Abstract
Aspergillus fungi are the cause of an array of diseases affecting humans, animals and plants. The triazole antifungal agents itraconazole, voriconazole, isavuconazole and posaconazole are treatment options against diseases caused by Aspergillus. However, resistance to azoles has recently emerged as a new therapeutic challenge in six continents. Although de novo azole resistance occurs occasionally in patients during azole therapy, the main burden is the aquisition of resistance through the environment. In this setting, the evolution of resistance is attributed to the widespread use of azole-based fungicides. Although ubiquitously distributed, A. fumigatus is not a phytopathogen. However, agricultural fungicides deployed against plant pathogenic moulds such as Fusarium, Mycospaerella and A. flavus also show activity against A. fumigatus in the environment and exposure of non-target fungi is inevitable. Further, similarity in molecule structure between azole fungicides and antifungal drugs results in cross-resistance of A. fumigatus to medical azoles. Clinical studies have shown that two-thirds of patients with azole-resistant infections had no previous history of azole therapy and high mortality rates between 50% and 100% are reported in azole-resistant invasive aspergillosis. The resistance phenotype is associated with key mutations in the cyp51A gene, including TR34/L98H, TR53 and TR46/Y121F/T289A resistance mechanisms. Early detection of resistance is of paramount importance and if demonstrated, either with susceptibility testing or through molecular analysis, azole monotherapy should be avoided. Liposomal amphotericin B or a combination of voriconazole and an echinocandin are recomended for azole-resistant aspergillosis.
This article is part of the themed issue ‘Tackling emerging fungal threats to animal health, food security and ecosystem resilience’.
Keywords: Aspergillus fumigatus, azole resistance, worldwide emergence, fungicides, epidemiology, treatment
1. Introduction
Aspergillus fungi cause a spectrum of clinical diseases in humans and animals. The disease spectrum ranges from colonization, allergic conditions such as allergic bronchopulmonary aspergillosis (ABPA) to invasive disease. ABPA is primarily seen in patients who manifest with chronic Aspergillus colonization, such as patients with cystic fibrosis and chronic granulomatous disease. Aspergillus can colonize a pre-existing cavity in the lung, such as occurs post-tuberculosis, which is then referred to as an aspergilloma. An aspergilloma can be asymptomatic; however, aspergilloma generally gives rise to persistent symptoms and lung haemorrhage. Patients with lung diseases such as chronic obstructive pulmonary disease (COPD) can develop chronic pulmonary aspergillosis (CPA), when the fungus becomes locally invasive with tissue damage [1]. This results in chronic inflammation, which can result in the occurrence of more cavities, or ultimately pulmonary fibrosis. At the other end of the spectrum is acute invasive aspergillosis (IA) primarily observed in patients with haematological malignancies such as acute myeloid leukemia (AML), and in patients who have undergone solid organ transplantation [2]. The disease is also increasingly diagnosed in critically ill patients with COPD and/or severe influenza [3,4].
2. Treatment of disease caused by Aspergillus infections
In the past 15 years, azoles have gained an important role in the prevention and treatment of Aspergillus diseases [5]. The azole antifungals target ergosterol that is present in the fungal cell membrane. This essential component is synthesized from lanosterol by removing the methyl group at C14, and is catalysed by the enzyme alpha-demethylase [6]. This enzyme, a member of the cytochrome P450 family, is encoded by the cyp51A gene, a coding region of 2 048 base pairs found on chromosome 4. Azoles inhibit the biosynthesis pathway for ergosterol, thereby interfering with the integrity of the fungal cell membrane [6]. Azole resistance can be an intrinsic phenotype, as it is known to occur in cryptic Aspergillus species related to A. fumigatus, specifically A. lentulus and A. pseudofischeri [7] whereas wild-type A. fumigatus and A. flavus are sensitive to these drugs. However, azole resistance is an acquired trait that occurs after azole exposure during medical treatment, or after fungicide exposure in the field. There are multiple mechanisms of acquired resistance to azoles, with the most common mutations that are involved being located in the cyp51A gene [8–10].
Primarily, itraconazole is used in the treatment of CPA [1], while voriconazole is used as first-line therapy of IA [5]. Recently, another azole, isavuconazole, was approved for the primary treatment of IA [11]. Posaconazole is indicated as prophylaxis in high-risk patients such as AML and stem cell transplant patients with graft-versus-host-disease [12]. Itraconazole, voriconazole, posaconazole and isavuconazole are available as oral and intravenous formulations, and azoles are the only class of antifungals available as oral options for treatment of Aspergillus diseases. Another class of compounds, the echinocandins, are also active against Aspergillus and have been used as salvage therapy [13,14]. This class has three registered drugs (caspofungin, anidulafungin and micafungin), which inhibit the synthesis of the fungal cell wall, a structure which is absent in human cells. The echinocandins have minimal side effects, but the effectiveness against Aspergillus is limited due to the fungistatic nature of the drug (fungistasis may be the net result of some cidal and some resistant growth). Clinical studies have shown that echinocandins are effective in patients with CPA [1], but they seem to be less effective as primary treatment of neutropenic patients with IA [15]. However, the echinocandins are suitable in combination with an azole for primary treatment against infections caused by Aspergillus [16].
