, no beneficial effects of atorvastatin were observed for DSS and atorvastatin co‐treatment in Rag1 −/− mice when compared with the DSS group, although atorvastatin induced G‐MDSC production (see Supplementary material, Fig. S4). Hence, atovastatin‐induced MDSCs probably attenuate DSS‐induced chronic colitis by suppressing T‐cell responses.
Figure 6.
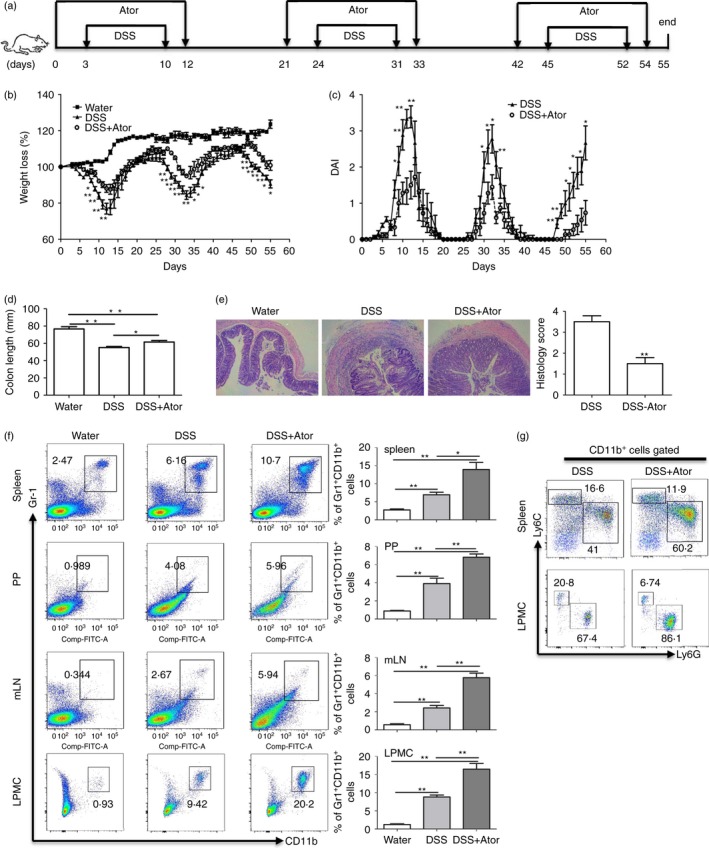
Atorvastatin attenuated dextran sodium sulphate (DSS) ‐induced murine chronic colitis. (a) The experimental design. Mice were killed at day 55. Chronic colitis was evaluated by: weight loss (b), disease activity index (DAI) (c), colon length (d) and colon histology (100 ×) and histology score (e). (f) Proportions of myeloid‐derived suppressor cells (MDSCs) (CD11b+ Gr‐1+) in different tissues of mice were evaluated by flow cytometry. Representative graph (left) and statistical graph (right) are shown (n = 5). (g) The representative flow cytometry plots of MDSCs subsets (with CD11b+ cells pre‐gated) in spleen and colon lamina propria mononuclear cells (LPMCs) for DSS and DSS+Ator treatment. For (b) and (c), DSS+Ator group was compared with DSS group. *P < 0·05, **P < 0·01.
To directly confirm the beneficial effects of atorvastatin‐induced G‐MDSCs in chronic colitis, we asked whether atorvastatin‐induced G‐MDSCs can attenuate chronic colitis in a T‐cell transfer model of colitis in Rag1 −/− mice.41 As expected, we observed that co‐transfer of CD4+ CD45RBhigh cells and atorvastatin‐induced G‐MDSCs, but not the immature myeloid cells, ameliorated the colitis as assessed by weight loss (Fig. 7a), colon length (Fig. 7b) and histological score (Fig. 7c). Meanwhile, the absolute numbers of CD4+ T cells in spleen, mLN and colon LPMCs of mice in the CD45RBhigh+MDSCs group were much lower than that of control groups (Fig. 7d). Therefore, we concluded that atorvastatin‐induced MDSCs attenuated chronic colitis mainly by inhibiting T‐cell responses.
Figure 7.
