Abstract
Chytridiomycosis is an emerging infectious disease of amphibians that affects over 700 species on all continents where amphibians occur. The amphibian–chytridiomycosis system is complex, and the response of any amphibian species to chytrid depends on many aspects of the ecology and evolutionary history of the amphibian, the genotype and phenotype of the fungus, and how the biological and physical environment can mediate that interaction. Impacts of chytridiomycosis on amphibians are varied; some species have been driven extinct, populations of others have declined severely, whereas still others have not obviously declined. Understanding patterns and mechanisms of amphibian responses to chytrids is critical for conservation and management. Robust estimates of population numbers are needed to identify species at risk, prioritize taxa for conservation actions, design management strategies for managing populations and species, and to develop effective measures to reduce impacts of chytrids on amphibians.
This article is part of the themed issue ‘Tackling emerging fungal threats to animal health, food security and ecosystem resilience’.
Keywords: amphibians, biodiversity, chytrid fungi, disease, populations
1. Introduction
Declining amphibian populations have been a concern of scientists ever since the first World Congress of Herpetology in 1988. There scientists realized many amphibians that had previously been common, abundant or reliably encountered had disappeared for no obvious reason, often from remote or protected areas [1]. The publication of the IUCN Global Amphibian Assessment (GAA) eventually revealed the extent of the problem. The GAA [2] showed that 43% of amphibian species were in decline and 32% of species threatened; not only was the problem widespread, it was recent, with 100 of the 168 presumably extinct species having disappeared since 1980. Threats varied regionally, with declines associated with habitat loss and alteration concentrated in the developed areas of Europe and North America, but a disproportionate number of severe ‘enigmatic’ declines, had occurred in species-rich tropical areas. Collins & Storfer [3] explored the various threats to amphibians and developed a framework that included traditional threats (e.g. land-use change, overexploitation and chemical contaminants) as well as novel threats (e.g. invasive species, emerging infectious diseases and climate change). These novel threats are more intractable than traditional threats because they are regional or global problems not contained within socio-political boundaries and whose solutions require global collaborations. The chytrid pathogen Batrachochytrium dendrobatidis (Bd; [4]) is a good example of these new threats, as it is both an emerging infectious disease and an invasive species. In a short period of time, Bd has contributed to the threatened status of almost 400 amphibian species—a greater impact than predation by established and well-known invasive species that affect all classes of vertebrates [5].
Bd infects over 700 species across three orders of vertebrates [6], causes species extinctions, mass mortality events and precipitous and persistent population declines where it has invaded [7–9]. Yet not all species respond equally to infection, with some species going extinct, others declining and persisting at low numbers, and some species declining very little or rebounding post-decline [10–12]. Today, we know that the amphibian–chytridiomycosis system is complex, and that the response of any amphibian species to chytrid depends on many aspects of the ecology and evolutionary history of the amphibians [10], the genotype and phenotype of the fungus [12,13], and how the biological and physical environment can mediate the interaction [14]). The interaction of host, pathogen and environment are thought to be key drivers of disease intensity, which ultimately determines mortality (figure 1) of host and the degree of response seen in host individuals, the population and the species.
Figure 1.
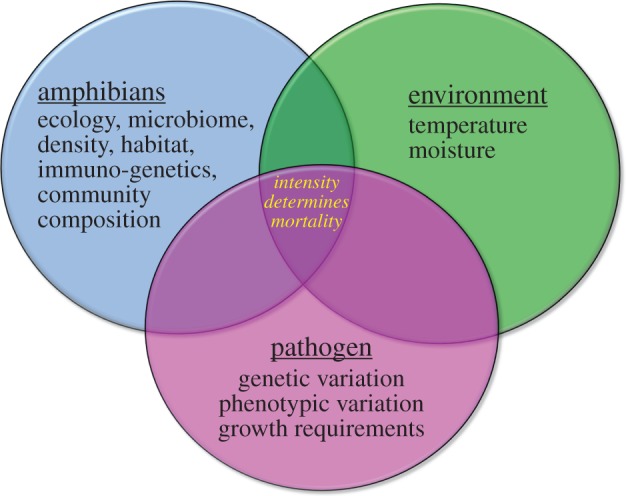
The interaction of host ecology and evolution, fungal genotype and phenotype, and environmental conditions are thought to be key drivers of disease intensity, which ultimately determines individual mortality and host response to infection. (Online version in colour.)
Our understanding of this new disease has changed greatly since it was first described in 1997 [4,15]. Then, it was thought that Bd was a clonal organism that had spread globally, and that we could expect to see many more epizootics as it invaded naive populations. Since then, we have observed only a few die-offs [7–9] although Bd has been found on every continent, often without any obvious impact on populations of amphibians, suggesting a long history or variation in virulence. Recent studies have shown that Bd is actually comprised of several genetically distinct lineages that vary in virulence and phenotype [16]. Of these lineages, the global pandemic lineage (GPL) is clearly an invasive species that has spread across the globe and caused mass mortality, population declines and extinctions.
Scientists have identified some traits that make some species more likely to decline by comparing responses among species from the same region and relating extirpation to species-specific traits. Lips et al. [10] described species-specific patterns of population decline among the amphibian communities at four sites where invasion by Bd-GPL was identified as the cause. They found that declining populations shared aquatic habitats, restricted elevational ranges and large body sizes. Most of these traits are similar to those associated with general conservation concern, meaning that traditional conservation prioritization efforts are likely to identify species at risk of chytridiomycosis, even when definitive data are lacking.
Few other comparisons of species response to chytrid invasion have been possible, because few other epidemics have been reported, or because where invasion has occurred, species richness is very low [9]. Comparing responses of species where Bd is endemic has been more difficult because of the lack of historic data on population response or on species composition. Still, some biotic (e.g. variation in pathogen virulence [12,16]; host ecology [10]; immunogenetic variation [17–19]; antimicrobial peptides [20]; skin bacteria [21,22]) and abiotic (e.g. temperature and moisture [23]; habitat [10]) factors have consistently been associated with higher probabilities of population persistence or decline across many locations.
Not only can Bd change community composition through unequal impacts on species, but the presence of species that amplify or dilute infection could also alter the response of a community to disease. Community composition is especially important in disease dynamics because of the broad host range of Bd. Bd infects over 700 species [6], which means that potentially all species of amphibians can serve as host, vector or reservoir. Laboratory studies have shown that the species composition of tadpole communities affects disease transmission [24,25] as do studies of artificial adult communities [25]. In these cases, some species reduced infection in co-occurring species. The lack of information on the ecology of many amphibians and insufficient information on the global distribution and history of chytrid lineages make predicting amphibian response difficult.