3. Resistance to azoles
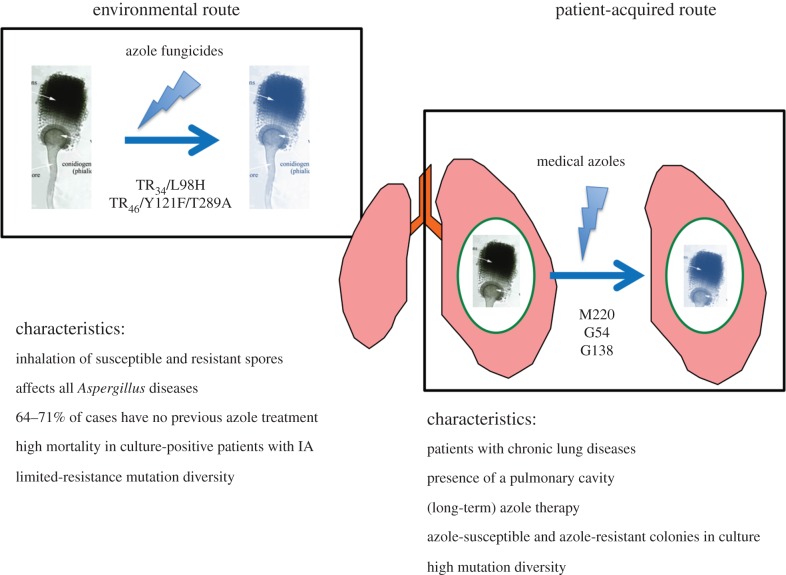
In retrospect, the first azole-resistant A. fumigatus isolates date back to the late 1980s on the West Coast of the USA in California, and were cultured from two patients treated with itraconazole [17]. Later, a Dutch study reported three itraconazole-resistant A. fumigatus recovered in 1997 from a lung transplant recipient after long-term itraconazole treatment [18]. Following these reports, a French study found four itraconazole-resistant isolates in 1999 [19]. Subsequently in 2007, a comprehensive series of nine cases of azole-resistant IA showed that four out of nine patients had previously never received azole therapy [20]. Further, the resistant A. fumigatus isolates exhibited the same resistance mechanism as that found in environmental and airborne isolates [21]. Remarkably, the genetic relatedness of European clinical and environmental azole-resistant A. fumigatus isolates measured using a panel of highly polymorphic genetic markers showed a close relationship, and it was hypothesized that these patients had acquired azole-resistant A. fumigatus strains from the environment [22]. This indicated that a second route for the induction of resistance had developed through a fungicide-driven route, due to exposure and selection of the fungus to azoles in the environment [8]. The characteristics of these two routes was clearly established in the last decade [23,24] (figure 1).
Figure 1.
Characteristics of azole-resistant Aspergillus infections by the environmental and patient-acquired route.
While a spectrum of resistance mechanisms to azoles has been characterized in A. fumigatus [25,26], azole resistance is frequently the result of mutations in the cyp51A gene. Many azole-resistant isolates have non-synonymous point mutations at codons in this gene, for example at positions G54, M220 and G138 [9], which are primarily found in patients who have been treated for long periods with azoles [24]. These point mutations induce changes in channels giving the azole access to a heme molecule to which the azole binds [27,28]. Protein models of lanosterol 14α-demethylase indicate that these mutations change the shape of the channel so that the azole molecule can no longer bind [27,28]. Many of these mutations result in resistance to multiple, if not all, anti-Aspergillus triazoles [24,29]. Patients harbouring azole-resistant A. fumigatus isolates with point mutations have primarily been observed in lung cavities, such as aspergilloma. Chronic cavitary lung diseases have been hypothesized as a risk factor for resistance development [30] because asexual sporulation, which takes place in a fungal lung cavity, could potentially facilitate the formation of mutations [31,32]. Within such patients, genotypically different colonies of A. fumigatus may grow that manifest varied resistance mutations and in vitro susceptibility tests suggest that multiple colonies should be investigated when exploring in vivo resistance [33,34]. It needs to be emphasized that, in comparision to the in-host development of azole resistance, the A. fumigatus strains from the environment have, in addition to the mutations in the cyp51A gene, a tandom repeat (TR) duplication in the promoter region. The presence of the TR repeat has been shown to drive overexpression of cyp51A by increasing the binding activity of the sterol regulatory element binding protein resulting in increased tolerance of azoles [35]. To date, three resistance mechanisms involving promotor duplications (TR34/L98H, TR53 and TR46/Y121F/T289A) and single mutations (G54 and M220) have been found in A. fumigatus isolates from soil and air samples and in clinical specimens of patients (table 1) [24,60–62].
Table 1.
Common resistance mechanisms reported in the cyp51A gene of clinical and environmental Aspergillus fumigatus.
| promotor tandem repeat (TR) insertion and point mutation | country | medical azoles affected | reference |
|---|---|---|---|
| TR34/L98H | The Netherlands, UK, Germany, Belgium, France, Spain, Italy, Austria, Denmark, Poland, Ireland China, USA, India, Japan, Colombia, Taiwan, Turkey, Kuwait, Iran, Pakistan, Australia, Tanzania, Romania | itraconazole, voriconazole, posaconazole, isavuconazole | [24,36–54] |
| TR46/Y121F/T289A | The Netherlands, UK, Germany, Belgium, France, Spain, Denmark, Ireland, China, USA, India, Japan, Colombia, Tanzania | itraconazole (variable), voriconazole, posaconazole (variable), isavuconazole | [24,36,37,41,54–58] |
| TR53 | The Netherlands, Colombia | itraconazole, voriconazole, posaconazole, isavuconazole | [24,58] |
| G54 | The Netherlands, UK, Germany, France, Spain, India, Romania, China, USA, Tanzania | itraconazole, posaconazole | [24] |
| M220 | The Netherlands, UK, Germany, Belgium, France, Spain, China, USA | itraconazole, posaconazole | [24] |
| Y121F | France | voriconazole | [59] |
Surveillance by University Medical Centers (UMCs) in The Netherlands increasingly show that the environmental route of infection is an important source for azole-resistant aspergillosis owing to the fact that between 80 and 90% of clinical drug-resistant strains contain one of the above mechanisms [63]. In addition to the cyp51A-mediated resistance mechanisms, about 10% of the resistant strains found in nature have no mutations in the cyp51A gene. It is highly likely that induced azole efflux mechanism is also involved in non-cyp51A-mediated resistance [26]. There are potentially (many) other, undiscovered, mechanisms that lead to azole resistance such as seen in the human fungal pathogen C. albicans where induced aneuploidy and isochromosome formations can occur [64]. One mechanism has been recently described in a patient with aspergillosis on long-term azole treatment, namely a P88 L substitution in HapE, but this mechanism currently appears to be rare and has not yet been found in the environment [65]. Although resistance to antifungals is expected to be accompanied by a ‘fitness cost’, it appears that for the cyp51A-mediated resistance, fitness is not affected and the virulence of resistant isolates is similar to that of wild-type strains [66,67].
