Abstract
Here, we report a case of insulin-derived amyloidosis in the lower abdomen. The mass continued to develop even after the patient ceased injecting insulin into the mass. Histological examination led to a diagnosis of insulin-derived amyloidosis. Excision is preferable in cases of insulin-derived amyloidosis if patient’s condition permits.
Keywords: Insulin-derived amyloidosis, insulin injection, lipohypertrophy
Introduction
Insulin-derived amyloidosis is defined as a subcutaneous amyloid mass at the site of insulin injection where the amyloid deposit shows positive staining with anti-insulin antibody.[1,2] This clinical entity is important because it causes deterioration of blood glucose control.[2] Despite its importance, it remains unclear whether the mass should be excised. Here, we report a case showing a progressive mass in the lower abdomen. We excised the mass and histological examination led to a diagnosis of insulin-derived amyloidosis. In this case, the mass continued developing even after cessation of insulin injection into it. These observations indicated that insulin-derived amyloidosis can enlarge even in the absence of insulin injection. Our experience suggests that surgeons should be aware of insulin-derived amyloidosis when examining subcutaneous tumors in diabetic patients.
Case report
A 78-year-old woman was referred to our department due to a mass in the lower abdomen. She had a history of type 2 diabetes mellitus and had been insulin-dependent for more than 7 years. She was admitted to the Internal Medicine Department because of deteriorating blood glucose control. Her HbA1c was 10.6% and total daily insulin dose was 58 units. On admission, physical examination revealed a firm mass in the lower abdomen with no pain or tenderness, and she was referred to our department for further treatment.
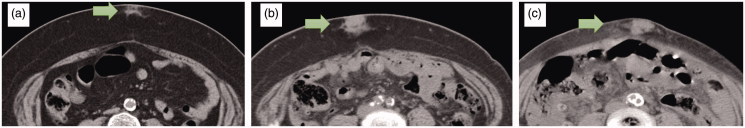
Computed tomography revealed a mass measuring 28 × 18 × 30 mm in the subcutaneous tissue (Figure 1(b)). She had injected insulin into the abdomen near the mass. We suspected that this was a skin-related complication of insulin therapy, and the patient was instructed to refrain from injecting insulin into the mass. To confirm the diagnosis, we planned excision of the mass. However, several days later, she fell on the floor and sustained right femoral neck fracture. She was admitted to the Orthopedic Department for surgical treatment, and excision of the mass was postponed. During this hospitalization, the floor nurses supervised her insulin injection. Her blood glucose control improved rapidly, and she had experienced hypoglycemia several times. Two months after visiting our department, her HbA1c was 7.8% and daily insulin dose was decreased to 33 units. She was discharged from our hospital, but she had not visited our department at that time.
Figure 1.
(a) Eight years before surgical excision (17 × 12 × 14 mm). (b) Four years before surgical excision (28 × 18 × 30 mm). (c) Preoperative (60 × 20 × 35 mm).
Four years later, she was admitted to the Internal Medicine Department because of renal anemia and referred to our department again due to the same mass in the lower abdomen, which was suspected to be a malignant soft tissue tumor. The mass was enlarged compared to her first visit. Her HbA1c was 7.4% and daily insulin dose was 22 units. Computed tomography revealed that the mass had expanded to 60 × 20 × 35 mm with unclear borders. We found her previous abdominal computed tomography results and confirmed that the mass had already existed 8 years previously, but it had been much smaller (17 × 12 × 14 mm) (Figure 1).
