Abstract
Invasive pathogens can cause considerable damage to forest ecosystems. Lack of coevolution is generally thought to enable invasive pathogens to bypass the defence and/or recognition systems in the host. Although mostly true, this argument fails to predict intermittent outcomes in space and time, underlining the need to include the roles of the environment and the phenotype in host–pathogen interactions when predicting disease impacts. We emphasize the need to consider host–tree imbalances from a phenotypic perspective, considering the lack of coevolutionary and evolutionary history with the pathogen and the environment, respectively. We describe how phenotypic plasticity and plastic responses to environmental shifts may become maladaptive when hosts are faced with novel pathogens. The lack of host–pathogen and environmental coevolution are aligned with two global processes currently driving forest damage: globalization and climate change, respectively. We suggest that globalization and climate change act synergistically, increasing the chances of both genotypic and phenotypic imbalances. Short moves on the same continent are more likely to be in balance than if the move is from another part of the world. We use Gremmeniella abietina outbreaks in Sweden to exemplify how host–pathogen phenotypic interactions can help to predict the impacts of specific invasive and emergent diseases.
This article is part of the themed issue ‘Tackling emerging fungal threats to animal health, food security and ecosystem resilience’.
Keywords: emergent disease, phenotypic plasticity, maladaptive phenotype, lack of coevolution
1. Predicting the impact of invasive and emerging diseases
Forest ecosystems are suffering from damage by pathogens worldwide [1]. Forest pathogens can attack all parts of the trees and may remain for extensive periods in an infected site. Armillaria spp., causing root rot of many tree species, can form individual mycelial networks that are among the oldest and largest of terrestrial organisms [2]. The economic impact of forest disease can be huge: Heterobasidion root rot to conifers alone causes losses in the order of 600 million euros annually in European forests [3]. The effects on ecosystems and their services can also be substantial [4]. Fungal and fungal-like pathogens are the main cause of both chronic and emerging infectious diseases, including examples of anthropogenically introduced fungal epidemics such as Dutch elm disease and chestnut blight [5]. Diebacks caused by members of the Oomycete genus Phytophthora can be exceedingly devastating, particularly when having a broad host range. For example, Phytophthora cinnamomi has killed plants in 34 genera in Australia [6], and P. ramorum is responsible for the recent sudden oak death in the USA and sudden larch death in the UK [7,8].
Over the past century, the amount of damage caused by invasive pathogens and emergent diseases to forest ecosystems has increased, with negative consequences in economic and ecological terms [5,9]. To understand the nature of these novel interactions, scientists have tried a number of theoretical approaches to predict new invasions [10,11]. The lack of coevolutionary history has been repeatedly suggested as the main reason why alien pathogens can severely damage naive hosts [12]. This idea has been used to explain cases where introduced pathogens have had huge impacts on naive hosts, causing diseases such as chestnut blight, Dutch elm disease, ash dieback and laurel wilt [13]. We appreciate that host–pathogen interactions can take several coevolutionary trajectories over time [14], but it is quite clear that introduced tree pathogens historically have been a major cause of disastrous epidemics [5]. However, concentrating solely on the lack of coevolution has failed to explain why certain invasive pathogens become epidemic on novel hosts in certain areas, whereas in other areas, the trees are undamaged (e.g. Dothistroma needle blight, Diplodia pinea or several soil-borne Phytophthora spp.). For these and many other pathogens, the impact of the pathogen is associated with either how stressing the conditions are for the host or how conducive the conditions are for the pathogen, underlining the need to include the role of the environment when predicting disease impacts for invasive pathogens [15].
Assuming that host and pathogens coevolve within certain environmental conditions, we propose that the disease impacts on a host can be explained as the interaction of two components: the lack of coevolution with the pathogen and the lack of evolution with the environment. Here, we have integrated these two components into one framework to examine the impacts caused by native, invasive and emerging pathogens (figure 1). By including the environment in the framework, we are able to focus on the role of the phenotype of the host and the pathogen and their physiology in determining the impact of the disease. This view expands current efforts to predict disease from a strict genetic incompatibility/compatibility perspective, in which unbalanced interactions result from a maladaptation of the defence system that does not enable the host to avoid/recognize the pathogen at the molecular level [11,12], and includes the interaction with environmental processes occurring at larger spatial scales such as tree, stand or landscape level [16]. It also complements an invasion biology perspective by trying to predict the likelihood of invasions (based on the capacity of the pathogen to establish and spread in a new environment) depending on the traits of the host or the pathogen [5,10], by focusing on the mechanisms leading to high disease impacts on the host [17].
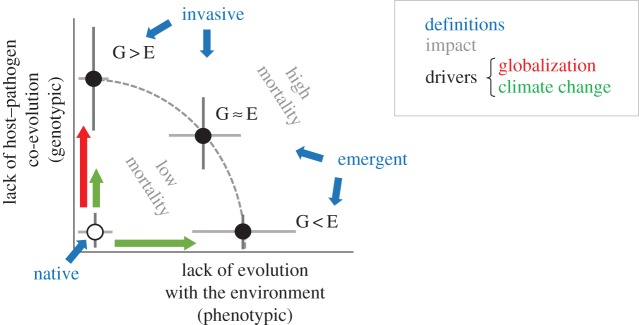
Figure 1.
Conceptual framework illustrating that disease can cause similar damage (mortality) with varying contributions of two components: lack of coevolution between host and pathogens (G), and lack of evolution with the environment (E). Including the environmental dimension in the disease takes into account the role of the phenotype of the host and the pathogen in the interaction. Red and green arrows indicate the contribution of globalization and climate change, respectively, to the two components. Including the environment also differentiates the concepts of emergence and invasiveness in relation to diseases caused by native pathogens.
We propose that an evolutionary approach considering the lack of interaction with the environment and the pathogen can explain why and where invasive and native pathogens may cause large impacts on tree hosts. Here, we review how trees adapt to environmental shifts and how this affects their capacity to deal with pathogens by putting special emphasis on the role of the phenotype. From this perspective, we also explore the current drivers of disease emergence, such as globalization and climate change, and how these can cause coevolutionary imbalances in different ways. We then explore how considering host–pathogen phenotypic interactions can help to predict the occurrence of specific invasive and emergent diseases by using Gremmeniella abietina in Sweden as an example. We conclude by suggesting research questions and experimental approaches to improve our capacity to predict disease emergence in forests.
2. Focusing on the environment: phenotypic interactions
Among the recent literature dealing with invasive forest pathogens, there are few articles that highlight the role of the phenotype in host–pathogen interactions. Common garden experiments are good illustrations of the relevance of phenotypic versus genotypic variation for determining host resistance. Disease resistance in plants is either genetically controlled by a few (R genes) or several loci (i.e. quantitative and qualitative resistance, respectively) [11]. For instance, in a system where resistance is controlled quantitatively, such as Pinus pinaster resistance against infection by the invasive fungus Fusarium circinatum, a large between-clone variation was observed, indicating significant differences in terms of resistance between genetically identical ramets of the same clone [18]. In the same study, a genetic correlation between growth traits and resistance was observed, indicating that resistant genotypes were also those that showed the greatest growth during the experiment. In a study of potato blight, which is a model disease for studying gene-for-gene interaction in plants, no changes in the expression of R genes were observed as plants became older and more resistant [19], illustrating the phenotypic dimension of disease in systems controlled by qualitative resistance.