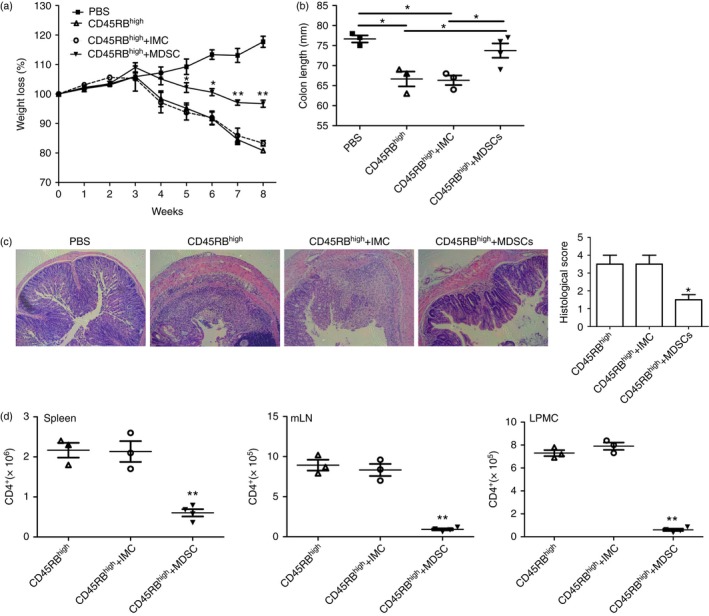
Atorvastatin‐derived granulocytic myeloid‐derived suppressor cells (G‐MDSCs) attenuated the T‐cell transfer of chronic colitis. Rag1 −/− mice were injected intraperitoneally with 5 × 105 CD4+ CD45RB high T cells from C57BL/6 mice, 5 × 105 CD4+ CD45RB high T cells + 5 × 105 G‐MDSCs or 5 × 105 immature myeloid cells (IMCs) with the same phenotype from the spleens of mice after atorvastatin or vehicle treatment. Mice were killed after 8 weeks. (a) Body weight loss of mice with different treatment. (b) Colon length of mice after 8 weeks adoptive transfer. (c) Representative colon histology (100 ×) and histology score. (d) Absolute numbers of CD4+ T cells in the spleen, mesenteric lymph nodes (mLNs) and colon lamina propria mononuclear cells (LPMCs) *P < 0·05, **P < 0·01.
Discussion
Compelling evidence generated from extensive clinical and laboratory studies now exists that statins have therapeutic benefits for the treatment of inflammatory and autoimmune disorders.1, 2 The mechanisms of statins therapy, however, are not fully understood. In this study, we show that atorvastatin promotes MDSC expansion and attenuates experimental murine colitis. Our finding therefore provides a novel mechanism for the immunomodulation of statins in the treatment of inflammatory bowel disease and perhaps other autoimmune disorders.
The MDSCs are derived from a differentiation deficiency of common myeloid progenitor cells and granulocyte and macrophage progenitor cells under pathological conditions, including autoimmune disease.25 In recent years, attention has been paid to statins therapy in autoimmune diseases because of their extensive immunomodulatory effects.1, 2 However, to our knowledge, little is known about the effect of statins on MDSC production and function. Our studies suggest that atorvastatin could remarkably promote G‐MDSC expansion both in vitro and in vivo. Meanwhile, along with the increase of MDSC frequency, a clear reduction in the proportion of immature dendritic cells was observed in the culture system in vitro (Fig. 1a). Previous studies also show that atorvastatin inhibits dendritic cell development and function.53, 54 Accordingly, atorvastatin may impair myeloid cell development, so enhancing the accumulation of MDSCs.
We further found that the effect of atorvastatin on MDSC expansion was dependent on its inhibition on the mevalonate pathway. Addition of mevalonate almost completely abrogated atorvastatin‐induced MDSC expansion. However, the mechanism of atorvastatin‐induced MDSC expansion still remains to be understood. Zoledronate, a nitrogen‐containing bisphosphonate blocking farnesylpyrophosphate synthesis in the mevalonate pathway, was shown to enhance the increase of immature myeloid cells with MDSC characteristics in a model of murine mesothelioma.55 The effect of atorvastatin on G‐MDSC expansion may be associated with statins' ability to inhibit protein prenylation mediated by farnesylpyrophosphate and geranylgeranylpyrophosphate, two important intermediate metabolites in the mevalonate pathway.1 The underlying mechanism requires further investigation.