4. Population structure of azole-resistant Aspergillus fumigatus
Aspergillus fumigatus is one of the most frequently occurring eukaryotes on the planet owing to its high prevalence as a saphrophyte on decaying plant matter; its airborne conidia are among the most numerous eukaryotes in air and soil samples [68]. The highly aerosolized and dispersive character of A. fumigatus has led to the hypothesis that it is a truly ‘globalized’ organism. Identification of the sexual cycle further suggested that this species may be a panmictic (globally unstructured and randomly interbreeding) organism [69]. The implication of being a cosmopolitan sexual organism is that there are little or no barriers to gene flow; the corollary being that a unique allele (such as TR34/L98H), that has a selective advantage over wild-type alleles, can theoretically penetrate all genetic backgrounds. If true, then this means that azole-resistance alleles that carry a selective advantage in nature have an essentially unlimited potential to disperse in space. A previous study by Rydholm et al. [70], analysing the genetic structure of A. fumigatus, suggested that A. fumigatus is cosmopolitan and randomly breeding across a global scale. A subsequent study, while confirming these aspects of the population genetic structure of A. fumigatus, also found evidence of a genetic barrier to gene flow, suggesting A. fumigatus contained two (or perhaps more) cryptic and morphologically identical species [68]. More recent studies have confirmed the occurrence of a separate sexual species, A. lentulus [71] and have hinted at the existence of further genetic diversity within A. fumigatus sensu stricto.
The advent of next-generation whole-genome sequencing (WGS) allows the sequencing of entire genomes for a cost that is similar to conventional multi-locus genotyping. WGS therefore heralds a new dawn in our ability, not only to genotype clinical and environmental genomes of A. fumigatus, but to also analyse patterns of natural selection and gene flow in nature. To this end, we recently sequenced and analysed 24 genomes of A. fumigatus from across the world. These isolates were rationally chosen to include an informative selection of clinical and environmental isolates that were wild-type, or known to carry the TR34/L98H allele [72]. This population genomic analysis showed that A. fumigatus was broadly panmictic, with as much genetic diversity found within a country as is found between continents. However, a striking exception to this pattern was shown in India, where isolates containing TR34/L98H were found to be highly related despite being isolated from both clinical and environmental sources across more than 1000 km. This high degree of relatedness across India suggested a recent selective sweep of a highly fit genotype that is associated with the TR34/L98H allele [73,74]. We found that the isolates that we sequenced all show the hallmarks of genetic recombination, showing that azole-resistant alleles are segregating into diverse genetic backgrounds. However, despite linkage equilibrium being high across the global sample (except for the Indian subset), TR34/L98H alleles did cluster suggesting that they are not in linkage equilibrium with the global set of allelic diversity.
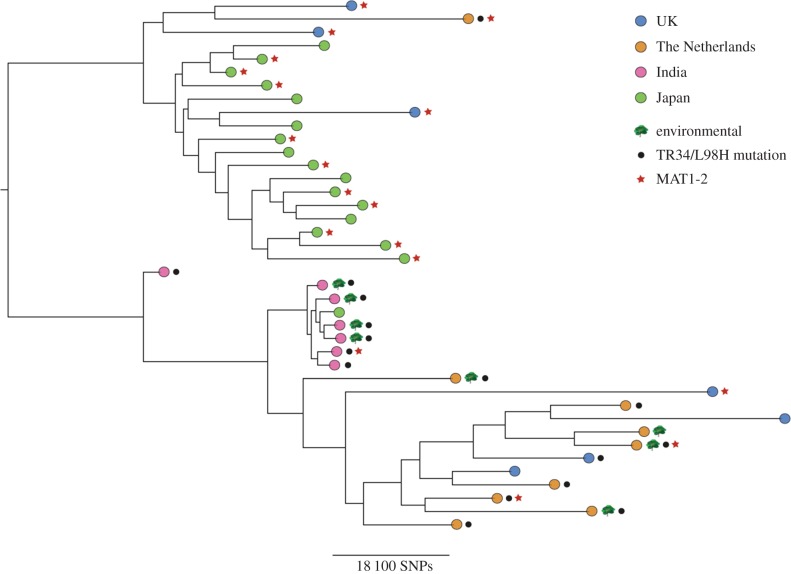
We then combined the 24 A. fumigatus genomes with 17 genomes recently published from clinical isolates in Japan [75]. Phylogenetic analysis (figure 2) revealed that the majority of Japanese isolates clustered together, with the exception of one isolate that clustered with the Indian isolates. These Japanese isolates were separated from the Indian isolates by over 50 000 SNPs. Further analysis revealed that none of the Japanese isolates contained known resistance mutations for azole drugs, and that the phylogeny showed evidence for two clades, only one of which was enriched for the TR34/L98H allele. Investigation into MAT-locus typing, which was carried out computationally, revealed that 58% of Japanese isolates were MAT1-2; indeed, 70% of the Japanese ‘cluster’ were typed as MAT1-2. This cluster also contained three UK isolates, one isolate from The Netherlands, and only 1/20 isolates carrying the TR34/L98H allele compared with 15/20 for the non-Japanese clade (figure 2). This preliminary population genomics analysis suggests that Pringle et al. [68] are correct and that cryptic and morphologically identical populations of A. fumigatus sensu stricto do occur, and that this genetic structure leads to substantial linkage disequilibrium for at least two important genetic markers, the mating types and azole-resistance alleles.
Figure 2.
Phylogenetic analysis of azole-susceptible and -resistant A. fumigatus isolates from UK, The Netherlands, India and Japan [68,71]. Bootstrap analysis using 100 replicated was performed on WGS SNP data to generate a maximum-likelihood phylogeny, with all branches supported to 68% or higher. Branch lengths represent the number of SNPs between taxa.
Although the sample of genomes that we analysed was small, our analysis shows that a broader analysis of globally occurring A. fumigatus genomes that are azole sensitive, azole resistant, and of known clinical or environmental provenance is urgently needed in order to determine worldwide patterns of linkage disequilibrium and the occurrence of cryptic species within A. fumigatus. At the time of writing, the evidence suggests that TR34/L98H at least is a relatively recent and novel evolutionary innovation, and that it is perturbing the natural population genetic structure of A. fumigatus as selective sweeps, which are imposed by this allele, occur. Dating the origin(s) of this and other azole-resistance alleles as they are discovered, is possible given a large enough sample of global A. fumigatus genomes. These data will also allow a description of the fundamental population genetic parameters that are needed to enable the use of genome-wide association studies to identify the contribution of SNP diversity to the generation and spread of azole resistance in this medically significant fungus, as well as describing where, when and how any genetic barriers to gene flow (cryptic species) occur.