I describe the range of responses of amphibians to chytrid pathogens and where additional research is needed. I divide this review into a description of case studies from (i) areas where chytrids are an invasive species, (ii) areas where impacts on amphibians are varied or unknown, and (iii) areas where chytrid is not present.
2. Impacts of chytrid on amphibian populations
(a). Chytrids as invasive pathogens
(i). Australia
Some of the earliest amphibian die-offs and unexplained declines were detected across many protected sites in tropical rainforest streams during the 1980s [7]. At least six species of frogs have not been seen for one to three decades and are presumed extinct, whereas six other species have declined so greatly that the probability of extinction is high [26]. Berger et al. [15] identified a skin infection associated with dead frogs and population declines from three regions that they would attribute to Bd, which had just been described [4]. In the decades to follow, extensive research on many of the surviving species has confirmed the role of Bd in contributing to population declines across Australia [27], and has shown how species-specific differences in ecology and behaviour contribute to variation in disease dynamics [14,28]. Despite continued declines in many species [25], others have developed ways to persist with infection. Retallick et al. [29] described a population of Taudactylus eungellensis persisting with stable infections that had experienced an epidemic die-off 10 years earlier. A 7-year capture–mark–recapture study of the endangered Mixophyes fleayi showed increased abundance after an initial population decline that was attributed to Bd [30]. Others have shown how warmer and drier microclimates might provide a refuge from Bd for some species [31]. Recent evidence suggests that some Australian amphibians may be adapting to Bd; in both laboratory [19] and field studies [32], populations of Litoria verreauxii alpina that had been previously exposed to Bd survived longer than those that had not.
(ii). Central America
Central America has played a key role in the Bd story for many reasons. First, it provided one of the early examples of enigmatic declines in the form of Monteverde's golden toad (Incilius periglenes) [33]. Second, this was one of the first sites where Bd was found infecting wild animals at sites of population decline and mass mortality, and served as definitive proof that a pathogen could cause mortality and declines in multiple species [30,34,35]. Third, the spatio-temporal spread of Bd through Central America provided definitive proof of an invasive pathogen moving in an epidemic wave [8,36]. Subsequent genetic studies have revealed that these die-offs were attributable to a single lineage, identified as the invasive Bd-GPL [37].
The Neotropics have been especially hard hit by these declines [38], with hundreds of species threatened with extinction. The high rates of loss are associated with the exceptionally high diversity and endemism in this region, with over 50% of all described amphibian species found in the Neotropics [38]. In the Neotropics amphibian endemism is concentrated in the montane areas, where the cool moist climate supports chytrid growth. For some tropical amphibians, an entire species could be eliminated by chytridiomycosis because of the small population size, small range and rapid spread of disease. The impact of Bd on Central American amphibians has been devastating [34,35,39]. In species-rich cloud forests of Lower Central America [8], mass die-offs were observed, followed by numerous population declines of many species, including extirpations of dozens of species in a matter of months [8]. To the north in Mexico, Guatemala and Honduras, die-offs were not seen, but unexplained disappearances of dozens of species were noted throughout the region in the 1990s and 2000s [39,40]. Decades later, scientists were able to revisit historic collecting sites and search for Bd [39,40] or to test museum specimens collected from these sites [40]. They found that the first appearance of Bd in the museum record was followed by the rapid loss of wild populations of both anurans [39] and salamanders [41], further extending the epidemic wave from Mexico through Panama.
In Central America, chytridiomycosis has reshaped the patterns of amphibian biodiversity, obscuring historical biogeographic patterns such that distance between sites is no longer correlated with community similarity. Community composition in the region was severely disrupted by epidemics at multiple sites and many of the unique species of these communities were eliminated, with disproportionate effects on endemic species. This resulted in a homogenization of the regional fauna and ecological homogenization of reproductive mode and habitat [42]. As a result, the impacts of chytridiomycosis have altered community structure and our ability to detect biogeographic patterns.
Among Neotropical amphibians, the harlequin frogs (genus Atelopus) are an iconic species in both research and conservation. As a group, the harlequin frogs (genus Atelopus) are one of the most threatened groups of amphibians in the world, having experienced severe population declines and extinctions from Bd throughout their range from Costa Rica and Panama to Colombia, Ecuador, Venezuela and Peru [43]. The similarity of response among such a large number of closely related species is the best example of taxonomy predicting response to disease. At least 40 of 97 described species have disappeared in the past 20 years, with three species listed as extinct and 82 species listed as endangered or critically endangered [38]; only 10 species are not threatened [36,43]. Declines have been so widespread and so obvious that Lips et al. [36] used the reports of losses to map the spatio-temporal pattern of population declines in Atelopus to represent the hypothesized spread of Bd through South American Andes. Atelopus species are also important because they may play a disproportionate role in disease dynamics. DiRenzo et al. [44] demonstrated that Atelopus zeteki is an acute supershedder, producing exceptionally high numbers of zoospores over several weeks prior to death. This led to the hypothesis that Atelopus species might contribute to increasing the pool of zoospores in the environment and the prediction that the presence of Atelopus species in a community might amplify disease and cause populations of other species to decline. This will be a key concern as conservation programmes consider reintroduction of Atelopus and other susceptible species into communities where they have been extirpated and where surviving species have achieved coexistence with Bd. While some species that had been missing for many years have been ‘rediscovered’, the low numbers, continued mortality and lack of large-scale recovery indicate that disease must still be causing unobserved mortality or lack of recruitment in amphibian populations at these sites. To effectively manage Atelopus or other species where Bd is present, we need to know how disease has altered demographic rates of species such as survivorship, recruitment and transitions between life stages. Quantitative data from four species of Atelopus [45–47] can provide the baseline data for reintroductions and help understand stages limiting to natural population recovery. As in Australia, there is some evidence that warmer regions where environmental conditions could mitigate the impacts of disease [48] might be the best option for reintroductions.
(iii). California
California has been important because it is the site of some of the earliest enigmatic declines and die-offs of amphibians from remote protected areas. One of the earliest, enigmatic population declines in the USA was noted in the Wyoming toad (Anaxyrus hemiophrys baxteri [49]) in the mid-1970s; it became extinct in the wild within 10 years [50], but no definitive cause has been attributed. The Yosemite toad (A. canorus) experienced population declines in 1976–1979 [51]. Some of those carcasses were later found to be infected with Bd [52], and it is presumed that Bd caused those declines. A mass die-off of mountain yellow-legged frogs (Lithobates muscosa) in the Sierra Nevada of California was seen in 1979, followed by population declines and extirpation by 1983 [53].