Additionally, atorvastatin‐derived MDSCs suppressed T‐cell proliferation by NO production as addition of the iNOS inhibitor l‐NMMA totally recovered T‐cell responses. As expected, we found that atorvastatin‐derived MDSCs expressed higher iNOS mRNA than control cells. Moreover, our preliminary study showed that atorvastatin‐derived MDSCs also expressed higher levels IL‐1β and IFN‐γ than controls, whereas the mRNA level of S100A8, S100A9, Cox‐2, IL‐10 and transforming growth factor‐β did not change (see Supplementary material, Fig. S5). Since the expression of iNOS could be induced by IFN‐γ and IL‐1β via JAK/STAT1 signalling,18 we speculate that atorvastatin may enhance the iNOS expression through this pathway but this requires further investigation. The up‐regulation of IL‐1β and IFN‐γ induced by statins has been reported previously.56, 57 Although simvastatin has been found to inhibit IFN‐γ‐induced CD40 gene expression by suppressing STAT1α in macrophages and microglia,58 mevastatin increases STAT1 activation and restores nitric oxide synthase (NOS2 or iNOS) expression in models of cystic fibrosis epithelium.59 However, how atorvastatin induces iNOS expression in MDSCs warrants further studies.
Consistent with previous studies,5, 9 we found that atorvastatin attenuated murine experimental colitis. To interpret the beneficial effects, early reports mainly focused on the negative regulation of statins on pro‐inflammatory factors (such as tumour necrosis factor‐α and IL‐17), or the up‐regulation of eNOS expression.5, 7 The mechanism, however, is not fully understood. Däbritz et al. found that the resistance to murine colitis in gp130757F/F mice is dependent on STAT3‐mediated G‐MDSC expansion, suggesting a beneficial role of G‐MDScs in inflammatory bowel disease.49 We found that the attenuation of both DSS‐induced acute and chronic colitis after atorvastatin treatment was coupled with a much higher level of G‐MDSCs than DSS control mice. Meanwhile, no beneficial effects were observed in DSS‐induced chronic colitis in Rag1 −/− mice that have no T and B cells. More importantly, we discovered that transfer of atorvastatin‐derived G‐MDSCs attenuated the severity of DSS‐induced acute colitis and T‐cell transfer of chronic colitis. Although T‐cell responses may also participate in DSS acute colits,50,51,a chronic colitis model mainly involves adaptive immune responses.37 Therefore our results showed that atorvastatin‐induced MDSCs attenuate murine colitis mainly by inhibiting T‐cell responses. Additionally, we also found that atorvastatin could promote human MDSCs (HLA‐DR−/low CD11b+ CD33+ cells) expansion in vitro (see Supplementary material, Fig. S6), implying that atorvastatin‐derived MDSCs may also ameliorate Crohn's disease and other autoimmune disorders in human.
In conclusion, our study provides information that atorvastatin promotes MDSC expansion, thus shedding new light on the immunomodulation of statins. The effect of atorvastatin on MDSC accumulation may represent another pathway for statins' therapeutic benefits in treating IBD and other autoimmune diseases.
Disclosures
The authors declare no conflict of interests.
Supporting information
Figure S1. Gating strategy for sorting mouse granulocytic myeloid‐derived suppressor cells (G‐MDSCs). CD11b+ Gr‐1high and CD11b+ Gr‐1low cells were gated, respectively. Then, the expression of Ly6G and Ly6C was analysed. CD11b+ Gr‐1high cells represents G‐MDSCs; CD11b+ Gr‐1low represents monocytic (M‐) MDSCs.
Figure S2. The suppressive function of granulocytic myeloid‐derived suppressor cells (G‐MDSCs; CD11b+ Gr‐1high) from bone marrow (BM), liver and peripheral blood mononuclear cells (PBMCs) of mice treated with atorvastatin or vehicle. G‐MDSCs (CD11b+ Gr‐1high) were purified by flow cytometric sorting. Allogeneic CD3+ T cells containing CD4+ and CD8+ T cells (from BALB/c mice) were stimulated with anti‐CD3/CD28 antibodies, and co‐cultured with isolated G‐MDSCs at the ratio of 2 : 1 for 3 days. CD4+ and CD8+ T‐cell proliferation was determined by CFSE dilution. Unstimulated T cells were used as a negative control. Representative data from a single experiment (left), as well as mean+SEM of three independent experiments (right) are shown. Ator, atorvastatin. **P < 0·01.
