Abstract
Since 2006, there has been a marked increase in the number of reports of severe and often fatal fungal skin infections in wild snakes in the eastern USA. The emerging condition, referred to as snake fungal disease (SFD), was initially documented in rattlesnakes, where the infections were believed to pose a risk to the viability of affected populations. The disease is caused by Ophidiomyces ophiodiicola, a fungus recently split from a complex of fungi long referred to as the Chrysosporium anamorph of Nannizziopsis vriesii (CANV). Here we review the current state of knowledge about O. ophiodiicola and SFD. In addition, we provide original findings which demonstrate that O. ophiodiicola is widely distributed in eastern North America, has a broad host range, is the predominant cause of fungal skin infections in wild snakes and often causes mild infections in snakes emerging from hibernation. This new information, together with what is already available in the scientific literature, advances our knowledge of the cause, pathogenesis and ecology of SFD. However, additional research is necessary to elucidate the factors driving the emergence of this disease and develop strategies to mitigate its impacts.
This article is part of the themed issue ‘Tackling emerging fungal threats to animal health, food security and ecosystem resilience’.
Keywords: dermatitis, emerging disease, fungal infection, North America, Ophidiomyces ophiodiicola, snake
1. Introduction
There has been an alarming increase in the number of fungal diseases affecting wildlife populations over the last several decades [1]. Although associated primarily with opportunistic and self-limiting infections in humans, fungal diseases of wildlife have caused some of the most important conservation crises in modern times. Of particular note are global loss of amphibian diversity due to chytridiomycosis and massive population declines of some bat species due to white-nose syndrome [2–4].
Beginning in 2006, severe skin infections were reported in association with a precipitous decline in a timber rattlesnake (Crotalus horridus) population in the northeastern USA [5]. In 2008, similar infections involving a fungal aetiology emerged in Illinois, USA in an imperiled population of massasaugas (Sistrurus catenatus) [6]. This infectious disease became known as snake fungal disease (SFD), and by 2015 SFD had been documented in wild snakes throughout most of the eastern USA. With the potential to cause lethal infections and contribute to extinction of localized snake populations, SFD is a major conservation concern in North America [7]. Here we provide a literature review of SFD and include novel findings about this emerging disease.
2. Causative agent
The general descriptor ‘SFD’ was coined early in the investigation. At the time, it was unclear whether the infections shared a common aetiology or whether multiple species of fungi were involved. The initial cases of SFD implicated Chrysosporium ophiodiicola as the possible causative agent [6]. Subsequent genetic studies revealed C. ophiodiicola to be a cryptic member of the Chrysosporium anamorph of Nannizziopsis vriesii (CANV), a complex of morphologically similar fungi associated with skin infections in reptiles [8]. Phylogenetic studies of CANV fungi revealed they were paraphyletic, and this resulted in the transfer of most taxa to other genera, including reassignment of C. ophiodiicola to the monotypic genus Ophidiomyces [8]. Onygenales includes some of the most medically significant fungal pathogens of animals, including Blastomyces, Histoplasma, Coccidioides, Paracoccidioides, Microsporum and Trichophyton.
To assess association of O. ophiodiicola with cases of fungal dermatitis in a broader sampling of wild snakes, we conducted a culture-based analysis of 82 snakes from the eastern USA (electronic supplementary material, table S1). Ophidiomyces ophiodiicola was associated with skin lesions in 76% of snakes with histologically confirmed fungal dermatitis (the electronic supplementary material). This probably underestimated the true proportion of O. ophiodiicola-associated cases as fungus culture lacks sensitivity and is prone to false-negative results (the electronic supplementary material). Although other fungi undoubtedly cause sporadic skin infections in wild snakes, O. ophiodiicola is the species most consistently associated with outbreaks of dermatitis. These findings are supported by additional studies, which demonstrate a strong relationship between SFD and the presence of O. ophiodiicola [8–10].
Owing to its association with skin lesions and taxonomic relatedness to other suspected fungal pathogens, O. ophiodiicola has been given ‘honorary primary pathogen’ status in some literature [8,11]. However, such circumstantial evidence did not preclude that O. ophiodiicola was part of the normal skin flora of snakes, acting merely as a secondary pathogen. Recently, an infection trial in which red corn snakes (Pantherophis guttatus) were challenged with a pure culture of O. ophiodiicola fulfilled Koch's postulates [12], demonstrating causality between exposure to the fungus and development of SFD [13]. We use the term SFD to refer specifically to infection caused by O. ophiodiicola to avoid confusion over changing the name of a disease widely adopted by the public and scientific community. However, further criteria are needed to facilitate consistency in how SFD is diagnosed and reported.
3. Distribution and host range
Since initial reports of SFD in Illinois and the northeastern USA, O. ophiodiicola was subsequently documented in wild snakes over a much larger area of the eastern USA (the electronic supplementary material; figure 1). Additionally, O. ophiodiicola was also isolated from an eastern foxsnake (Pantherophis vulpinus) with skin lesions in Ontario, Canada (UAMH Centre for Global Microfungal Biodiversity, isolate UAMH 11863), the first detection of the fungus in a wild snake outside the USA. The lack of O. ophiodiicola records in western North America (and perhaps in other parts of the world) may be due to survey bias and lower disease prevalence or severity rather than the absence of the fungus. Projects are underway to assess the global distribution of O. ophiodiicola on wild snakes.