Hosts and pathogens interact through their phenotype. Trees and pathogens have coevolved to adapt to each other under certain environmental conditions. Conceptually, unbalanced interactions could occur and produce a similar impact when: (i) the host is evolutionarily limited by the environment but not by the pathogen; (ii) the host is neither evolutionarily limited by the environment nor by the pathogen; and (iii) the host and pathogen share an evolutionary history that was forged under different environmental conditions to those currently being experienced, so the host and pathogen, respectively, display either novel or maladaptive phenotypes (figure 1).
Several novel aspects emerge from this model (figure 1). On the one hand, it puts focus on the capacity of the host to adapt to environmental changes and how that influences its capability to cope with pathogens. On the other hand, it aligns both the lack of host–pathogen coevolution and evolution with the environment with two global processes that are currently driving the decline in forest health: globalization and climate change [20]. The addition of these two drivers to the framework shown in figure 1 enables us to differentiate between those pathogens that have jumped host owing to novel climatic conditions (range expansions) and those pathogens that have arrived in a new location owing to human-mediated long-distance dispersal (figure 1). These two components also segregate the ‘invasive’ component of a disease (caused by exotic pathogens) from the ‘emergence’ component (a recent increase in the prevalence of the disease owing to environmental changes).
3. Lack of coevolution with the environment: the role of phenotypic plasticity and plastic responses
Trees have specific ways of adapting to environmental shifts, which is why evolutionary imbalances with the environment can occur that can favour disease. Trees are non-mobile, long-lived organisms that often belong to large outbreeding populations. These features, coupled with slow mutation rates, mean that trees have a low capacity to adapt to rapid environmental shifts via natural selection. Small plants with a short lifespan, for example, rely on genetic diversity to cope with environmental and pathogen shifts [21]. By contrast, trees seem to rely on their phenotypic plasticity [22], although examples of local genetic adaptation and positive selection have been reported [23,24]. Phenotypic plasticity is believed to be a key mechanism to enable trees to cope with novel stressors such as climate change [25]. However, flexibility comes at a cost, and phenotypic plasticity may further reduce the already low genetic adaptation capacity of trees [26]. Overall, trees may have difficulties adapting fast enough to novel biotic or abiotic factors that cannot be handled within their phenotypic span. Trees are therefore likely to experience short- and mid-term situations for which they lack a coevolutionary background.
Trees adapt to average environmental conditions by regulating morphological and life-history traits such as needle size or leaf shedding. In order to cope with the amplitude of those conditions, they use physiological mechanisms, such as stomatal control against drought, or winter hardening against frost damage. These reversible responses are referred to as phenotypic flexibility as opposed to developmental irreversible phenotypic responses determined by growth conditions during the life of the tree [27]. Developmental and physiological responses can be correlated, so that, for instance, drought-adapted genotypes may not only show structural adaptations to drought, but also more fine-tuned responses to water deficit [28]. Depending on how limiting or variable the environmental conditions are, trees may evolve different strategies, so they rely more on developmental plasticity versus phenotypic flexibility [29]. This trade-off is well exemplified in the genus Pinus in which species adapted to more limiting conditions tend to rely more on constitutive than on induced defence responses [30]. A novel environment may, therefore, be unfavourable for the set adaptive strategies inherited throughout evolutionary history.
Adaptations to certain factors (abiotic and biotic) can be maladaptive for others, especially when extreme values of certain stressors are experienced [22,31]. This situation can occur in cases where trees are simultaneously exposed to novel or more aggressive pathogens and to novel environmental conditions that cannot be handled within their phenotypic plasticity and flexibility span (figure 1). Several examples of current invasive/emergent forest pathogens fall into this category, especially when affecting tree species on their southern- and northernmost distribution limits [32,33]. A good example of conflicts between adaptations to different stressors is shown by how different strategies to cope with drought affect the tree's capacity to defend itself against pathogens [34]. Tree species or genotypes can be classified as more anisohydric or more isohydric, depending on their level of stomatal control when subjected to soil water deficits. Isohydric species have strict stomatal control, thus in the case of moderate water deficits they reduce transpiration and C assimilation. By contrast, anisohydric species are able to maintain transpiration for longer because they are better adapted to avoid the occurrence of xylem embolism [35], which, in turn, allows them to maintain a positive carbon balance for longer. If moderate drought conditions are prolonged for long enough, then isohydric species are predicted to deplete their reserves faster, potentially limiting the amount of non-structural carbon (NSC) for defence [36,37]. NSC availability is one of several requirements for eliciting a defence against pathogens; therefore, each stressor/pathogen combination may have different mechanisms behind phenotypic susceptibility.
A phenotype may have varying levels of maladaptation against different types of pathogens. Depending on their trophic interaction with the host, pathogens are classified as biotrophs (feeding on living host cells), necrotrophs (killing host cells before consuming their content) and vascular wilts (interfering with the water transportation system in the xylem of trees). These trophic types are fundamentally different in the way that they interact with the carbon and water storage and transport systems of the tree and, therefore, may benefit differently from the type of plastic and developmental responses used by trees to cope with the environment [38]. Linked to the previous example, biotrophic pathogens, for instance, feed directly on NSC stored in tissues and may have a greater impact on imbalanced trees with an anisohydric strategy (i.e. those trees that maintain larger NSC reserves under drought conditions) [34]. However, an isohydric strategy may favour necrotrophic pathogens whose damage depends on the capacity of the host to mount strong defences, which, in turn, depends on the amount and the accessibility of the NSC available for defence at the site of infection [38,39]. Vascular wilts may benefit from trees maintaining a higher water potential in the tissues [40].
Phenotypic plasticity may be maladaptive to events happening simultaneously or at different times during the life of the tree. An example of this is shown by trees allocating the ‘wrong’ amount of resources to growth and to defence when facing novel environmental conditions. Although the allocation of resources to growth or defence may be the net result of competing sinks, it can also be actively regulated [41,42] and, therefore, is part of the plastic and flexible phenotypic repertoire of the tree. A conflict between growth and defence has been observed in Populus tremuloides when resources were most limiting but not under normal conditions [43], indicating that under stressing conditions a tendency to allocate more resources towards growth could increase the risk of being damaged by pathogens.
Trees are long-lived organisms and during their life they experience a series of events for which their phenotype may or may not be well adapted. In either case, the timing, duration and intensity of events prior to an interaction with a novel pathogen will shape the host phenotype and affect its outcome. In other words, a lack of adaptation to the environment can be expressed in a cumulative way in the long term, ultimately predisposing trees to damage by pathogens. This is the case for tree declines [1] in which not only native, but also invasive pathogens play a role [44]. Under the same environmental shift, certain developmental phenotypes may be more resilient than others, as a result of stresses suffered earlier [45,46]. Likewise, the outcome of plastic responses to stressors such as defoliation or carbon storage may affect the tree's survival [47] and susceptibility to pathogens years after the stressing event [48,49].
When faced with a new environment, hosts may develop a phenotype that increases disease susceptibility by showing a maladaptation to the average environmental conditions, which are determined by more or less constant parameters such as light conditions, soil, temperature or precipitation. In this situation, morphological and life-history traits such as growth or phenology could influence the impact of a pathogen. Phenological mismatch is a well-known mechanism that can limit the damage caused by invasive pathogens [50,51]. Powdery mildew ascospore production, for example, occurs 30–50 days after the appearance of susceptible new shoots of Quercus robur. A climatic shift could close the gap between the time when new shoots are developing and when spore release is greatest, increasing the impact of this invasive pathogen [50].