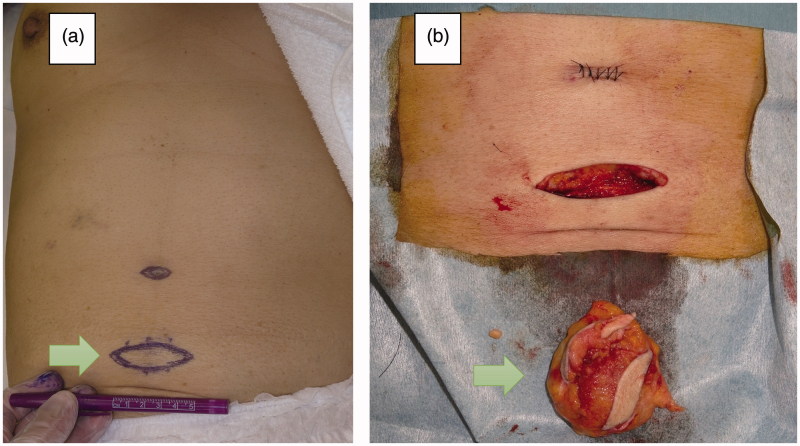
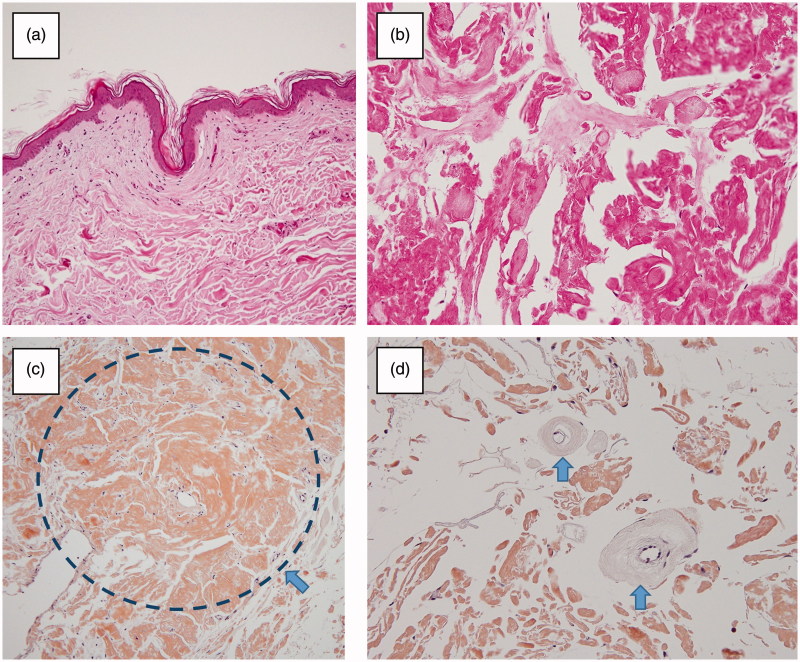
We excised the mass under local anesthesia. The mass was yellowish elastic hard, and had unclear boundaries. We excised the mass with indurated subcutaneous fat (Figure 2). On pathological examination, hematoxylin and eosin staining showed broadly degenerated subcutaneous tissue and large amounts of homogeneous eosinophilic material in the tumor tissue. Congo red staining confirmed that they were amyloid deposits. There was no evidence of vascular involvement. Amyloid-AA, kappa-chain and lambda-chain were not detected immunohistochemically (Figure 3). These findings confirmed the pathological diagnosis of insulin injection-related local amyloidosis. The patient recovered uneventfully. No specific change was observed in her blood glucose level. She was treated her renal anemia in the Internal Medicine Department and discharged from our hospital at 25 days postoperatively. No wound complication was observed.
Figure 2.
(a) Preoperative appearance. (b) The excised mass with indurated subcutaneous fat.
Figure 3.
(a,b) The subcutaneous tissue was degenerated broadly (Haematoxylin and eosin staining). (c) Extensive amyloid deposition in the subcutaneous tissue (arrow) (Congo red staining). (d) No evidence of vascular involvement (arrow: subcutaneous artery without amyloid deposition) (Congo red staining).
Discussion
Insulin-derived amyloidosis, also known as ‘insulin ball’,[1] is a rare skin-related complication of insulin therapy. Insulin injections at the same site may be a risk factor of amyloidosis formation,[1–7] but the mechanism underlying insulin-derived amyloidosis formation is still unknown. The differential diagnoses of local site reactions in patients on insulin therapy include lipohypertrophy, which has many symptoms in common with insulin-derived amyloidosis. Distinction between the two entities may not be possible on clinical grounds without histological confirmation.[3] However, lipohypertrophy is usually diagnosed physically by palpation, and this may result in misinterpretation and misdiagnosis.[4] In Nagase’s report, they experienced seven cases in only one institute although only 11 cases have been reported in the English language literature.[2] Nagase suggested that insulin-derived amyloidosis may be a more common complication of insulin therapy than previously thought and a more common manifestation of amyloid-related disease. Injection into lipohypertrophy can reduce the effectiveness of insulin, although less markedly than injection into amyloid.[1] Lipohypertrophy shows regression after cessation of insulin injection.[3,4] Therefore, if a subcutaneous mass at the site of insulin injection does not regress after cessation of insulin injection, histological examination should be performed to make an accurate diagnosis.