Figure 1.
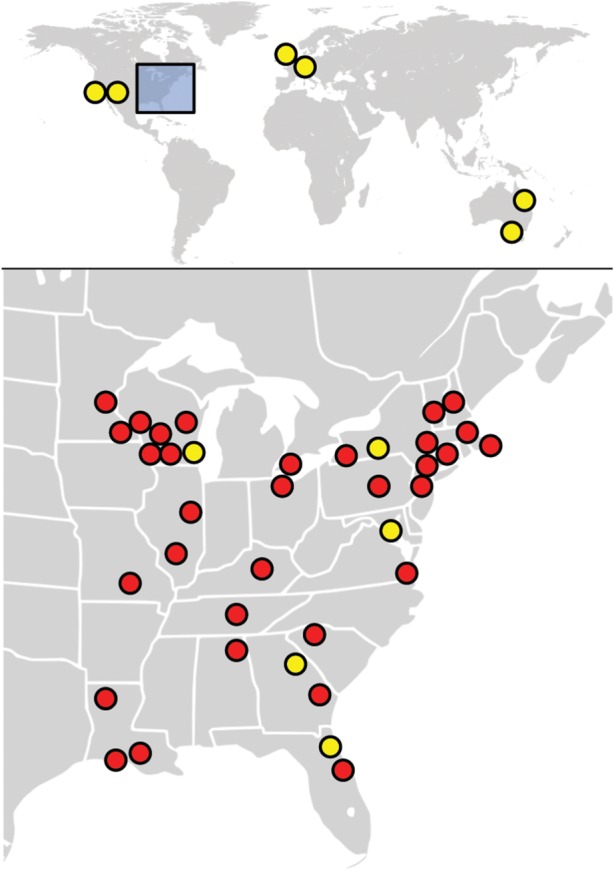
Known distribution (as of April 2016) of Ophidiomyces ophiodiicola based on recovery of fungal isolates. Yellow dots depict records from captive snakes; red dots represent isolates from wild snakes. Note that some locations in close proximity may be represented by a single dot.
The documented geographical distribution of O. ophiodiicola is broader among captive snakes than wild snakes. In the USA, isolates have been recovered from captive snakes in California, Georgia, Maryland, New Mexico, New York and Wisconsin (summarized by [8]; figure 1). Some infected snakes were originally collected from the wild [8,14,15] making it unclear where animals were exposed to the fungus. Outside North America, O. ophiodiicola has been cultured from lesions of captive snakes in the United Kingdom, Germany and Australia [8,16]. Some snakes are asymptomatic carriers of O. ophiodiicola [17,18], and possible transmission between animals within collections makes it difficult to trace where the fungus originated. To date, O. ophiodiicola has been isolated from over 30 species, representing six families of snakes (table 1). Species of all three families of snakes native to the eastern USA are vulnerable. The diversity of susceptible host species will probably broaden as this disease garners more attention.
Table 1.
Known host range of Ophidiomyces ophiodiicola as determined by recovery of isolates.
| host species | host origin | references |
|---|---|---|
| Family: Acrochordidae | ||
| Acrochordus sp., Java wart snake sp. | captive | [8] |
| Family: Boidae | ||
| Eunectes murinus, green anaconda | captive | [8] |
| Family: Colubridae | ||
| Boiga irregularis, brown treesnake | captive | [8,15] |
| Coluber constrictor, North American racer | wild | [10]; this study |
| Farancia abacura, red-bellied mudsnake | wild | this study |
| Lampropeltis nigra, eastern black kingsnake | wild | this study |
| Lampropeltis triangulum, eastern milksnake | wild | this study |
| Lampropeltis sp., milksnake sp. | captive | [8] |
| Nerodia clarkii taeniata, Atlantic saltmarsh watersnake | captivea | [8] |
| Nerodia fasciata confluens, broad-banded watersnake | wild | [19] |
| Nerodia sipedon, common watersnake | wild | [10]; this study |
| Nerodia taxispilota, brown watersnake | wild | [10] |
| Pantherophis alleghaniensis, | captivea | [14] |
| eastern ratsnake | wild | this study |
| Pantherophis guttatus, red cornsnake | captive | [8] |
| Pantherophis vupinus, eastern foxsnake | wild | this study |
| Pantherophis sp., foxsnake sp. | wild | this study |
| Pituophis catenifer sayi, bullsnake | wild | this study |
| Pituophis ruthveni, Louisiana pinesnake | wild | this study |
| Regina septemvittata, queensnake | wild | [20]; this study |
| Thamnophis sp., gartersnake sp. | captive | [8,16] |
| Thamnophis proximus, western ribbonsnake | wild | this study |
| Thamnophis radix, plains gartersnake | wild | [21] |
| Thamnophis sirtalis, common gartersnake | wild | this study |
| Virginia valeriae, smooth earthsnake | wild | this study |
| Family: Elapidae | ||
| Hoplocephalus bungaroides, broad-headed snake | captive | [8] |
| Family: Pythonidae | ||
| Python regius, ball python | captive | [8] |
| Python sebae, African rock python | captive | [8,17] |
| Family: Viperidae | ||
| Agkistrodon contortrix, copperhead | wild | this study |
| Agkistrodon piscivorus, cottonmouth | captive | this study |
| Crotalus adamanteus, eastern diamond-backed rattlesnake | not specified | [8] |
| Crotalus horridus, timber rattlesnake | wild | [9,22]; this study |
| Sistrurus catenatus, massasauga | wild | [6] |
| Sistrurus miliarius barbouri, dusky pygmy rattlesnake | wild | this study |
aCaptured from wild; may have developed SFD in captivity.