However, disease may occur because of a maladaptation to cope with novel extreme events such as frost, heat or drought conditions by, for example, limiting the amount/access to resources for defence, or increasing repair costs for damaged tissues [37]. Adaptation to novel extreme events may depend on the previous history of events and how the trees reacted to these events. For instance, Quercus ilex seedlings that suffered combinations of drought and waterlogging pre- and post-infection with Phytophthora cinnamomi resulted in different levels of seedling mortality ranging from 40% to 100% [32]. Higher levels of damage by P. cinnamomi were observed in well-watered seedlings that were then subjected to drought than in drought-stressed seedlings that had not previously been well watered.
The environment not only affects the impact of a novel interaction by affecting the capacity of the host to defend itself, but can also bring higher-than-expected inoculum doses. The disease impact depends on the amount of inoculum [52]. Novel conditions may, for example, extend the length of conducive periods, when the pathogen can increase the number of propagules (more cycles) and their viability (e.g. optimal temperature and moisture conditions) [53]. The new environment can affect survival and either favour range expansions or increase the transfer of inoculum across years. Cold winters, for instance, have been shown to limit the distribution of Phytophthora alni and P. cinnamomi as well as the damage caused by these pathogens [54]. These Phytophthora species lack the ability to form cold-resistant structures because the hybrid nature of P. alni impedes the formation of viable oospores, and P. cinnamomi lacks the complementary mating type to undergo sex and form thick-walled oospores. At present, the winter temperatures in the northern latitudes of Europe are possibly not high enough to enable these two Phytophthora species to achieve sizeable populations; however, the impacts of P. alni and P. cinnamomi infections could increase if milder winters become more recurrent [55].
4. Globalization and climate change interfere with coevolution differently
The two components of disease shown in figure 1 indicate that coevolution can be arrested when either a host or a pathogen with no recent coevolutionary history are moved into close contact with one another, or the environment changes (figure 1). These two processes are connected to two current global human-mediated processes: globalization and climate change (figure 2).
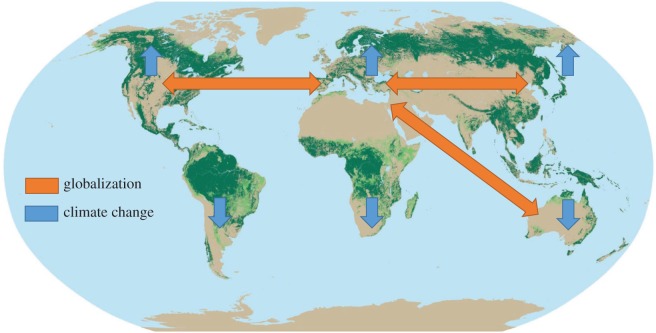
Figure 2.
Globalization and climate change. Globalization and international trade can potentially bring plants from geographically distant but climatically similar locations into contact with new pathogens (orange arrows). Predicted climate changes could enable pathogens to move short distances along climatic gradients, bringing them into contact with new host trees (blue arrows). Map of global forest distribution (green areas) from © FAO 2005 The world's forest, http://foris.fao.org/static/data/fra2005/maps/2.2.jpg, accessed 9 June 2016. This is an adaptation of an original work by the FAO. The views and opinions expressed in the adaptation are the sole responsibility of the authors of the adaptation and are not endorsed by the FAO.
Globalization has brought together organisms that have similar climatic requirements, but that have been previously separated geographically by substantial distances, for example, organisms from around the world have been introduced into the Mediterranean area (figure 2) [56]. To maximize the survival of an introduced plant from one continent on another continent, we try to match the environmental requirements of the native environment. The desire to have new and interesting plants in our gardens and parks is driving the interest in growing exotic plants. When production plants are introduced into a new area, there is also often a gain in productivity [57]. This might partly be because the plants have been released from the pathogen and pest load in their native environment [58]. However, minor pathogens or even endophytic fungi can be passive passengers on the introduced plants. Occasionally, such harmless organisms can unintentionally move to another species and end up in an unbalanced pathogenic relationship with a new host species [59].
Such long-distance spread of pathogenic fungi is not a regular feature of natural ecosystems [53]. However, long-distance wind-borne dispersal of fungal spores can occur, especially in species with pigmented thick-walled spores (e.g. rust spores). In agricultural systems, such as wheat growing, this is thought to be the main way that fungal genotypes are distributed over continental scales during a growing season [60]. Although between-continent spread of rust spores has been reported, this is thought to be exceptional [61]. For species producing hyaline spores, wind dispersal is also important; however, spore viability is reduced under UV light and, therefore, the effective dispersal distance for species producing hyaline spores is normally shorter than that for rusts [62].
Movement of infected plant material is the main source of inoculum responsible for the introduction of new diseases [63]. The likelihood of bringing in a new species and its successful establishment in a new environment depends on the amount of plants brought into an area [2]. This, in turn, depends on the trade networks and the size of the economies involved in trade. Consequently, large import/export harbours are key points for the potential entry of diseases. Horticultural trade networks have been shown to be centred on distribution hubs [64].
In contrast to the situation for new pathogens brought in from another continent where coevolution is unlikely to have occurred, we argue that emerging disease problems arising from climate change are more likely to involve host–pathogen interactions that have at least a partly shared evolutionary history. Pathogens would presumably move more quickly than hosts into new areas in response to a change in climatic conditions. This may end up in scenarios where trees left stressed by new climatic challenges encounter a slightly different set of pathogens that could have moved from areas where they had already adapted to the new climatic conditions (figure 1). It is plausible that over evolutionary time there have been encounters between similar host–pathogen combinations and, therefore, that they may have a more balanced interaction pattern than would be the case for the intercontinental movement of a challenger fungal species. Taking all available data into consideration, it would be plausible to expect a higher risk for large outbreaks of forest disease under the global transportation scenario than under the climate change relatively short-distance movements scenario (table 1). However, climate change may also lead to a higher incidence of extreme weather events that can incite outbreaks of forest epidemics. It would be of interest to further explore the magnitude of these different forces from an evolutionary viewpoint.
Table 1.
Differences between novel interactions originating from globalization compared with climate change.
| globalization | climate change | |
|---|---|---|
| climate matching | new organisms that fit into a specific climate envelope | adaptation to slightly different conditions |
| host/competitors | new | changing |
| movement | along trading routes | along climatic gradients |
| coevolution | none | partial |
| range expansions | latitude and longitudinal | latitudinal (towards the poles) |
| main component | pathogen control | environmental control |
| overall risk | high–medium | medium–low |
5. Case study: Gremmeniella abietina in Sweden
An evolutionary perspective, considering both the lack of adaptation of the host with the pathogen and the environment (phenotype), can help to understand current diseases and anticipate future damage. This view may be especially relevant to explain the impacts of invasive and emerging pathogens that cause damage intermittently in space and time, such as Gremmeniella abietina, Dothistroma needle blight, Diplodia pinea, Erysiphe alphitoides and Phytophthora spp. These pathogens represent cases in which non-native hosts show some level of native resistance, and where the interactions with the environment play a major role, i.e. where both lack of coevolution with the pathogen and the environment take place. The role of the environment is not exclusive to pathogens causing intermittent damage; it also influences the impact of highly damaging invasive pathogens. Phenology, for instance, can enable elm trees to escape Ophiostoma novoulmi attacks in Dutch elm disease because of an asynchrony between the life cycle of the disease vector and the period when the twig is most susceptible [65]. Furthermore, the impact of chestnut blight is reduced in situations where the host is subjected to less stressing conditions [66].