Insulin-derived amyloidosis causes deterioration of blood glucose control. Patients with this disease suffer from hyperglycemia because insulin absorption is markedly impaired at the mass.[2] Consequently, their insulin dose requirements are increased. In contrast, severe hypoglycemia will occur when patients inject an increased dose of insulin into a normal site.[2] In a previous report, one patient had routinely injected insulin into such lesions, as they could be grasped easily, and injection was less painful than elsewhere.[1] It is possible that patients tend to inject into such lesions because of these features. Once the diagnosis was confirmed, clinicians should ensure that patients avoid injecting into the lesion. In our case, floor nurses supervised insulin injection and prevented the patient from injecting into inappropriate areas, including the mass. It is notable that changing insulin injection site to normal site from the mass will cause severe hypoglycemia, as in our case. Therefore, it is advisable to closely monitor blood glucose level when changing the insulin injection site.
Excision of the lesion is preferable for insulin-derived amyloidosis. According to Gupta’s review, treatment for insulin-derived amyloidosis involves surgical excision of the lesion or avoiding injection at sites of amyloidosis.[5] In several reports,[1,2,6] the lesion was not completely resected after confirmation of the diagnosis of insulin-derived amyloidosis by incisional biopsy or needle biopsy. In these cases, the patients were instructed not to inject insulin into sites of amyloidosis. In other reports,[3,4,7] the insulin-derived amyloidosis was resected and the diagnosis was histologically confirmed. Changing the insulin injection site from the mass to a normal site will improve blood glucose control. In our case, we confirmed that the tumor enlarged even after the patient had stopped injecting insulin into it and even after her blood glucose control had improved. These observations indicated that insulin-derived amyloidosis lesions can enlarge even in the absence of insulin injection into the mass. Moreover, resection of the tumor eliminates the possibility of accidentally injecting insulin into the lesion. Excision of the tumor will achieve a more effective treatment than incisional or needle biopsy only. On the other hand, excision of the tumor can lead to postoperative complications such as wound infection or delayed wound healing, especially when it was done while blood glucose control was poor. We think that excision of the lesion will achieve better result for insulin-derived amyloidosis, but further studies are needed to determine the treatment of choice.
It is important for surgeons who routinely examine subcutaneous tumors to recognize insulin-derived amyloidosis. As mentioned above, insulin-derived amyloidosis may be a more common complication of insulin therapy than previously thought.[2] Insulin-derived amyloidosis may be misdiagnosed as lipohypertrophy because it may not be possible to distinguish between these two entities by physical examination only.[3] When diabetic patients visit the plastic surgery department due to a subcutaneous tumor at the site of insulin injection, the plastic surgeons should actively perform excision of the mass and confirm the histological diagnosis if patient’s condition permits. This will lead not only to accurate diagnosis but also to improvement of the patient’s blood glucose control. In addition, surgeons should be aware of the risk of hypoglycemia after tumor resection. When surgeons extirpate the tumor, it is advisable to cooperate with diabetologists for management of blood glucose control. Successful excision of insulin-derived amyloidosis will achieve good blood glucose control, and consequently result in good patient prognosis. Surgeons should be aware of insulin-derived amyloidosis when examining subcutaneous tumors in diabetic patients.
Conclusion
Surgeons should be aware of insulin-derived amyloidosis when examining subcutaneous tumors in diabetic patients. From our case, we recommend excision of insulin-derived amyloidosis to prevent patients from injecting insulin into the mass, to achieve good blood glucose control, and to make an accurate pathological diagnosis if patient’s condition permits. Further studies are needed in order to determine the treatment of choice.
Acknowledgments
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Nagase T, Katsura Y, Iwaki Y, et al. The insulin ball. Lancet. 2009;373:184. doi: 10.1016/S0140-6736(09)60041-6. [DOI] [PubMed] [Google Scholar]
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450–454. doi: 10.1016/j.amjmed.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19:881–882. doi: 10.1046/j.1464-5491.2002.07581.x. [DOI] [PubMed] [Google Scholar]
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655–1660. doi: 10.1016/j.humpath.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metabol. 2015;19:174–177. doi: 10.4103/2230-8210.146879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunes D, Rapkiewicz A, Simsir A. Amyloidoma secondary to insulin injection: cytologic diagnosis and pitfalls. CytoJournal. 2015;12:15. doi: 10.4103/1742-6413.161602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama Y, Kitazawa J, Yagihashi N, et al. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397–401. doi: 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]