Based on initial reports, it appeared that rattlesnakes (Crotalus and Sistrurus spp.) were more prone to developing SFD—or at least developing more severe infections—than other types of snakes [5,6,9]. This assumption, however, is probably the result of more intensive monitoring and sampling of rattlesnake populations compared with other snake species. Outbreaks of severe disease in Lake Erie watersnake (Nerodia sipedon insularis) and eastern fox snake populations (the electronic supplementary material) demonstrate that other snake taxa are vulnerable. Nonetheless, host species probably vary in their susceptibility to developing life-threatening infections; however, the genetic, physiological, behavioural and ecological factors underpinning these differences have not yet been investigated.
4. Pathogenesis
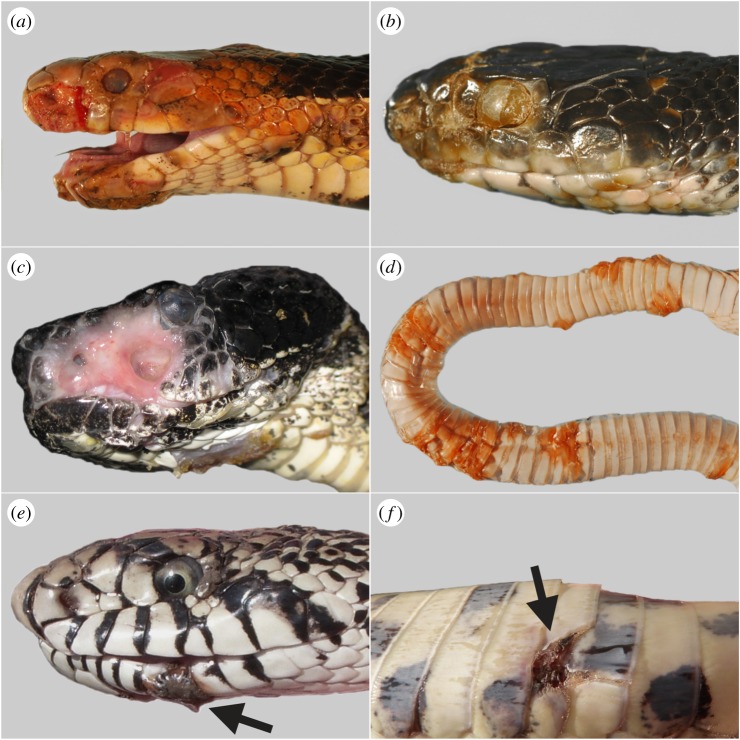
Infection by O. ophiodiicola initiates when the stratum corneum (outermost layer of skin) of a susceptible snake host is breached, permitting the fungus entry into the epidermis. Mechanical abrasion of the stratum corneum facilitates infection, although infections have been known to develop in the absence of skin scarification [13]. Integrity of the stratum corneum is frequently compromised in snakes as a result of natural abrasion or injury. Once O. ophiodiicola has breached the epidermis, the host mounts an immune response that includes oedema and recruitment of immune cells to the site of infection [13]. Within days, the infected epidermis becomes necrotic and thickened, producing the conspicuous yellow to brown crusts that are characteristic of SFD [13] (figure 2). These crusts may break off, resulting in erosion or ulceration (figure 2c). Within necrotic skin, the fungus proliferates and lesions may gradually expand in size. Wild snakes often present with several distinct lesions on various parts of the body, head or tail (electronic supplementary material, table S1). Histopathologically, fungal invasion is generally limited to the epidermis with occasional hyphae penetrating into the dermis. In severe cases, the dermis and subcutis can be more heavily infected and hyphae may invade underlying skeletal muscle. Fungi in deeper tissues are often encased within granulomas that may present clinically as nodules. Invasion of the cornea, maxillary bone and lungs have been reported [6,16,21], but disseminated infections caused by O. ophiodiicola are relatively uncommon in wild snakes, perhaps because most animals succumb to secondary disease processes prior to the fungal infection reaching such an advanced state.
Figure 2.
Snakes with Ophidiomyces ophiodiicola infections of varying severity. Severe infections include (a) eastern foxsnake (Pantherophis vulpinus) with disfigured head, (b) eastern ratsnake (P. alleghaniensis) with lesions on the eye, snout and lower jaw, (c) timber rattlesnake (Crotalus horridus) with skin ulceration and (d) Lake Erie watersnake (Nerodia sipedon insularis) with areas of thickened, necrotic skin on ventral surface. Mild infections include bullsnakes (Pituophis catenifer sayi) with small lesions on (e) the lower jaw and (f) ventral scale (arrows).
Snakes also respond to O. ophiodiicola infection by increasing moult frequency [13] (the electronic supplementary material). During a moult, necrotic tissue and fungal elements within the old epidermis are cast off, and new skin often appears clinically normal with the exception of occasional deformed scales [13] (figure 3). If the infection was limited to the superficial epidermis, moulting presumably clears the infection and the snake may recover. However, if O. ophiodiicola invades the new epidermis prior to moulting, disease may recur. Thus, a snake with SFD may need to moult several times in rapid succession to completely rid itself of the infection. Portions of the old infected epidermis sometimes adhere to the new skin, potentially facilitating reinfection [13].