The needle pathogen Gremmeniella abietina, which attacks the native Pinus sylvestris and the exotic Pinus contorta in Sweden, is a good illustration of the importance of the two components: that is, the lack of adaptation of both P. sylvestris and P. contorta to the environment, and the lack of adaptation of P. contorta to the pathogen. Both P. sylvestris and P. contorta are more susceptible to the pathogen when planted on more fertile ‘spruce sites’ than when planted on ‘pine sites’ [60]. The mechanisms behind this higher susceptibility are not known, but it is plausible that pines develop a maladaptive phenotype specific for this pathogen on fertile sites. Site fertility not only increases growth, but also reduces the number of needle cohorts on the branches, i.e. there is a greater proportion of young needles [67]. Gremmeniella abietina kills the 1 and 2 year old shoots and needles, thus, when growing under fertile conditions, pines develop a phenotype that makes them more susceptible, leaving fewer cohorts of surviving needles than when growing under normal conditions. The link between fertility and susceptibility has been seen in other invasive pathogens such as Diplodia, Dothistroma or Phytophthora [68–70], although little is known about the phenotypic mechanism mediating these effects.
In Sweden, in cases where southern varieties of P. sylvestris and P. contorta have been planted in northern locations, greater levels of damage as a result of G. abietina infection have been recorded [71]. The higher level of susceptibility has been linked to winter hardening in connection with frost damage events [72]. Like the situation with G. abietina, susceptibility differences between northern and southern provenances of Pinus greggii to Diplodia pinea seem to be mediated by their resistance to hail damage [73]. In Australia, Eucalyptus provenances with a lower phloem moisture content were more resistant to Phytophthora cinnamomi [74], although the exact mechanisms are unknown.
Pinus contorta generally suffers more damage as a result of G. abietina infection than P. sylvestris [75], which seems to be mainly because of greater maladaptation to the Swedish environment. The higher levels of damage suffered by P. contorta could also indicate a lack of coevolutionary history with the pathogen, although inoculation trials suggest that there are mainly qualitative and not quantitative susceptibility differences between these two species [76]. A variant of G. abietina exists in North America, which could explain why P. contorta is not fully naive to infection by the European race of G. abietina. Both species also show similar levels of susceptibility to Dothistroma septosporum, supporting the finding that they share some coevolutionary past [77]. Overall, the higher level of resistance of P. sylvestris to native pathogens such as G. abietina, Heterobasidion annosum and Phacidium infestans than that shown by P. contorta under field conditions [78,79] suggests either a general mechanism of environmental maladaptation by P. contorta or indeed a certain degree of lack of coevolution with European pathogens.
Gremmeniella abietina is also a good example of an emergent disease driven by climate change. Under very specific conditions, G. abietina becomes highly virulent and moves from tips to branches, causing cankers and killing trees [80]. The crown dieback disease is caused by a different race (large tree type, LTT) of the fungus to the one described above for P. contorta (small tree type) [81]. This pathogen LTT ‘phenotype’ of crown dieback and tree killing is believed to occur when the inoculum potential is high enough, which depends on a rare 3 year cycle in which a rainy and cool summer occurs in areas where a lot of inoculum is also present because of an epidemic 2 years earlier [82]. Climate change is predicted to increase the frequency of conducive conditions in Sweden, i.e. milder winters and wetter summers [83] and, hence, the risk of epidemics could potentially increase, as seen in the past decades (figure 3). Forest disease emergence owing to novel climatic conditions causing higher than normal inoculum potential is also well documented for Dothistroma needle blight [90].
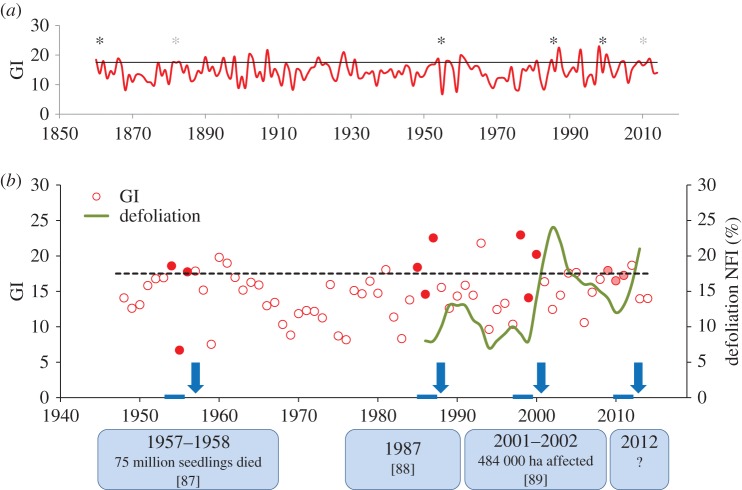
Figure 3.
(a) Gremmeniella index (GI) for central Sweden (Svealand, SMHI Swedish Meteorological and Hydrological Institute) between 1860 and 2015. The mm of rainfall during June–August for the year divided by the average temperature in degrees above 0°C was used to calculate the index, following the method described in Hansson & Karlman [75]. A solid grey line representing 17.5 on the GI indicates higher than normal values. Black asterisks indicate years following a reinforcement of the risk of infection, which happens in the second year of conditions conducive for Gremmeniella infection since sporulation peaks 2 years after a first outbreak. Grey asterisks indicate the periods when the GI was over 17.2. Between 1860 and 2015, six such high-risk years occurred in this area, more frequently in recent times. (b) The GI for central Sweden (Svealand, SMHI Swedish Meteorological and Hydrological Institute) between 1945 and 2015 linked to major G. abietina epidemics [84–86]. A dashed black line representing 17.5 on the GI indicates higher than normal values. Red and pale-red dots indicate the period when conditions conducive for infection occurred (GI > 17.5 and GI > 17.2, respectively). The green line indicates the average needle loss in pine trees in the same region as determined from the National Forest Inventory (NFI). Blue arrows indicate the year after a two year period of inciting and reinforcement of Gremmeniella attack, and the blue boxes include information on documented major outbreaks of the disease. Peaks in needle loss coincide with reports of major G. abietina epidemics at the local scale. GI was above 17.2 in 2010 and 2011, and even though no major outbreaks were reported, a needle loss peak was also found, suggesting that a mild G. abietina epidemic may have struck central Sweden during this period [87–89].
6. Further research on phenotypic resistance mechanisms is needed
Through several examples, we have shown that the phenotype and, hence, phenotypic maladaptation, play a large role in tree disease. Biotic factors are key in limiting the capacity of trees to use all their potential phenotypic plasticity and plastic responses [22] and, therefore, are likely candidates to contribute to imbalances with the physical environment. Although understanding the genetic determinants of disease has been the focus of many research studies, we still know little about the physiological mechanisms behind phenotypic tree resistance.