Understanding the mechanisms that drive a chytridiomycosis epidemic came from long-term studies of mountain yellow-legged frogs in alpine lakes across the California Sierra Nevada [9,54]. These researchers documented the invasion of Bd-GPL into naive populations of amphibians where it caused die-offs, population declines and extirpations. They identified intensity of infection as the key predictor of mortality, with higher intensities associated with individual mortality and the average intensity of the population determining whether a population persists or is extirpated [9]. For mountain yellow-legged frogs in the Sierra Nevada, individuals die at 10 000 zoospores, and populations are extirpated when average infection is 10 000 zoospores [9]. However, these values cannot be applied to all systems, as the mortality threshold varies among species [44,55]. Higher frog density was identified as the mechanism causing more infections, greater numbers of zoospores and a larger environmental pool of zoospores that caused higher intensities and mortality [54]. Similar data do not exist for any other species or system, although other studies have shown that higher intensity of disease is generally associated with greater mortality or morbidity. The large number of ponds spread out across a large area has allowed repeated observations of epidemics, to document the epidemic spread of Bd across the landscape. These results are consistent with the epidemic wave hypothesis, and provide an example independent of those from Central America, Andean South America and Australia.
The single-species system of the California Sierra Nevada also offers a contrast to the multiple-species, highly complex tropical cloud forest amphibian fauna of Central America. For two systems that are so different in terms of climate, habitat and biological composition, it is remarkable that the patterns of disease spread and epizootics are so similar. The next step is to determine whether disease dynamics of the epidemics described in Panama [8] match those reported for the Sierra Nevada system, and whether frog density and disease load are the key predictors of tropical epizootics and species response to infection.
(iv). Puerto Rico
In Puerto Rico, three endemic species of amphibians became extinct in the 1970s [56]. No die-offs were ever observed, but museum specimens showed that Bd has been present since 1976. Like species that disappeared from Panama and Costa Rica, these species were habitat specialists, and at least one was associated with streams. Using capture–mark–recapture and disease surveillance, researchers were able to document ongoing population declines associated with disease despite the lack of detectable mortality. Today, surviving species are at lower abundance than historically, and populations fluctuate with climate patterns [56]. Burrowes et al. [56] proposed a synergism between disease and climatic conditions to explain fluctuations. They proposed that during the dry season when these frogs aggregate in retreat sites, the localized high density promotes the spread of Bd, and highly infected individuals die in the retreat site. It is not known how this Bd-GPL lineage got to the island; however, severe outbreaks of chytridiomycosis on other Caribbean islands such as the extirpation of mountain chicken frogs (Leptodactylus fallax) on Dominica and Montserrat [57] have been caused by Bd-GPL, showing that this lineage is actively island-hopping across this region.
(v). Salamander chytrid
Batrachochytrium salamandrivorans (Bsal) is a recently discovered species of salamander-specific chytrid [58] that has been introduced into Europe, where it is causing mass die-offs and population declines in several species [58,59]. Field and museum sampling has shown that Bsal has been present in Asia for over 150 years and is present in the wild in at least three countries [58–60], although the impact on Asian populations is not known. Bsal has not been found in North America [46,61], although several species are highly susceptible in the laboratory. Because North America is a global hotspot for salamanders, with 10 families and 675 species [48], the US Fish and Wildlife Service has taken preventative measures and imposed a moratorium on imports of 201 species belonging to genera known to be carriers of Bsal. Because the USA lacks a formal policy requiring inspection or testing of live imports of most wildlife species for diseases or pathogens, native species remain at risk of invasion by these and other pathogens. Switzerland has taken similar steps and passed a law banning the import of all salamanders into that country.
(b). Chytrid present, but impacts on amphibians varied or unknown
(i). Africa
At least two lineages of Bd are present in Africa, Bd-Cape, an endemic lineage from South Africa, and the invasive Bd-GPL [16]. Neither the geographical nor taxonomic distribution of either lineage has been described for Africa, and no demographic studies have assessed impacts of either lineage on wild populations. Bd has been present in South Africa since at least 1938 and is commonly found in Ghana, Kenya, South Africa and Western Africa [62]. Bd is widespread in many of the highland regions [63], whereas tropical West African areas regions are reported to be clear of Bd [64]. Recent reports from Cameroon [65] found Bd present in several species, several of which had shown recent declines and they suggested Bd as a likely cause. One epidemic of chytridiomycosis has been reported from Africa, that of the Kihansi Gorge in Tanzania. In 2003, a mass mortality event was followed by population declines of many species that was attributed to Bd [66]. It is not known which lineage of Bd caused this mortality event, although patterns suggest the invasive Bd-GPL. It was hypothesized that infected frogs arrived at the site in construction material, although how the site remained Bd-free after a century of Bd presence in surrounding areas is curious. Luckily, a captive population of the endemic Kihansi spray toad (Nectophrynoides asperginis) had been established in captivity, and more than 2000 animals were reintroduced to the site in 2013, despite the fact that chytrid has never been eliminated from any site where it occurs. Many kinds of laboratory and field studies are needed to explain amphibian–chytrid history at Kihansi Gorge, and across Africa.
(ii). Asia
Studies in Asia are likely to be very enlightening in understanding the history of the amphibian–chytrid system. Bd is widely distributed throughout Asia [67] and has been present in the region since at least 1911 [68], but no reports exist of disease-driven mortality or population declines [67]. Many distinct genotypes are being described from the regions, including Japan [69], Korea [70] and India [71] as well as Bsal [58]. The growing diversity of chytrid pathogens of amphibians from Asia suggests a likely site of origin for these pathogens. Unfortunately, Asia has one of the poorest records of amphibian demographic research both in general and in relation to chytrid infection, and this greatly limits conclusions on the evolutionary history of the system and hampers effective conservation and management.