Figure 3.
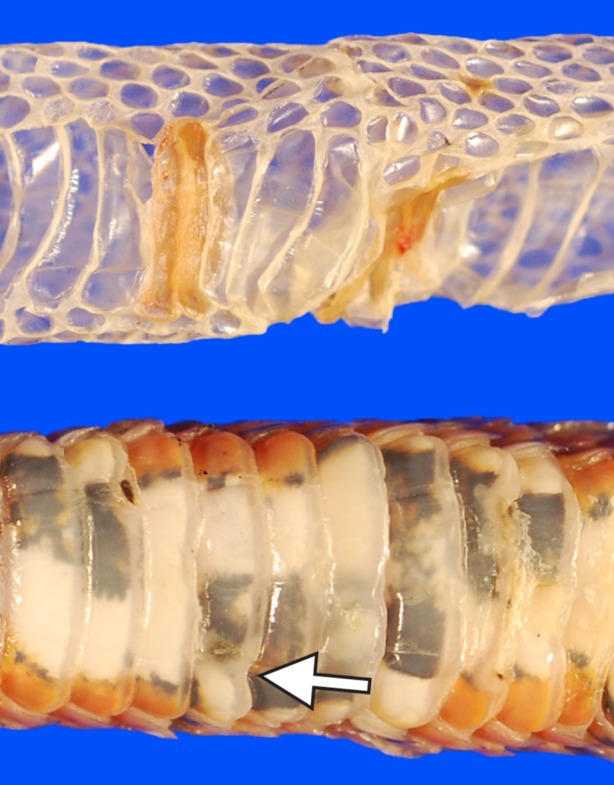
Moulting appears to be an important host response to infection by Ophidiomyces ophiodiicola. Most of the infected epidermis (seen here as thickened areas of yellow-brown skin) is cast off with the old skin (top). Post-moult, the skin at the site of a previous lesion is often grossly normal with the exception of some misshapen scales (arrow; bottom).
Severe cases of SFD frequently result in mortality [6,14–16,21]. The mechanism(s) by which death occurs is probably multifactorial. Disseminated infections in the lungs may result in tissue damage sufficient to kill the host [16,21]. However, evidence suggests SFD is a chronic disease in which many wild snakes die from complications of the infection rather than from direct fungal damage. Infections of the head that affect vision, olfaction and infrared sensing (in the case of pit vipers) probably impact the ability to procure food. Indeed, anorexia has been observed in experimentally infected captive snakes, and emaciation is a common finding in wild snakes with SFD [13] (electronic supplementary material, table S1). As an infected snake's health declines, the animal may be subject to opportunistic infections or other secondary disease processes. Although primary infection by O. ophiodiicola may initiate a chain of events that lead to death, it is also plausible that in some instances fungal colonization of tissues occurs because the host's health is compromised.
SFD may predispose snakes to additional forms of mortality by eliciting ‘risky’ behaviours. In the laboratory, experimentally infected captive-bred snakes were more likely to be observed resting in conspicuous areas (as opposed to under provided shelters), and wild snakes with SFD have been found basking at times of year when the animals would normally be hibernating [9,13]. These snakes were exhibiting behaviours that maintain a body temperature conducive to fighting infection, but that also increase their vulnerability to mortality from predation or exposure. For example, several snake carcasses found near an infected hibernaculum were thought to have prematurely emerged from the den site and succumbed to night-time frosts (the electronic supplementary material). Additional research focused on the relationship between host body temperature and SFD progression may explain why infected snakes exhibit unusual behaviours and whether such ‘sickness behaviours’ are effective in combatting infection as has been proposed in other animals [23].
Mild cases of SFD are frequently observed in snakes shortly after emergence from hibernation (the electronic supplementary material; figure 2e,f). The hibernation period may predispose snakes to infection by increasing exposure to or transmission of O. ophiodiicola (i.e. large numbers of snakes concentrated in small areas) and reducing host defences. Although growth of O. ophiodiicola is significantly decreased at the low temperatures at which snakes hibernate in the northern USA, reptile immune function and moulting frequency are also depressed at cooler temperatures (reviewed by [24,25]). Most snakes that developed SFD after being brought out of hibernation at a rehabilitation facility seemingly cleared superficial infections within weeks (the electronic supplementary material). Recurrence of SFD in some individuals in autumn (the electronic supplementary material) further suggests that hibernation-induced physiological changes may be important for infection. However, hibernation is not essential for disease development; infections by O. ophiodiicola have been documented in captive snakes that were presumably not subjected to a cooling period [8,14–16] and in wild snakes from warmer climates where activity occurs year round [19,26]. Studies are underway to investigate O. ophiodiicola infection dynamics, temporal patterns and disease remission and progression in various geographical areas.
Fungi within Onygenales show significant genomic adaptations for using animal substrates [27]. The virulence factors possessed by O. ophiodiicola have not been fully characterized, but Allender et al. [11] demonstrated that O. ophiodiicola has gelatinase, β-glucosidase, lipase, esterase, urease and keratinase activities in vitro. Although these enzymes probably play a role in saprotrophic growth, some may also contribute to pathogenicity. For example, gelatinase, keratinase and lipase activities could facilitate infection of skin through breakdown of collagen, keratin and lipids, respectively. Generation of toxic ammonia by ureases could result in host tissue death (reviewed by [28]). Whether the extensive epidermal necrosis observed in SFD is caused directly by O. ophiodiicola or is the result of ‘collateral damage’ from the host's own immune response requires further investigation.