The most striking knowledge gap relates to our understanding of how pathogens kill trees. This is a complex process, because pathogens can establish a large array of interactions with the host with differing physiological consequences [37]. Our understanding of how pathogens kill trees is further complicated (i) by a lack of agreement in the scientific community on the mechanisms that lead to tree death, which seem to involve carbon starvation or hydraulic failure, or a combination of both, and (ii) because pathogens often interact with other abiotic factors to kill trees. The complexity of this process is well illustrated in the case of drought and pathogens [36]. Trees can defend themselves against pathogens by compartmentalizing the infection within carbon-expensive barriers [91,92], the creation of which requires access to and mobilization of C reserves from source (storage) to sink (infected tissues) [38]. Drought stress reduces assimilation and increases the demand for C owing to reduced assimilation and increased osmotic refilling needs, decreasing the amount of C available for defence [37]. Drought can also reduce phloem transport, limiting the capacity to mobilize C from source to sink tissues [39]. Pathogens can, therefore, benefit from or accelerate damage caused by drought in an interactive way; however, the key drivers of mortality have yet to be determined.
Understanding the mechanisms behind phenotypic maladaptation is crucial in the light of predicted climate changes. In summary, a novel environment may, for instance, increase the conflict between adaptive traits to abiotic and biotic factors, and iso-/anisohydric strategies against drought may influence the capacity of trees to defend themselves from pathogen infections. Pathogens that establish different trophic interactions with the host may be affected by iso-/anisohydric strategies in different ways [34,37]. Other specific cases of maladaptation, such as higher levels of damage caused by G. abietina infections in fertilized areas potentially mediated by trees having a lower number of needle cohorts, also deserve attention. The number of needle cohorts is especially relevant in conifer forests in northern latitudes, where much of the year's growth happens during a short period of time, and where early needle losses may have large impacts on fitness [93]. Testing such hypotheses can help to predict which type of trees may be more susceptible to invaders, and what type of pathogens are more likely to emerge and have a negative impact on forest trees following introductions.
Resistance to different pathogens is genetically correlated in plants [94], which means that predictions beyond species level are possible. For instance, it remains to be understood whether a higher level of adaptation to a certain environment also confers a better general adaptation to pathogens thriving under those conditions. In other words, is there an environmental signal in host–pathogen coevolution? Northern populations of Pinus muricata growing in cooler and moister conditions, such as the conditions needed for D. septosporum development, were found to be more resistant to the pathogen than southern populations growing in warmer and drier conditions, which are less favourable for the pathogen [95]. Similar results have been found for a native pathogen causing Mycosphaerella leaf disease in Eucalyptus [96]. Trees, as long-lived organisms, have both signs of purifying and diversifying selection in defence loci [84]. Plants are able to recognize and defend themselves against closely related pathogens that they have not previously encountered [85]. Likewise, pathogens are able to colonize novel plants as long as they are phylogenetically similar to some of their coevolutionary hosts [86]. Understanding how much these associations vary with environmental conditions could help to quantify the contribution of the lack of coevolution with the environment in tree disease.
Tolerance is emerging as a novel mechanism of defence against invasive pathogens [18]. In theory, a trade-off between resistance and tolerance exists in plants, so that resistance to a certain type/amount of damage is associated with a low tolerance to the same form of damage [97]. Evolutionary benefits of resistance versus tolerance are defined by their relative costs. We have shown that novel conditions may increase the competition between different traits and, thus, could favour tolerant versus resistant strategies, although little is known about the tolerance and resistance determinants in trees [98]. Likewise, the tree microbiome is also part of the phenotype and may be responsible for the lack of coevolution with the environment. The endophytic community in trees plays an important role in helping trees to sense shifts in the environment and mediating responses against biotic and abiotic stressors [53]. Questions such as whether a maladapted phenotype is less likely to recruit a beneficial microbial community or, vice versa, whether recruiting a certain community causes a tree to develop the wrong phenotype, may also help to predict future impacts.
Exotics represent a good study system for testing the importance of a phenotypic maladaptation. Exotics often have novel phenotypes as a result of an interaction with the new environment, which may bring, for example, changes in fertility and rainfall. Exotic pines such as Pinus radiata are an extreme example of this, showing spectacular growth when planted outside their native range. However, many exotics planted outside their native range become highly affected by native or exotic pathogens [99]. It is plausible that exotic trees are more susceptible to pathogens in their new environment because they are showing a novel phenotype, for which they have experienced little selection, because the conditions have rarely (or never) been experienced in their native environment. Little selection may also have occurred because of a limited number of pathogens that have coevolved pathogenicity under these conditions in their native environment. Having no coevolutionary memory could make trees show maladaptive phenotypes and plastic responses, for instance, unbalanced growth–defence trade-offs, or unbalanced constitutive versus induced defence responses [100].
Acknowledgement
We thank Caroline Woods for linguistic revision and two anonymous referees for helping to clarify the manuscript.
Data accessibility
Data on climate and Gremmeniella abietina epidemics is available on Dryad: doi:10.5061/dryad.1182k.
Authors' contributions
J.S. and J.O. contributed equally to the study.
Competing interests
We have no competing interests.
Funding
This research has been partly supported by the RESIPATH-Biodiversa project, the Future Forests research programme and by the Swedish Research Council FORMAS.
References
- 1.Manion PD. 1981. Tree disease concepts. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- 2.Smith ML, Bruhn JN, Anderson JB. 1992. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356, 428–431. ( 10.1038/356428a0) [DOI] [Google Scholar]