Figure S3. Another two representative flow cytometry plots of myeloid‐derived suppressor cell (MDSC) subsets (CD11b+ cells pre‐gated) in the mouse spleen and colon lamina propria mononuclear cells (LPMCs) were evaluated by flow cytometry in Fig. 4(h).
Figure S4. Atorvastatin did not attenuate dextran sodium sulphate (DSS) ‐induced chronic colitis in Rag1 −/− mice. (a) The experimental design. Rag1 −/− mice were injected daily intraperitoneally with atorvastatin (50 mg/kg/day) or vehicle control. Mice were killed at day 33. Chronic colitis was evaluated by: weight loss (b), disease activity index (DAI) (c), colon length (d) and colon histology (100 ×) and histology score (e). (f) Proportions of myeloid‐derived suppressor cells (MDSCs; CD11b+ Gr‐1+) in spleen and colon lamina propria mononuclear cells (LPMCs) were evaluated by flow cytometry. Representative graph (left) and statistical graph (right) are shown (n = 4). (g) The representative flow cytometry plots of MDSC subsets (with CD11b+ cells pregated) in spleen and colon LPMCs for DSS and DSS+Atorvastatin treatment. *P < 0·05, **P < 0·01.
Figure S5. The effect of atorvastatin on interferon‐γ (IFN‐γ) and interleukin‐1β (IL‐1β) mRNA expression. (a) mRNA expression of S100A8, S100A9, IL‐1β, IFN‐γ, Cox‐2, IL‐10 and transforming growth factor‐β (TGF‐β) was evaluated by quantitative RT‐PCR using samples as described in Fig. 3(a). (b) Mouse bone marrow cells were treated with atorvastatin (5 μm) or DMSO for 24 hr. Then, the cells were collected and the mRNA expression of S100A8 and S100A9, IL‐1β, IFN‐γ, Cox‐2, IL‐10 and TGF‐β was measured by quantitative RT‐PCR.
Figure S6. Atorvastatin promoted human myeloid‐derived suppressor cell (MDSC) expansion in vitro. Human peripheral blood mononuclear cells (PBMCs) from healthy volunteers were cultured in the presence of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and interleukin‐6 (IL‐6) (10 ng/mL) and treated with atorvastatin (5 μm) or mevalonolactone (100 μm) for 6 days. The proportions of human MDSCs (HLA‐DR−/low CD11b+ CD33+) were evaluated by flow cytometry. (a) Gating strategy of monocytic (M‐) MDSCs by flow cytometry analysis. HLA‐DR−/low cells were first selected from live PBMCs, and the CD11b+ CD33+ population was further gated as human MDSCs. (b) Representative data from a single experiment (left), as well as mean+SEM of three independent experiments (right) are shown. Ctrl, control; Meva, mevalonolactone; Ator, atorvastatin. *P < 0·05; **P < 0·01.
Table S1. Sequences of primers used in this study.
Acknowledgements
LA and QY designed and performed the experiments, analysed the results and drafted the figures and manuscript. XL, HC, MS, QX, YC and YH participated in experiments and analysis of the data. JZ designed the study, interpreted the data and edited the manuscript. This work was supported by the following grants: National Key Basic Research Programme of China (No. 2012CB524900), Guangdong Innovative Research Team Programme (No. 2009010058), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (GDUPS, 2014), Programme for the 12th Five‐year Plan (No. 2012ZX10001003), National Natural Science Foundation of China (No. 81072397; No. 31270921; No. 301500740), Natural Science Foundation of Guangdong (No. S2011020006072), the Fundamental Research Funds for the Central Universities, the Provincial Talents Cultivated by “Thousand‐Hundred‐Ten” programme of Guangdong Province, 111 Project (No. B12003), Natural Science Foundation of Guangdong Province (No. 42030118), China Postdoctoral Science Foundation (No. 41090137).