5. Cause of emergence
Fungal diseases in wild plants and animals typically emerge following introduction of an exotic pathogen to an area with naive hosts [1,29]. Examination of historical fungal isolates demonstrated that O. ophiodiicola was present in captive snakes in the eastern USA since at least 1986 [8]. No wild snake isolates are known prior to 2008; thus introduction by spillover of O. ophiodiicola from captive to wild snake populations represented a plausible explanation for the sudden emergence of SFD. Alternatively, the lack of verified detections of O. ophiodiicola may have resulted from insufficient health monitoring in wild snake populations and technical limitations related to O. ophiodiicola isolation and identification. For example, Cheatwood et al. [30] reported Geotrichum candidum (a fungus that is often confused with O. ophiodiicola based on morphology [8]) as the possible cause of fungal dermatitis in pygmy rattlesnakes (Sistrurus miliarius) in Florida in the mid 1990's. In 2012, we resampled snakes with lesions from the same Florida population and found that O. ophiodiicola (not Geotrichum) was associated with the infections. If the initial identification of the causative agent was in error, O. ophiodiicola may have occurred in wild snakes over a decade earlier than previously reported.
Historical observations of skin disease in wild snakes further challenge the hypothesis that O. ophiodiicola was recently introduced to eastern North America. Cases of dermatitis are often referred to as ‘hibernation blisters’ or ‘hibernation sores’ by field biologists because lesions are most often seen as snakes emerge from hibernation. Observations of such skin lesions have been reported for decades although the aetiologies were only rarely explored [31–35]. We investigated the prevalence of skin lesions compatible with ‘hibernation sores’ and found that 41% of wild snakes at our study sites had signs of dermatitis post-emergence from hibernation (the electronic supplementary material). We collected samples from a subset of these affected snakes and detected O. ophiodiicola from lesions in 74% of the animals tested; furthermore, histopathologic findings were consistent with SFD (the electronic supplementary material). Similarly, snakes examined from Virginia had a 38% prevalence of gross skin lesions, most of which were mild despite being associated with O. ophiodiicola [10]. Although O. ophiodiicola cannot be definitively implicated as the cause of disease in older reports of ‘hibernation sores,’ these findings raise the possibility that the fungus could have been present in North America prior to recent reports of severe disease. Furthermore, cases of SFD do not exhibit a systematic dispersal pattern on the landscape typical of an introduced pathogen [29]. Specifically, the sequence of documented cases in wild snakes appears random with successive cases sometimes occurring 500–1000 km away from one another. That cases lack an obvious pattern of spread may further support that O. ophiodiicola is not a recent arrival to the eastern USA. However, lack of an organized surveillance plan immediately following the first description of SFD and possible bias in disease monitoring complicate interpretation of existing data.
The alternative to the ‘introduced pathogen hypothesis’ is that O. ophiodiicola has been present in North America for a long time, and recent environmental changes are driving SFD emergence. The 2006 outbreak of severe dermatitis (now thought to have been SFD) in a timber rattlesnake population in the northeastern USA was associated with extremely wet conditions [5]. Ophidiomyces ophiodiicola is thought to be able to survive in the environment without a host [11], and moist conditions could play an important role in disease by promoting fungal growth and persistence in the environment. In addition, precipitation and cloud cover could negatively impact host thermoregulation mechanisms for fighting infection. Hibernation appears to be important in SFD, and slight temperature increases during the hibernation season (resulting from climate change) may allow O. ophiodiicola to grow at a faster rate [11] and establish more severe infections. However, elucidating the role of climate in SFD dynamics is challenging. Host species vary in their tolerance to different environmental conditions, and thus one specific set of climatic parameters may not facilitate emergence of disease across host species and locations. For example, whereas unusually cool and wet weather may increase the prevalence and severity of SFD, so too might hot dry conditions that force snakes to spend more time underground (where the microclimate is humid and environmental reservoirs of O. ophiodiicola are likely to be higher). For these reasons, examining the role of climate in SFD emergence must consider changes at multiple scales, including microclimates available to, and used by, snakes at a given site.
Documented outbreaks of severe disease have typically occurred in relatively small or isolated snake populations. Such imperiled populations are more likely to be monitored, which may explain this trend. However, it is also plausible that factors associated with small population size contribute to disease. Suspected infections by O. ophiodiicola were thought to act in concert with habitat destruction and inbreeding depression to cause the decline of a timber rattlesnake population in New Hampshire, USA [5]. In that study, the authors speculated that loss of genetic diversity may have resulted in increased disease susceptibility. Furthermore, habitat degradation and fragmentation could influence SFD dynamics by limiting microclimates, such as suitable basking sites, necessary for snakes to fend off fungal infections, or by forcing snakes to congregate in common hibernacula or other areas contaminated with large amounts of the pathogen. Habitat degradation could additionally limit prey abundance or facilitate other disease processes that diminish overall health of snakes and exacerbate the effects of SFD.