- 3.Woodward S, Stenlid J, Karjalainen R, Hüttermann A. 1998. Heterobasidion annosum biology, ecology, impact and control. Wallingford, UK: CAB International. [Google Scholar]
- 4.Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ. 2013. The consequence of tree pests and diseases for ecosystem services. Science 342, 1235773 ( 10.1126/science.1235773) [DOI] [PubMed] [Google Scholar]
- 5.Santini A, et al. 2013. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 197, 238–250. ( 10.1111/j.1469-8137.2012.04364.x) [DOI] [PubMed] [Google Scholar]
- 6.Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougal KL. 2008. Phytophthora cinnamomi and Australia's biodiversity: impacts, predictions and progress towards control. Aust. J. Bot. 56, 279–310. ( 10.1071/BT07159) [DOI] [Google Scholar]
- 7.Grünwald NJ, Goss EM, Press CM. 2008. Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Mol. Plant Pathol. 9, 729–740. ( 10.1111/j.1364-3703.2008.00500.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasier C, Webber J. 2010. Sudden larch death. Nature 486, 824–825. ( 10.1038/466824a) [DOI] [PubMed] [Google Scholar]
- 9.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desprez-Loustau M-L, Robin C, Buée M, Courtecuisse R, Garbaye J, Suffert F, Sache I, Rizzo DM. 2007. The fungal dimension of biological invasions. Trends Ecol. Evol. 22, 472–480. ( 10.1016/j.tree.2007.04.005) [DOI] [PubMed] [Google Scholar]
- 11.Ennos RA. 2015. Resilience of forests to pathogens: an evolutionary ecology perspective. Forestry 88, 41–52. ( 10.1093/forestry/cpu048) [DOI] [Google Scholar]
- 12.Parker IM, Gilbert GS. 2004. The evolutionary ecology of novel plant–pathogen interactions. Annu. Rev. Ecol. Evol. Syst. 35, 675–700. ( 10.1146/annurev.ecolsys.34.011802.132339) [DOI] [Google Scholar]
- 13.Stenlid J, Oliva J, Boberg JB, Hopkins AJM. 2011. Emerging diseases in European forest ecosystems and responses in society. Forests 2, 486–504. ( 10.3390/f2020486) [DOI] [Google Scholar]
- 14.Burdon JJ, Thrall PH. 2009. Co-evolution of plants and their pathogens in natural habitats. Science 324, 755–756. ( 10.1126/science.1171663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dukes JS, et al. 2009. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: what can we predict? Can. J. Forest Res. 39, 231–248. ( 10.1139/X08-171) [DOI] [Google Scholar]
- 16.Holdenrieder O, Pautasso M, Weisberg PJ, Lonsdale D. 2004. Tree diseases and landscape processes: the challenge of landscape pathology. Trends Ecol. Evol. 19, 446–452. ( 10.1016/j.tree.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 17.Simberloff D, et al. 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28, 58–66. ( 10.1016/j.tree.2012.07.013) [DOI] [PubMed] [Google Scholar]
- 18.Elvira-Recuenco M, Iturritxa E, Majada J, Alia R, Raposo R. 2014. Adaptive potential of maritime pine (Pinus pinaster) populations to the emerging pitch canker pathogen, Fusarium circinatum. PLoS ONE 9, e114971 ( 10.1371/journal.pone.0114971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millett BP, Mollov DS, Iorizzo M, Carputo D, Bradeen JM. 2009. Changes in disease resistance phenotypes associated with plant physiological age are not caused by variation in R gene transcript abundance. Mol. Plant Microbe Interact. 22, 362–368. ( 10.1094/MPMI-22-3-0362) [DOI] [PubMed] [Google Scholar]
- 20.Trumbore S, Brando P, Hartmann H. 2015. Forest health and global change. Science 349, 814–818. ( 10.1126/science.aac6759) [DOI] [PubMed] [Google Scholar]
- 21.Laine A-L, Burdon JJ, Dodds PN, Thrall PH. 2011. Spatial variation in disease resistance: from molecules to metapopulations. J. Ecol. 99, 96–112. ( 10.1111/j.1365-2745.2010.01738.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763. ( 10.1111/j.1469-8137.2007.02275.x) [DOI] [PubMed] [Google Scholar]
- 23.Savolainen O, Pyhäjärvi T, Knürr T. 2007. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 38, 595–619. ( 10.1146/annurev.ecolsys.38.091206.095646) [DOI] [Google Scholar]
- 24.Eckert AJ, et al. 2013. The evolutionary genetics of the genes underlying phenotypic associations for loblolly pine (Pinus taeda, Pinaceae). Genetics 195, 1353–1372. ( 10.1534/genetics.113.157198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franks SJ, Weber JJ, Aitken SN. 2014. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 7, 123–139. ( 10.1111/eva.12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price TD, Qvarnström A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440. ( 10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsman A. 2015. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–284. ( 10.1038/hdy.2014.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen-Queyrens A, Bouchet-Lannat F. 2003. Osmotic adjustment in three-year-old seedlings of five provenances of maritime pine (Pinus pinaster) in response to drought. Tree Physiol. 23, 397–404. ( 10.1093/treephys/23.6.397) [DOI] [PubMed] [Google Scholar]
- 29.Sampedro L, Moreira X, Zas R. 2011. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J. Ecol. 99, 818–827. ( 10.1111/j.1365-2745.2011.01814.x) [DOI] [Google Scholar]
- 30.Moreira X, Sampedro L, Zas R, Pearse IS. 2016. Defensive traits in young pine trees cluster into two divergent syndromes related to early growth rate. PLoS ONE 11, e0152537 ( 10.1371/journal.pone.0152537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 32.Corcobado T, Cubera E, Juárez E, Moreno G, Solla A. 2014. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agr. For. Meteorol. 192–193, 1–8. ( 10.1016/j.agrformet.2014.02.007) [DOI] [Google Scholar]
- 33.Woods AJ, et al. 2016. Dothistroma needle blight, weather and possible climatic triggers for the disease's recent emergence. For. Pathol. ( 10.1111/efp.12248) [DOI] [Google Scholar]
- 34.Sade N, Gebremedhin A, Moshelion M. 2012. Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 7, 767–770. ( 10.4161/psb.20505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Forner N, et al. 2016. Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant Cell Environ. 39, 38–49. ( 10.1111/pce.12588) [DOI] [PubMed] [Google Scholar]
- 36.McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. 2011. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 26, 523–532. ( 10.1016/j.tree.2011.06.003) [DOI] [PubMed] [Google Scholar]
- 37.Oliva J, Stenlid J, Martínez-Vilalta J. 2014. The effect of fungal pathogens on the water and carbon economy of trees: implications for drought-induced mortality. New Phytol. 203, 1028–1035. ( 10.1111/nph.12857) [DOI] [PubMed] [Google Scholar]
- 38.Guérard N, Maillard P, Bréchet C, Lieutier F, Dreyer E. 2007. Do trees use reserve or newly assimilated carbon for their defense reactions? A 13C labeling approach with young Scots pines inoculated with a bark-beetle-associated fungus (Ophiostoma brunneo ciliatum). Ann. Forest Sci. 64, 601–608. ( 10.1051/forest:2007038) [DOI] [Google Scholar]