(iii). Europe
In their meta-analysis of published studies, Houlahan et al. [72] identified rapid and widespread population declines in many amphibians in the UK and western Europe starting in the late 1950s and lasting into the late 1960s. Further research is needed to assess causes and patterns of these historic declines, but disease is one of several possible causes. Europe is home to at least three species of chytrid: an endemic Swiss lineage, the invasive Bd-GPL lineage and invasive Bsal. Despite extensive research across the region, evidence for epidemics is limited to several recent die-offs and population declines in montane regions of Spain and the French Pyrenees. The earliest confirmed case of mass mortality and subsequent population declines from Bd was during 1997–1999 [73] from Central Spain. By 2007, populations of Salamandra salamandra also began to decline in this park and mass mortalities of Bufo bufo were observed [74,75]. Garner et al. [76] surveyed field and museum samples collected across Europe between 1994 and 2004, and found evidence for infections in Spain, Portugal, Italy, Switzerland and Great Britain from as early as 1998. They concluded that chytrid is widespread, even though epidemics and mass mortalities have rarely been reported. Walker et al. [77] modelled the geographical distribution of Bd in Iberia and looked for evidence of both spread and environmental forcing of disease emergence. They found multiple distinct genotypes consistent with either a history of multiple introductions or of a single ancient introduction of Bd into Iberia. The impact of chytrids on amphibians in other parts of Europe is less clear. Switzerland has its own unique, ancient lineage of Bd (Bd-CH [16]) that may be hypovirulent as there are no confirmed chytrid-related die-offs or population declines from that region [78]. Many Swiss populations of amphibians are missing from many historical localities, although causes are complex and none have definitively been attributed to disease. The situation is similar to that in Italy, where Bd has been present for several decades and populations of some species are declining, but no causative link has been established [79,80]. Spitzen-Van Der Sluijs et al. [81] showed that in the Netherlands Bd was present in many species but at low prevalence and low intensity. They concluded that in Europe host responses are geographically and taxonomically inconsistent and are influenced by environmental factors or strain-dependent variation in virulence. For example, they found that Alytes obstetricans in the Netherlands is infected but not declining, whereas populations of that species in upland areas of Spain are highly susceptible. As Bsal continues to spread in the region [59], researchers will have opportunity to establish capture–mark–recapture studies to quantify the response of native amphibians to co-infection by multiple chytrid lineages.
(iv). Brazil
Brazil presents one of the most complex amphibian–chytrid stories and has contributed to our understanding of the amphibian–chytrid system in several unique ways. Brazil was among the first sites to show evidence of mass die-offs of a diverse community of amphibians. Heyer et al. [82] observed a mass die-off of an entire community of amphibians in a protected site in the Atlantic Coastal Forest of Brazil in 1979. They attributed the loss to a particularly bad winter, but Bd has since been found throughout the Atlantic coastal forest [83], although no definitive link has been made between losses at this site and chytridiomycosis. This is the general situation for most of the country, where reports of mass mortality are lacking [84] and definitive impacts of chytrids on amphibian populations are inconsistent or lacking. Like Europe, Brazil hosts multiple lineages of chytrid but is the first to show evidence of hybridization between lineages [83,85,86]. Brazil is unique in having amphibians infected by both the endemic lineage (Bd-Brazil) and the invasive Bd-GPL for over 100 years. Importantly, the long history of Bd-GPL in Brazil suggests additional vectors of pathogen introduction beyond the trade in amphibians. The lack of clear evidence of population declines, the long occurrence of Bd in Brazil and the low but steady prevalence of infection in Brazil suggests that these amphibians have evolved ways of coexisting with chytrid [83] and may produce insights for long-term planning in other regions of the world.
(v). Eastern United States
Bd is widespread in North America [6] and generally occurs at low prevalence and low intensity in the eastern US [37]. Bd has been present in the USA for at least 140 years, with the oldest records from southern leopard frogs (Lithobates sphenocephala) collected in Illinois in 1888 [87]. In this region, no die-offs have been reported, no species have been lost, and no recent dramatic population declines have been identified. Disease prevalence in Illinois today is some of the highest reported, and infection intensity is sufficient to cause death in California ranids. It is not known which lineage of Bd is present in Illinois, and whether it is native or introduced. Such a long coexistence between host and pathogen in Illinois suggests that amphibians and chytrids may have a long coevolutionary history there or that the USA is home to an endemic lineage [61].
That is not to say populations have not experienced declines or are at risk of future declines. An older, slower, silent wave of population declines occurred in the USA with little note, and is continuing today. Houlahan et al. [72] described widespread declines in amphibians in North America and Europe in the 1950–1960s. Most of these populations did not recover, but experienced a second round of declines in the 1970s and 1980s. To date, no cause of those declines has been put forward. A pattern of slow, unnoted declines has been shown for amphibians across the USA [88]. These authors synthesized amphibian monitoring data and detected a 3.7% annual decline in occupancy, with southern species of amphibians (especially salamanders) showing the greatest declines. They concluded that declines may be more widespread and severe here than previously recognized, but did not assign any specific cause to any of these declines. Grant et al. [89] compared the spatial patterns and intensities of four threats—including chytridiomycosis—to declines in species occupancy for those US Geological Survey data and concluded that no single threat was consistent in explaining observed trends and that amphibian response to the threats varied spatially.
One of the largest and most widespread reports of salamander declines comes from the Appalachian Mountains. Declines in populations in terrestrial forest salamanders (Family Plethodontidae: Plethodon) occurred throughout the eastern US in the late 1970s to early 1980s [90], although die-offs were not noted and no definitive cause has been identified. Caruso & Lips [91] resurveyed many of Highton's historic collecting sites and found that occupancy and detection were lower for many species of Plethodon in the Great Smoky Mountain National Park. They resurveyed more species at Highton's historic sites and found that many populations of multiple genera have declined in both occupancy and detection (Caruso et al. 2012, unpublished data). Extensive testing for disease in living animals and in preserved museum specimens from these sites produced very few animals infected with Bd. The lack of mortality, species extinction or disease epidemics all suggest either that the eastern US lineage of Bd has been present for a long time, and native species have evolved to avoid infection, that it is hypovirulent like Bd-CH, or that undiscovered lineages of chytrid fungi are present but not detected with current molecular assays.
(c). Chytrid-free areas
Places where Bd has not yet arrived are rare, but generally are oceanic islands such as Papua New Guinea, Fiji, the Solomon Islands [67,92] and the Seychelles [93]. The Seychelles support an amphibian fauna that is 92% endemic [93], which makes it impossible to predict responses based on phylogeny. However, in the case of Papua New Guinea, the fauna has strong connections to that of Australia, which was severely affected by Bd in the 1980s [7], suggesting that impacts of introduced chytrids there are likely to be devastating. Understanding how are these areas have not become infected can inform efforts to prevent its establishment. Human movements and commercial trade have been linked to international movement of chytrid fungi across large scales [16,94]. The recent detection of Bd on Madagascar [95]—which is 1800 km from the Seychelles—is especially worrisome. Conservationists were prepared for a devastating effect when Bd was first detected on Madagascar, but so far, these have not materialized [95]. Unfortunately, the Madagascar chytrid has not been sequenced, so we do not know which lineage it is, or how it got there. Also lacking are experimental data on the response of Madagascar amphibians to infections. It will be important to institute disease surveillance and amphibian monitoring in these uninfected places to provide information on the arrival and spread of disease. Testing species in the laboratory for susceptibility will be critical to predict the response in the field and design conservation measures.