6. Conservation implications
Emergence of SFD in eastern North America has raised concerns about the viability of some imperiled snake populations. The suspected SFD outbreak in New Hampshire resulted in a more than 50% decline in an affected population of timber rattlesnakes within 1 year [5]. In Illinois, severe cases of SFD have caused mortality within an endangered population of massasaugas [6]. The United States Fish and Wildlife Service has proposed listing eastern populations of the massasauga as a threatened species under the Endangered Species Act, citing SFD as a potential threat to the species' survival [36]. In 2009, a mortality event in Lake Erie watersnakes was linked to O. ophiodiicola infections. Although the Lake Erie watersnake population had generally been increasing since 2001, it declined by an estimated 18% in the year following the outbreak [37]. The Lake Erie watersnake was removed from the Threatened Species List in 2011, but with a population of around 10 000 individuals and a geographically restricted distribution [37], the subspecies may be at risk if outbreaks of SFD increase in frequency and severity.
Impacts due to SFD are variable and not all snake populations are thought to decline as a result of the disease. Namely, declines are not suspected in snake populations in Minnesota or Virginia where infections by O. ophiodiicola are frequently mild [10,22]. Nevertheless, baseline data on population health prior to a documented outbreak are rare, and qualitative reports of snake population stability should be interpreted cautiously. Multiple factors influence population impacts of disease, including the life history of the host. Timber rattlesnakes in the northeastern USA, for example, have a low reproductive output [38]. In addition to infected animals that are lost directly to disease, chronic infections by O. ophiodiicola may also affect host fitness and reproduction [9]. Thus, the ability of northern timber rattlesnake populations to recover from outbreaks of severe SFD is probably more limited than for rapidly maturing snake species that produce young on an annual basis.
There are currently few options for managing SFD. To date, most efforts have focused on rehabilitating individual snakes. Such a strategy is impractical for many snake populations because it can be difficult to locate the majority of individuals within the population, is resource intensive, and fails to prevent reinfection. However, for highly endangered populations where the survival of each individual snake is vital, rehabilitation might represent a feasible option. Although treatments with antifungal agents have thus far not had clear results [6,9,39], supportive care alone may facilitate recovery (the electronic supplementary material). As temporary resolution of clinical signs may not mean that infection has been eliminated, caution should be exercised to ensure that infections have been completely cleared prior to releasing animals. From a preventive standpoint, individuals handling wild snakes should observe appropriate biosecurity procedures, including frequent disinfection of hands, tools and working surfaces, and dedication of gear and work spaces for wild versus captive reptiles. Future elucidation of environmental contributors to SFD may provide additional disease control strategies by informing habitat management actions that limit disease prevalence and progression.
7. Conclusion
Emerging fungal diseases pose a significant threat to wildlife health. Fungi, more so than other pathogens, possess characteristics that make them capable of causing massive population declines and extinction of their hosts [1]. Ophidiomyces ophiodiicola has many traits of a well-adapted pathogen, including a broad host range and the ability to survive in the environment. As a result, SFD poses a major challenge for snake conservation. The focus thus far has been impacts on imperiled snake populations in the eastern USA, and the threat to snakes on a global scale has yet to be assessed. Snake populations are declining worldwide, and although the declines are multifaceted [40], the role of disease may be overlooked in species where available data on health or long-term population trends are lacking. Snakes provide substantial economic benefits and play critical roles in ecosystems, preying on animals that destroy agricultural crops and carry zoonotic disease as well as serving as an important food base for many other species of vertebrates [41]. Future studies to quantify the benefits of snakes, as has been done for other previously maligned wildlife such as bats [42], will be essential in gauging the impacts that the loss of snakes could have and will also help the public to understand the importance of protecting these reptiles from emerging threats such as SFD.
Recent attention to SFD demonstrates a growing interest in conserving snakes. While this review describes progress that has been made in understanding SFD in a relatively short period of time, much remains to be learned regarding pathogen virulence mechanisms, host susceptibility, population-level impacts and role of the environment in infection dynamics. Studies are currently underway to address many of these outstanding questions, including the mechanism by which SFD is emerging. Such information will assist with predicting which snake populations are most at risk and facilitate development of management strategies aimed at mitigating disease impacts.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We acknowledge NWHC, Southeastern Cooperative Wildlife Disease Study, Cornell University Animal Health Diagnostic Center and University of Florida Veterinary Medical Center personnel for providing expertise in disease diagnostics, pathology and epidemiology related to SFD. We are grateful to numerous state and federal biologists and wildlife health staff for collecting data, samples and sharing their observations from the field. We thank James Condon and Kevin McCurley for their efforts to bring public attention to this disease early in the investigation. We credit D.E. Green (NWHC) for the photographs used in figure 2b,d and Stephanie Steinfeldt (NWHC) for the photograph used in figure 2a. Original maps modified for figure 1 are attributed to Crates (world map) and Alan Rockefeller (North American map), are available on Wikimedia Commons and are used under the terms of the GNU Free Documentation Licence. Additional acknowledgements related to the novel data referenced in this manuscript can be found in the electronic supplementary material.
Data accessibility
Novel data described in this article are available in the electronic supplementary material or in machine readable format at http://dx.doi.org/10.5066/F7Z31WRB. Use the following citation for the dataset: Lorch JM et al. Snake dermatitis data. USGS data release. 2016. http://dx.doi.org/10.5066/F7Z31WRB.