- 39.Hartmann H, Ziegler W, Trumbore S. 2013. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 27, 413–427. ( 10.1111/1365-2435.12046) [DOI] [Google Scholar]
- 40.Venturas M, López R, Martín JA, Gascó A, Gil L. 2014. Heritability of Ulmus minor resistance to Dutch elm disease and its relationship to vessel size, but not to xylem vulnerability to drought. Plant Pathol. 63, 500–509. ( 10.1111/ppa.12115) [DOI] [Google Scholar]
- 41.Sala A, Woodruff DR, Meinzer FC. 2012. Carbon dynamics in trees: feast or famine? Tree Physiol. 32, 764–775. ( 10.1093/treephys/tpr143) [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Vilalta J. 2014. Carbon storage in trees: pathogens have their say. Tree Physiol. 34, 215–217. ( 10.1093/treephys/tpu010) [DOI] [PubMed] [Google Scholar]
- 43.Donaldson JR, Kruger EL, Lindroth RL. 2006. Competition- and resource-mediated tradeoffs between growth and defensive chemistry in trembling aspen (Populus tremuloides). New Phytol. 169, 561–570. ( 10.1111/j.1469-8137.2005.01613.x) [DOI] [PubMed] [Google Scholar]
- 44.Marcais B, Bréda N. 2006. Role of an opportunistic pathogen in the decline of stressed oak trees. J. Ecol. 94, 1214–1223. ( 10.1111/j.1365-2745.2006.01173.x) [DOI] [Google Scholar]
- 45.Sangüesa-Barreda G, Camarero JJ, Oliva J, Montes F, Gazol A. 2015. Past logging, drought and pathogens interact and contribute to forest dieback. Agr. For. Meteorol. 208, 85–94. ( 10.1016/j.agrformet.2015.04.011) [DOI] [Google Scholar]
- 46.Oliva J, Colinas C. 2007. Decline of silver fir (Abies alba Mill.) stands in the Spanish Pyrenees: role of management, historic dynamics and pathogens. For. Ecol. Manage. 252, 84–97. ( 10.1016/j.foreco.2007.06.017) [DOI] [Google Scholar]
- 47.Galiano L, Martínez-Vilalta J, Sabaté S, Lloret F. 2012. Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean holm oak forest. Tree Physiol. 32, 478–489. ( 10.1093/treephys/tps025) [DOI] [PubMed] [Google Scholar]
- 48.Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. 2015. To die or not to die: early warnings of tree dieback in response to a severe drought. J. Ecol. 103, 44–57. ( 10.1111/1365-2745.12295) [DOI] [Google Scholar]
- 49.Aguadé D, Poyatos R, Gómez M, Oliva J, Martínez-Vilalta J. 2015. The role of defoliation and root rot pathogen infection in driving the mode of drought-related physiological decline in Scots pine (Pinus sylvestris L.). Tree Physiol. 35, 229–242. ( 10.1093/treephys/tpv005) [DOI] [PubMed] [Google Scholar]
- 50.Marçais B, Kavkova M, Desprez-Loustau M-L. 2009. Phenotypic variation in the phenology of ascospore production between European populations of oak powdery mildew. Ann. Forest Sci. 66, 814 ( 10.1051/forest/2009077) [DOI] [Google Scholar]
- 51.Dodd RS, Huberli D, Mayer W, Harnik TY, Afzal-Rafli Z, Garbelotto M. 2008. Evidence for the role of synchronicity between host phenology and pathogen activity in the distribution of sudden oak death canker disease. New Phytol. 179, 505–514. ( 10.1111/j.1469-8137.2008.02450.x) [DOI] [PubMed] [Google Scholar]
- 52.van der Plank JE. 1963. Plant diseases: epidemics and control. New York, NY: Academic Press. [Google Scholar]
- 53.Oliva J, Boberg JB, Hopkins AJ, Stenlid J. 2013. Concepts of epidemiology of forest diseases. In Infectious forest diseases (eds Gonthier P, Niccolotti G), pp. 1–28. Wallingford, UK: CAB International. [Google Scholar]
- 54.Marçais B, Dupuis F, Desprez-Loustau M. 1996. Modelling the influence of winter frosts on the development of the stem canker of red oak, caused by Phytophthora cinnamomi. Ann. Forest Sci. 53, 369–382. ( 10.1051/forest:19960219) [DOI] [Google Scholar]
- 55.Redondo MA, Boberg J, Olsson CH, Oliva J. 2015. Winter conditions correlate with Phytophthora alni subspecies distribution in Southern Sweden. Phytopathology 105, 1191–1197. ( 10.1094/PHYTO-01-15-0020-R) [DOI] [PubMed] [Google Scholar]
- 56.Garbelotto M, Pautasso M. 2012. Impacts of exotic forest pathogens on Mediterranean ecosystems: four case studies. Eur. J. Plant Pathol. 133, 101–116. ( 10.1007/s10658-011-9928-6) [DOI] [Google Scholar]
- 57.Wingfield MJ. 1999. Pathogens in exotic plantation forestry. Int. Forest Rev. 1, 163–168. [Google Scholar]
- 58.Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17, 164–170. ( 10.1016/S0169-5347(02)02499-0) [DOI] [Google Scholar]
- 59.Cleary M, Nguyen D, Marčiulynienė D, Berlin A, Vasaitis R, Stenlid J. 2016. Friend or foe? Biological and ecological traits of the European ash dieback pathogen Hymenoscyphus fraxineus in its native environment. Sci. Rep. 6, 21895 ( 10.1038/srep21895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolmer JA. 2005. Tracking wheat rust on a continental scale. Curr. Opin. Plant Biol. 8, 441–449. ( 10.1016/j.pbi.2005.05.001) [DOI] [PubMed] [Google Scholar]
- 61.Brown JKM, Hovmøller MS. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297, 537–541. ( 10.1126/science.1072678) [DOI] [PubMed] [Google Scholar]
- 62.Fitt BDL, Gregory PH, Todd AD, McCartney HA, Macdonald OC. 1987. Spore dispersal and plant disease gradients; a comparison between two empirical models. J. Phytopathol. 118, 227–242. ( 10.1111/j.1439-0434.1987.tb00452.x) [DOI] [Google Scholar]
- 63.Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO. 2012. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 10, 135–143. ( 10.1890/110198) [DOI] [Google Scholar]
- 64.Dehnen-Schmutz K, Holdenrieder O, Jeger MJ, Pautasso M. 2010. Structural change in the international horticultural industry: some implications for plant health. Sci. Hort. 125, 1–15. ( 10.1016/j.scienta.2010.02.017) [DOI] [Google Scholar]
- 65.Santini A, Fagnani A, Ferrini F, Ghelardini L, Mittempergher L. 2005. Variation among Italian and French elm clones in their response to Ophiostoma novo-ulmi inoculation. For. Pathol. 35, 183–193. ( 10.1111/j.1439-0329.2005.00401.x) [DOI] [Google Scholar]
- 66.Waldboth M, Oberhuber W. 2009. Synergistic effect of drought and chestnut blight (Cryphonectria parasitica) on growth decline of European chestnut (Castanea sativa). For. Pathol. 39, 43–55. ( 10.1111/j.1439-0329.2008.00562.x) [DOI] [Google Scholar]
- 67.Balster NJ, Marshall JD. 2000. Decreased needle longevity of fertilized Douglas-fir and grand fir in the northern Rockies. Tree Physiol. 20, 1191–1197. ( 10.1093/treephys/20.17.1191) [DOI] [PubMed] [Google Scholar]
- 68.Blodgett JT, Herms DA, Bonello P. 2005. Effects of fertilization on red pine defense chemistry and resistance to Sphaeropsis sapinea. For. Ecol. Manage. 208, 373–382. ( 10.1016/j.foreco.2005.01.014) [DOI] [Google Scholar]
- 69.Lambert MJ. 1986. Sulphur and nitrogen nutrition and their interactive effects on Dothistroma infection in Pinus radiata. Can. J. Forest Res. 16, 1055–1062. ( 10.1139/x86-183) [DOI] [Google Scholar]
- 70.Hummel RL, Elliott M, Chastagner G, Riley RE, Riley K, DeBauw A. 2013. Nitrogen fertility influences growth and susceptibility of rhododendrons to Phytophthora ramorum. HortScience 48, 601–607. [Google Scholar]