5. The environmental route of dissemination
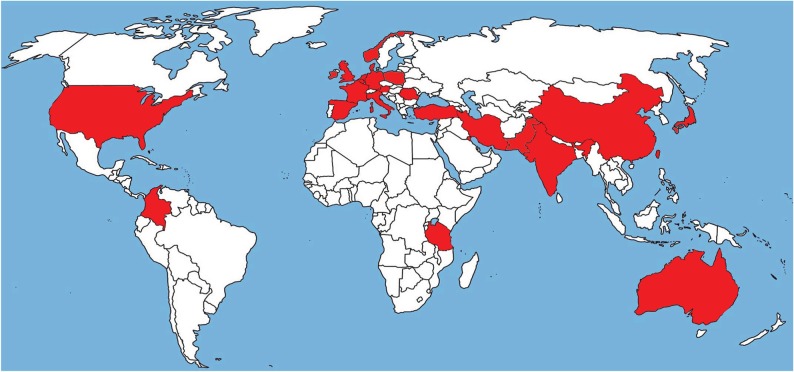
Evidence for an environmental route of azole-resistant A. fumigatus acquisition was first found in The Netherlands. Azoles are widely used outside medicine for crop protection and as biocides, with the aim of deterring mould infestation of crops and materials. Aspergillus fumigatus is a saprophyte and not a plant pathogen, and is therefore an ‘innocent bystander’ when exposure of crops to fungicides occurs. Although azole fungicides are not employed to target A. fumigatus, it transpires that many of these fungicides have activity against A. fumigatus, a condition that led to the development of resistance [76,77]. More than 30 azole fungicides have been tested for their in vitro activity against A. fumigatus [76,77]. Many of these fungicides were able to inhibit wild-type strains and some azole fungicides were active against wild-type strains but ineffective against isolates with the TR34/L98H mutations. Structural modelling showed that there is a spatial molecular similarity between medical triazoles and the agricultural triazole fungicides such as propiconazole, bromuconazole, epoxiconazole, difenconazole and tebuconazole [76]. These fungicides were introduced in The Netherlands between 1990 and 1996, just before the first clinical TR34/L98H strain was found in 1997. Azoles are also frequently used as biocides in paint and coatings, wall-paper paste, clothing, and wood preservation. Currently, the hypothesis is that A. fumigatus becomes resistant through the inevitable exposure to these agents in the environment [77,78]. Vulnerable patients can become exposed to resistant spores in the air and, if these hosts develop Aspergillus disease, the medical azoles are no longer effective due to the structural similarity of the molecules. It is still unclear how and where resistance develops in the environment and which applications of fungicides are inducing the development of resistance most successfully. It has not been routine practice in microbiological laboratories to carry out in vitro susceptibility testing of filamentous fungi. However, this has changed in the last few years and presently many centres now test A. fumigatus for resistance to azoles. As a result, it has become evident that azole resistance has a potentially global distribution, and is a worldwide problem [24,36]. The mutations associated with the environmental route are found in many geographically diverse countries and continents (figure 3). In Europe, many countries including Belgium, France, Spain, Denmark, Italy, The Netherlands, Norway, Germany, Ireland, United Kingdom, Poland, Romania and Austria have reported azole resistance [37–45,55,56]. Resistance is also reported outside Europe in Turkey, Iran, Kuwait, Japan, China, Taiwan, Pakistan, India, Tanzania and Australia [46–53,57], so far mainly associated with TR34/L98H but increasingly also TR46/Y121F/T289A. In the United States and Colombia, azole resistance owing to environmental mutations was reported recently [54,58]. Extensive asexual sporulation, the ability to survive in very different environmental conditions, and the hydrophobicity of spores, faciliating airborne dispersibility, probably enable the global spread of resistance from its centres of origin [31,79]. At present, the development of azole resistance is mainly associated with A. fumigatus and less so with human pathogens A. flavus [80] and A. terreus [81]; however, further surveillance is warranted.
Figure 3.
Worldwide map of azole resistance in Aspergillus fumigatus. The red highlighted countries have reported azole resistance.
6. Clinical implications of resistance
Although no randomized studies have been conducted, it is increasingly clear that patients with azole-resistant Aspergillus infections have a high risk for therapy failure. In a Dutch surveillance study, eight patients were identified with azole-resistant IA, seven of whom died within two months after diagnosis [60]. Five patients had a culture-positive pulmonary aspergillosis and failed treatment with voriconazole. Other case series confirmed this observation, such as a recent study from Germany where seven of eight patients with azole-resistant IA failed on therapy and died, [44] and it was also shown that patients with CPA due to azole resistance failed azole therapy [45]. However, there are many other factors that can cause failure of therapy for aspergillosis such as patients who fail to come into remission of the underlying leukemia, have insufficient voriconazole exposure, or have an already far advanced infection, among others [82]. As a result, at present, the contribution of a high MIC in therapy failure is unclear, warranting case-control investigations to ascertain the exact nature of the relationship. Animal models, however, support the observation that an increased MIC leads to loss of effectiveness of azoles [83].
7. Diagnosis of azole resistance
Azole resistance in Europe and particularly in The Netherlands is widespread. The National Institute for Public Health and the Environment in The Netherlands is supporting surveillance of resistance in UMCs and the data are published annually in Nethmap [63]. On average, in 7% of the patients who are culture-positive, azole resistance has been detected. This percentage differs between the UMCs ranging from 4.3% to 13.3% in 2014, and from 6.7% to 16.3% in 2015 [63]. The reason for this variation between the centres is unclear at present. In a first surveillance study, in large teaching hospitals, similar high resistance rates were observed [84].