3. Conclusion
This paper along with other recent reviews [37,81] of global patterns of amphibian responses to chytridiomycosis all show that host responses are context-dependent. In particular, four key factors seem to be the primary drivers of host responses to disease: (i) the genetic lineage of the fungal pathogen, (ii) the length of time the host and pathogen have been interacting (i.e. is chytrid epizootic or enzootic), (iii) host population and species ecology and evolutionary biology, and (iv) environmental conditions.
In particular, two recent advances have greatly altered our understanding of amphibian–chytridiomycosis dynamics: the recognition that Bd is not one species, but is instead many genotypically and phenotypically distinct lineages that vary in distribution and virulence, and the recognition that these chytrid fungi have been infecting amphibians for much longer than we thought. Thus, host–pathogen interactions are much more complicated than we originally thought (figure 2), and we now must include the possibility that long co-occurrence might have allowed amphibians to develop resistance or tolerance to the pathogens, or that the pathogens have lost virulence. This is borne out by laboratory and field studies showing that prior exposure can increase survival [18,32,96] and that inherent immunogenetic variation in species [17] and populations [97] is correlated with response to disease.
Figure 2.
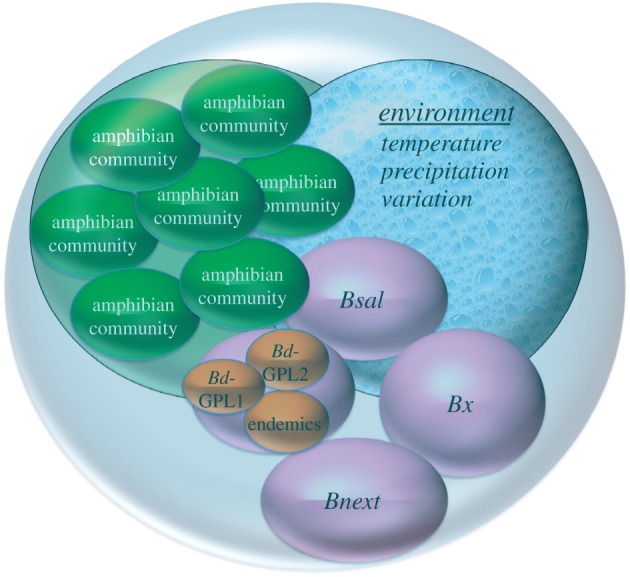
The evolving view of amphibian–chytrid interactions that includes responses of the entire amphibian community, multiple pathogens infecting a host community, and the effects of both abiotic environment and microbiome on host response. (Online version in colour.)
Just as individual species respond variously to different pathogens, so too will communities comprised of different host species. Moving forward, we need to understand how multiple infections by multiple pathogens will affect species and communities, especially as these pathogens will represent mixtures of endemic, established and invasive lineages and effects may vary depending on the order in which they arrive and their previous history with amphibian hosts.
In this review, I have highlighted what we know about the impacts of chytrids on amphibian populations. Yet even in the best-studied cases, we are still lacking basic information on numbers of amphibian species, population sizes, trends in occupancy and abundance, and how chytrids are impacting any of these estimates. Ultimately, governments and conservation organizations base decisions on species endangerment, funding and conservation priority on the numbers of individuals and populations and how quickly those numbers are going up or down. We need robust data on all these if we wish to accurately identify species at risk, prioritize taxa for conservation actions, design management strategies for or develop measures to reduce impacts of chytrids on amphibians.
We have learned a lot since Bd was first described in 1997, but the story is changing rapidly with new molecular techniques, access to new geographical areas and sharing of information among research groups. Novel statistical approaches and modelling techniques provide new tools to better estimate amphibian abundance, and it is time we made a concerted effort to obtain quantitative field data on the numbers and trends of amphibian populations from around the world if we wish to do more than document the problem.
Acknowledgements
Thanks to conference organizers Mat Fisher, Sarah Gurr and Neil Gow for the invitation to participate. Thanks to G. Direnzo, A. Longo, J. Mendelson, M. Fisher and two anonymous reviewers for comments on previous drafts. This work was supported by the National Science Foundation (DEB 1120161). The author declares no competing financial interests.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Blaustein AR, Wake DB. 1990. Declining amphibian populations: a global phenomenon? Trends Ecol. Evol. 7, 203–204. ( 10.1016/0169-5347(90)90129-2) [DOI] [Google Scholar]
- 2.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RL. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. ( 10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 3.Collins JP, Storfer A. 2003. Global amphibian declines: sorting the hypotheses. Divers. Distribs. 9, 89–98. ( 10.1046/j.1472-4642.2003.00012.x) [DOI] [Google Scholar]
- 4.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227. ( 10.2307/3761366) [DOI] [Google Scholar]
- 5.Bellard C, Genovesi P, Jeschke JM. 2016. Global patterns in threats to vertebrates by biological invasions. Proc. R. Soc. B 283, 20152454 ( 10.1098/rspb.2015.2454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Fisher MC. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 8, e56802 ( 10.1371/journal.pone.0056802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurance WF, McDonald KR, Speare R. 1996. Epidemic disease and the catastrophic decline of Australian rainforest frogs. Conserv. Biol. 10, 406–413. ( 10.1046/j.1523-1739.1996.10020406.x) [DOI] [Google Scholar]
- 8.Lips KR, et al. 2006. Infectious disease and global biodiversity loss: pathogens and enigmatic amphibian extinctions. Proc. Natl Acad. Sci. USA 103, 3165–3170. ( 10.1073/pnas.0506889103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vredenburg VT, Knapp RA, Tunstall TA, Briggs CJ. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694. ( 10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lips KR, Reeve J, Witters L. 2003. Ecological factors predicting amphibian population declines in Central America. Conserv. Biol. 17, 1078–1088. ( 10.1046/j.1523-1739.2003.01623.x) [DOI] [Google Scholar]