- 71.Karlman M, Hansson P, Witzell J. 1994. Scleroderris canker on lodgepole pine introduced in northern Sweden. Can. J. Forest Res. 24, 1948–1959. ( 10.1139/x94-250) [DOI] [Google Scholar]
- 72.Petäistö R-L, Kurkela T. 1993. The susceptibility of Scots pine seedlings to Gremmeniella abietina: effect of growth phase, cold and drought stress. Eur. J. Forest Pathol. 23, 385–399. ( 10.1111/j.1439-0329.1993.tb00819.x) [DOI] [Google Scholar]
- 73.Smith H, Coutinho TA, Wolfaardt FW, Wingfield MJ. 2002. Relative susceptibility of northern and southern provenances of Pinus greggii to infection by Sphaeropsis sapinea. For. Ecol. Manage. 166, 331–336. ( 10.1016/S0378-1127(01)00667-3) [DOI] [Google Scholar]
- 74.Shearer BL, Michaelsen BJ, Somerford PJ, Williams M. 2014. Forest environment mediated intraspecific resistance of Eucalyptus marginata to Phytophthora cinnamomi. Austral. Plant Pathol. 43, 245–255. ( 10.1007/s13313-013-0263-6) [DOI] [Google Scholar]
- 75.Hansson P, Karlman M. 1997. Survival, height and health status of 20-year-old Pinus sylvestris and Pinus contorta after different scarification treatments in a harsh boreal climate. Scand. J. Forest Res. 12, 340–350. ( 10.1080/02827589709355421) [DOI] [Google Scholar]
- 76.Hansson P. 1998. Susceptibility of different provenances of Pinus sylvestris, Pinus contorta and Picea abies to Gremmeniella abietina. Eur. J. Forest Pathol. 28, 21–32. ( 10.1111/j.1439-0329.1998.tb01162.x) [DOI] [Google Scholar]
- 77.Millberg H, Hopkins AJM, Boberg J, Davydenko K, Stenlid J. 2015. Disease development of Dothistroma needle blight in seedlings of Pinus sylvestris and Pinus contorta under Nordic conditions. For. Pathol. ( 10.1111/efp.12242) [DOI] [Google Scholar]
- 78.Zaluma A, Arhipova N, Sisenis A, Jansons A, Baumanis T, Gaitnieks R, Vasaitis R. 2011. Resistance of Pinus contorta and Pinus sylvestris to Heterobasidion annosum. In XIII Conference ‘Root and Butt Rot of Forest Trees’ IUFRO Working Party 70201 (eds Capretti P, Comparini C, Garbelotto M, La Porta N, Santini A), p. 110 Firenze, Italy: Firenze University Press. [Google Scholar]
- 79.Karlman M. 1986. Damage to Pinus contorta in northern Sweden with special emphasis on pathogens. Stud. Forest Suec. 176, 1–42. [Google Scholar]
- 80.Sikström U, Jansson G, Weslien J. 2005. Predicting the mortality of Pinus sylvestris attacked by Gremmeniella abietina and occurrence of Tomicus piniperda colonization. Can J. Forest Res. 35, 860–867. ( 10.1139/X08-157) [DOI] [Google Scholar]
- 81.Hellgren M, Högberg N. 1995. Ecotypic variation of Gremmeniella abietina in northern Europe: disease patterns reflected by DNA variation. Can. J. Bot. 73, 1531–1539. ( 10.1139/b95-166) [DOI] [Google Scholar]
- 82.Thomsen IM. 2009. Precipitation and temperature as factors in Gremmeniella abietina epidemics. For. Pathol. 39, 56–72. ( 10.1111/j.1439-0329.2008.00561.x) [DOI] [Google Scholar]
- 83.Lind P, Kjellström E. 2008. Temperature and precipitation changes in Sweden; a wide range of model-based projections for the 21st century. SMHI Rep. Meteorol. Climatol. 113, 1–50. See http://www.smhi.se/content/1/c6/04/16/54/attatchments/RMK113_rapport_090421.pdf. [Google Scholar]
- 84.Ersoz ES, Wright MH, González-Martínez SC, Langley CH, Neale DB. 2010. Evolution of disease response genes in loblolly pine: insights from candidate genes. PLoS ONE 5, e14234 ( 10.1371/journal.pone.0014234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Wit PJGM, et al. 2012. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 8, e1003088 ( 10.1371/journal.pgen.1003088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parker IM, Saunders M, Bontrager M, Weitz AP, Hendricks R, Magarey R, Suiter K, Gilbert GS. 2015. Phylogenetic structure and host abundance drive disease pressure in communities. Nature 520, 542–544. ( 10.1038/nature14372) [DOI] [PubMed] [Google Scholar]
- 87.Björkman E. 1959. Ny svampsjukdom i skogträdsplantskolor. Skogen 46, 292–293. [In Swedish.] [Google Scholar]
- 88.Hellgren M, Stenlid J. 1997. Diseases of conifers caused by Gremmeniella abietina. In Compendium of forest pathology (eds Hansen EM, Lewis K), pp. 43–45. St. Paul, MN: APS Press. [Google Scholar]
- 89.Wulff S, Hansson P, Witzell J. 2006. The applicability of national forest inventories for estimating forest damage outbreaks – experiences from a Gremmeniella outbreak in Sweden. Can. J. Forest Res. 36, 2605–2613. ( 10.1139/x06-148) [DOI] [Google Scholar]
- 90.Woods A, Coates KD, Hamann A. 2005. Is an unprecedented Dothistroma needle blight epidemic related to climate change? BioScience 55, 761–769. ( 10.1641/0006-3568(2005)055%5B0761:iaudnb%5D2.0.co;2) [DOI] [Google Scholar]
- 91.Oliva J, Thor M, Stenlid J. 2010. Reaction zone and periodic increment decrease in Picea abies trees infected by Heterobasidion annosum s.l. For. Ecol. Manage. 260, 692–698. ( 10.1016/j.foreco.2010.05.024) [DOI] [Google Scholar]
- 92.Oliva J, Camarero JJ, Stenlid J. 2012. Understanding the role of sapwood loss and reaction zone formation on radial growth of Norway spruce (Picea abies) trees decayed by Heterobasidion annosum s.l. For. Ecol. Manage. 274, 201–209. ( 10.1016/j.foreco.2012.02.026) [DOI] [Google Scholar]
- 93.Kurkela T, Drenkhan R, Vuorinen M, Hanso M. 2009. Growth response of young Scots pines to needle loss assessed from productive foliage. For. Stud. 50, 5–22. ( 10.2478/v10132-011-0066-x) [DOI] [Google Scholar]
- 94.Leimu R, Koricheva J. 2006. A meta-analysis of genetic correlations between plant resistances to multiple enemies. Am. Nat. 168, 15–37. ( 10.1086/505766) [DOI] [PubMed] [Google Scholar]
- 95.Ades PK, Simpson JA, Eldridge KG, Eldridge RH. 1992. Genetic variation in susceptibility to Dothistroma needle blight among provenances and families of Pinus muricata. Can. J. Forest Res. 22, 1111–1117. ( 10.1139/x92-147) [DOI] [Google Scholar]
- 96.Hamilton MG, Williams DR, Tilyard PA, Pinkard EA, Wardlaw TJ, Glen M, Vaillancourt RE, Potts BM. 2013. A latitudinal cline in disease resistance of a host tree. Heredity 110, 372–379. ( 10.1038/hdy.2012.106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fineblum WL, Rausher MD. 1995. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377, 517–520. ( 10.1038/377517a0) [DOI] [Google Scholar]
- 98.Desprez-Loustau M-L, et al. 2016. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann. Forest Sci. 73, 45–67. ( 10.1007/s13595-015-0487-4) [DOI] [Google Scholar]
- 99.Lombardero MJ, Vázquez-Mejuto P, Ayres MP. 2008. Role of plant enemies in the forestry of indigenous vs. nonindigenous pines. Ecol. Appl. 18, 1171–1181. ( 10.1890/07-1048.1) [DOI] [PubMed] [Google Scholar]
- 100.Zas R, Moreira X, Sampedro L. 2011. Tolerance and induced resistance in a native and an exotic pine species: relevant traits for invasion ecology. J. Ecol. 99, 1316–1326. ( 10.1111/j.1365-2745.2011.01872.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on climate and Gremmeniella abietina epidemics is available on Dryad: doi:10.5061/dryad.1182k.