It is recommended also to perform in vitro susceptibility testing on multiple colonies when patients have a positive A. fumigatus culture and the clinical intention is to treat. The problem with this approach is that MIC determinations of Aspergillus are not performed routinely in many microbiological laboratories, at least not in The Netherlands [85]. Therefore, a screening method based on an agar-based test has the advantage of selecting resistant strains with unknown mechanisms of resistance. Multiple colonies are sub-cultured on a four-well plate with a growth control and itraconazole, voriconazole and posaconazole added to the agar (VIP Check™, Beneden-Leeuwen, The Netherlands). This approach detects with high sensitivity and specificity potential resistance in the isolates in a simplified way, i.e. isolates growing only on the growth-control well excludes resistance. However, growth on drug-containing wells very probably represent a resistant isolate and a MIC-determination should be done. This approach is, in addition to surveillance studies, also useful for clinical management of patients with aspergillosis. The agar-based test can help to select the strains for in vitro susceptibility testing. Furthermore, growth of A. fumigatus on the agar with added azoles makes the likelihood of azole resistance so high that modification of therapy should be considered, even without confirmation with MIC testing. Alternatively, isolates can also be screened with the Etest method. This screening can best be done with itraconazole, although some resistance mechanisms can be missed. Both the EUCAST and CLSI have a published reference method for in vitro susceptibility testing of conidia-producing fungi [86,87]. The CLSI used an epidemiological cutoff value for detection of non-wild-types while the EUCAST, based on a number of parameters such as standard dose, pharmacokinetics/dynamics and clinical outcome of treatment, defined clinical breakpoints.
8. Detection of azole resistance in patients with negative cultures
Conventional cultures of bronchoalveolar lavage (BAL) fluid and other respiratory samples are negative in the majority (up to 90%) of aspergillosis patients [88]. Thus, determination of resistance in culture-negative patients is difficult. Although tests such as galactomannan antigen and beta-D-glucan are indicative of aspergillosis, they give no information about azole sensitivity of the infecting species. The detection of resistance mechanisms by means of PCR directly from tissue was first described in 2010 in a patient with culture-negative cerebral aspergillosis [89]. Other researchers have confirmed that in culture-negative patients, direct detection of mutations in clinical samples is possible [90]. Recently, a multiplex real-time PCR for detection of some Aspergillus species became available (AsperGenius®, PathoNostics, Maastricht, The Netherlands) which also detects two mechanisms of resistance associated with the environment, TR34/L98H and TR46/Y121F/T289A. This test showed a good sensitivity and specificity for the detection of Aspergillus in BAL fluid [91]. In patients with haematologic malignancies, the sensitivity and specificity was 88.9% and 89.3%, respectively, and in ICU patients 80% and 93.3%. Of 77 BAL fluid samples, 22 patients with IA (2 proven, 9 probable and 11 non-classifiable) were included. In another study, AsperGenius-PCR in serum of patients with IA [92] reported 100% sensitivity and 78.6% specificity, but no cases of azole-resistant IA were diagnosed. Because the cyp51A gene exists in a single copy per cell, the detection of mutations is difficult in serum due to the limited sensitivity of the test. In addition, the AsperGenius-PCR only allows two mechanisms of resistance to be identified. Therefore, a negative test result does not rule out the presence of an azole-resistant infection. Yet the results of molecular tests are encouraging and may be of added value in clinical settings, particularly in the rapid analysis of BAL fluid samples. Excepting a recent consensus document from an expert panel [23], there are currently no guidelines available in which the treatment of azole-resistant aspergillosis is described. Guidelines on the treatment of Aspergillus diseases with ESCMID and ECMM are in preparation, including treatment of azole-resistant disease [93].
9. Future trends for treating azole-resistant Aspergillus disease
The use of azoles in different application areas, namely crop protection, material conservation and treatment of fungal diseases in humans and animals, has resulted in a complicated policy situation. Azole-resistance mechanisms found in the environment are also involved in incurable human infections. Arguably, without a change of policy the successful use of azoles as a first line of defence against diseases caused by Aspergillus is threatened, constituting a broad impact on public health. Clearly, it is very important to investigate the development of azole resistance by A. fumigatus in the environment and to focus on the questions ‘where’, ‘when’, ‘how’ and ‘how often’ it occurs. This could, for instance, identify high-risk situations (hot spots) in which wild-type isolates are able to develop resistance in each of the application areas of azoles. This may lead to insights into how to decrease the rate at which resistance develops. Given the huge worldwide applications of azole fungicides in farming practices, measures should also be considered on a worldwide scale to achieve any effect and to curb the selection for resistance. However, this policy intervention is complicated, as food security against fungal epidemics is predicated on the widespread deployment of azoles on crop monocultures, and trade-offs occur depending on policy recommendations.
Regarding the diagnosis and treatment of patients with aspergillosis, if possible, the presence of azole resistance should be vigorously investigated as there are no obvious patient risk factors known which predict infection with Aspergillus that may harbour an environmentally acquired mutation. Therefore, intensive monitoring of patients treated with azole monotherapy is necessary and when there is suspicion of clinical failure, new diagnostic interventions should (rapidly) be considered. When azole resistance is demonstrated therapy should be adjusted to include liposomal amphotericin B, or alternatively an echinocandin should be added to voriconazole. If no proof of azole resistance has been obtained and there is a high prevalence of resistance in the hospital or the specific department, the proper approach to (empirical) therapy is currently unclear and is a public health concern that should be debated. During a recently held consensus meeting, most experts felt that first- choice (empirical) therapy should not consist of voriconazole monotherapy when a prevalence of 10% resistance due to environmental azole resistance was present [23] and such reflections may constitute the public health response that will be needed to combat this infection into the future.
Authors' contributions
Preparation of the first draft was done by J.F.M., preparation of figures by A.C., J.L.R. and P.E.V.; concept of the review, commenting and editing subsequent versions of the manuscript were done by all authors.
Competing interests
We have no competing interests.
Funding
J.F.M. received grants from Astellas, Basilea and Merck. He has been a consultant to Astellas and Merck and received speaker's fees from Gilead Sciences, Merck, Pfizer and United Medical. P.E.V. received research grants from Astellas, Basilea, F2G, Gilead Sciences, Merck and Pfizer. He has been a consultant to Basilea, F2G, Gilead Sciences, Merck and Pfizer and received speaker's fees from Basilea, Gilead Sciences and Merck.