- 11.Crawford AJ, Lips KR, Bermingham E. 2010. Epidemic disease reduces evolutionary diversity of amphibians. Proc. Natl Acad. Sci. USA 107, 13 777–13 782. ( 10.1073/pnas.0914115107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger L, Roberts AA, Voyles J, Longcore JE, Murray KA, Skerratt L. 2016. History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 19, 89–99. ( 10.1016/j.funeco.2015.09.007) [DOI] [Google Scholar]
- 13.Rosenblum EB, et al. 2013. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc. Natl Acad. Sci. USA 110, 9385–9390. ( 10.1073/pnas.1300130110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley JL, Alford RA. 2013. Hot bodies protect amphibians against chytrid infection in nature. Sci. Rept. 3, 1515 ( 10.1038/srep01515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rainforests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031–9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer RA, et al. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl Acad. Sci. USA 108, 18 732–18 736. ( 10.1073/pnas.1111915108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison AR, et al. 2014. More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol. Evol. 7, 286–298. ( 10.1093/gbe/evu285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon TA, et al. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227. ( 10.1038/nature13491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bataille A, et al. 2015. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc. R. Soc. B 282, 20143127 ( 10.1098/rspb.2014.3127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodhams DC, et al. 2014. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 9, e96375 ( 10.1371/journal.pone.0096375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RN, et al. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3, 818–824. ( 10.1038/ismej.2009.27) [DOI] [PubMed] [Google Scholar]
- 22.Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ. 2013. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 23, 1238–1250. ( 10.1111/mec.12510) [DOI] [PubMed] [Google Scholar]
- 23.Kriger KM, Hero JM. 2007. Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J. Zool. 271, 352–359. [Google Scholar]
- 24.Han BA, Kerby JL, Searle CL, Storfer A, Blaustein AR. 2015. Host species composition influences infection severity among amphibians in the absence of spillover transmission. Ecol. Evol. 5, 1432–1439. ( 10.1002/ece3.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker CG, Rodriguez D, Toledo LF, Longo AV, Lambertini C, Correa DT, Leite DS, Haddad CFB, Zamudio KR. 2014. Partitioning the net effect of host diversity on an emerging amphibian pathogen. Proc. R. Soc. B 281, 0141796 ( 10.1098/rspb.2014.1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skerratt LF, et al. 2016. Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildl. Res. 43, 105–120. ( 10.1071/WR15071) [DOI] [Google Scholar]
- 27.Murray K, et al. 2010. The distribution and host range of the pandemic disease chytridiomycosis in Australia, spanning surveys from 1956–2007. Ecology 91, 1557–1558. ( 10.1890/09-1608.1) [DOI] [Google Scholar]
- 28.Rowley JL, Alford RA. 2007. Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis. Aq. Org. 77, 1–9. ( 10.3354/dao01830) [DOI] [PubMed] [Google Scholar]
- 29.Retallick RWR, McCallum H, Speare R. 2004. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2, e351 ( 10.1371/journal.pbio.0020351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newell DA, Goldingay RL, Brooks LO. 2013. Population recovery following decline in an endangered stream-breeding frog (Mixophyes fleayi) from subtropical Australia. PLoS ONE 8, e58559 ( 10.1371/journal.pone.0058559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puschendorf R, Hoskin CJ, Cashins SD, McDonald K, Skerratt LF, Vanderwal J, Alford RA. 2011. Environmental refuge from disease-driven amphibian extinction. Conserv. Biol. 25, 956–964. ( 10.1111/j.1523-1739.2011.01728.x) [DOI] [PubMed] [Google Scholar]
- 32.Brannelly LA, Hunter DA, Skerratt LF, Scheele BC, Lenger D, McFadden MS, Harlow PS, Berger L. 2016. Chytrid infection and post-release fitness in the reintroduction of an endangered alpine tree frog. Anim. Conserv. 19, 153–162. ( 10.1111/acv.12230) [DOI] [Google Scholar]
- 33.Pounds JA, Crump ML. 1994. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv. Biol. 8, 72–85. ( 10.1046/j.1523-1739.1994.08010072.x) [DOI] [Google Scholar]
- 34.Lips KR. 1998. Decline of a tropical montane amphibian fauna. Conserv. Biol. 12, 106–117. ( 10.1046/j.1523-1739.1998.96359.x) [DOI] [Google Scholar]
- 35.Lips KR. 1999. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv. Biol. 13, 117–125. ( 10.1046/j.1523-1739.1999.97185.x) [DOI] [Google Scholar]
- 36.Lips KR, Diffendorfer J, Mendelson JM, Sears MW. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLOS Biol. 6, e72 ( 10.1371/journal.pbio.0060072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James TY, et al. 2015. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol. Evol. 5, 4079–4097. ( 10.1002/ece3.1672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.IUCN Redlist. 2008. Amphibian initiative. http://www.iucnredlist.org/initiatives/amphibians/analysis (accessed 17 Aril 2016).