Authors' contributions
A.E.B., D.S.B. and J.M.L. coordinated with field personnel and helped procure the majority of samples and data used in this study. S.K. and J.S.L. performed necropsies and contributed to the understanding of SFD pathogenesis. J.M.L. and K.Z.S. conducted fungus culture and molecular analyses. J.L.E., J.M.K., J.M.L., R.A.S. and E.R.W. collected samples and field data on disease prevalence. K.M. collected and summarized data on disease progression in snakes being rehabilitated. D.B., T.M.F., B.M.G., L.A.L., S.J.P., K.L.S., C.E.S., J.F.X.W. provided additional samples, data and observations for various analyses. J.M.L. analysed novel data presented in this manuscript and wrote the initial draft. All authors provided substantial edits to the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was funded by the US Geological Survey, the US Fish and Wildlife Service, the Minnesota Department of Natural Resources and the Wisconsin Department of Natural Resources—Endangered Resources Fund.
Disclaimer
The use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US government.
References
- 1.Fisher MC, Henk DA, Briggs CJ, Brownstein LC, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031–9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blehert DS, et al. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227 ( 10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 4.Turner GG, Reeder DM, Coleman JTH. 2011. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Res. News 52, 13–27. [Google Scholar]
- 5.Clark RW, Marchand MN, Clifford BJ, Stechert R, Stephens S. 2011. Decline of an isolated timber rattlesnake (Crotalus horridus) population: interactions between climate change, disease, and loss of genetic diversity. Biol. Conserv. 144, 886–891. ( 10.1016/j.biocon.2010.12.001) [DOI] [Google Scholar]
- 6.Allender MC, Dreslik M, Wylie S, Phillips C, Wylie DB, Maddox C, Delaney MA, Kinsel MJ. 2011. Chrysosporium sp. infection in eastern massasauga rattlesnakes. Emerg. Infect. Dis. 17, 2383–2384. ( 10.3201/eid1712.110240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland WJ, et al. 2014. A horizon scan of global conservation issues for 2014. Trends Ecol. Evol. 29, 15–22. ( 10.1016/j.tree.2013.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigler L, Hambleton S, Paré JA. 2013. Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J. Clin. Microbiol. 51, 3338–3357. ( 10.1128/JCM.01465-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride MP, Wojick KB, Georoff TA, Kimbro J, Garner MM, Wang X, Childress AL, Wellehan JFX Jr. 2015. Ophidiomyces ophiodiicola dermatitis in eight free-ranging timber rattlesnakes (Crotalus horridus) from Massachusetts. J. Zoo Wildl. Med. 46, 86–94. ( 10.1638/2012-0248R2.1) [DOI] [PubMed] [Google Scholar]
- 10.Guthrie AL, Knowles S, Ballmann AE, Lorch JM. 2016. Detection of snake fungal disease due to Ophidiomyces ophiodiicola in Virginia, USA. J. Wildl. Dis. 52, 143–149. ( 10.7589/2015-04-093.1) [DOI] [PubMed] [Google Scholar]
- 11.Allender MC, Raudabaugh DB, Gleason FH, Miller AN. 2015. The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecol. 17, 187–196. ( 10.1016/j.funeco.2015.05.003) [DOI] [Google Scholar]
- 12.Koch R. 1884. Die Aetiologie der Tuberkulosa. Mitt Kaiser Gesundh 2, 1–88. [Google Scholar]
- 13.Lorch JM, Lankton J, Werner K, Falendysz EA, McCurley K, Blehert DS. 2015. Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. mBio 6, e01534-15. ( 10.1128/mBio.01534-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajeev S, et al. 2009. Isolation and characterization of a new fungal species, Chrysosporium ophiodiicola, from a mycotic granuloma of a black rat snake (Elaphe obsoleta obsoleta). J. Clin. Microbiol. 47, 1264–1268. ( 10.1128/JCM.01751-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols DK, Weyant RS, Lamirande EW, Sigler L, Mason RT. 1999. Fatal mycotic dermatitis in captive brown tree snakes (Boiga irregularis). J. Zoo Wildl. Med. 30, 111–118. [PubMed] [Google Scholar]
- 16.Vissiennon T, Schüppel K-F, Ullrich E, Kuijpers AFA. 1999. Case report. A disseminated infection due to Chrysosporium queenslandicum in a garter snake (Thamnophis). Mycoses 42, 107–110. ( 10.1046/j.1439-0507.1999.00409.x) [DOI] [PubMed] [Google Scholar]
- 17.Paré JA, Sigler L, Rypien KL, Gibas CC. 2003. Cutaneous mycobiota of captive squamate reptiles with notes on the scarcity of Chrysosporium anamorph of Nannizziopsis vriesii. J. Herpetol. Med. Surg. 13, 10–15. [Google Scholar]
- 18.Bohuski E, Lorch JM, Griffin KM, Blehert DS. 2015. TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease. BMC Vet. Res. 11, 95 ( 10.1186/s12917-015-0407-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glorioso BM, Waddle JH, Green DE, Lorch JM. 2016. First documented case of snake fungal disease in a free-ranging wild snake in Louisiana. Southeast Nat. 15, N4–N6. ( 10.1656/058.015.0111) [DOI] [Google Scholar]