References
- 1.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 47, 45–68. ( 10.1183/13993003.00583-2015) [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. 2015. The clinical spectrum of pulmonary aspergillosis. Thorax 70, 270–277. ( 10.1136/thoraxjnl-2014-206291) [DOI] [PubMed] [Google Scholar]
- 3.Taccone FS, et al. 2015. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit. Care 19, 7 ( 10.1186/s13054-014-0722-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wauters J, et al. 2012. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 38, 1761–1768. ( 10.1007/s00134-012-2673-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson TF, et al. 2016. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63, e1–e60. ( 10.1093/cid/ciw326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodríguez-Tudela JL. 2003. A point mutation in the 14-alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 47, 1120–1124. ( 10.1128/AAC.47.3.1120-1124.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Linden JWM, Warris A, Verweij PE. 2011. Aspergillus species intrinsically resistant to antifungal agents. Med. Mycol. 49, S82–S99. ( 10.3109/13693786.2010.499916) [DOI] [PubMed] [Google Scholar]
- 8.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side effect of environmental fungicide use? Lancet Infect. Dis. 9, 789–795. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 9, 697–711. ( 10.2217/fmb.14.27) [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Sharma C, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front. Microbiol. 6, 428 ( 10.3389/fmicb.2015.00428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maertens JA, et al. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387, 760–769. ( 10.1016/S0140-6736(15)01159-9) [DOI] [PubMed] [Google Scholar]
- 12.Liss B, et al. 2015. Our 2015 approach to invasive pulmonary aspergillosis. Mycoses 58, 375–382. ( 10.1111/myc.12319) [DOI] [PubMed] [Google Scholar]
- 13.Hiemenz JW, et al. 2010. Efficacy of caspofungin as salvage therapy for invasive aspergillosis compared to standard therapy in a historical cohort. Eur. J. Clin. Microbiol. Infect. Dis. 29, 1387–1394. ( 10.1007/s10096-010-1013-0) [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Meems L, Herbrecht R, Viscoli C, van Amsterdam RG, Ruhnke M. 2015. Randomised, multicentre trial of micafungin vs. an institutional standard regimen for salvage treatment of invasive aspergillosis. Mycoses 58, 58–64. ( 10.1111/myc.12274) [DOI] [PubMed] [Google Scholar]
- 15.Viscoli C, et al. 2009. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J. Antimicrob. Chemother. 64, 1274–1281. ( 10.1093/jac/dkp355) [DOI] [PubMed] [Google Scholar]
- 16.Marr KA, et al. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann. Intern. Med. 162, 81–89. ( 10.7326/M13-2508) [DOI] [PubMed] [Google Scholar]
- 17.Denning DW, Venkateswarlu K, Oakley KL, Anderson MJ, Manning NJ, Stevens DA, Warnock DW, Kelly SL. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41, 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweij PE, Te Dorsthorst DT, Rijs AJ, De Vries-Hospers HG, Meis JF. 2002. Nationwide survey of in vitro activities of itraconazole and voriconazole against clinical Aspergillus fumigatus isolates cultured between 1945 and 1998. J. Clin. Microbiol. 40, 2648–2650. ( 10.1128/JCM.40.7.2648-2650.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dannaoui E, Persat F, Monier MF, Borel E, Piens MA, Picot S. 1999. In-vitro susceptibility of Aspergillus spp. isolates to amphotericin B and itraconazole. J. Antimicrob. Chemother. 44, 553–555. ( 10.1093/jac/44.4.553) [DOI] [PubMed] [Google Scholar]
- 20.Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole–resistant aspergillosis. N. Engl. J. Med. 356, 1481–1483. ( 10.1056/NEJMc061720) [DOI] [PubMed] [Google Scholar]
- 21.Snelders E, et al. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5, e219 ( 10.1371/journal.pmed.0050219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camps SM, Rijs AJ, Klaassen CH, Meis JF, O'Gorman CM, Dyer PS, Melchers WJ, Verweij PE. 2012. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J. Clin. Microbiol. 50, 2674–2680. ( 10.1128/JCM.00335-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij PE, et al. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Update 21–22, 30–40. ( 10.1016/j.drup.2015.08.001) [DOI] [PubMed] [Google Scholar]
- 24.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin. Infect. Dis. 62, 362–368. ( 10.1093/cid/civ885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, Ramage G, Denning DW, Bowyer P. 2013. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 68, 1486–1496. ( 10.1093/jac/dkt075) [DOI] [PubMed] [Google Scholar]
- 26.Meneau I, Coste AT, Sanglard D. 2016. Identification of Aspergillus fumigatus multidrug transporter genes and their potential involvement in antifungal resistance. Med. Mycol. 54, 616–627. ( 10.1093/mmy/myw005) [DOI] [PubMed] [Google Scholar]
- 27.Monk BC, et al. 2014. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc. Natl Acad. Sci. USA 111, 3865–3870. ( 10.1073/pnas.1324245111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus cyp51A based on protein homology modeling. Antimicrob. Agents Chemother. 54, 2425–2430. ( 10.1128/AAC.01599-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob. Agents Chemother. 56, 10–16. ( 10.1128/AAC.05088-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard SJ, Pasqualotto AC, Anderson MJ, Leatherbarrow H, Albarrag AM, Harrison E, Gregson L, Bowyer P, Denning DW. 2013. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses 56, 434–441. ( 10.1111/myc.12047) [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Debets AJ, Verweij PE, Melchers WJ, Zwaan BJ, Schoustra SE. 2015. Asexual sporulation facilitates adaptation: the emergence of azole resistance in Aspergillus fumigatus. Evolution 69, 2573–2586. ( 10.1111/evo.12763) [DOI] [PubMed] [Google Scholar]
- 32.Verweij PE, Zhang J, Debets AJM, Meis JF, van de Veerdonk FL, Schoustra SE, Zwaan B, Melchers WJG. 2016. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect. Dis. 16, In press, S1473-3099(16)30138-4. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad S, Joseph L, Hagen F, Meis JF, Khan Z. 2015. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J. Antimicrob. Chemother. 70, 412–415. ( 10.1093/jac/dku410) [DOI] [PubMed] [Google Scholar]