- 39.Lips KR, Mendelson JR III, Muñoz Alonso A, Canseco-Marquez L, Mulcahy DG. 2004. Direct evidence of declines in amphibian populations in montane southern Mexico. Biol. Conserv. 119, 555–564. ( 10.1016/j.biocon.2004.01.017) [DOI] [Google Scholar]
- 40.Rovito SM, Parra-Olea G, Vásquez-Almazán CR, Papenfuss TJ, Wake DB. 2009. Dramatic declines in Middle American salamanders are an important component of the global amphibian crisis. Proc. Natl Acad. Sci. USA 106, 3231–3236. ( 10.1073/pnas.0813051106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. 2011. Coincident mass extirpation of neotropical amphibians with the emerging infectious fungal pathogen Batrachochytrium dendrobatidis. Proc. Natl Acad. Sci. USA 108, 9502–9507. ( 10.1073/pnas.1105538108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KG, Lips KR, Chase J. 2009. Epidemic disease homogenizes amphibian communities. Ecol. Lett. 12, 1069–1078. ( 10.1111/j.1461-0248.2009.01363.x) [DOI] [PubMed] [Google Scholar]
- 43.LaMarca E, et al. 2005. Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37, 190–201. ( 10.1111/j.1744-7429.2005.00026.x) [DOI] [Google Scholar]
- 44.DiRenzo G, Langhammer PF, Zamudio KR, Lips KR. 2014. Fungal infection intensity and zoospore output of Atelopus zeteki, a potential chytrid supershedder. PLoS ONE 9, e93356 ( 10.1371/journal.pone.0093356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaffery RM, Richards-Zawacki C, Lips KR. 2015. The demography of Atelopus decline: harlequin frog survival and abundance in Central Panama prior to and during a disease outbreak. Glob. Ecol. Conserv. 4, 232–242. ( 10.1016/j.gecco.2015.07.003) [DOI] [Google Scholar]
- 46.Tarvin RD, Pena P, Ron SR. 2014. Changes in population size and survival in Atelopus spumarius (Anura: Bufonidae) are not correlated with chytrid prevalence. J. Herpetol. 48, 291–297. ( 10.1670/11-269) [DOI] [Google Scholar]
- 47.Lampo M, Celsa SJ, Rodriguez-Contreras A, Rojas-Runjaic F, Garcia CZ. 2012. High turnover rates in remnant populations of the harlequin frog Atelopus cruciger (Bufonidae): low risk of extinction? Biotropica 44, 420–426. ( 10.1111/j.1744-7429.2011.00830.x) [DOI] [Google Scholar]
- 48.Flechas SV, Sarmiento C, Amézquita A. 2012. Bd on the beach: high prevalence of Batrachochytrium dendrobatidis in the lowland forests of Gorgona Island (Colombia, South America). Ecohealth 9, 298–302. ( 10.1007/s10393-012-0771-9) [DOI] [PubMed] [Google Scholar]
- 49.Baxter GT, Stromberg MR, Dodd CK Jr. 1982. The status of the Wyoming toad, Bufo hemiophrys baxteri. Environ. Conserv. 9, 338 ( 10.1017/S0376892900020920) [DOI] [Google Scholar]
- 50.Lewis DL, Baxter GT, Johnson KM, Stone MD. 1985. Possible extinction of the Wyoming toad, Bufo hemiophrys baxteri. J. Herpetol. 19, 166–168. ( 10.2307/1564434) [DOI] [Google Scholar]
- 51.Kagarise Sherman C, Morton ML. 1993. Population declines of Yosemite toads in the eastern Sierra Nevada of California. J. Hepetol. 27, 186–198. ( 10.2307/1564935) [DOI] [Google Scholar]
- 52.Green DE, Kagarise Sherman C. 2001. Diagnostic histological findings in Yosemite toads (Bufo canorus) from a die-off in the 1970s. J. Herpetol. 35, 92–103. ( 10.2307/1566028) [DOI] [Google Scholar]
- 53.Bradford DF. 1991. Mass mortality and extinction in a high-elevation population of Rana muscosa. J. Herpetol. 25, 174–177. ( 10.2307/1564645) [DOI] [Google Scholar]
- 54.Briggs CJ, Knapp RA, Vredenburg VT. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc. Natl Acad. Sci. USA 107, 9695–9700. ( 10.1073/pnas.0912886107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clare F, Daniel O, Garner TWJ, Fisher MC. 2016. Assessing the ability of swab data to determine the true burden of infection for the amphibian pathogen Batrachochytrium dendrobatidis. Ecohealth 13, 360–367. ( 10.1007/s10393-016-1114-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burrowes PA, Joglar RL, Green DE. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60, 141–154. ( 10.1655/03-50) [DOI] [Google Scholar]
- 57.Malhotra A, Thorpe RS, Hypolite E, James A. 2007. A report on the status of the herpetofauna of the Commonwealth of Dominica, West Indies. Appl. Herpetol. 4, 177–194. ( 10.1163/157075407780681365) [DOI] [Google Scholar]
- 58.Martel A, et al. 2014. Recent introduction of a chytrid fungus endangers western Palearctic salamanders. Science 346, 630–631. ( 10.1126/science.1258268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spitzen-van der Sluijs A. et al. 2016. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 22, 1286–1288. ( 10.3201/eid2207.160109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martel A, et al. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl Acad. Sci. USA 110, 15 325–15 329. ( 10.1073/pnas.1307356110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muletz C, Caruso NM, Fleischer RC, McDiarmid RW, Lips KR. 2014. Unexpected rarity of the pathogen Batrachochytrium dendrobatidis in Appalachian Plethodon salamanders: 1957–2011. PLoS ONE 9, e103728 ( 10.1371/journal.pone.0103728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weldon C, Du Preez LH, Hyatt AD, Muller R, Speare R. 2004. Origin of the amphibian chytrid fungus. Emerg. Infect. Dis 10, 2100–2105. ( 10.3201/eid1012.030804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gower DJ, et al. 2012. High prevalence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) across multiple taxa and localities in the highlands of Ethiopia. Herpetol. J. 22, 225–233. [Google Scholar]
- 64.Penner J, et al. 2013. West Africa - a safe haven for frogs? A subcontinental assessment of the chytrid fungus (Batrachochytrium dendrobatidis). PLoS ONE 8, e56236 ( 10.1371/journal.pone.0056236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirschfeld M, Blackburn DC, Doherty-Bone TM, Gonwouo LN, Ghose S, Rödel M-O. 2016. Dramatic declines of montane frogs in a central African biodiversity hotspot. PLoS ONE 11, e0155129 ( 10.1371/journal.pone.0155129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krajick K. 2006. Conservation biology – the lost world of the Kihansi toad. Science 311, 1230–1232. ( 10.1126/science.311.5765.1230) [DOI] [PubMed] [Google Scholar]
- 67.Swei A, et al. 2011. Is chytridiomycosis an emerging infectious disease in Asia? PLoS ONE 6, e23179 ( 10.1371/journal.pone.0023179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fong JJ, Cheng TL, Bataille A, Pessier AP, Waldman B, Vredenburg VT. 2015. Early 1900s detection of Batrachochytrium dendrobatidis in Korean amphibians. PLoS ONE 10, e0115656 ( 10.1371/journal.pone.0115656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goka K, et al. 2009. Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol. Ecol. 18, 4757–4774. ( 10.1111/j.1365-294X.2009.04384.x) [DOI] [PubMed] [Google Scholar]