References
- 1. Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006; 6:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ulivieri C, Baldari CT. Statins: from cholesterol‐lowering drugs to novel immunomodulators for the treatment of Th17‐mediated autoimmune diseases. Pharmacol Res 2014; 88:41–52. [DOI] [PubMed] [Google Scholar]
- 3. Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med 2008; 14:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, et al The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta‐analysis. Clin Exp Rheumatol 2015; 33:69–76. [PubMed] [Google Scholar]
- 5. Aktunc E, Kayhan B, Arasli M, Gun BD, Barut F. The effect of atorvastatin and its role on systemic cytokine network in treatment of acute experimental colitis. Immunopharmacol Immunotoxicol 2011; 33:667–75. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki M, Bharwani S, Jordan P, Joh T, Manas K, Warren A, et al The 3‐hydroxy‐3‐methylglutaryl‐CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran‐sulfate induced colitis. J Pharmacol Exp Ther 2003; 305:78–85. [DOI] [PubMed] [Google Scholar]
- 7. Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, et al Rosuvastatin, a new HMG‐CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium‐induced colitis in mice. Int J Mol Med 2006; 17:997–1004. [PubMed] [Google Scholar]
- 8. Lee JY, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Simvastatin inhibits NF‐κB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int Immunopharmacol 2007; 7:241–8. [DOI] [PubMed] [Google Scholar]
- 9. Kanagarajan N, Nam JH, Noah ZA, Murthy S. Disease modifying effect of statins in dextran sulfate sodium model of mouse colitis. Inflamm Res 2008; 57:34–8. [DOI] [PubMed] [Google Scholar]
- 10. Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al The HMG‐CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002; 420:78–84. [DOI] [PubMed] [Google Scholar]
- 11. Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, et al Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med 2003; 197:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al A novel anti‐inflammatory role for simvastatin in inflammatory arthritis. J Immunol 2003; 170:1524–30. [DOI] [PubMed] [Google Scholar]
- 13. Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein MR. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J Immunol 2004; 173:7641–6. [DOI] [PubMed] [Google Scholar]
- 14. Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn's disease. PLoS One 2009; 4:e5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grip O, Janciauskiene S, Bredberg A. Use of atorvastatin as an anti‐inflammatory treatment in Crohn's disease. Br J Pharmacol 2008; 155:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grip O, Janciauskiene S, Lindgren S. Circulating monocytes and plasma inflammatory biomarkers in active Crohn's disease: elevated oxidized low‐density lipoprotein and the anti‐inflammatory effect of atorvastatin. Inflamm Bowel Dis 2004; 10:193–200. [DOI] [PubMed] [Google Scholar]
- 17. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talmadge JE, Gabrilovich DI. History of myeloid‐derived suppressor cells. Nat Rev Cancer 2013; 13:739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid‐derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013; 138:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al Myeloid‐derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 2010; 22:238–44. [DOI] [PubMed] [Google Scholar]
- 22. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid‐derived suppressor cells in tumor‐bearing mice. J Immunol 2008; 181:5791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol 2011; 11:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid‐derived suppressor cell differentiation and function. Trends Immunol 2011; 32:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid‐derived suppressor cells. J Leukoc Biol 2015; 98:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guan Q, Moreno S, Qing G, Weiss CR, Lu L, Bernstein CN, et al The role and potential therapeutic application of myeloid‐derived suppressor cells in TNBS‐induced colitis. J Leukoc Biol 2013; 94:803–11. [DOI] [PubMed] [Google Scholar]
- 27. Zhang R, Ito S, Nishio N, Cheng Z, Suzuki H, Isobe KI. Dextran sulphate sodium increases splenic Gr1+ CD11b+ cells which accelerate recovery from colitis following intravenous transplantation. Clin Exp Immunol 2011; 164:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, et al Myeloid‐derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 2008; 135:871–81, 881 e1–5. [DOI] [PubMed] [Google Scholar]
- 29. Shi M, Shi G, Tang J, Kong D, Bao Y, Xiao B, et al Myeloid‐derived suppressor cell function is diminished in aspirin‐triggered allergic airway hyperresponsiveness in mice. J Allergy Clin Immunol 2014; 134:1163–74 e16. [DOI] [PubMed] [Google Scholar]
- 30. Fujii W, Ashihara E, Hirai H, Nagahara H, Kajitani N, Fujioka K, et al Myeloid‐derived suppressor cells play crucial roles in the regulation of mouse collagen‐induced arthritis. J Immunol 2013; 191:1073–81. [DOI] [PubMed] [Google Scholar]
- 31. Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, et al Crucial role of granulocytic myeloid‐derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol 2012; 188:1136–46. [DOI] [PubMed] [Google Scholar]