- 34.Kolwijck E, van der Hoeven H, de Sévaux RG, Ten Oever J, Rijstenberg LL, van der Lee HA, Zoll J, Melchers WJ, Verweij PE. 2016. Voriconazole-susceptible and voriconazole-resistant Aspergillus fumigatus coinfection. Am. J. Respir. Crit. Care Med. 193, 927–929. ( 10.1164/rccm.201510-2104LE) [DOI] [PubMed] [Google Scholar]
- 35.Gsaller F, et al. 2016. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog. 12, e1005775 ( 10.1371/journal.ppat.1005775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Linden JW, et al. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 21, 1041–1044. ( 10.3201/eid2106.140717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen E, Maertens J, De Bel A, Nulens E, Boelens J, Surmont I, Mertens A, Boel A, Lagrou K. 2015. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob. Agents Chemother. 59, 4569–4576. ( 10.1128/AAC.00233-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhren J, Voskuil WS, Boel CH, Haas PJ, Hagen F, Meis JF, Kusters JG. 2015. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J. Antimicrob. Chemother. 70, 2894–2898. ( 10.1093/jac/dkv177) [DOI] [PubMed] [Google Scholar]
- 39.de Fontbrune FS, Denis B, Meunier M, Garcia-Hermoso D, Bretagne S, Alanio A. 2014. Iterative breakthrough invasive aspergillosis due to TR(34)/ L98H azole-resistant Aspergillus fumigatus and Emericella sublata in a single hematopoietic stem cell transplant patient. Transpl. Infect. Dis. 16, 687–691. ( 10.1111/tid.12231) [DOI] [PubMed] [Google Scholar]
- 40.Burgel PR, et al. 2012. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 56, 869–874. ( 10.1128/AAC.05077-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen RH, Hagen F, Astvad KM, Tyron A, Meis JF, Arendrup MC. 2016. Azole resistant Aspergillus fumigatus in Denmark: a laboratory based study on resistance mechanisms and genotypes. Clin. Microbiol. Infect. 22, 570e1-9. ( 10.1016/j.cmi.2016.04.001) [DOI] [PubMed] [Google Scholar]
- 42.Brillowska-Dąbrowska A, Mroczyńska M, Nawrot U, Włodarczyk K, Kurzyk E. 2015. Examination of cyp51A and cyp51B expression level of the first Polish azole resistant clinical Aspergillus fumigatus isolate. Acta Biochim. Pol. 62, 837–839. ( 10.18388/abp.2015_1143) [DOI] [PubMed] [Google Scholar]
- 43.Lazzarini C, Esposto MC, Prigitano A, Cogliati M, De Lorenzis G, Tortorano AM. 2015. Azole resistance in Aspergillus fumigatus clinical isolates from an Italian culture collection. Antimicrob. Agents Chemother. 60, 682–685. ( 10.1128/AAC.02234-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinmann J, et al. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J. Antimicrob. Chemother. 70, 1522–1526. ( 10.1093/jac/dku566) [DOI] [PubMed] [Google Scholar]
- 45.Howard SJ, et al. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15, 1068–1076. ( 10.3201/eid1507.090043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 67, 362–366. ( 10.1093/jac/dkr443) [DOI] [PubMed] [Google Scholar]
- 47.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J. Antimicrob. Chemother. 69, 2979–2983. ( 10.1093/jac/dku259) [DOI] [PubMed] [Google Scholar]
- 48.Badali H, et al. 2013. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses 56, 659–563. ( 10.1111/myc.12089) [DOI] [PubMed] [Google Scholar]
- 49.Ahmad S, Khan Z, Hagen F, Meis JF. 2014. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ Res. 133, 20–26. ( 10.1016/j.envres.2014.05.009) [DOI] [PubMed] [Google Scholar]
- 50.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58, 350–355. ( 10.1111/myc.12324) [DOI] [PubMed] [Google Scholar]
- 51.Özmerdiven GE, Ak S, Ener B, Ağca H, Cilo BD, Tunca B, Akalın H. 2015. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J. Infect. Chemother. 21, 581–586. ( 10.1016/j.jiac.2015.04.012) [DOI] [PubMed] [Google Scholar]
- 52.Wu C-J, Wang H-C, Lee J-C, Lo H-J, Dai C-T, Chou P-H, Ko W-C, Chen Y-C. 2015. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses 58, 544–549. ( 10.1111/myc.12354) [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, et al. 2016. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus from China. Antimicrob. Agents Chemother. Antimicrob. Agents Chemother. 60, In press, pii: AAC.01005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiederhold NP, et al. 2016. First detection of TR34/L98H and TR46/Y121F/ T289A cyp51 mutations in Aspergillus fumigatus isolates in the United States. J. Clin. Microbiol. 54, 168–171. ( 10.1128/JCM.02478-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. 2015. First detection of Aspergillus fumigatus azole-resistant strain due to cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect. 6, 33–34. ( 10.1016/j.nmni.2015.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavergne R-A, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, Verweij PE, Le Pape P. 2015. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob. Agents Chemother. 59, 4331–4335. ( 10.1128/AAC.00127-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagiwara D, Takahashi H, Fujimoto M, Sugahara M, Misawa Y, Gonoi T, Itoyama S, Watanabe A, Kamei K. 2016. Multi-azole resistant Aspergillus fumigatus harboring cyp51A TR46/Y121F/T289A isolated in Japan. J. Infect. Chemother. 22, 577–579. ( 10.1016/j.jiac.2016.01.015) [DOI] [PubMed] [Google Scholar]
- 58.Le Pape P, Lavergne RA, Morio F, Alvarez-Moreno C. 2016. Multiple Fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg. Infect. Dis. 22, 156–157. ( 10.3201/eid2201.150978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lescar J, Meyer I, Akshita K, Srinivasaraghavan K, Verma C, Palous M, Mazier D, Datry A, Fekkar A. 2014. Aspergillus fumigatus harbouring the sole Y121F mutation shows decreased susceptibility to voriconazole but maintained susceptibility to itraconazole and posaconazole. J. Antimicrob. Chemother. 69, 3244–3247. ( 10.1093/jac/dku316) [DOI] [PubMed] [Google Scholar]
- 60.Van der Linden JW, et al. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 17, 1846–1854. ( 10.3201/eid1710.110226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Linden JW, et al. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 57, 513–520. ( 10.1093/cid/cit320) [DOI] [PubMed] [Google Scholar]
- 62.Lavergne RA, Chouaki T, Hagen F, Toublanc B, Dupont H, Jounieaux V, Meis JF, Morio F, Le Pape P. 2016. Home environment as a source of life-threatening azole-resistant Aspergillus fumigatus in immunocompromised patients. Clin. Infect. Dis. accepted for publication (CID 83151). [DOI] [PubMed] [Google Scholar]