- 70.Bataille A, Fong JJ, Cha M, Wogan GOU, Baek H-J, Lee H, Min M-S, Waldman B. 2013. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol. Ecol. 22, 4196–4209. ( 10.1111/mec.12385) [DOI] [PubMed] [Google Scholar]
- 71.Dahanukar N, Krutha K, Paingankar MS, Padhye AD, Modak N, Molur S. 2013. Endemic Asian chytrid strain infection in threatened and endemic anurans of the northern Western Ghats, India. PLoS ONE 8, e77528 ( 10.1371/journal.pone.0077528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL. 2000. Quantitative evidence for global amphibian population declines. Nature 404, 752–755. ( 10.1038/35008052) [DOI] [PubMed] [Google Scholar]
- 73.Bosch J, Martinez-Solano I, Garcia-Paris M. 2001. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 97, 331–337. ( 10.1016/S0006-3207(00)00132-4) [DOI] [Google Scholar]
- 74.Bosch J, Martínez-Solano I. 2006. Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the Peñalara Natural Park (Central Spain). Oryx. 40, 84–89. ( 10.1017/S0030605306000093) [DOI] [Google Scholar]
- 75.Bosch J, Carrascal JM, Durán L, Walker S, Fisher MC. 2007. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. R. Soc. B 274, 253–260. ( 10.1098/rspb.2006.3713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garner TWJ, Walker S, Bosch J, Hyatt AD, Cunningham AA, Fisher MC. 2005. Widespread European distribution of a global amphibian pathogen. Emerg. Infect. Dis. 11, 1639–1641. ( 10.3201/eid1110.050109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walker SF, et al. 2010. Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol. Letts. 13, 372–382. ( 10.1111/j.1461-0248.2009.01434.x) [DOI] [PubMed] [Google Scholar]
- 78.Tobler U, Borgula A, Schmidt BR. 2012. Populations of a susceptible amphibian species can grow despite the presence of a pathogenic chytrid fungus. PLoS ONE 7, e34667 ( 10.1371/journal.pone.0034667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canessa S, Martel A, Pasmans F. 2013. No detection of chytrid in first systematic screening of Bombina variegata pachypus (Anura: Bombinatoridae) in Liguria, northern Italy. Acta Herpetol. 8, 59–63. [Google Scholar]
- 80.Canestrelli D, Zampiglia M, Nascetti G. 2013. Widespread occurrence of Batrachochytrium dendrobatidis in contemporary and historical samples of the endangered Bombina pachypus along the Italian peninsula. PLoS ONE 8, e63349 ( 10.1371/journal.pone.0063349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spitzen-Van Der Sluijs A, Martel A, Hallmann CA, Bosman W, Garner TWJ, Van Rooij P, Jooris R, Haesebrouck F, Pasmans F. 2014. Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks: environmental determinants promote Bd endemism. Conserv. Biol. 28, 1302–1311. ( 10.1111/cobi.12281) [DOI] [PubMed] [Google Scholar]
- 82.Heyer WR, Rand AS, Cruz CAG, Peixoto OL. 1988. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica 20, 230–235. ( 10.2307/2388238) [DOI] [Google Scholar]
- 83.Rodriguez D, Becker CG, Pupin NC, Haddad CFB, Zamudio KR. 2014. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol. Ecol. 23, 774–787. ( 10.1111/mec.12615) [DOI] [PubMed] [Google Scholar]
- 84.Eterovick PC, Carnaval AC, Borges-Nojosa DM, Silvano DL, Segalla MV, Sazima I. 2005. Amphibian declines in Brazil: an overview. Biotropica 37, 166–179. ( 10.1111/j.1744-7429.2005.00024.x) [DOI] [Google Scholar]
- 85.Schloegel LM, et al. 2012. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol. Ecol. 21, 5162–5177. ( 10.1111/j.1365-294X.2012.05710.x) [DOI] [PubMed] [Google Scholar]
- 86.Jenkinson TS, et al. 2016. Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol. Ecol. 28, 1302–1311. ( 10.1111/mec.13599) [DOI] [PubMed] [Google Scholar]
- 87.Talley BL, Muletz C, Vredenburg VT, Fleischer R, Lips KR. 2015. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol. Conserv. 182, 254–261. ( 10.1016/j.biocon.2014.12.007) [DOI] [Google Scholar]
- 88.Adams MJ, et al. 2013. Trends in amphibian occupancy in the United States. PLoS ONE 8, e64347 ( 10.1371/journal.pone.0064347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grant EHC, et al. 2016. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 6, 25625 ( 10.1038/srep25625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Highton R. 2005. Declines of eastern North American woodland salamanders (Plethodon). In Status and conservation of U.S. amphibians (ed. Lannoo MJ.), pp. 34–46. Berkeley, CA: University of California Press. [Google Scholar]
- 91.Caruso NM, Lips KR. 2013. Truly enigmatic declines in terrestrial salamander populations in Great Smoky Mountains National Park. Divers. Distrib. 19, 38–48. ( 10.1111/j.1472-4642.2012.00938.x) [DOI] [Google Scholar]
- 92.Narayan E, Molinia F, Hero JM. 2011. Absence of invasive chytrid fungus (Batrachochytrium dendrobatidis) in native Fijian ground frog (Platymantis vitiana) populations on Viwa-Tailevu, Fiji Islands. Acta Herpetol. 6, 261–266. [Google Scholar]
- 93.Labisko J, et al. 2015. Chytrid fungus (Batrachochytrium dendrobatidis) undetected in the two orders of Seycehelles amphibians. Herpetol. Rev. 46, 41–45. [Google Scholar]
- 94.Schloegel LM, Picco A, Kilpatrick AM, Davies AJ, Hyatt A, Daszak P. 2009. Magnitude of the US trade in amphibians and presence of presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 142, 1420–1426. ( 10.1016/j.biocon.2009.02.007) [DOI] [Google Scholar]
- 95.Bletz MC, et al. 2015. Widespread presence of the pathogenic fungus Batrachochytrium dendrobatidis in wild amphibian communities in Madagascar. Sci. Rep. 5, 8633 ( 10.1038/srep08633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Savage AE, Zamudio KR. 2016. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc. R. Soc. B 283, 20153115 ( 10.1098/rspb.2015.3115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Savage AE, Terrell KA, Gratwicke B, Mattheus NM, Augustine L, Fleischer RC. 2016. Reduced immune function predicts disease susceptibility in frogs infected with a deadly fungal pathogen. Conserv. Physiol. 4, cow011. ( 10.1093/conphys/cow011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Searle CL, Biga LM, Spatafora JW, Blaustein AR. 2011. A dilution effect in the emerging amphibian pathogen, Batrachochytrium dendrobatidis. Proc. Natl Acad. Sci. USA 108, 16 322–16 326. ( 10.1073/pnas.1108490108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duellman WE. 1999. Patterns of distribution of amphibians: a global perspective. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 100.AmphibiaWeb. See http://amphibiaweb.org/ (accessed 17 April 2016).