- 32. Kostlin N, Kugel H, Spring B, Leiber A, Marme A, Henes M, et al Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T‐cell responses. Eur J Immunol 2014; 44:2582–91. [DOI] [PubMed] [Google Scholar]
- 33. Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al Tumor‐induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 2010; 32:790–802. [DOI] [PubMed] [Google Scholar]
- 34. Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, et al Polyunsaturated fatty acids promote the expansion of myeloid‐derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 2013; 43:2943–55. [DOI] [PubMed] [Google Scholar]
- 35. Lozanoska‐Ochser B, Barone F, Pitzalis C, Peakman M. Atorvastatin fails to prevent the development of autoimmune diabetes despite inhibition of pathogenic β‐cell‐specific CD8 T‐cells. Diabetes 2006; 55:1004–10. [DOI] [PubMed] [Google Scholar]
- 36. Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, et al Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol 2003; 171:5507–13. [DOI] [PubMed] [Google Scholar]
- 37. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007; 2:541–6. [DOI] [PubMed] [Google Scholar]
- 38. Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL‐1β‐converting enzyme (caspase‐1) in intestinal inflammation. Proc Natl Acad Sci USA 2001; 98:13249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oh SY, Cho KA, Kang JL, Kim KH, Woo SY. Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med 2014; 33:333–40. [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Wang B, Zhang W, Wei Y, Bian Z, Zhang CY, et al Protein tyrosine phosphatase 1B deficiency ameliorates murine experimental colitis via the expansion of myeloid‐derived suppressor cells. PLoS One 2013; 8:e70828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ostanin DV, Bao J, Koboziev I, Gray L, Robinson‐Jackson SA, Kosloski‐Davidson M, et al T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol 2009; 2:G135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, et al Expansion of monocytic myeloid‐derived suppressor cells dampens T cell function in HIV‐1‐seropositive individuals. J Virol 2013; 87:1477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity 2009; 30:845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine‐induced myeloid‐derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 2010; 185:2273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen‐specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004; 172:989–99. [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Analysis of splenic Gr‐1int immature myeloid cells in tumor‐bearing mice. Microbiol Immunol 2008; 52:47–53. [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Tian J, Tang X, Rui K, Tian X, Ma J, et al Exosomes released by granulocytic myeloid‐derived suppressor cells attenuate DSS‐induced colitis in mice. Oncotarget 2016; 7:15356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leal MC, Däbritz J. Immunoregulatory role of myeloid‐derived cells in inflammatory bowel disease. Inflamm Bowel Dis 2015; 21:2936–47. [DOI] [PubMed] [Google Scholar]
- 49. Däbritz J, Judd LM, Chalinor HV, Menheniott TR, Giraud AS. Altered gp130 signalling ameliorates experimental colitis via myeloid cell‐specific STAT3 activation and myeloid‐derived suppressor cells. Sci Rep 2016; 6:20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, et al Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998; 114:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, Kim JY, et al Involvement of lymphocytes in dextran sulfate sodium‐induced experimental colitis. World J Gastroenterol 2006; 12:302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015; 1:154–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, et al HMG‐CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis 2004; 172:85–93. [DOI] [PubMed] [Google Scholar]
- 54. Leuenberger T, Pfueller CF, Luessi F, Bendix I, Paterka M, Prozorovski T, et al Modulation of dendritic cell immunobiology via inhibition of 3‐hydroxy‐3‐methylglutaryl‐CoA (HMG‐CoA) reductase. PLoS One 2014; 9:e100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JP, et al Zoledronic acid impairs myeloid differentiation to tumour‐associated macrophages in mesothelioma. Br J Cancer 2010; 103:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuijk LM, Mandey SH, Schellens I, Waterham HR, Rijkers GT, Coffer PJ, et al Statin synergizes with LPS to induce IL‐1β release by THP‐1 cells through activation of caspase‐1. Mol Immunol 2008; 45:2158–65. [DOI] [PubMed] [Google Scholar]
- 57. Montero MT, Hernandez O, Suarez Y, Matilla J, Ferruelo AJ, Martinez‐Botas J, et al Hydroxymethylglutaryl‐coenzyme A reductase inhibition stimulates caspase‐1 activity and Th1‐cytokine release in peripheral blood mononuclear cells. Atherosclerosis 2000; 153:303–13. [DOI] [PubMed] [Google Scholar]
- 58. Lee SJ, Qin H, Benveniste EN. Simvastatin inhibits IFN‐γ‐induced CD40 gene expression by suppressing STAT‐1α . J Leukoc Biol 2007; 82:436–47. [DOI] [PubMed] [Google Scholar]
- 59. Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin‐mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003; 285:L1286–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy for sorting mouse granulocytic myeloid‐derived suppressor cells (G‐MDSCs). CD11b+ Gr‐1high and CD11b+ Gr‐1low cells were gated, respectively. Then, the expression of Ly6G and Ly6C was analysed. CD11b+ Gr‐1high cells represents G‐MDSCs; CD11b+ Gr‐1low represents monocytic (M‐) MDSCs.