- 63.Verweij PE. 2016. Azole resistance in Aspergillus fumigatus. Chapter 4.5.7 In NethMap 2015. Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands (eds SC de Greeff, JW Mouton, AF Schoffelen). Bergen, The Netherlands: SWAB, pp. 128–131. [Google Scholar]
- 64.Selmecki A, Forche A, Berman J. 2006. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313, 367–370. ( 10.1126/science.1128242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. 2012. Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7, e50034 ( 10.1371/journal.pone.0050034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valsecchi I, Mellado E, Beau R, Raj S, Latgé JP. 2015. Fitness studies of azole-resistant strains of Aspergillus fumigatus. Antimicrob. Agents Chemother. 59, 7866–7869. ( 10.1128/AAC.01594-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mavridou E, Meletiadis J, Jancura P, Abbas S, Arendrup MC, Melchers WJ, Heskes T, Mouton JW, Verweij PE. 2013. Composite survival index to compare virulence changes in azole-resistant Aspergillus fumigatus clinical isolates. PLoS ONE 8, e72280 ( 10.1371/journal.pone.0072280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pringle A, Baker DM, Platt JL, Latge JP, Taylor JW. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59, 1886–1899. ( 10.1111/j.0014-3820.2005.tb01059.x) [DOI] [PubMed] [Google Scholar]
- 69.O'Gorman CM, Fuller H, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457, 471–474. ( 10.1038/nature07528) [DOI] [PubMed] [Google Scholar]
- 70.Rydholm C, Szakacs G, Lutzoni F. 2006. Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot. Cell 5, 650–657. ( 10.1128/EC.5.4.650-657.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swilaiman SS, O'Gorman CM, Balajee SA, Dyer PS. 2013. Discovery of a sexual cycle in Aspergillus lentulus, a close relative of A. fumigatus. Eukaryot. Cell 12, 962–969. ( 10.1128/EC.00040-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. 2015. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 6, e00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chowdhary A, et al. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS ONE 7, e52871 ( 10.1371/journal.pone.0052871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang H, Ashu E, Sharma C, Kathuria S, Chowdhary A, Xu J. 2016. Diversity and origins of Indian multi-triazole resistant strains of Aspergillus fumigatus. Mycoses 59, 450–466. ( 10.1111/myc.12494) [DOI] [PubMed] [Google Scholar]
- 75.Takahashi-Nakaguchi A, et al. 2015. Genome sequence comparison of Aspergillus fumigatus strains isolated from patients with pulmonary aspergilloma and chronic necrotizing pulmonary aspergillosis. Med. Mycol. 53, 353–360. ( 10.1093/mmy/myv003) [DOI] [PubMed] [Google Scholar]
- 76.Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, van der Lee HA, Klaassen CH, Melchers WJ, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 7, e31801 ( 10.1371/journal.pone.0031801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snelders E, Huis In't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75, 4053–4057. ( 10.1128/AEM.00231-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 9, e1003633 ( 10.1371/journal.ppat.1003633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwon-Chung KJ, Sugui JA. 2013. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 9, e1003743 ( 10.1371/journal.ppat.1003743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul RA, Rudramurthy SM, Meis JF, Mouton JW, Chakrabarti A. 2015. A novel Y319H substitution in Cyp51c associated with azole resistance in Aspergillus flavus. Antimicrob. Agents Chemother. 59, 6615–6619. ( 10.1128/AAC.00637-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arendrup MC, Jensen RH, Grif K, Skov M, Pressler T, Johansen HK, Lass-Flörl C. 2012. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a cyp51A M217I alteration. J. Infect. Dis. 206, 981–985. ( 10.1093/infdis/jis442) [DOI] [PubMed] [Google Scholar]
- 82.Nivoix Y, et al. 2008. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 47, 1176–1184. ( 10.1086/592255) [DOI] [PubMed] [Google Scholar]
- 83.Seyedmousavi S, Brüggemann RJ, Meis JF, Melchers WJ, Verweij PE, Mouton JW. 2015. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob. Agents Chemother. 59, 2855–2866. ( 10.1128/AAC.04907-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meis JF, et al. 2015. Multicenter surveillance of azole-resistant Aspergillus fumigatus isolates in non-academic teaching hospitals in the Netherlands. Mycoses 58(Suppl. 4), 60. [Google Scholar]
- 85.Lestrade PP, et al. 2016. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses 59, 101–107. ( 10.1111/myc.12440) [DOI] [PubMed] [Google Scholar]
- 86.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd edn Document M38-A2. Wayne PA, USA: Clinical Laboratory Standards Institute. [Google Scholar]
- 87.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ.2016. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST DEFINITIVE DOCUMENT E.DEF 9.3. See http://www.eucast.org/ast_of_fungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_moulds/ .
- 88.Langridge PJ, Sheehan RL, Denning DW. 2016. Microbial yield from physiotherapy assisted sputum production in respiratory outpatients. BMC Pulm. Med. 16, 23 ( 10.1186/s12890-016-0188-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. 2010. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. J. Clin. Microbiol. 48, 1478–1480. ( 10.1128/JCM.02221-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Denning DW, et al. 2011. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 52, 1123–1129. ( 10.1093/cid/cir179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong GL, van de Sande WW, Dingemans GJ, Gaajetaan GR, Vonk AG, Hayette MP, van Tegelen DW, Simons GF, Rijnders BJ. 2015. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J. Clin. Microbiol. 53, 868–874. ( 10.1128/JCM.03216-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White PL, Posso RB, Barnes RA. 2015. An analytical and clinical evaluation of the PathoNostics AsperGenius® Assay for detection of invasive aspergillosis and resistance to azole antifungal drugs when testing serum samples. J. Clin. Microbiol. 53, 2115–2121. ( 10.1128/JCM.00667-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verweij PE, Arendrup MC, Denning DW, Kullberg BJ, Meis JF, Arikan-Akdagli S.. 2017 Aspergillus guideline: Azole and amphotericin B resistance in Aspergillus, in preparation.