- 20.Price SJ, Oldham CR, Boys WM, Fleckenstein LJ. 2015. First record of snake fungal disease in Kentucky. J. Ky. Acad. Sci. 76, in press. [Google Scholar]
- 21.Dolinski AC, Allender MC, Hsiao V, Maddox CW. 2014. Systemic Ophidiomyces ophiodiicola infection in a free-ranging plains garter snake (Thamnophis radix). J. Herpetol. Med. Surg. 24, 7–10. ( 10.5818/1529-9651-24.1.7) [DOI] [Google Scholar]
- 22.Smith CE, Edwards J, Lorch JM. 2013. Crotalus horridus (Timber Rattlesnake), fungal pathogens. Herpetol. Rev. 44, 519–520. [Google Scholar]
- 23.Shakhar K, Shakhar G. 2015. Why do we feel sick when infected—can altruism play a role? PLoS Biol. 13, e1002276 ( 10.1371/journal.pbio.1002276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman LM, Vogel LA, Bowden RM. 2009. Understanding the vertebrate immune system: insights from the reptilian perspective. J. Exp. Biol. 213, 661–671. ( 10.1242/jeb.038315) [DOI] [PubMed] [Google Scholar]
- 25.Semlitsch RD. 1979. The influence of temperature on ecdysis rates in snakes (genus Natrix) (Reptilia, Serpentes, Colubridae). J. Herpetol. 13, 212–214. ( 10.2307/1563932) [DOI] [Google Scholar]
- 26.May PG et al. 1996. The seasonal abundance and activity of a rattlesnake (Sistrurus miliarius barbouri) in central Florida. Copeia 1996, 389–400. ( 10.2307/1446855) [DOI] [Google Scholar]
- 27.Desjardins CA, et al. 2011. Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet. 7, e1002345 ( 10.1371/journal.pgen.1002345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutherford JC. 2014. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 10, e1004062 ( 10.1371/journal.ppat.1004062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rachowicz LA, Hero J-M, Alford RA, Taylor JW, Morgan JAT, Vredenburg VT, Collins JP, Briggs CJ. 2005. The novel and endemic pathogen hypotheses: competing explanations for the origin of emerging infectious diseases of wildlife. Conserv. Biol. 19, 1441–1448. ( 10.1111/j.1523-1739.2005.00255.x) [DOI] [Google Scholar]
- 30.Cheatwood JL, Jacobson ER, May PG, Farrell TM, Homer BL, Samuelson DA, Kimbrough JW. 2003. An outbreak of fungal dermatitis and stomatitis in a free-ranging population of pigmy rattlesnakes (Sistrurus miliarius barbouri) in Florida. J. Wildl. Dis. 39, 329–337. ( 10.7589/0090-3558-39.2.329) [DOI] [PubMed] [Google Scholar]
- 31.Page LA. 1966. Diseases and infections of snakes: a review. Bull. Wildl. Dis. Assoc. 2, 111–126. ( 10.7589/0090-3558-2.4.111) [DOI] [Google Scholar]
- 32.Fitch HS. 1963. Natural history of the black rat snake (Elaphe o. obsoleta) in Kansas. Copeia 1963, 649–658. ( 10.2307/1440967) [DOI] [Google Scholar]
- 33.Branson BA, Baker EC. 1974. An ecological study of the queen snake, Regina septemvittata (Say) in Kentucky. Tulane Stud. Zool. Bot. 18, 153–171. [Google Scholar]
- 34.Jacobson ER. 1980. Necrotizing mycotic dermatitis in snakes: clinical and pathologic features. J. Am. Vet. Med. Assoc. 177, 838–841. [PubMed] [Google Scholar]
- 35.Reinert HK, Rupert RR Jr. 1999. Impacts of translocation on behavior and survival of timber rattlesnakes, Crotalus horridus. J. Herpetol. 33, 45–61. ( 10.2307/1565542) [DOI] [Google Scholar]
- 36.Endangered and Threatened Wildlife and Plants; Threatened Species Status for the Eastern Massasauga Rattlesnake, 80 FR 58688. Federal Register 80, 58688–58701 (September 30, 2015).
- 37.Endangered and Threatened Wildlife and Plants; Removal of the Lake Erie Watersnake (Nerodia sipedon insularum) From the Federal List of Endangered and Threatened Wildlife, 76 FR 50680. Federal Register 78, 50680–50702 (August 16, 2011).
- 38.Brown WS. 1991. Female reproductive ecology in a northern population of the timber rattlesnake, Crotalus horridus. Herpetologica 47, 101–115. [Google Scholar]
- 39.Tetzlaff S, Allender M, Ravesi M, Smith J, Kingsbury B. 2015. First report of snake fungal disease from Michigan, USA involving massasaugas, Sistrurus catenatus (Rafinesque 1818). Herpetol. Notes 8, 31–33. [Google Scholar]
- 40.Reading CJ, et al. 2010. Are snake populations in widespread decline? Biol. Lett. 6, 777–780. ( 10.1098/rsbl.2010.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullin SJ, Seigel RA. 2009. Snakes: ecology and conservation. Ithaca, NY: Cornell University Press. [Google Scholar]
- 42.Boyles JG, Cryan PM, McCracken GF, Kunz TH. 2011. Economic importance of bats in agriculture. Science 332, 41–42. ( 10.1126/science.1201366) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Novel data described in this article are available in the electronic supplementary material or in machine readable format at http://dx.doi.org/10.5066/F7Z31WRB. Use the following citation for the dataset: Lorch JM et al. Snake dermatitis data. USGS data release. 2016. http://dx.doi.org/10.5066/F7Z31WRB.