Figure S2. The suppressive function of granulocytic myeloid‐derived suppressor cells (G‐MDSCs; CD11b+ Gr‐1high) from bone marrow (BM), liver and peripheral blood mononuclear cells (PBMCs) of mice treated with atorvastatin or vehicle. G‐MDSCs (CD11b+ Gr‐1high) were purified by flow cytometric sorting. Allogeneic CD3+ T cells containing CD4+ and CD8+ T cells (from BALB/c mice) were stimulated with anti‐CD3/CD28 antibodies, and co‐cultured with isolated G‐MDSCs at the ratio of 2 : 1 for 3 days. CD4+ and CD8+ T‐cell proliferation was determined by CFSE dilution. Unstimulated T cells were used as a negative control. Representative data from a single experiment (left), as well as mean+SEM of three independent experiments (right) are shown. Ator, atorvastatin. **P < 0·01.
Figure S3. Another two representative flow cytometry plots of myeloid‐derived suppressor cell (MDSC) subsets (CD11b+ cells pre‐gated) in the mouse spleen and colon lamina propria mononuclear cells (LPMCs) were evaluated by flow cytometry in Fig. 4(h).
Figure S4. Atorvastatin did not attenuate dextran sodium sulphate (DSS) ‐induced chronic colitis in Rag1 −/− mice. (a) The experimental design. Rag1 −/− mice were injected daily intraperitoneally with atorvastatin (50 mg/kg/day) or vehicle control. Mice were killed at day 33. Chronic colitis was evaluated by: weight loss (b), disease activity index (DAI) (c), colon length (d) and colon histology (100 ×) and histology score (e). (f) Proportions of myeloid‐derived suppressor cells (MDSCs; CD11b+ Gr‐1+) in spleen and colon lamina propria mononuclear cells (LPMCs) were evaluated by flow cytometry. Representative graph (left) and statistical graph (right) are shown (n = 4). (g) The representative flow cytometry plots of MDSC subsets (with CD11b+ cells pregated) in spleen and colon LPMCs for DSS and DSS+Atorvastatin treatment. *P < 0·05, **P < 0·01.
Figure S5. The effect of atorvastatin on interferon‐γ (IFN‐γ) and interleukin‐1β (IL‐1β) mRNA expression. (a) mRNA expression of S100A8, S100A9, IL‐1β, IFN‐γ, Cox‐2, IL‐10 and transforming growth factor‐β (TGF‐β) was evaluated by quantitative RT‐PCR using samples as described in Fig. 3(a). (b) Mouse bone marrow cells were treated with atorvastatin (5 μm) or DMSO for 24 hr. Then, the cells were collected and the mRNA expression of S100A8 and S100A9, IL‐1β, IFN‐γ, Cox‐2, IL‐10 and TGF‐β was measured by quantitative RT‐PCR.
Figure S6. Atorvastatin promoted human myeloid‐derived suppressor cell (MDSC) expansion in vitro. Human peripheral blood mononuclear cells (PBMCs) from healthy volunteers were cultured in the presence of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and interleukin‐6 (IL‐6) (10 ng/mL) and treated with atorvastatin (5 μm) or mevalonolactone (100 μm) for 6 days. The proportions of human MDSCs (HLA‐DR−/low CD11b+ CD33+) were evaluated by flow cytometry. (a) Gating strategy of monocytic (M‐) MDSCs by flow cytometry analysis. HLA‐DR−/low cells were first selected from live PBMCs, and the CD11b+ CD33+ population was further gated as human MDSCs. (b) Representative data from a single experiment (left), as well as mean+SEM of three independent experiments (right) are shown. Ctrl, control; Meva, mevalonolactone; Ator, atorvastatin. *P < 0·05; **P < 0·01.
Table S1. Sequences of primers used in this study.


